EBV in Hodgkin Lymphoma
Giuseppina Massini1, Doerte Siemer2 and Stefan Hohaus1
1Istituto di Ematologia, Universita’ Cattolica S. Cuore, Rome, Italy
2University of Duisburg-Essen, Medical School, Institute for Cell Biology (Tumor Research), Essen, Germany.
Correspondence
to:
Dr Stefan Hohaus, Istituto di Ematologia, Universita’ Cattolica S.
Cuore, L.go A. Gemelli, 1, 00168 ROMA, Tel: +39-06-30154180,
Fax:+39-06-35503777, E-mail: stefan.hohaus@rm.unicatt.it
Published: November 24, 2009
Received: October 06, 2009
Accepted: November 22, 2009
Medit J Hemat Infect Dis 2009, 1(2): e2009013 DOI 10.4084/MJHID.2009.013
This article is available from: http://www.mjhid.org/article/view/5114
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Up
to 40% of Hodgkin lymphoma (HL) cases are associated with the
Epstein-Barr virus (EBV). Clonal viral genomes can be found in the HL
tumor cells, the Hodgkin Reed-Sternberg cells (HRS). The latent
infection results in expression of the viral oncogenes LMP1 and LMP2A
which contribute to generate the particular phenotype of the HRS cells.
EBV does not only undergo epigenetic changes of its genome during
latency, but also induces epigenetic changes in the host genome. The
presence of EBV may alter the composition and activity of the immune
cells surrounding the HRS cells. EBV favours a Th1 reaction, but
this attempt at a cell mediated immune response appears to be
ineffective. The presence of EBV in HL is associated with several
clinicopathological characteristics: It is more frequent in cases with
mixed cellular histology, in males, in children and older adults, and
in developing countries, while the young-adult onset HL of nodular
sclerosis type in industrialized countries is typically EBV-negative.
Countries in the Mediterranean area often show an intermediate
epidemiological pattern. Recent studies suggest a genetic
predisposition to develop EBV-associated HL. Circulating EBV-DNA may
serve as a biomarker to monitor response to therapy, and eventually,
EBV will become a target for therapeutic intervention also in HL.
Introduction
Since
its first description by Sir Thomas Hodgkin in 1832, the nature and
cellular origin of Hodgkin’s disease has been an enigma. There was a
long-lasting controversy as to whether Hodgkin’s disease was a
malignant, inflammatory or infectious disease. Hodgkin’s disease is
characterized by a rare population of Hodgkin Reed-Sternberg (HRS)
cells which are surrounded by a massive inflammatory infiltrate. The
paucity of the HRS cells hampered their biological characterization [1].
In the 1990s, finally, amplification of immunoglobulin genes by the polymerase chain reaction from isolated HRS cells helped to clarify the nature of the HRS cells [2]. Clonally related rearrangements of Ig VH genes carrying a high load of somatic mutations indicated an origin of the HRS cells from germinal center B lymphocytes [3]. Recently, a small clonotypic B cell in the peripheral blood of HL patients was identified raising the question for a HRS precursor cell population4. These biological studies led to the current consensus that the HRS cells in Hodgkin’s disease are neoplastic, and now, the term Hodgkin lymphoma (HL) instead of Hodgkin’s disease is recommended [5].
An infectious aetiology has long been suspected in HL, and so far, Epstein-Barr virus (EBV) is the only candidate for the infectious agent causing HL. There are several lines of evidence linking EBV to the aetiology of some HL: the biological plausibility of EBV-mediated B cell transformation and presence of clonal EBV genomes within HL tumor cells, which implies that infection occurred before malignant transformation, epidemiologic associations with infectious mononucleosis (IM), representing symptomatic primary EBV infection, distinctive EBV antibody titer profiles and viral loads both pre- and post-HL diagnosis, and differing demographic, clinical, and epidemiologic characteristics of EBV+ and EBV- HL. Together this evidence strongly suggests that these virally defined variants of HL are distinct entities and that their pathogenesis should be considered separately. However, only a varying proportion of HL cases are EBV-associated. Despite extensive research, no other infectious agent has been so far identified [6-8].
The large majority of Hodgkin lymphoma arising in the setting of HIV infection are pathogenetically linked to EBV, with rates of EBV positivity ranging from 80 to 100% [9]. The distinctive features HIV-related HL will not be discussed in this review, as it is the topic of another review in this issue of the journal.
This review will discuss the role of EBV in the pathogenesis of HL, the impact of EBV on immunological and clinical characteristics, the epidemiological evidences for a role of EBV in HL, and potential clinical applications. There have been excellent reviews on this topic, and therefore, we will focus on data from more recent publications [10-16].
The role of EBV in transforming B cells to HRS cells: EBV or human herpes virus 4 is closely associated with the GC B cell malignancies Burkitt lymphoma, post transplantation lymphomas and HL. HL is one of the most frequent lymphomas in the western world and can be divided into lymphocyte predominant (LP-HL) and classical (cHL) including mixed cellularity, nodular sclerosis and lymphocyte-rich subtypes [17]. Most cases of cHL and LP-HL carry clonal somatically mutated Ig V-gene rearrangement. Characteristically HRS cells make only 1% of the tumor mass and show a strong NF-B overexpression18-2). HRS cells of HL have lost the expression of typical B cell lineage genes and instead show a strong expression of signaling molecules and transcription factors of other cell types [22,23]. About 40% of HL cases are infected with EBV. Clonal viral genomes can be found in all of the tumor cells and the virus remains in the malignant cells during the whole course of disease [24,25].
EBV preferentially infects human B cells, in which it mainly persists as a harmless passenger. Over 90% of people worldwide are latently infected with the virus [14]. In early childhood infection with EBV usually remains asymptomatic and leads to a lifelong persistency of the virus within the B cell compartment with 1 - 10 of 106 B cells being latently-infected [26-28]. However, delayed infection in puberty or early adulthood can cause the self-limiting disease “infectious mononucleosis (IM)” [29,30]. In addition, EBV was originally identified in a Burkitt lymphoma biopsy and therefore, was described as the first human tumor virus [31]. Tumorigenic properties are demonstrated by the transformation of normal B cells into immortal lymphoblastoid cell lines (LCLs) after in vitro infection with EBV [32]. EBV can also infect epithelial cells, which likely plays a role in virus propagation and release into the saliva [33,34].
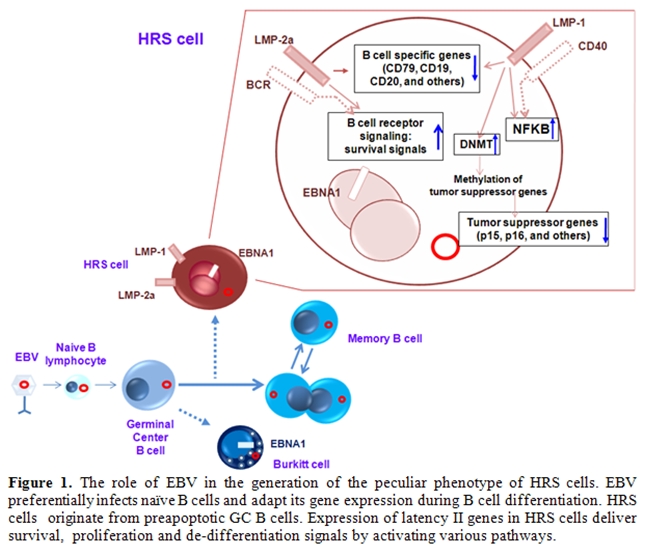
After infection of B cells the 172 kbp viral DNA genome circularizes to an episome in the nucleus and is subsequently amplified to 10-100 copies per cell [35,36]. (Figure 1).
In the 1990s, finally, amplification of immunoglobulin genes by the polymerase chain reaction from isolated HRS cells helped to clarify the nature of the HRS cells [2]. Clonally related rearrangements of Ig VH genes carrying a high load of somatic mutations indicated an origin of the HRS cells from germinal center B lymphocytes [3]. Recently, a small clonotypic B cell in the peripheral blood of HL patients was identified raising the question for a HRS precursor cell population4. These biological studies led to the current consensus that the HRS cells in Hodgkin’s disease are neoplastic, and now, the term Hodgkin lymphoma (HL) instead of Hodgkin’s disease is recommended [5].
An infectious aetiology has long been suspected in HL, and so far, Epstein-Barr virus (EBV) is the only candidate for the infectious agent causing HL. There are several lines of evidence linking EBV to the aetiology of some HL: the biological plausibility of EBV-mediated B cell transformation and presence of clonal EBV genomes within HL tumor cells, which implies that infection occurred before malignant transformation, epidemiologic associations with infectious mononucleosis (IM), representing symptomatic primary EBV infection, distinctive EBV antibody titer profiles and viral loads both pre- and post-HL diagnosis, and differing demographic, clinical, and epidemiologic characteristics of EBV+ and EBV- HL. Together this evidence strongly suggests that these virally defined variants of HL are distinct entities and that their pathogenesis should be considered separately. However, only a varying proportion of HL cases are EBV-associated. Despite extensive research, no other infectious agent has been so far identified [6-8].
The large majority of Hodgkin lymphoma arising in the setting of HIV infection are pathogenetically linked to EBV, with rates of EBV positivity ranging from 80 to 100% [9]. The distinctive features HIV-related HL will not be discussed in this review, as it is the topic of another review in this issue of the journal.
This review will discuss the role of EBV in the pathogenesis of HL, the impact of EBV on immunological and clinical characteristics, the epidemiological evidences for a role of EBV in HL, and potential clinical applications. There have been excellent reviews on this topic, and therefore, we will focus on data from more recent publications [10-16].
The role of EBV in transforming B cells to HRS cells: EBV or human herpes virus 4 is closely associated with the GC B cell malignancies Burkitt lymphoma, post transplantation lymphomas and HL. HL is one of the most frequent lymphomas in the western world and can be divided into lymphocyte predominant (LP-HL) and classical (cHL) including mixed cellularity, nodular sclerosis and lymphocyte-rich subtypes [17]. Most cases of cHL and LP-HL carry clonal somatically mutated Ig V-gene rearrangement. Characteristically HRS cells make only 1% of the tumor mass and show a strong NF-B overexpression18-2). HRS cells of HL have lost the expression of typical B cell lineage genes and instead show a strong expression of signaling molecules and transcription factors of other cell types [22,23]. About 40% of HL cases are infected with EBV. Clonal viral genomes can be found in all of the tumor cells and the virus remains in the malignant cells during the whole course of disease [24,25].
EBV preferentially infects human B cells, in which it mainly persists as a harmless passenger. Over 90% of people worldwide are latently infected with the virus [14]. In early childhood infection with EBV usually remains asymptomatic and leads to a lifelong persistency of the virus within the B cell compartment with 1 - 10 of 106 B cells being latently-infected [26-28]. However, delayed infection in puberty or early adulthood can cause the self-limiting disease “infectious mononucleosis (IM)” [29,30]. In addition, EBV was originally identified in a Burkitt lymphoma biopsy and therefore, was described as the first human tumor virus [31]. Tumorigenic properties are demonstrated by the transformation of normal B cells into immortal lymphoblastoid cell lines (LCLs) after in vitro infection with EBV [32]. EBV can also infect epithelial cells, which likely plays a role in virus propagation and release into the saliva [33,34].
After infection of B cells the 172 kbp viral DNA genome circularizes to an episome in the nucleus and is subsequently amplified to 10-100 copies per cell [35,36]. (Figure 1).

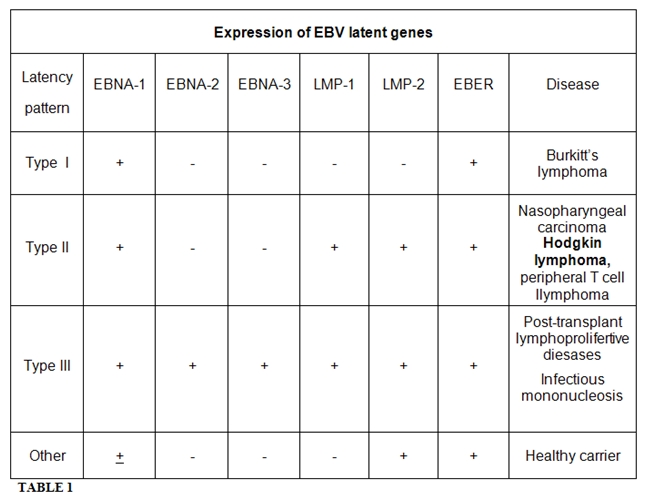
EBV can express different
latency programs and thereby shows a very high adaptation to the B cell
physiology (Table 1).

The first latency program to be
expressed after B cell infection is
latency III [37]. Here the dependency of the viral
latency III promotor
for the B cell specific transcription factor PAX5 guarantees B cell
specificity of the virus [38]. In latency III the
latent membrane proteins
LMP1, LMP2A and LMP2B, the nuclear proteins EBNA1, -2, -3A, -3B, -3C
and EBNA-LP as well as two non-polyadenylated RNAs, EBER1 and EBER2 and
the BART RNAs are expressed. In latency II only LMP1, LMP2A and -2B,
EBNA1 and the EBER and BART transcripts are expressed [14].
Latency I is
characterized by the expression of EBER and BART transcripts as well as
of EBNA1 as the only latent EBV protein. Latency I is mainly found in
Burkitt lymphoma [14]. In latency 0 only the EBER
and BART transcripts and
sometimes LMP2A are expressed [14,39-42].
It is speculated that EBV
preferentially infects naïve B cells that get activated and pass
through a normal germinal center (GC) reaction. During this
differentiation process latency programs change from latency III to
eventually latency 0 in the long-living memory B cell population [43].
Thereby, EBV can persist in memory B cells unrecognizable by cytotoxic
T cells41. However, this scenario of EBV persistency remains
controversely discussed. In IM tonsils EBV infected B cell clones do
not participate in the GC reaction [44,45]. In
addition, histological
stainings of GC in EBV persistency only rarely showed EBV + B cells
within GC [46,47].
It is believed that the tumor cells in cHL originate from preapoptotic GC B cells. This is supported by the finding that in 25% of cHL cases the HRS cells carry B cell receptor (BCR)-destructive mutations in originally functional V-gene rearrangements [3]. Normally GC B cells that acquire BCR-destructive mutations or mutations that decrease the affinity towards the antigen during affinity maturation in the GC die quickly by apoptosis [48,49]. However, in cHL BCR-deficient tumor cell precursor GC B cells survive due to a transformation event. This transformation event likely is the infection with EBV, since it was shown by in vitro infection of GC B cells that EBV can rescue BCR-deficient GC B cells from apoptosis and transform them into long-lived cell lines [50-52]. This rescue is thought to be due to the expression of the BCR-mimic LMP2A that is also expressed in EBV+ HRS cells [53]. In addition, Mancao and Hammerschmidt showed that in long-term cultures LMP2A replaces the BCR-signal and BCR+ cells even become dependent on the expression of LMP2A [54]. The rescue of BCR-deficient GC B cells by EBV is further supported by the fact that the BCR-deficient HL cases are all EBV+, whereas in total only 40% of HL shows an association with EBV [17]. The role of EBV LMP2A in HL pathogenesis likely confers to early lymphomagenesis, where the signaling molecules of LMP2A and BCR were still expressed.
The transcription factor NF-B seems to play a major role in the rescue of HRS precursor cells. NF-B activates the expression of the anti-apoptotic DISC-inhibitor c-FLIP and induces the expression of pro-inflammatory cytokines [55-57]. In EBV+ HL the virus displays a latency II gene expression pattern, including the expression of LMP1, LMP2A and EBNA1. LMP1 mimics a constitutively active CD40 receptor, thereby acting as a strong activator of NF-B, and is presumably responsible for the constitutive NF-B expression in EBV+ HL cases [23,58-60]. Until recently the cause of the NF-B overexpression in the EBV- HL cases was largely unknown. Genetic aberrations of the NF-B inhibitors IB and IB and amplifications of c-rel could be found in only some cases [61-65]. Recently Schmitz and colleagues showed that the constitutive activity of NF-B in about 50% of primary EBV- cHL is likely caused by inactivating mutations in both of the A20 tumor suppressor gene alleles [66]. A20 inactivating mutations exclusively in EBV- HL cases seem to replace the transforming and NF-B-activating role of LMP1 in EBV+ HL cases and demonstrate the essential role of EBV in the pathogenesis of EBV+ HL.
Recent papers addressed the effect of EBV gene products on the expression of B cell-specific differentiation antigens. Vockerodt and colleagues used a non-viral vector-based method to express LMP1 in primary human GC B cells [67]. Gene expression profiling revealed that LMP1 induced in GC B cells transcriptional changes characteristic of HL cell lines. Strikingly, LMP1 down-regulated the expression of B-cell-specific genes including B-cell receptor components such as CD79A, CD79B, CD19, CD20, CD22, and BLNK. LMP1 also induced the expression of ID2, a negative regulator of B-cell differentiation. As well, expression of LMP2A as a transgene resulted in global down-regulation of gene transcription necessary for proper B-cell development [68].
A tissue microarray analysis on 288 HL biopsies showed a specific gene expression profile for EBV+ cases. The presence of EBV correlated strongly with the expression of STAT1 and STAT3, but inversely with the expression of cyclin E, CDK6, p27, p53, HDM2, and BCL-XL [69].
EBV and epigenetic changes: EBV may modulate cellular gene expression also by induction of epigenetic changes which result in inducing gene silencing [70,71]. LMP1 is able to induce the expression of the DNA methyltransferases DNMT1, 3A, and 3B in latently infected cells [72]. In nasopharyngeal carcinoma (NPC) cells, LMP1 activates DNMT1 through the activation of c-Jun NH2-terminal kinase (JNK)-activator protein-1 (AP-1) signaling [73]. Seo and colleagues also suggested a role for the Rb-E2F pathway in LMP1-induced DNMT1 activation in NPC cells [74].
Modulation of the host methylation system may have implications for the pathogenesis of EBV-associated tumors. For gastric carcinomas, it has been reported that the methylation frequency of tumor suppressor genes is much higher in EBV+ than EBV- cases, indicating a possibility that EBV induces the hypermethylation of cellular genes critical to tumor pathogenesis. Interestingly, activation of DNMT1 appears not to be limited to EBV-induced tumorigenesis, but it was recently suggested to play an essential role in aberrant de novo methylation in various tumors which are associated with viral infection, such as tumors infected with the human papillomavirus-16 or the hepatitis B virus [75,76].
The EBV genome undergoes changes in CpG methylation which appear to play an important role in regulating viral latency and limiting viral gene expression in normal lymphocytes and in tumors [77,78]. The EBV genome is highly unmethylated in infectious virions and in latency III. During B cell transformation, the viral genome is increasingly methylated upon cell propagation, which may reflect the in vivo situation of a transition from an EBV-infected lymphoblastoid proliferating B cell to latently infected B-cells (latency II, I or 0) in normal lymphoid tissues in healthy individuals [79]. In normal lymphocytes and tumors from immune competent patients, some of the EBV latent promoters need to be downregulated, in order to silence or limit viral immunodominant gene expression and thus evade the host immune surveillance [78]. A complex transcriptional regulation of these EBV genes allows the virus to persist in the host with or without a potent immune response. Methylation of EBV genes is achieved by taking advantage of the host cell DNA methylation system. Treatment with demethylating agents such as the DNA methyltransferase inhibitor 5-azacytidine can reactivate the transcription of methylated EBV genes along with the demethylation of their promoters [80]. This may have implications for EBV-directed therapeutic approaches.
Interaction of EBV + - HRS cells with the microenvironment and the immune system: The clinical and pathologic features of cHL reflect an abnormal immune response that is thought to be due to the elaboration of a variety of cytokines by the malignant Reed-Sternberg (RS) cells or surrounding tissues [81]. The majority of cHL cases are characterized by expression of tumor necrosis factor receptor (TNFR) family members and their ligands, as well as an unbalanced production of Th2 cytokines and chemokines. The production of cytokines play a pivotal role in the immunopathogenesis of HL, as these factors can act both as autocrine growth factors or as factors initiating and sustaining the reactive infiltrate. In addition, cytokines produced by the surrounding cells of the microenvironment may contribute to stimulate HRS cell survival and proliferation.
The presence of EBV may alter the expression of cytokines and chemokines. In fact, EBV favors a Th1 reaction in the HL microenvironment. The expression of IL-12, which is responsible for Th1-cell differentiation, and chemokines that support a Th1 response (IP-10, Mig, MIP-1) are expressed at higher levels in EBV+ HL cases than in EBV- cases [82]. Accordingly, CD8+ T cells are more numerous in the reactive infiltrate of EBV+ cases. However, this attempt at a cell mediated immune response in EBV+ cases appears to be ineffective, because there is a local suppression of cytotoxic T cells specifically targeting EBV antigens. This suppression may be due to the presence of IL-10, a potent anti-inflammatory cytokine frequently produced by RS cells in EBV+ cHL cases. LMP1 can induce cellular IL-10 expression in EBV+ cells [83]. Herling et al, studying 577 HL patients, reported higher IL-10 levels in EBV+ cases [84].
IL-6 is another cytokine whose expression has been reported to be increased in EBV+ tumors [85]. However, associations between cytokine levels in peripheral blood and EBV tumor status have not always produced unequivocal correlations [86,87].
A recent study demonstrated an increased expression of CCL20, a chemokine capable of attracting Treg cells, in the microenvironment of EBV+ versus EBV- RS cells [88]. Another cytokine whose expression has been reported to be increased in EBV+ tumors is autotoxin [89]. Autotaxin is an autocrine motility factor with lysophospholipase-D activity generating lysophosphatidic acid (LPA) which enhances growth and survival of HL cells, whereas specific down-regulation of autotaxin decreased LPA levels and reduced cell growth and viability.
The development of molecular profiling techniques made it possible to establish more comprehensive gene expression patterns of EBV+ HL tissues. EBV+ tumors are characterized by a robust gene signature involving innate immunity and antiviral responses [90]. The molecular profiling confirms that EBV favors a Th1 reaction with simultaneous overexpression of IFN, CXCL9, CXCL10, and CXCL11/ITAC and observes an antiviral response with overexpression of genes such as IVNS1ABP (NS1BP), PLSCR1, and OAS.
Histopathological differences between EBV+ and EBV- HL: Weiss et al were the first to demonstrate the presence of EBV DNA in HL tissue specimens using the cloned BamHI-W fragment of EBV, as a probe for in-situ hybridization (ISH) [25]. The subsequent development of ISH targeting the highly abundant EBERs provided a reliable and simple method for the detection of EBV in archival HL specimens [91]. HL can be classified into two clinicopathological entities: the nodular lymphocyte predominant type (NLPHL) which represents 5% of cases and are virtually never associated with EBV, and the classical HL, which can be further subdivided in four morphological subtypes: nodular sclerosis (NSHL) which accounts for the majority of cases, mixed cellularity (MCHL), lymphocyte-rich (LRHL) and lymphocyte-depleted (LDHL). As LHRL and LDHL each comprise less than 5 % of cases of HL, most of the data on EBV are derived from studies on NSHL and MCHL. There is a strong association between EBV-positivity and histologic subtype: cases of MCHL are EBV + in about 75 %, whereas the cases of NSHL are positive in less than 20%.
EBV genome products was shown to be clonal in the neoplastic Hodgkin-Reed-Sternberg (HRS) cells25. Its presence is not associated with immunophenotypic characteristics of the HRS cells.
Epidemiological Aspects of EBV+ HL: The complex epidemiology of HL suggests a multifactorial aetiology. Interactions between genetic and environmental factors are probable. The contribution of EBV to HL aetiology differs according to age group and geographic origin [92-94].
The individual’s age at the time of primary EBV-infection in combination with his genetic background appears to be important factors for the clinical manifestations of the infection. When primary infection with the virus occurs in early childhood, as is typical of developing countries, it is accompanied by, few if, any symptoms. If primary infection in contrast is delayed to adolescence, as often is seen in industrialized countries, it is associated with the clinical syndrome, infectious mononucleosis.
A wealth of cohort and case–control studies has shown that mononucleosis is followed by an increased risk of HL [95]. In the largest study to date, a Scandinavian cohort study of 38000 mononucleosis patients, mononucleosis was associated with a more than 2.5-fold increased HL risk, which although it decreased with time remained significantly elevated for up to two decades [96]. Moreover, because mononucleosis typically occurs in adolescence, HL risk was particularly high, 3.5-fold increased, in young adults. Paradoxically, EBV is particularly infrequently encountered in HL in young adults, the age group for which the IM association is the strongest. It appears, that earlier exposure to EBV protects against development of adolescent mononucleosis, and as a consequence protects against young-adult onset EBV+ HL.
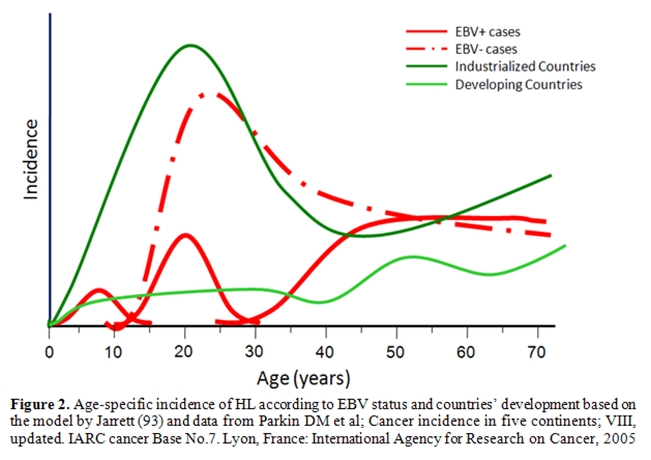
At variance from the typical bimodal age distribution of HL in most developed nations, with an initial peak occurring among young adults aged 15 to 39 years, in developing nations, the first peak occurs earlier, among children under age 15 years. (Figure 2). HL among children in developing countries are characterized by a particularly high proportion of EBV+ cases.
It is believed that the tumor cells in cHL originate from preapoptotic GC B cells. This is supported by the finding that in 25% of cHL cases the HRS cells carry B cell receptor (BCR)-destructive mutations in originally functional V-gene rearrangements [3]. Normally GC B cells that acquire BCR-destructive mutations or mutations that decrease the affinity towards the antigen during affinity maturation in the GC die quickly by apoptosis [48,49]. However, in cHL BCR-deficient tumor cell precursor GC B cells survive due to a transformation event. This transformation event likely is the infection with EBV, since it was shown by in vitro infection of GC B cells that EBV can rescue BCR-deficient GC B cells from apoptosis and transform them into long-lived cell lines [50-52]. This rescue is thought to be due to the expression of the BCR-mimic LMP2A that is also expressed in EBV+ HRS cells [53]. In addition, Mancao and Hammerschmidt showed that in long-term cultures LMP2A replaces the BCR-signal and BCR+ cells even become dependent on the expression of LMP2A [54]. The rescue of BCR-deficient GC B cells by EBV is further supported by the fact that the BCR-deficient HL cases are all EBV+, whereas in total only 40% of HL shows an association with EBV [17]. The role of EBV LMP2A in HL pathogenesis likely confers to early lymphomagenesis, where the signaling molecules of LMP2A and BCR were still expressed.
The transcription factor NF-B seems to play a major role in the rescue of HRS precursor cells. NF-B activates the expression of the anti-apoptotic DISC-inhibitor c-FLIP and induces the expression of pro-inflammatory cytokines [55-57]. In EBV+ HL the virus displays a latency II gene expression pattern, including the expression of LMP1, LMP2A and EBNA1. LMP1 mimics a constitutively active CD40 receptor, thereby acting as a strong activator of NF-B, and is presumably responsible for the constitutive NF-B expression in EBV+ HL cases [23,58-60]. Until recently the cause of the NF-B overexpression in the EBV- HL cases was largely unknown. Genetic aberrations of the NF-B inhibitors IB and IB and amplifications of c-rel could be found in only some cases [61-65]. Recently Schmitz and colleagues showed that the constitutive activity of NF-B in about 50% of primary EBV- cHL is likely caused by inactivating mutations in both of the A20 tumor suppressor gene alleles [66]. A20 inactivating mutations exclusively in EBV- HL cases seem to replace the transforming and NF-B-activating role of LMP1 in EBV+ HL cases and demonstrate the essential role of EBV in the pathogenesis of EBV+ HL.
Recent papers addressed the effect of EBV gene products on the expression of B cell-specific differentiation antigens. Vockerodt and colleagues used a non-viral vector-based method to express LMP1 in primary human GC B cells [67]. Gene expression profiling revealed that LMP1 induced in GC B cells transcriptional changes characteristic of HL cell lines. Strikingly, LMP1 down-regulated the expression of B-cell-specific genes including B-cell receptor components such as CD79A, CD79B, CD19, CD20, CD22, and BLNK. LMP1 also induced the expression of ID2, a negative regulator of B-cell differentiation. As well, expression of LMP2A as a transgene resulted in global down-regulation of gene transcription necessary for proper B-cell development [68].
A tissue microarray analysis on 288 HL biopsies showed a specific gene expression profile for EBV+ cases. The presence of EBV correlated strongly with the expression of STAT1 and STAT3, but inversely with the expression of cyclin E, CDK6, p27, p53, HDM2, and BCL-XL [69].
EBV and epigenetic changes: EBV may modulate cellular gene expression also by induction of epigenetic changes which result in inducing gene silencing [70,71]. LMP1 is able to induce the expression of the DNA methyltransferases DNMT1, 3A, and 3B in latently infected cells [72]. In nasopharyngeal carcinoma (NPC) cells, LMP1 activates DNMT1 through the activation of c-Jun NH2-terminal kinase (JNK)-activator protein-1 (AP-1) signaling [73]. Seo and colleagues also suggested a role for the Rb-E2F pathway in LMP1-induced DNMT1 activation in NPC cells [74].
Modulation of the host methylation system may have implications for the pathogenesis of EBV-associated tumors. For gastric carcinomas, it has been reported that the methylation frequency of tumor suppressor genes is much higher in EBV+ than EBV- cases, indicating a possibility that EBV induces the hypermethylation of cellular genes critical to tumor pathogenesis. Interestingly, activation of DNMT1 appears not to be limited to EBV-induced tumorigenesis, but it was recently suggested to play an essential role in aberrant de novo methylation in various tumors which are associated with viral infection, such as tumors infected with the human papillomavirus-16 or the hepatitis B virus [75,76].
The EBV genome undergoes changes in CpG methylation which appear to play an important role in regulating viral latency and limiting viral gene expression in normal lymphocytes and in tumors [77,78]. The EBV genome is highly unmethylated in infectious virions and in latency III. During B cell transformation, the viral genome is increasingly methylated upon cell propagation, which may reflect the in vivo situation of a transition from an EBV-infected lymphoblastoid proliferating B cell to latently infected B-cells (latency II, I or 0) in normal lymphoid tissues in healthy individuals [79]. In normal lymphocytes and tumors from immune competent patients, some of the EBV latent promoters need to be downregulated, in order to silence or limit viral immunodominant gene expression and thus evade the host immune surveillance [78]. A complex transcriptional regulation of these EBV genes allows the virus to persist in the host with or without a potent immune response. Methylation of EBV genes is achieved by taking advantage of the host cell DNA methylation system. Treatment with demethylating agents such as the DNA methyltransferase inhibitor 5-azacytidine can reactivate the transcription of methylated EBV genes along with the demethylation of their promoters [80]. This may have implications for EBV-directed therapeutic approaches.
Interaction of EBV + - HRS cells with the microenvironment and the immune system: The clinical and pathologic features of cHL reflect an abnormal immune response that is thought to be due to the elaboration of a variety of cytokines by the malignant Reed-Sternberg (RS) cells or surrounding tissues [81]. The majority of cHL cases are characterized by expression of tumor necrosis factor receptor (TNFR) family members and their ligands, as well as an unbalanced production of Th2 cytokines and chemokines. The production of cytokines play a pivotal role in the immunopathogenesis of HL, as these factors can act both as autocrine growth factors or as factors initiating and sustaining the reactive infiltrate. In addition, cytokines produced by the surrounding cells of the microenvironment may contribute to stimulate HRS cell survival and proliferation.
The presence of EBV may alter the expression of cytokines and chemokines. In fact, EBV favors a Th1 reaction in the HL microenvironment. The expression of IL-12, which is responsible for Th1-cell differentiation, and chemokines that support a Th1 response (IP-10, Mig, MIP-1) are expressed at higher levels in EBV+ HL cases than in EBV- cases [82]. Accordingly, CD8+ T cells are more numerous in the reactive infiltrate of EBV+ cases. However, this attempt at a cell mediated immune response in EBV+ cases appears to be ineffective, because there is a local suppression of cytotoxic T cells specifically targeting EBV antigens. This suppression may be due to the presence of IL-10, a potent anti-inflammatory cytokine frequently produced by RS cells in EBV+ cHL cases. LMP1 can induce cellular IL-10 expression in EBV+ cells [83]. Herling et al, studying 577 HL patients, reported higher IL-10 levels in EBV+ cases [84].
IL-6 is another cytokine whose expression has been reported to be increased in EBV+ tumors [85]. However, associations between cytokine levels in peripheral blood and EBV tumor status have not always produced unequivocal correlations [86,87].
A recent study demonstrated an increased expression of CCL20, a chemokine capable of attracting Treg cells, in the microenvironment of EBV+ versus EBV- RS cells [88]. Another cytokine whose expression has been reported to be increased in EBV+ tumors is autotoxin [89]. Autotaxin is an autocrine motility factor with lysophospholipase-D activity generating lysophosphatidic acid (LPA) which enhances growth and survival of HL cells, whereas specific down-regulation of autotaxin decreased LPA levels and reduced cell growth and viability.
The development of molecular profiling techniques made it possible to establish more comprehensive gene expression patterns of EBV+ HL tissues. EBV+ tumors are characterized by a robust gene signature involving innate immunity and antiviral responses [90]. The molecular profiling confirms that EBV favors a Th1 reaction with simultaneous overexpression of IFN, CXCL9, CXCL10, and CXCL11/ITAC and observes an antiviral response with overexpression of genes such as IVNS1ABP (NS1BP), PLSCR1, and OAS.
Histopathological differences between EBV+ and EBV- HL: Weiss et al were the first to demonstrate the presence of EBV DNA in HL tissue specimens using the cloned BamHI-W fragment of EBV, as a probe for in-situ hybridization (ISH) [25]. The subsequent development of ISH targeting the highly abundant EBERs provided a reliable and simple method for the detection of EBV in archival HL specimens [91]. HL can be classified into two clinicopathological entities: the nodular lymphocyte predominant type (NLPHL) which represents 5% of cases and are virtually never associated with EBV, and the classical HL, which can be further subdivided in four morphological subtypes: nodular sclerosis (NSHL) which accounts for the majority of cases, mixed cellularity (MCHL), lymphocyte-rich (LRHL) and lymphocyte-depleted (LDHL). As LHRL and LDHL each comprise less than 5 % of cases of HL, most of the data on EBV are derived from studies on NSHL and MCHL. There is a strong association between EBV-positivity and histologic subtype: cases of MCHL are EBV + in about 75 %, whereas the cases of NSHL are positive in less than 20%.
EBV genome products was shown to be clonal in the neoplastic Hodgkin-Reed-Sternberg (HRS) cells25. Its presence is not associated with immunophenotypic characteristics of the HRS cells.
Epidemiological Aspects of EBV+ HL: The complex epidemiology of HL suggests a multifactorial aetiology. Interactions between genetic and environmental factors are probable. The contribution of EBV to HL aetiology differs according to age group and geographic origin [92-94].
The individual’s age at the time of primary EBV-infection in combination with his genetic background appears to be important factors for the clinical manifestations of the infection. When primary infection with the virus occurs in early childhood, as is typical of developing countries, it is accompanied by, few if, any symptoms. If primary infection in contrast is delayed to adolescence, as often is seen in industrialized countries, it is associated with the clinical syndrome, infectious mononucleosis.
A wealth of cohort and case–control studies has shown that mononucleosis is followed by an increased risk of HL [95]. In the largest study to date, a Scandinavian cohort study of 38000 mononucleosis patients, mononucleosis was associated with a more than 2.5-fold increased HL risk, which although it decreased with time remained significantly elevated for up to two decades [96]. Moreover, because mononucleosis typically occurs in adolescence, HL risk was particularly high, 3.5-fold increased, in young adults. Paradoxically, EBV is particularly infrequently encountered in HL in young adults, the age group for which the IM association is the strongest. It appears, that earlier exposure to EBV protects against development of adolescent mononucleosis, and as a consequence protects against young-adult onset EBV+ HL.
At variance from the typical bimodal age distribution of HL in most developed nations, with an initial peak occurring among young adults aged 15 to 39 years, in developing nations, the first peak occurs earlier, among children under age 15 years. (Figure 2). HL among children in developing countries are characterized by a particularly high proportion of EBV+ cases.

With respect to geographic
variation, EBV rates in HL tumors from North
America and Europe have been reported to vary between 20-50%,
while several studies from Central and South America showed an
incidence rates varying from 50 to 95% [97-99]. In a
report from China,
evidence of EBV expression in cases of HL reached 65% [100],
and in a
large series from Kenya, EBV was detected in 92% of HL cases [101].
A study on 277 biopsies from 10 countries revealed a particular high frequency of EBV-positivity (80-100%) in childhood HL from Kenya, Costa Rica, Iran, but also from Greece, while EBV was present in about 50% of pediatric HL cases from Egypt, Jordan, South Africa and the United Kingdom [102]. A case series of childhood HL form Southeast Turkey showed the typical prevalence of male sex, mixed cellularity and EBV positivity [103].
The pattern of HL in Israel is intermediate between the bimodal pattern of Western countries and the pediatric peak seen in developing countries. Patients born in Europe had a slightly lower rate of positivity to EBV (21.8%). This rate was higher in patients from Asia and Africa (27%). In contrast to these groups of Jews, the Bedouin patients, although representing a small group, showed a 66.7% rate of EBV infection [104]. Studies form the middle East indicate incidence rates of EBV in HL varying form 38-56 % with patterns of early-industrialized countries [105,106]. These epidemiologic studies led to the proposal of a model by Jarrett et al that EBV-associated HL cases can be divided into three groups according to age at exposure to EBV and age at HL diagnosis [93]. This model recognises a childhood group, accounting for almost all cases of HL in early childhood, a young adult group, which epidemiological data suggest is associated with delayed exposure to EBV, and an older adult group, which might result from loss of the normal balance between latent EBV infection and host immunity.
Three epidemiological patterns can be discerned. The type I pattern which is prevalent in developing countries, shows a relatively high incidence in male children, a low incidence in the third decade and a second peak of high incidence in older age groups. Type III, which is usually seen in developed countries, is characterised by a low rate in children and a pronounced initial peak in young adults. The third pattern (Type II), which is described in many Asian countries, is intermediate and reflects a transition between types I and III. In this pattern there is both a childhood and a third decade peak.
An interesting question is whether the socioeconomic progress will change the incidence patterns of HL in developing countries. Hjalgrim and colleagues analysed HL incidence patterns in Singapore between 1968 and 2004, during which time period a socioeconomic transition towards Western World lifestyles took place [107]. A HL incidence peak emerged among adolescents and young adults in Singapore with annual increase rates up to 13.7%, in particular in females. However, the incidence peak remained considerably lower than what can be observed in young adults in the Western World. It remains to be determined to what extent the current lower incidence of HL in young Asian adults should be attributed to birth cohort phenomena, as would be suggested by continued increase in incidence, and to ethnic variation in HL susceptibility between Asian and non-Asian populations, respectively.
The impact of socioeconomic and racial factors on the risk to develop EBV+ HL was studied by Glaser et al in a Californian population [108]. Tumor EBV-positivity was associated with Hispanic and Asian/Pacific Islander (API) but not black race/ethnicity, irrespective of demographic and clinical factors. In Hispanics, EBV+ HL was associated not only with young and older age, male sex, and mixed cellularity histology, but also with foreign birth and lower neighbourhood socioeconomic status in females. The racial/ethnic variation suggests that EBV+ HL results from an intricate interplay of early- and later-life environmental, hormonal, and genetic factors leading to depressed immune function and poorly controlled EBV infection.
Genetic predisposition to develop EBV+ HL: Genetic predisposition to develop EBV+ HL is supported by the association of EBV+ HL with the highly polymorphic human leukocyte antigen (HLA) genes, which vary by racial/ethnic group. Genetic association of EBV+ HL was found with the HLA class I region, including the HLA-A gene [109,110]. HLA-A*02 was underrepresented in patients with EBV+ HL (15%), and HLA-A*01 was overrepresented in patients with EBV+ HL (37.1%). These data may suggest functional differences in the HLA-A alleles in the context of presentation of EBV-derived peptides. HLA-A*02 can present various immunogenic EBV-derived peptides of the latency type II antigens, and can mediate a cellular immune response, and thereby mediate a protective effect.
The distinct manifestations of EBV-infection are thought to be affected by the host’s different immune response to EBV, especially by cytokine production. The levels of interleukin (IL)-1a, IL-2, IL-6, and interferon (IFN)- have been reported to be elevated in the serum of patients with infectious mononucleosis. There is also increasing evidence indicating that cytokine gene polymorphisms, such as those of the IL-10 and IL-1a genes, have an impact on susceptibility to EBV infection. We studied several polymorphic allele variants of the cytokine genes IL-10 (T-3575A, G-2849A, C-2763A, A-1082G and C-592A) in HL patients [111]. A subgroup of 71 samples were studied for the EBV status. EBV was detected in HRS cells in 20 of 71 (28%) cases tested. No associations between EBV and cytokine polymorphisms were detected.
In Japanese individuals, the polymorphism of TGF-1 at codon 10 was associated with the development of EBV-related hematologic diseases, such as infectious mononucleosis, however associations with the development of EBV+ HL have not been reported so far [112]. Chang et al investigated whether polymorphic variation in genes involved in NF-kB activation and inhibition, other inflammatory pathways influenced HL risk [113]. HL risk was significantly associated with rs1585215 in NF-kB1 and with NF-kB1 haplotypes, with similar associations regardless of the tumor EBV status.
EBV and prognosis: The impact of the tumor cell EBV status on the prognosis of patients with HL remains controversial. Some of the inter-study variation may be attributable to the different epidemiological features of the disease in different geographical settings and some may be related to case selection.
Considering the age-stratified model discussed above seems to be important when analyzing the impact of EBV status on the outcome for HL patients. Thus, in young adults, there seems to be a marginal prognostic advantage when patients carry the EBV genome in their tumor [114-117]. Yet among patients aged more than 50 years, EBV positivity was associated with a significantly poorer outcome [115,118-120]. In children aged < 15 years, some studies suggested that EBV presence was associated with favorable survival120 while others suggested a negative impact of EBV-positivity on outcome [121]. Thus, the influence of EBV on survival in HL might reflect differences at the oncogenic capacity of the virus or in the immune response. The presence EBV might reflect a poor immune status, which in turn means that patients might tolerate disease and its treatment less well.
Circulating EBV-DNA as Biomarker: Detection and quantitation of free plasma EBV viral DNA could potentially be used as a biomarker of disease activity in EBV+ HL. A number of groups have explored the value of cell-free EBV-DNA viral load quantification in EBV-associated malignancies including HL [122-125]. The frequency of EBV-infected circulating memory B cells is increased in pretreatment samples of EBV+ HL patients compared with EBV- HL cases [126].
EBV genomes are detectable in the serum and plasma of EBV-associated HL cases. The origin of EBV genomes in serum/plasma varies in different disease states, in HL viral genomes are present as naked DNA and are probably shed from tumors. Consistent with the notion that cell-free viral DNA may be shed from circulating apoptotic malignant cells, it has been shown that cell-free DNA is present as ‘‘naked’’ DNA rather than as virions [123]. Using conventional PCR, Gallagher et al. reported that EBV-DNA was detected in 91% of serum samples from patients with EBV+ HL, whereas 23% of EBV- HL patients had detectable viral DNA. Using real-time PCR for EBV+ HL results were similar, and only 10% of patients with EBV- HL had a quantifiable (low level) load, consistent with lysis of bystander EBV+ B cells within the diseased lymph node. Using quantitative (but not real-time) PCR, Drouet and colleagues confirmed the observation that EBV-DNA was more frequently detected in serum from EBV+ HL than EBV- HL [127]. Wagner et al. detected plasma EBV-DNA by real-time PCR prior to therapy in 13 of 24 pediatric patients with EBV seropositive HL, and in none of the patients in stable remission, suggesting that viral load monitoring may be useful in disease evaluation [128]. However, this study did not test tissue samples for the presence of EBV within Hodgkin Reed-Sternberg cells and therefore was not able to stratify between EBV+ HL and EBV- HL cases. Gandhi detected EBV-DNA in the plasma of all EBV+ patients with HL prior to therapy, while it was detected in peripheral blood monocytes only in 50% of EBV+ HL patients [124]. Plasma EBV-DNA was not detected in all patients with EBV- HL, and those with long-term remission. Serial analysis done in EBV+ HL patients who presented with active disease showed that response to chemotherapy was associated with decline in viral load to undetectable levels.
The variation in sensitivity and specificity of EBV-DNA as a biomarker between these studies and ours may in part be reflected by technical differences in the assays employed. In this regard, the use of new generation reagents are likely to improve results. The sensitivity of real-time PCR varies with the efficiency and purity of DNA extraction, the segment of DNA amplified, the fluorogenic probe used, and potentially, the source of DNA (serum versus plasma). Gandhi et al amplified a region of BALF5, a subunit of the EBV-DNA polymerase gene, from plasma samples [124]. By contrast, the study by Wagner used primers to detect the BamH1-W region in plasma. This region is repeated a variable number of times, and therefore, their results cannot not be used to directly compare viral copy number [128].
EBV genome copy number in serum/plasma may provide an indication of tumor burden and may prove to be a useful marker for monitoring HL patients. Additional prospective studies are required to further evaluate the use of free plasma EBV-DNA as a biomarker for monitoring response to treatment in patients with EBV+ HL.
A study on 277 biopsies from 10 countries revealed a particular high frequency of EBV-positivity (80-100%) in childhood HL from Kenya, Costa Rica, Iran, but also from Greece, while EBV was present in about 50% of pediatric HL cases from Egypt, Jordan, South Africa and the United Kingdom [102]. A case series of childhood HL form Southeast Turkey showed the typical prevalence of male sex, mixed cellularity and EBV positivity [103].
The pattern of HL in Israel is intermediate between the bimodal pattern of Western countries and the pediatric peak seen in developing countries. Patients born in Europe had a slightly lower rate of positivity to EBV (21.8%). This rate was higher in patients from Asia and Africa (27%). In contrast to these groups of Jews, the Bedouin patients, although representing a small group, showed a 66.7% rate of EBV infection [104]. Studies form the middle East indicate incidence rates of EBV in HL varying form 38-56 % with patterns of early-industrialized countries [105,106]. These epidemiologic studies led to the proposal of a model by Jarrett et al that EBV-associated HL cases can be divided into three groups according to age at exposure to EBV and age at HL diagnosis [93]. This model recognises a childhood group, accounting for almost all cases of HL in early childhood, a young adult group, which epidemiological data suggest is associated with delayed exposure to EBV, and an older adult group, which might result from loss of the normal balance between latent EBV infection and host immunity.
Three epidemiological patterns can be discerned. The type I pattern which is prevalent in developing countries, shows a relatively high incidence in male children, a low incidence in the third decade and a second peak of high incidence in older age groups. Type III, which is usually seen in developed countries, is characterised by a low rate in children and a pronounced initial peak in young adults. The third pattern (Type II), which is described in many Asian countries, is intermediate and reflects a transition between types I and III. In this pattern there is both a childhood and a third decade peak.
An interesting question is whether the socioeconomic progress will change the incidence patterns of HL in developing countries. Hjalgrim and colleagues analysed HL incidence patterns in Singapore between 1968 and 2004, during which time period a socioeconomic transition towards Western World lifestyles took place [107]. A HL incidence peak emerged among adolescents and young adults in Singapore with annual increase rates up to 13.7%, in particular in females. However, the incidence peak remained considerably lower than what can be observed in young adults in the Western World. It remains to be determined to what extent the current lower incidence of HL in young Asian adults should be attributed to birth cohort phenomena, as would be suggested by continued increase in incidence, and to ethnic variation in HL susceptibility between Asian and non-Asian populations, respectively.
The impact of socioeconomic and racial factors on the risk to develop EBV+ HL was studied by Glaser et al in a Californian population [108]. Tumor EBV-positivity was associated with Hispanic and Asian/Pacific Islander (API) but not black race/ethnicity, irrespective of demographic and clinical factors. In Hispanics, EBV+ HL was associated not only with young and older age, male sex, and mixed cellularity histology, but also with foreign birth and lower neighbourhood socioeconomic status in females. The racial/ethnic variation suggests that EBV+ HL results from an intricate interplay of early- and later-life environmental, hormonal, and genetic factors leading to depressed immune function and poorly controlled EBV infection.
Genetic predisposition to develop EBV+ HL: Genetic predisposition to develop EBV+ HL is supported by the association of EBV+ HL with the highly polymorphic human leukocyte antigen (HLA) genes, which vary by racial/ethnic group. Genetic association of EBV+ HL was found with the HLA class I region, including the HLA-A gene [109,110]. HLA-A*02 was underrepresented in patients with EBV+ HL (15%), and HLA-A*01 was overrepresented in patients with EBV+ HL (37.1%). These data may suggest functional differences in the HLA-A alleles in the context of presentation of EBV-derived peptides. HLA-A*02 can present various immunogenic EBV-derived peptides of the latency type II antigens, and can mediate a cellular immune response, and thereby mediate a protective effect.
The distinct manifestations of EBV-infection are thought to be affected by the host’s different immune response to EBV, especially by cytokine production. The levels of interleukin (IL)-1a, IL-2, IL-6, and interferon (IFN)- have been reported to be elevated in the serum of patients with infectious mononucleosis. There is also increasing evidence indicating that cytokine gene polymorphisms, such as those of the IL-10 and IL-1a genes, have an impact on susceptibility to EBV infection. We studied several polymorphic allele variants of the cytokine genes IL-10 (T-3575A, G-2849A, C-2763A, A-1082G and C-592A) in HL patients [111]. A subgroup of 71 samples were studied for the EBV status. EBV was detected in HRS cells in 20 of 71 (28%) cases tested. No associations between EBV and cytokine polymorphisms were detected.
In Japanese individuals, the polymorphism of TGF-1 at codon 10 was associated with the development of EBV-related hematologic diseases, such as infectious mononucleosis, however associations with the development of EBV+ HL have not been reported so far [112]. Chang et al investigated whether polymorphic variation in genes involved in NF-kB activation and inhibition, other inflammatory pathways influenced HL risk [113]. HL risk was significantly associated with rs1585215 in NF-kB1 and with NF-kB1 haplotypes, with similar associations regardless of the tumor EBV status.
EBV and prognosis: The impact of the tumor cell EBV status on the prognosis of patients with HL remains controversial. Some of the inter-study variation may be attributable to the different epidemiological features of the disease in different geographical settings and some may be related to case selection.
Considering the age-stratified model discussed above seems to be important when analyzing the impact of EBV status on the outcome for HL patients. Thus, in young adults, there seems to be a marginal prognostic advantage when patients carry the EBV genome in their tumor [114-117]. Yet among patients aged more than 50 years, EBV positivity was associated with a significantly poorer outcome [115,118-120]. In children aged < 15 years, some studies suggested that EBV presence was associated with favorable survival120 while others suggested a negative impact of EBV-positivity on outcome [121]. Thus, the influence of EBV on survival in HL might reflect differences at the oncogenic capacity of the virus or in the immune response. The presence EBV might reflect a poor immune status, which in turn means that patients might tolerate disease and its treatment less well.
Circulating EBV-DNA as Biomarker: Detection and quantitation of free plasma EBV viral DNA could potentially be used as a biomarker of disease activity in EBV+ HL. A number of groups have explored the value of cell-free EBV-DNA viral load quantification in EBV-associated malignancies including HL [122-125]. The frequency of EBV-infected circulating memory B cells is increased in pretreatment samples of EBV+ HL patients compared with EBV- HL cases [126].
EBV genomes are detectable in the serum and plasma of EBV-associated HL cases. The origin of EBV genomes in serum/plasma varies in different disease states, in HL viral genomes are present as naked DNA and are probably shed from tumors. Consistent with the notion that cell-free viral DNA may be shed from circulating apoptotic malignant cells, it has been shown that cell-free DNA is present as ‘‘naked’’ DNA rather than as virions [123]. Using conventional PCR, Gallagher et al. reported that EBV-DNA was detected in 91% of serum samples from patients with EBV+ HL, whereas 23% of EBV- HL patients had detectable viral DNA. Using real-time PCR for EBV+ HL results were similar, and only 10% of patients with EBV- HL had a quantifiable (low level) load, consistent with lysis of bystander EBV+ B cells within the diseased lymph node. Using quantitative (but not real-time) PCR, Drouet and colleagues confirmed the observation that EBV-DNA was more frequently detected in serum from EBV+ HL than EBV- HL [127]. Wagner et al. detected plasma EBV-DNA by real-time PCR prior to therapy in 13 of 24 pediatric patients with EBV seropositive HL, and in none of the patients in stable remission, suggesting that viral load monitoring may be useful in disease evaluation [128]. However, this study did not test tissue samples for the presence of EBV within Hodgkin Reed-Sternberg cells and therefore was not able to stratify between EBV+ HL and EBV- HL cases. Gandhi detected EBV-DNA in the plasma of all EBV+ patients with HL prior to therapy, while it was detected in peripheral blood monocytes only in 50% of EBV+ HL patients [124]. Plasma EBV-DNA was not detected in all patients with EBV- HL, and those with long-term remission. Serial analysis done in EBV+ HL patients who presented with active disease showed that response to chemotherapy was associated with decline in viral load to undetectable levels.
The variation in sensitivity and specificity of EBV-DNA as a biomarker between these studies and ours may in part be reflected by technical differences in the assays employed. In this regard, the use of new generation reagents are likely to improve results. The sensitivity of real-time PCR varies with the efficiency and purity of DNA extraction, the segment of DNA amplified, the fluorogenic probe used, and potentially, the source of DNA (serum versus plasma). Gandhi et al amplified a region of BALF5, a subunit of the EBV-DNA polymerase gene, from plasma samples [124]. By contrast, the study by Wagner used primers to detect the BamH1-W region in plasma. This region is repeated a variable number of times, and therefore, their results cannot not be used to directly compare viral copy number [128].
EBV genome copy number in serum/plasma may provide an indication of tumor burden and may prove to be a useful marker for monitoring HL patients. Additional prospective studies are required to further evaluate the use of free plasma EBV-DNA as a biomarker for monitoring response to treatment in patients with EBV+ HL.
EBV
as target for therapeutic intervention in HL:
As the immunotherapeutic approach to EBV+ lymphoproliferative diseases
including HL is the topic of another review in this journal, we will
limit this subject to some few considerations. The presence of EBV
latent antigens in EBV+ HL appear to be an excellent opportunity both
for targeted cellular immunotherapy and antiviral strategies. These
antigens could act as tumor-associated antigens for EBV-specific
cytotoxic T cells (CTL). Antigen processing and presentation appear to
remain intact in EBV+ HL, however an impaired CTL response is observed
in cases of HL. The study by Gandhi et al. suggests that Gal-1hi
expression in HRS cells is an important negative regulator of HL
tumour-associated EBV antigen-specific CD8+ T-cell immunity in cHL, and
thus enables HRS cells to avoid T cell dependent immune attack [129].
Galectin-1 (Gal-1) is a soluble lectin which inhibits proliferation and
IFN-γ expression by EBV-specific T-cells. Targeted inhibition of Gal-1
expression in tumor cells has been shown to potentiate anti-tumor
effector T cells. The Gal-1 mediated immunosuppressive pathway may
represent a target to enhance efficacy of immunotherapeutic strategies
for HL.
Like other herpesviruses, EBV encodes a thymidine kinase (TK) enzyme which can be a target for purine nucleoside analogs, such as acyclovir (ACV) and ganciclovir (GCV). Latently infected B cells however do not express the EBV-TK transcript or protein, and are unaffected by these antiviral agents. However, exposure of these cells in vitro to arginine butyrate (or the sodium salt) results in modest induction of some lytic-phase genes and gene products, including TK [130]. Butyrate has been shown to sensitize EBV+ lymphoma cells in vitro to apoptosis induced by ganciclovir. Perrine et al administered arginine butyrate in combination with ganciclovir in 15 patients with refractory EBV+ lymphoid malignancies including one patient with EBV+ HL to evaluate the drug combination for toxicity, pharmacokinetics, and clinical responses [131]. Ganciclovir was administered twice daily at standard doses, and arginine butyrate was administered by continuous infusion in an intrapatient dose escalation, from 500 mg/kg/day escalating to 2000 mg/kg/day, as tolerated, for a 21-day cycle. The MTD for arginine butyrate in combination with ganciclovir was established as 1000 mg/kg/day. Ten of 15 patients showed significant antitumor responses, with 4 CRs and 6 PRs within one treatment cycle. The single patient with HL demonstrated no response to the protocol. Review of pathology before therapy was instituted showed that only a single lymph node was positive for EBV antigens, whereas the patient’s large central mediastinal masses were negative for EBV.
Like other herpesviruses, EBV encodes a thymidine kinase (TK) enzyme which can be a target for purine nucleoside analogs, such as acyclovir (ACV) and ganciclovir (GCV). Latently infected B cells however do not express the EBV-TK transcript or protein, and are unaffected by these antiviral agents. However, exposure of these cells in vitro to arginine butyrate (or the sodium salt) results in modest induction of some lytic-phase genes and gene products, including TK [130]. Butyrate has been shown to sensitize EBV+ lymphoma cells in vitro to apoptosis induced by ganciclovir. Perrine et al administered arginine butyrate in combination with ganciclovir in 15 patients with refractory EBV+ lymphoid malignancies including one patient with EBV+ HL to evaluate the drug combination for toxicity, pharmacokinetics, and clinical responses [131]. Ganciclovir was administered twice daily at standard doses, and arginine butyrate was administered by continuous infusion in an intrapatient dose escalation, from 500 mg/kg/day escalating to 2000 mg/kg/day, as tolerated, for a 21-day cycle. The MTD for arginine butyrate in combination with ganciclovir was established as 1000 mg/kg/day. Ten of 15 patients showed significant antitumor responses, with 4 CRs and 6 PRs within one treatment cycle. The single patient with HL demonstrated no response to the protocol. Review of pathology before therapy was instituted showed that only a single lymph node was positive for EBV antigens, whereas the patient’s large central mediastinal masses were negative for EBV.
Conclusion:
In conclusion, studies on the role of EBV in the
immunopathogenesis of
HL have delivered important insights which form the basis for
therapeutic approaches targeting EBV. Recent progress may help to
overcome obstacles encountered on the way to a targeted therapy in EBV+
HL.
References
- Küppers R. The biology of Hodgkin's
lymphoma. Nat Rev Cancer. 2009, Jan,9(1):15-27. Epub 2008 Dec 11. Review

- Küppers R, Rajewsky K, Zhao M, Simons G,
Laumann R, Fischer R, Hansmann ML. Hodgkin disease: Hodgkin and
Reed-Sternberg cells picked from histological sections show clonal
immunoglobulin gene rearrangements and appear to be derived from B
cells at various stages of development. Proc Natl Acad Sci U S A. 1994,
Nov 8,91(23):10962-6.

- Kanzler H, Küppers R, Hansmann ML, Rajewsky
K. Hodgkin and Reed-Sternberg cells in Hodgkin's disease represent the
outgrowth of a dominant tumor clone derived from (crippled) germinal
center B cells. J Exp Med. 1996 Oct 1,184(4):1495-505

- Jones RJ, Gocke CD, Kasamon YL, Miller CB,
Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M, Lemas
MV, Brennan S, Ambinder RF, Matsui W. Circulating clonotypic B cells in
classic Hodgkin lymphoma. Blood. 2009 Jun 4,113(23):5920-6

- Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008). WHO Classification of tumors of Hematopoietic and Lymphoid Tissues. IARC, Lyon
- Gallagher A, Perry J, Shield L, Freeland J,
MacKenzie J, Jarrett RF. Viruses and Hodgkin disease: no evidence of
novel herpesviruses in non-EBV-associated lesions. Int J Cancer. 2002
Sep 20,101(3):259-64

- Wilson KS, Gallagher A, Freeland JM, Shield
LA, Jarrett RF. Viruses and Hodgkin lymphoma: no evidence of
polyomavirus genomes in tumor biopsies. Leuk Lymphoma. 2006
Jul,47(7):1315-21

- Wilson KS, Freeland JM, Gallagher A, Cosby
SL, Earle JA, Alexander FE, Taylor GM, Jarrett RF. Measles virus and
classical Hodgkin lymphoma: no evidence for a direct association. Int J
Cancer. 2007 Jul 15,121(2):442-7

- Dolcetti R, Boiocchi M, Gloghini A, Carbone
A. Pathogenetic and histogenetic features of HIV-associated Hodgkin's
disease. Eur J Cancer. 2001 Jul,37(10):1276-87.

- Andersson J. Epstein-Barr virus and
Hodgkin's lymphoma. Herpes. 2006 May,13(1):12-6. Review

- Gandhi MK, Tellam JT, Khanna R.
Epstein-Barr virus-associated Hodgkin's lymphoma. Br J Haematol. 2004
May,125(3):267-81. Review

- Hammerschmidt W, Sugden B. Epstein-Barr
virus sustains Burkitt's lymphomas and Hodgkin's disease. Trends Mol
Med. 2004 Jul,10(7):331-6. Review

- Kapatai G, Murray P. Contribution of the
Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J
Clin Pathol. 2007 Dec,60(12):1342-9. Review

- Küppers R. B cells under influence:
transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003
Oct,3(10):801-12. Review.

- Rezk SA, Weiss LM. Epstein-Barr
virus-associated lymphoproliferative disorders. Hum Pathol. 2007
Sep,38(9):1293-304. Review

- Weiss LM. Epstein-Barr virus and Hodgkin's
disease. Curr Oncol Rep. 2000 Mar,2(2):199-204. Review.

- Bräuninger A, Schmitz R, Bechtel D, Renné
C, Hansmann ML, Küppers R. Molecular biology of Hodgkin's and
Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer. 2006 Apr
15,118(8):1853-61. Review

- Bargou RC, Emmerich F, Krappmann D,
Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A,
Scheidereit C, Dörken B. Constitutive nuclear factor-kappaB-RelA
activation is required for proliferation and survival of Hodgkin's
disease tumor cells. J Clin Invest. 1997 Dec 15,100(12):2961-9

- Jundt F, Kley K, Anagnostopoulos I,
Schulze Pröbsting K, Greiner A, Mathas S, Scheidereit C, Wirth T, Stein
H, Dörken B. Loss of PU.1 expression is associated with defective
immunoglobulin transcription in Hodgkin and Reed-Sternberg cells of
classical Hodgkin disease. Blood. 2002 Apr 15,99(8):3060-2

- Re D, Müschen M, Ahmadi T, Wickenhauser C,
Staratschek-Jox A, Holtick U, Diehl V and Wolf J. Oct-2 and Bob-1
deficiency in Hodgkin and Reed Sternberg cells. Cancer Res. 2001,
1:2080-2084.

- Torlakovic E, Tierens A, Dang HD and
Delabie J. The transcription factor PU.1, necessary for B-cell
development is expressed in lymphocyte predominance, but not classical
Hodgkin’s disease. Am J Path 2001, 159:1807-1814.

- Schwering I, Bräuninger A, Klein U,
Jungnickel B, Tinguely M, Diehl V, Hansmann ML, Dalla-Favera R,
Rajewsky K and Küppers R. Loss of the B-lineage-specific gene
expression program in Hodgkin and Reed-Sternberg cells of Hodgkin
lymphoma. Blood 2003, 101:1505-1512.

- Schmitz R, Stanelle J, Hansmann ML,
Küppers R. Pathogenesis of classical and lymphocyte-predominant Hodgkin
lymphoma. Annu Rev Pathol 2009a, 4:151-174. Review

- Brousset P, Schlaifer D, Meggetto F,
Bachmann E, Rothenberger S, Pris J, Delsol G and Knecht H. Persistence
of the same viral strain in early and late relapses of Epstein-Barr
virus-associated Hodgkin’s disease. Blood 1994, 84:2447-2451.

- Weiss LM, Movahed LA, Warnke RA, Sklar J.
Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of
Hodgkin's disease. N Engl J Med. 1989 Feb 23,320(8):502-6

- Fleisher G, Henle W, Henle G, Lennette ET
and Biggar RJ. Primary infection with Epstein-Barr virus in infants in
the United States: clinical and serologic observations. J Infect Dis
1979, 139 :553-558

- Golden HD, Chang RS, Prescott W, Simpson E
and Cooper TY. Leukocyte-transforming agent: prolonged excretion by
patients with mononucleosis and excretion by normal individuals. J
Infect Dis 1973, 127 :471-473

- Khan G, Miyashita EM, Yang B, Babcock GJ
and Thorley-Lawson DA. Is EBV persistence in vivo a model for B cell
homeostasis? Immunity 1996, 5:173-179

- Henle G, Henle W and Diehl V. Relation of
Burkitt’s tumor-associated herpes-type virus to infectious
mononucleosis. Proc Natl Acad Sci U S A 1968, 59:94-101

- Sawyer RN, Evans AS, Niederman JC and
McCollum RW. Prospective studies of a group of Yale University
freshman. I. Occurrence of infectious mononucleosis. J Infect Dis 1971,
123 :263-270

- Epstein MA, Barr YM and Achong BG. Studies
with Burkitt’s lymphoma. Wistar Inst Symp Monogr 1965, 4:69-82

- Henle W, Diehl V, Kohn G, Zur Hausen
H and Henle G. Herpes-type virus and chromosome marker in normal
leukocytes after growth with irradiated Burkitt cells. Science 1967,
157:1064-1065

- Thorley-Lawson DA. EBV the
prototypical human tumor virus-just how bad is it? J Allergy Clin
Immunol 2005, 116:251-261. Review

- Tugizov SM, Berline JW and Palefsky JM.
Epstein-Barr virus infection of polarized tongue and nasopharyngeal
epithelial cells. Nat Med 2003, 9:307-314

- Hurley EA and Thorley-Lawson DA. B cell
activation and the establishment of Epstein-Barr virus latency. J Exp
Med 1988, 168:2059-2075

- Kintner CR and Sugden B. The structure of
the termini of the DNA of Epstein-Barr virus. Cell 1979, 17:661-671

- Alfieri C, Birkenbach M and Kieff E. Early
events in Epstein-Barr virus infection of human B lymphocytes. Virology
1991, 181:595-608

- Tierney R, Nagra J, Hutchings I,
Shannon-Lowe C, Altmann M, Hammerschmidt W, Rickinson A and Bell A.
Epstein-Barr virus exploits BSAP/Pax5 to achieve the B-cell specificity
of its growth-transforming program. J Virol 2007, 81:10092-10100

- Chen F, Zou JZ, di Renzo L, Winberg G, Hu
LF, Klein E, Klein G and Ernberg I. A subpopulation of normal B cells
latently infected with Epstein-Barr virus resembles Burkitt lymphoma
cells in expressing EBNA-1 but not EBNA-2 or LMP1. J Virol 1995,
69:3752-3758

- Hochberg D, Middeldorp JM, Catalina M,
Sullivan JL, Luzuriaga K and Thorley-Lawson DA. Demonstration of the
Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently
infected memory cells in vivo. Proc Natl Acad Sci U S A 2004,
101:239-244

- Miyashita EM, Yang B, Babcock GJ and
Thorley-Lawson DA. Identification of the site of Epstein-Barr virus
persistence in vivo as a resting B cell. J Virol 1997, 71:4882-4891

- Tierney RJ, Steven N, Young LS, Rickinson
AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of
viral gene transcription during primary infection and in the carrier
state. J Virol 1994, 68:7374-7385

- Thorley-Lawson DA. Epstein-Barr virus:
exploiting the immune system. Nat Rev Immunol 2001, 1:75-82. Review

- Kurth J, Spieker T, Wustrow J, Strickler
GJ, Hansmann ML, Rajewsky K and Küppers R. EBV-infected B cells in
infectious mononucleosis: viral strategies for spreading in the B cell
compartment and establishing latency. Immunity 2000, 13:485-495

- Kurth J, Hansmann ML, Rajewsky K and
Küppers R. Epstein-Barr virus-infected B cells expanding in germinal
centers of infectious mononucleosis patients do not participate in the
germinal center reaction. Proc Natl Acad Sci U S A 2003, 100:4730-4735

- Araujo I, Foss HD, Hummel M,
Anagnostopoulos I, Barbosa HS, Bittencourt A and Stein H. Frequent
expansion of Epstein-Barr virus (EBV) infected cells in germinal
centres of tonsils from an area with a high incidence of EBV-associated
lymphoma. J Pathol 1999, 187:326-330

- Kobayashi R, Takeuchi H, Sasaki M,
Hasegawa M and Hirai K. Detection of Epstein-Barr virus infection in
the epithelial cells and lymphocytes of non-neoplastic tonsils by in
situ hybridization and in situ PCR. Arch Virol 1998, 143:803-813

- Liu YJ, Joshua DE, Williams GT, Smith CA,
Gordon J and MacLennan IC. Mechanism of antigen-driven selection in
germinal centres. Nature 1989, 342:929-931

- Rajewsky K. Clonal selection and learning
in the antibody system. Nature 1996, 381, 751-758. Review

- Bechtel D, Kurth J, Unkel C, Küppers R.
Transformation of BCR-deficient germinal-center B cells by EBV supports
a major role of the virus in the pathogenesis of Hodgkin and
posttransplantation lymphomas. Blood 2005, 106:4345-4350

- Mancao C, Altmann M, Jungnickel B and
Hammerschmidt W. Rescue of ”crippled” germinal center B cells from
apoptosis by Epstein-Barr virus. Blood 2005, 106:4339-4344

- Chaganti S, Bell AI, Pastor NB, Milner AE,
Drayson M, Gordon J and Rickinson AB. Epstein-Barr virus infection in
vitro can rescue germinal center B cells with inactivated
immunoglobulin genes. Blood 2005, 106:4249-4252

- Niedobitek G, Kremmer E, Herbst H,
Whitehead L, Dawson CW, Niedobitek E, von Ostau C, Rooney N, Grasser FA
and Young LS. Immunohistochemical detection of the Epstein-Barr
virus-encoded latent membrane protein 2A in Hodgkin’s disease and
infectious mononucleosis. Blood 1997, 90:1664-1672

- Mancao C and Hammerschmidt W. Epstein-Barr
virus latent membrane protein 2A is a B-cell receptor mimic and
essential for B-cell survival. Blood 2007, 110:3715-3721

- Dutton A, O’Neill JD, Milner AE, Reynolds
GM, Starczynski J, Crocker J, Young LS and Murray P. Expression of the
cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin’s lymphoma
cells from autonomous Fas-mediated death. Proc Natl Acad Sci U S A
2004, 101:6611-6616

- Hinz M, Lemke P, Anagnostopoulos I, Hacker
C, Krappmann D, Mathas S, Dorken B, Zenke M, Stein H and Scheidereit C.
Nuclear factor kappaB-dependent gene expression profiling of Hodgkin’s
disease tumor cells, pathogenetic significance, and link to
constitutive signal transducer and activator of transcription 5a
activity. J Exp Med 2002, 196:605-617

- Kreuz S, Sigmund D, Scheurich P and Wajant
H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive
inhibitor of death receptor signaling. Mol Cell Biol 2001, 21:3964-3973

- Gires O, Zimber-Strobl U, Gonnella R,
Ueffing M, Marschall G, Zeidler R, Pich D and Hammerschmidt W. Latent
membrane protein 1 of Epstein-Barr virus mimics a constitutively active
receptor molecule. EMBO 1997, 16:6131-6140

- Kilger E, Kieser A, Baumann M and
Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is
dependent upon latent membrane protein 1, which simulates an activated
CD40 receptor. EMBO J 1998, 17:1700-1709

- Cahir McFarland ED, Izumi KM and Mosialos
G. Epstein-Barr virus transformation: involvement of latent membrane
protein 1-mediated activation of NF-kappaB. Oncogene 1999, 18:6959-6964a

- Cabannes E, Khan G, Aillet F, Jarrett RF
and Hay RT. Mutations in the IkBa gene in Hodgkin’s disease suggest a
tumour suppressor role for IkappaBalpha. Oncogene 1999, 18:3063-3070

- Emmerich F, Meiser M, Hummel M, Demel G,
Foss HD, Jundt F, Mathas S, Krappmann D, Scheidereit C, Stein H and
Dorken B. Overexpression of I kappa B alpha without inhibition of
NF-kappaB acitivity and mutations in the I kappa B alpha gene in
Reed-Sternberg cells. Blood 1999, 94:3129-3134

- Emmerich F, Theurich S, Hummel M, Haeffker
A, Vry MS, Dohner K, Bommert K, Stein H and Dorken B. Inactivating I
kappa B epsilon mutations in Hodgkin/Reed-Sternberg cells. J Pathol
2003, 201:413-420

- Jungnickel B, Staratschek-Jox A,
Bräuninger A, Spieker T, Wolf J, Diehl V, Hansmann ML, Rajewsky K and
Küppers R. Clonal deleterious mutations in the ikBa gene in the
malignant cells in Hodgkin’s disease. J Exp Med 2000, 191:395-401

- Martin-Subero JI, Gesk S, Harder L, Sonoki
T, Tucker PW, Schlegelberger B, Grote W, Novo FJ, Calasanz MJ, Hansmann
ML, Dyer MJ and Siebert R. Recurrent involvement of the REL and BCL11A
loci in classical Hodgkin lymphoma. Blood 2002, 99:1474-1477

- Schmitz R, Hansmann ML, Bohle V,
Martin-Subero JI, Hartmann S, Mechtersheimer G, Klapper W, Vater I,
Giefing M, Gesk S, Stanelle J, Siebert R, Küppers R. TNFAIP3 (A20) is a
tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B
cell lymphoma. J Exp Med. 2009b May 11,206(5):981-9

- Vockerodt M, Morgan SL, Kuo M, Wei W,
Chukwuma MB, Arrand JR, Kube D, Gordon J, Young LS, Woodman CB, Murray
PG. The Epstein-Barr virus oncoprotein, latent membrane protein-1,
reprograms germinal centre B cells towards a Hodgkin's
Reed-Sternberg-like phenotype. J Pathol. 2008 Sep,216(1):83-92

- Portis T, Dyck P, Longnecker R.
Epstein-Barr Virus (EBV) LMP2A induces alterations in gene
transcription similar to those observed in Reed-Sternberg cells of
Hodgkin lymphoma. Blood. 2003 Dec 1,102(12):4166-78

- García JF, Camacho FI, Morente M, Fraga M,
Montalbán C, Alvaro T, Bellas C, Castaño A, Díez A, Flores T, Martin C,
Martinez MA, Mazorra F, Menárguez J, Mestre MJ, Mollejo M, Sáez AI,
Sánchez L, Piris MA, Spanish Hodgkin Lymphoma Study Group. Hodgkin and
Reed-Sternberg cells harbor alterations in the major tumor suppressor
pathways and cell-cycle checkpoints: analyses using tissue microarrays.
Blood. 2003 Jan 15,101(2):681-9

- Tao Q, Young LS, Woodman CB, Murray PG.
Epstein-Barr virus (EBV) and its associated human cancers--genetics,
epigenetics, pathobiology and novel therapeutics. Front Biosci. 2006
Sep 1,11:2672-713. Review

- Niller HH, Wolf H, Minarovits J.
Epigenetic dysregulation of the host cell genome in Epstein-Barr
virus-associated neoplasia. Semin Cancer Biol. 2009 Jun,19(3):158-64

- Tsai CN, Tsai CL, Tse KP, Chang HY, Chang
YS. The Epstein-Barr virus oncogene product, latent membrane protein 1,
induces the downregulation of E-cadherin gene expression via activation
of DNA methyltransferases. Proc Natl Acad Sci U S A. 2002 Jul
23,99(15):10084-9

- Tsai CL, Li HP, Lu YJ, Hsueh C, Liang Y,
Chen CL, Tsao SW, Tse KP, Yu JS, Chang YS. Activation of DNA
methyltransferase 1 by EBV LMP1 Involves c-Jun NH(2)-terminal kinase
signaling. Cancer Res. 2006 Dec 15,66(24):11668-76

- Seo SY, Kim EO, Jang KL. Epstein-Barr
virus latent membrane protein 1 suppresses the growth-inhibitory effect
of retinoic acid by inhibiting retinoic acid receptor-beta2 expression
via DNA methylation. Cancer Lett. 2008 Oct 18,270(1):66-76

- Jung JK, Arora P, Pagano JS, Jang KL.
Expression of DNA methyltransferase 1 is activated by hepatitis B virus
X protein via a regulatory circuit involving the p16INK4a-cyclin D1-CDK
4/6-pRb-E2F1 pathway. Cancer Res. 2007 Jun 15,67(12):5771-8

- Burgers WA, Blanchon L, Pradhan S, de
Launoit Y, Kouzarides T, Fuks F. Viral oncoproteins target the DNA
methyltransferases. Oncogene. 2007 Mar 8,26(11):1650-5

- Minarovits J. Epigenotypes of latent
herpesvirus genomes. Curr Top Microbiol Immunol. 2006,310:61-80. Review

- Tao Q, Robertson KD. Stealth technology:
how Epstein-Barr virus utilizes DNA methylation to cloak itself from
immune detection. Clin Immunol. 2003 Oct,109(1):53-63. Review

- Tierney RJ, Kirby HE, Nagra JK, Desmond J,
Bell AI, Rickinson AB. Methylation of transcription factor binding
sites in the Epstein-Barr virus latent cycle promoter Wp coincides with
promoter down-regulation during virus-induced B-cell transformation. J
Virol. 2000 Nov,74(22):10468-79

- Chan AT, Tao Q, Robertson KD, Flinn IW,
Mann RB, Klencke B, Kwan WH, Leung TW, Johnson PJ, Ambinder RF.
Azacitidine induces demethylation of the Epstein-Barr virus genome in
tumors. J Clin Oncol. 2004 Apr 15,22(8):1373-81

- Skinnider BF, Mak TW. The role of
cytokines in classical Hodgkin lymphoma. Blood. 2002 Jun
15,99(12):4283-97. Review

- Teichmann M, Meyer B, Beck A, Niedobitek
G. Expression of the interferon-inducible chemokine IP-10 (CXCL10), a
chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma
and nasopharyngeal carcinoma. J Pathol. 2005 May,206(1):68-75

- Lambert SL, Martinez OM. Latent membrane
protein 1 of EBV activates phosphatidylinositol 3-kinase to induce
production of IL-10. J Immunol. 2007 Dec 15,179(12):8225-34

- Herling M, Rassidakis GZ, Medeiros LJ,
Vassilakopoulos TP, Kliche KO, Nadali G, Viviani S, Bonfante V,
Giardini R, Chilosi M, Kittas C, Gianni AM, Bonadonna G, Pizzolo G,
Pangalis GA, Cabanillas F, Sarris AH. Expression of Epstein-Barr virus
latent membrane protein-1 in Hodgkin and Reed-Sternberg cells of
classical Hodgkin's lymphoma: associations with presenting features,
serum interleukin 10 levels, and clinical outcome. Clin Cancer Res.
2003 Jun,9(6):2114-20

- Herbst H, Samol J, Foss HD, Raff T,
Niedobitek G.Modulation of interleukin-6 expression in Hodgkin and
Reed-Sternberg cells by Epstein-Barr virus. J Pathol. 1997
Jul,182(3):299-306

- Biggar RJ, Johansen JS, Smedby KE,
Rostgaard K, Chang ET, Adami HO, Glimelius B, Molin D, Hamilton-Dutoit
S, Melbye M, Hjalgrim H. Serum YKL-40 and interleukin 6 levels in
Hodgkin lymphoma. Clin Cancer Res. 2008 Nov 1,14(21):6974-8

- Hohaus S, Giachelia M, Massini G, Vannata
B, Criscuolo M, Martini M, D'Alo' F, Voso MT, Larocca LM, Leone G.
Clinical significance of interleukin-10 gene polymorphisms and plasma
levels in Hodgkin lymphoma. Leuk Res. 2009 Oct,33(10):1352-6.

- Baumforth KR, Birgersdotter A, Reynolds
GM, Wei W, Kapatai G, Flavell JR, Kalk E, Piper K, Lee S, Machado L,
Hadley K, Sundblad A, Sjoberg J, Bjorkholm M, Porwit AA, Yap LF, Teo S,
Grundy RG, Young LS, Ernberg I, Woodman CB, Murray PG. Expression of
the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in
Hodgkin's lymphoma cells mediates Up-regulation of CCL20 and the
migration of regulatory T cells. Am J Pathol. 2008 Jul,173(1):195-204

- Baumforth KR, Flavell JR, Reynolds GM,
Davies G, Pettit TR, Wei W, Morgan S, Stankovic T, Kishi Y, Arai H,
Nowakova M, Pratt G, Aoki J, Wakelam MJ, Young LS, Murray PG. Induction
of autotaxin by the Epstein-Barr virus promotes the growth and survival
of Hodgkin lymphoma cells. Blood. 2005 Sep 15,106(6):2138-46

- Chetaille B, Bertucci F, Finetti P,
Esterni B, Stamatoullas A, Picquenot JM, Copin MC, Morschhauser F,
Casasnovas O, Petrella T, Molina T, Vekhoff A, Feugier P, Bouabdallah
R, Birnbaum D, Olive D, Xerri L. Molecular profiling of classical
Hodgkin lymphoma tissues uncovers variations in the tumor
microenvironment and correlations with EBV infection and outcome.
Blood. 2009 Mar 19,113(12):2765-3775

- Gulley ML, Tang W.Laboratory assays for
Epstein-Barr virus-related disease.J Mol Diagn. 2008 Jul,10(4):279-92

- Hjalgrim H, Engels EA. Infectious
aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the
epidemiological evidence. J Intern Med. 2008 Dec,264(6):537-48. Review

- Jarrett RF. Viruses and Hodgkin's lymphoma. Ann Oncol. 2002,13 Suppl 1:23-9.
- Jarrett RF, Krajewski AS, Angus B,
Freeland J, Taylor PR, Taylor GM, Alexander FE. The Scotland and
Newcastle epidemiological study of Hodgkin's disease: impact of
histopathological review and EBV status on incidence estimates. J Clin
Pathol. 2003 Nov,56(11):811-6.

- Hjalgrim H, Askling J, Sĝrensen P, Madsen
M, Rosdahl N, Storm HH, Hamilton-Dutoit S, Eriksen LS, Frisch M, Ekbom
A, Melbye M. Risk of Hodgkin's disease and other cancers after
infectious mononucleosis. J Natl Cancer Inst. 2000 Sep 20,92(18):1522-8

- Hjalgrim H, Askling J, Rostgaard K,
Hamilton-Dutoit S, Frisch M, Zhang JS, Madsen M, Rosdahl N, Konradsen
HB, Storm HH, Melbye M. Characteristics of Hodgkin's lymphoma after
infectious mononucleosis. N Engl J Med. 2003 Oct 2,349(14):1324-32

- Chang KL, Albújar PF, Chen YY, Johnson RM,
Weiss LM. High prevalence of Epstein-Barr virus in the Reed-Sternberg
cells of Hodgkin's disease occurring in Peru. Blood. 1993 Jan
15,81(2):496-501

- Zarate-Osorno A, Roman LN, Kingma DW,
Meneses-Garcia A, Jaffe ES. Hodgkin's disease in Mexico. Prevalence of
Epstein-Barr virus sequences and correlations with histologic subtype.
Cancer. 1995 Mar 15,75(6):1360-6

- Quintanilla-Martínez L, Gamboa-Domńquez A,
Gamez-Ledesma I, Angeles-Angeles A, Mohar A. Association of
Epstein-Barr virus latent membrane protein and Hodgkin's disease in
Mexico. Mod Pathol. 1995 Aug,8(6):675-9

- Zhou XG, Hamilton-Dutoit SJ, Yan QH,
Pallesen G. The association between Epstein-Barr virus and Chinese
Hodgkin's disease. Int J Cancer. 1993 Sep 30,55(3):359-63

- Leoncini L, Spina D, Nyong'o A, Abinya O,
Minacci C, Disanto A, De Luca F, De Vivo A, Sabattini E, Poggi S,
Pileri S, Tosi P. Neoplastic cells of Hodgkin's disease show
differences in EBV expression between Kenya and Italy. Int J Cancer.
1996 Mar 15,65(6):781-4

- Weinreb M, Day PJ, Niggli F, Powell JE,
Raafat F, Hesseling PB, Schneider JW, Hartley PS,
Tzortzatou-Stathopoulou F, Khalek ER, Mangoud A, El-Safy UR, Madanat F,
Al Sheyyab M, Mpofu C, Revesz T, Rafii R, Tiedemann K, Waters KD,
Barrantes JC, Nyongo A, Riyat MS, Mann JR. The role of Epstein-Barr
virus in Hodgkin's disease from different geographical areas. Arch Dis
Child. 1996 Jan,74(1):27-31

- Yilmaz F, Uzunlar AK, Sogutcu N, Ozaydin
M. Hodgkin's disease and association with Epstein-Barr virus in
children in Southeast Turkey. Saudi Med J. 2005 Apr,26(4):571-5

- Benharroch D, Brousset P, Goldstein J,
Prinsloo I, Rabinovitch D, Shendler Y, Ariad S, Levy A, Delsol G, Gopas
J. Association of the Epstein-Barr virus with Hodgkin's disease in
Southern Israel. Int J Cancer. 1997 Apr 10,71(2):138-41

- Makar RR, Saji T, Junaid TA. Epstein-Barr
virus expression in Hodgkin's lymphoma in Kuwait. Pathol Oncol Res.
2003,9(3):159-65

- Al-Salam S, John A, Daoud S, Chong SM,
Castella A. Expression of Epstein-Barr virus in Hodgkin lymphoma in a
population of United Arab Emirates nationals. Leuk Lymphoma. 2008
Sep,49(9):1769-77

- Hjalgrim H, Seow A, Rostgaard K, Friborg
J. Changing patterns of Hodgkin lymphoma incidence in Singapore. Int J
Cancer. 2008 Aug 1,123(3):716-9

- laser SL, Gulley ML, Clarke CA, Keegan
TH, Chang ET, Shema SJ, Craig FE, Digiuseppe JA, Dorfman RF, Mann RB,
Anton-Culver H, Ambinder RF. Racial/ethnic variation in EBV-positive
classical Hodgkin lymphoma in California populations. Int J Cancer.
2008 Oct 1,123(7):1499-507.

- Diepstra A, Niens M, Vellenga E, van
Imhoff GW, Nolte IM, Schaapveld M, van der Steege G, van der Berg A,
Kibbelaar RE, te Meerman GJ, Poppema S. Association with HLA class I in
Epstein-Barr-virus-positive and with HLA class III in
Epstein-Barr-virus-negative Hodgkin’s lymphoma. Lancet. 2005 Jun 25-Jul
1,365(9478): 2216-24

- Niens M, Jarrett RF, Hepkema B, Nolte IM,
Diepstra A, Platteel M, Kouprie N, Delury CP, Gallagher A, Visser L,
Poppema S, te Meerman GJ, van den Berg A. HLA-A*02 is associated with a
reduced risk and HLA-A*01 with an increased risk of developing EBV+
Hodgkin lymphoma. Blood. 2007 Nov 1,110(9):3310-5

- Hohaus S, Giachelia M, Di Febo A, Martini
M, Massini G, Vannata B, D'Alo' F, Guidi F, Greco M, Pierconti F,
Larocca LM, Voso MT, Leone G. Polymorphism in cytokine genes as
prognostic markers in Hodgkin's lymphoma. Ann Oncol. 2007
Aug,18(8):1376-81

- Hatta K, Morimoto A, Ishii E, Kimura H,
Ueda I, Hibi S, Todo S, Sugimoto T, Imashuku S. Association of
transforming growth factor-beta1 gene polymorphism in the development
of Epstein-Barr virus-related hematologic diseases. Haematologica. 2007
Nov,92(11):1470-4

- Chang ET, Birmann BM, Kasperzyk JL, Conti
DV, Kraft P, Ambinder RF, Zheng T, Mueller NE. Polymorphic variation in
NFKB1 and other aspirin-related genes and risk of Hodgkin lymphoma.
Cancer Epidemiol Biomarkers Prev. 2009 Mar,18(3):976-86

- Flavell KJ, Billingham LJ, Biddulph JP,
Gray L, Flavell JR, Constandinou CM, Young LS, Murray PG. The effect of
Epstein-Barr virus status on outcome in age- and sex-defined subgroups
of patients with advanced Hodgkin's disease. Ann Oncol. 2003
Feb,14(2):282-90

- Glavina-Durdov M, Jakic-Razumovic J,
Capkun V, Murray P. Assessment of the prognostic impact of the
Epstein-Barr virus-encoded latent membrane protein-1 expression in
Hodgkin's disease. Br J Cancer. 2001 May 4,84(9):1227-34.

- Jarrett RF, Stark GL, White J, Angus B,
Alexander FE, Krajewski AS, Freeland J, Taylor GM, Taylor PR, Scotland
and Newcastle Epidemiology of Hodgkin Disease Study Group. Impact of
tumor Epstein-Barr virus status on presenting features and outcome in
age-defined subgroups of patients with classic Hodgkin lymphoma: a
population-based study. Blood. 2005 Oct 1,106(7):2444-51

- Kwon JM, Park YH, Kang JH, Kim K, Ko YH,
Ryoo BY, Lee SS, Lee SI, Koo HH, Kim WS. The effect of Epstein-Barr
virus status on clinical outcome in Hodgkin's lymphoma. Ann Hematol.
2006 Jul,85(7):463-8

- Clarke CA, Glaser SL, Dorfman RF, Mann R,
DiGiuseppe JA, Prehn AW, Ambinder RF. Epstein-Barr virus and survival
after Hodgkin disease in a population-based series of women. Cancer.
2001 Apr 15,91(8):1579-87

- Diepstra A, van Imhoff GW, Schaapveld M,
Karim-Kos H, van den Berg A, Vellenga E, Poppema S. Latent Epstein-Barr
virus infection of tumor cells in classical Hodgkin's lymphoma predicts
adverse outcome in older adult patients. J Clin Oncol. 2009 Aug
10,27(23):3815-21.

- Keegan TH, Glaser SL, Clarke CA, Gulley
ML, Craig FE, Digiuseppe JA, Dorfman RF, Mann RB, Ambinder RF.
Epstein-Barr virus as a marker of survival after Hodgkin's lymphoma: a
population-based study. J Clin Oncol. 2005 Oct 20,23(30):7604-13. Epub
2005 Sep 26. Review.

- Claviez A, Tiemann M, Lüders H, Krams M,
Parwaresch R, Schellong G, Dörffel W. Impact of latent Epstein-Barr
virus infection on outcome in children and adolescents with Hodgkin's
lymphoma. J Clin Oncol. 2005 Jun 20,23(18):4048-56

- Au WY, Pang A, Choy C, Chim CS, Kwong YL.
Quantification of circulating Epstein-Barr virus (EBV) DNA in the
diagnosis and monitoring of natural killer cell and EBV-positive
lymphomas in immunocompetent patients. Blood. 2004 Jul 1,104(1):243-9

- Gallagher A, Armstrong AA, MacKenzie J,
Shield L, Khan G, Lake A, Proctor S, Taylor P, Clements GB, Jarrett RF.
Detection of Epstein-Barr virus (EBV) genomes in the serum of patients
with EBV-associated Hodgkin's disease. Int J Cancer. 1999 Aug
20,84(4):442-8.

- Gandhi MK, Lambley E, Burrows J, Dua U,
Elliott S, Shaw PJ, Prince HM, Wolf M, Clarke K, Underhill C, Mills T,
Mollee P, Gill D, Marlton P, Seymour JF, Khanna R. Plasma Epstein-Barr
virus (EBV) DNA is a biomarker for EBV-positive Hodgkin's lymphoma.
Clin Cancer Res. 2006 Jan 15,12(2):460-4

- Lei KI, Chan LY, Chan WY, Johnson PJ, Lo
YM. Quantitative analysis of circulating cell-free Epstein-Barr virus
(EBV) DNA levels in patients with EBV-associated lymphoid malignancies.
Br J Haematol. 2000 Oct,111(1):239-46

- Khan G, Lake A, Shield L, Freeland J,
Andrew L, Alexander FE, Jackson R, Taylor PR, McCruden EA, Jarrett RF.
Phenotype and frequency of Epstein-Barr virus-infected cells in
pretreatment blood samples from patients with Hodgkin lymphoma. Br J
Haematol. 2005 May,129(4):511-9

- Drouet E, Brousset P, Fares F, Icart J,
Verniol C, Meggetto F, Schlaifer D, Desmorat-Coat H, Rigal-Huguet F,
Niveleau A, Delsol G. High Epstein-Barr virus serum load and elevated
titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor
cells of Hodgkin's disease. J Med Virol. 1999 Apr,57(4):383-9.

- Wagner HJ, Schläger F, Claviez A, Bucsky
P. Detection of Epstein-Barr virus DNA in peripheral blood of
paediatric patients with Hodgkin's disease by real-time polymerase
chain reaction. Eur J Cancer. 2001 Oct,37(15):1853-7

- Gandhi MK, Moll G, Smith C, Dua U,
Lambley E, Ramuz O, Gill D, Marlton P, Seymour JF, Khanna R. Galectin-1
mediated suppression of Epstein-Barr virus specific T-cell immunity in
classic Hodgkin lymphoma. Blood. 2007 Aug 15,110(4):1326-9.

- Faller DV, Mentzer SJ, Perrine SP.
Induction of the Epstein-Barr virus thymidine kinase gene with
concomitant nucleoside antivirals as a therapeutic strategy for
Epstein-Barr virus-associated malignancies. Curr Opin Oncol. 2001
Sep,13(5):360-7

- Perrine SP, Hermine O, Small T, Suarez F,
O'Reilly R, Boulad F, Fingeroth J, Askin M, Levy A, Mentzer SJ, Di
Nicola M, Gianni AM, Klein C, Horwitz S, Faller DV. A phase 1/2 trial
of arginine butyrate and ganciclovir in patients with Epstein-Barr
virus-associated lymphoid malignancies. Blood.
2007 Mar 15;109(6):2571-8. Epub 2006 Nov 21.
