Invasive Candidiasis in Non-Hematological Patients
Malgorzata Mikulska, Matteo Bassetti, Sandra Ratto and Claudio Viscoli2
1Division of
Infectious Diseases, San Martino University Hospital, Genoa, Italy.
Correspondence
to: Malorzata
Mikulska , Division of Infectious Diseases, San Martino University
Hospital, Genoa, Italy. m_mikulska@yahoo.com,
claudio.viscoli@unige.it
Published: 2011
Received: January 4, 2011
Accepted: January 17, 2011
Medit J Hemat Infect Dis 2011, 3: e2011007, DOI 10.4084/MJHID.2011.007
This article is available from: http://www.mjhid.org/article/view/7453
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Candida is one of the most
frequent pathogens isolated in bloodstream infections, and is
associated with significant morbidity and mortality. In addition to
haematological patients, there are several other populations with a
substantial risk of developing invasive candidiasis (IC). These include
patients undergoing prolonged hospitalisation with the use of
broad-spectrum antibiotics, those fitted with intravascular catheters,
admitted to both adult and neonate intensive care units (ICU) or
gastrointestinal surgery wards and subjects with solid tumours
undergoing cytotoxic chemotherapy. As a general rule, every
immunocompromised patient might be at risk of Candida infection,
including, for example, diabetic patients.
The epidemiology of species responsible for IC has been changing, both at local and worldwide level, shifting from C. albicans to non-albicans species, that can be intrinsically resistant to fluconazole (C. krusei and, to some extent, C. glabrata), difficult to eradicate because of biofilm production (C. parapsilosis) or than might acquire resistance to azole during therapy.
Delaying the specific therapy has been shown to increase morbidity and mortality, but traditional microbiological diagnosis is poorly sensitive and slow. Thus, culture-based treatment may result in therapy started too late. In order to reduce the mortality in IC, several management strategies have been developed: prophylaxis, empirical and pre-emptive therapy. Compared to prophylaxis, the latter approaches allow to reduce the use of antifungals by targeting only patients at very high risk of IC. Non-invasive serological markers and scores based on clinical prediction rules such as the presence of risk factors or Candida colonisation, have been developed with the aim of allowing prompt initiation of treatment. Although the use of these diagnostic tools in pre-emptive strategies is promising, the performance and cost-effectiveness should be tested in large trials.
Agents recommended for initial treatment of candidemia in severely ill patients include echinocandins and lipid formulations of amphotericin B, while stable patients without risk factors for azole-resistance might be treated with fluconazole.
The epidemiology of species responsible for IC has been changing, both at local and worldwide level, shifting from C. albicans to non-albicans species, that can be intrinsically resistant to fluconazole (C. krusei and, to some extent, C. glabrata), difficult to eradicate because of biofilm production (C. parapsilosis) or than might acquire resistance to azole during therapy.
Delaying the specific therapy has been shown to increase morbidity and mortality, but traditional microbiological diagnosis is poorly sensitive and slow. Thus, culture-based treatment may result in therapy started too late. In order to reduce the mortality in IC, several management strategies have been developed: prophylaxis, empirical and pre-emptive therapy. Compared to prophylaxis, the latter approaches allow to reduce the use of antifungals by targeting only patients at very high risk of IC. Non-invasive serological markers and scores based on clinical prediction rules such as the presence of risk factors or Candida colonisation, have been developed with the aim of allowing prompt initiation of treatment. Although the use of these diagnostic tools in pre-emptive strategies is promising, the performance and cost-effectiveness should be tested in large trials.
Agents recommended for initial treatment of candidemia in severely ill patients include echinocandins and lipid formulations of amphotericin B, while stable patients without risk factors for azole-resistance might be treated with fluconazole.
Introduction
Candida is a yeast responsible for the majority of fungal infections in humans. This fungus causes pathologies of different severity, ranging from mucocutaneous infections to invasive disease that can involve any organ. The incidence of invasive candidiasis (IC), particularly candidemia, has increased significantly in recent years and Candida spp. is now the fourth most common pathogen isolated in blood cultures in the US.[1] In Europe it ranks among the ten most frequently isolated pathogens.[2,3] Candidemia is a life-threatening infection with high morbidity and mortality.[4-7] Even in the most recent studies, crude mortality rates reached 50-60% in critically ill patients,[8-10] although attributable mortality can be substantially lower.
Immunocompromised patients, such as those affected by solid tumours or haematological malignancies are at high risk for developing Candida infection. However, the widespread use of fluconazole prophylaxis in haematological and stem cell transplant settings might be responsible for a decreased incidence of invasive Candida infections in these populations.[11] On the contrary, patients with multiple severe comorbidities, undergoing gastrointestinal surgery or admitted to ICU constitute now the largest population at risk for developing candidemia.[12] In fact, IC can affect up to about 10% of all critically ill subjects.[13,14] Fungal infections are being increasingly diagnosed in these patients, because advances in medical science now allow patients in desperate underlying conditions to survive. However, this is not obtained without a price, such as the development of infectious complications. Therefore, the population of subjects vulnerable to a range of infections is increasing and this trend will likely continue.
From a clinical point of view, Candida causes bloodstream infections, sometimes with endophtalmitis, followed by peritonitis and other abdominal infection and endocarditis. A matter of debate can be how often a blood culture positive for Candida represents the external sign of a deep-seated infection, or it is simply a bloodstream infection without localisation. Most of the patients included in studies on epidemiology or treatment of invasive candidiasis had candidemia (approximately 68-90%), with or without localisation, while peritonitis was the second most common disease (approximately 7-30% of subjects).[9,15,16] In a recent French study, isolated candidemia, IC with candidemia and IC without candidemia accounted each for 1/3 of all episodes of IC.[9] Additionally, Candida accounted for approximately 3% of all surgery-related peritoneal infections, both community-acquired and nosocomial.[9]
On the of main points regarding invasive Candida infection is the fact that delaying antifungal treatment significantly increases mortality.[17-20] Even 12-24 hours delay can result in twofold increase in crude mortality rate in candidemia.[21,22] However, nosocomial fungal infections have one of the highest rates of inappropriate therapy, that consists mostly of omission of including an antifungal in the initial empirical therapy and use the of inadequate doses, all of which have been associated with increased mortality.[12,21-23] Additionally, the estimated cost of each episode of IC in hospitalised adults is tremendous.[24,25] Thus, high awareness of this infection, early diagnosis and appropriate prompt therapy remain the cornerstone of treatment.
During the last decade several new antifungal drugs have been developed and obtained approval for treatment of Candida infections. Therefore, treating a candidemia has become a difficult exercise, because of the need to make the appropriate choice at the appropriate time. In the following lines we will try to discuss epidemiology, risk factors, diagnosis and management of IC in non-haematological patients.
Epidemiology of invasive candidiasis
The epidemiology of Candida infections, both on a worldwide scale, and more importantly on the local level, has significant implications for the management of these infections.
During the past two decades, most hospitals have reported a progressive shift in the species of Candida. In the past, almost all the isolates responsible for bloodstream infections were C. albicans, whereas in recent years a growing proportion of episodes of candidemia have been caused by Candida species other than albicans.[26-31] Although, C. albicans remains the predominant strain in most countries,[9,32,33] non-albicans species are increasingly common and in some adult ICUs they were responsible for over 50% of candidemias.[29,34] The most common non-albicans species are C. parapsilosis and C. glabrata, followed by C. tropicalis and C. krusei.[9,29,35-37] Rare species reported to cause candidemia include C. lusitaniae, C. guilliermondii, and C. rugosa.[12,35]
Numerous studies have tried to find reasons for this shift and several risk factors have been associated with the emergence of non-albicans species.[30,38,39] It is likely that the widespread use of fluconazole can predispose patients to the development of infections due to species that are intrinsically resistant to azoles or have developed resistance during treatment. Indeed, the previous use of fluconazole has been found to be a risk factor for the presence of non-albicans fungemia in many studies,[29,30,40] even though others did not find the same association.[28] In particular, risk factors for candidemia due to C. parapsilosis include the presence of in-dwelling devices, hyperalimentation and neonatal age.[35] The specific risk factors associated with IC and with different Candida species are outlined in table 1.
The overall rise in the incidence of non-albicans strains is alarming, since there are important differences among species. Specifically, the main difference between C. albicans and C. krusei or C. glabrata is the resistance to the most frequently used antifungal, i.e. fluconazole.[41] Therefore, species identification and the knowledge of local epidemiology of Candida strains causing candidemia is of utmost importance for guiding appropriate empirical therapy. In vitro susceptibility testing of clinical isolates of Candida might prove valuable for guiding therapy in patients who have received prior antifungal treatment or who are not responding to first line therapy, especially if performed by experience microbiologists.
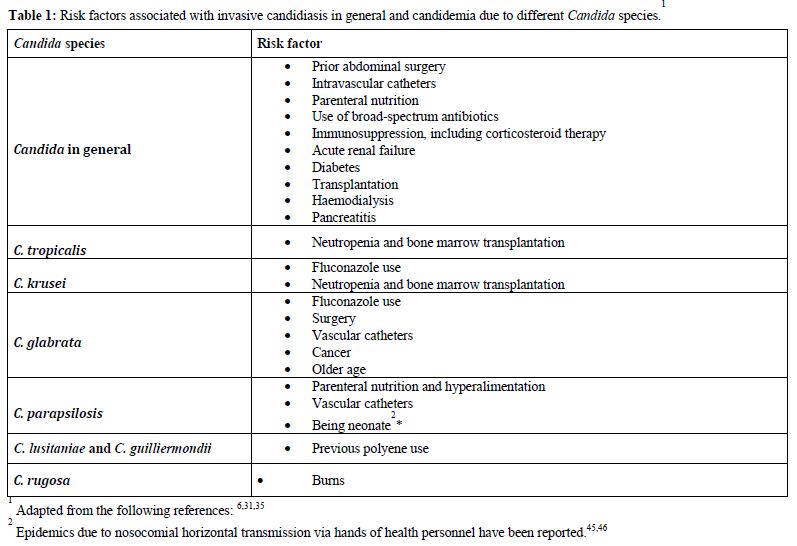
Table 1. Risk factors associated with invasive candidiasis in general and candidemia due to different Candida species
Risk factors for invasive candidiasis and predictive scores
The predominant source of invasive Candida infections is endogenous, from superficial mucosal and cutaneous colonisation to haematogenous dissemination,42 although cases of exogenous transmission due to contaminated materials or transmission from healthcare workers to patients and from patients to patients have been described.[43-46] The suppression of the normal bacterial flora of the gastrointestinal tract by broad spectrum antibiotic therapy allows the yeast to proliferate and long-term and high density colonisation has been shown to predispose to candidemia.[47,48] Numerous other conditions, frequent in hospitalised patients, such as steroid treatment and poor control of blood glucose concentrations (diabetes) have been described. In addition, parenteral nutrition, intravascular catheters or ischemia and reperfusion, may damage the integrity of the skin or gastrointestinal mucosa, with traslocation and bloodstream invasion. In particular, as much as one third of patients with recurrent gastrointestinal perforations, anastomotic leaks or necrotising pancreatitis develop IC (table 1).[49,50]
The effort to identify patients who are at high risk of developing IC has been made in order to reduce mortality by offering them prophylaxis, empirical or pre-emptive treatment. Once risk factors have been reported, they were combined to create reliable risk prediction scores.
Candida colonisation index (CI), reported in 1994, was studied in a surgical population with the aim of predicting patients who would develop IC,[48] and was used as a base for pre-emptive therapy.[51] Although it is highly predictive for IC, its routine use has been limited by workload required and consequent costs.
In 2006, Leon and colleagues described their Candida Score (CS) system, that was helpful to select patients who could benefit from early antifungal therapy (those with CS > 2.5 were almost 8 times more likely to develop IC than those with CS < 2.5).[52] Subsequently, the same group validated their CS in a prospective multicenter trial that included 1107 patients admitted for at least 7 days to ICU.[53] CS was calculated as follows: 1 point for the presence of parenteral nutrition, surgery or multifocal Candida colonization, 2 points for severe sepsis. In patients with Candida Score <3, the incidence of IC was 2.3%, thus allowing to withhold empirical antifungal treatment. On the contrary, one of four patients with a CS of 5 developed IC.[53]
Another clinical risk prediction score was developed by Ostrosky-Zeichner and colleagues. In this study, systemic antibiotic treatment or central venous catheter, combined with two or more of additional five parameters (parenteral nutrition, dialysis, major surgery, pancreatitis, treatment with steroids or other immunosuppressive agents), were able to identify patients with candidemia, with positive and negative predictive values of 10% and 97%, respectively.[54]
Finally, Dupont and colleagues studied a predictive score for peritoneal Candida infection in an ICU population and found that the presence of 3 out of 4 factors (female gender, upper gastrointestinal tract origin of peritonitis, intraoperative cardiovascular failure and previous antibiotic therapy) had positive and negative predictive values of 67% and 72%, respectively.[55]
Diagnosis of candidemia
Blood cultures remain the mainstay for the diagnosis of candidemia, although sensitivity is not optimal and the time from the blood sample collection to the microbiological response of a growing yeast is long. Furthermore, at least 24-48 hours are required for species identification and susceptibility testing. Traditional cultures from sterile sites other than the bloodstream (e.g. peritoneum), remain useful for the diagnosis IC, but more sensitive and more rapid diagnostic methods are needed.
In recent years, non-invasive markers have been investigated, which include serological markers (mannan, antimannan and (1,3)-beta-D-glucan) and polymerase chain reaction. Although the mannan and antimannan commercially available ELISA tests have been marketed for almost 10 years, the only data derive from a single-centre studies that differ significantly in terms of sensitivity and specificity.[56-59] The (1,3)-beta-D-glucan test has been marketed more recently in Europe and in the US. Despite promising results in various cohorts, no large prospective study able to evaluate sensitivity, specificity, and especially cost-effectiveness, has been performed.[60,61] The main problems of the routine use of (1,3)-beta-D-glucan are its high cost and high rate of false positive results. Indeed, (1,3)-beta-D-glucan is ubiquitous in nature contamination can be caused by concomitant bacterial bloodstream infections, presence of surgical gauzes, use of glucan-containing membranes for haemofiltration and use of albumin or immunoglobulins.[62] For example, in a study that focused on the validation of the Candida Score, (1,3)-beta-D-glucan testing was performed in a subgroup of 240 patients with Candida species colonisation or invasive fungal infection.[53] For a cut-off of 75 pg/ml, good sensitivity of 77.8% was reported, but the specificity was low (52.7%). In particular, among patients with a positive result, only 12% developed documented invasive candidiasis. However, a positive (1,3)-beta-D-glucan result is one of microbiological criteria defining a probable invasive fungal infection according to 2008 definitions of invasive fungal disease published by the European Organization for Research and Treatment of Cancer and the Infectious Diseases Mycoses Study Group (EORTC/MSG).[63]
Finally, two new rapid methods are available for species identification and they include matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and fluorescence in-situ hybridization (FISH).
Management of candidemia in non-haematological setting
Different management strategies cen be used for managing suspected or documented IC, including prophylaxis, empirical or pre-emptive therapy and treatment of a culture-proven infection. Based on the incidence of IC, prophylaxis may be judged appropriate in patients with high risk of IC (incidence > 10%). In settings with lower incidence rate, patients might benefit from pre-emptive strategies based on predictive scores. Obviously, the knowledge of local epidemiology helps to define the most appropriate antifungal therapy, based on the most frequent species and susceptibility patterns of Candida isolated in a single centre.
Prophylaxis, defined as administration of an antifungal to a patient with no evidence of infection, has been evaluated in surgical and critically ill patients in several studies and metaanalyses.[33,64-73] Fluconazole prophylaxis reduced by approximately 50% the incidence of IC, and seemed associated with improved outcome.[70-72] Naturally, antifungal prophylaxis is efficacious and cost-effective in populations with high prevalence of IC, when the number of patients that need to receive the prophylactic treatment in order to prevent one episode of IC (number needed to treat) is low. On the other hand, the disadvantages of fluconazole prophylaxis include overtreatment, possible toxicity and profound influence on local epidemiology with the emergence of azole-resistant isolates.[74] Therefore, antifungal prophylaxis might be indicated only for patients or procedures in which the rate of IC is higher than 10%, as compared to the normal rates of 1-2%.[41,54,75] In such populations, the number needed to treat is less than 20, as compared with over 100 in an average population of ICU patients with the incidence of IC of 2%.
Empirical treatment is defined as the administration of antifungals in the presence of persistent or refractory fever in subjects who are at high risk of developing a fungal infection. This strategy has been developed almost 3 decades ago for neutropenic cancer patients, when it became evident that the lack of sensitivity of microbiological and clinical findings was likely resulting in delayed diagnosis and increased morbidity and mortality.[76] Although the first studies on empirical therapy had numerous methodological flaws, this fever-driven strategy is being used in different clinical settings and various antifungals are recommended for empirical treatment of invasive candidiasis, both in neutropenic and non-neutropenic patients.[41] However, in ICU or surgery patients, there are several causes of protracted fever and, probably for this reason, in a randomised multicenter study in critically ill patients, the empirical therapy with fluconazole was not more beneficial than placebo.[77]
With the availability of diagnostic tools such as radiological imaging, invasive diagnostic procedures, improved cultures techniques and serological markers, it became evident that a diagnosis-driven approach was possible and should be pursued. Pre-emptive treatment is characterised by starting antifungal therapy when one or more microbiological or clinical markers result positive. Microbiological markers include multiple colonisation, positivity of mannan, (1,3)-beta-D-glucan or molecular testing.[60,62] However, there is a certain degree of confusion between prophylaxis, empirical and pre-emptive treatment in patients with high risk of IC, as defined by high Candida colonisation index. In fact, the IDSA guidelines recommend a pre-emptive approach (although they continue to call it empirical treatment) based on clinical assessment of risk factors, serologic markers, and/or culture data from nonsterile sites, rather than fever.[41]
Despite all the advances in diagnostic tools, it is to be remembered that repeated blood cultures, both from CVC and peripheral line, remain the cornerstone of diagnosis of candidemia, and that any positive blood culture for Candida must be taken seriously and needs appropriate treatment.
Broad spectrum antifungals are recommended for the first line treatment while species identification is pending, but when species is known, a de-escalation can be recommended.[41] The initial choice of antifungals depends on patient’s clinical condition and the risk of azole-resistant strain, due to previous azole exposure or local epidemiology.[41] For patients in severe or moderately severe clinical conditions (e.g. hemodynamically unstable, or with suspected concomitant organ involvement), echinocandins are the first choice because of their cidal activity against Candida and excellent toxicity profile.[41] Liposomal amphotericin B - another fungicidal agent indicated for first line treatment in critically ill patients, is more expensive and probably associated with a higher toxicity.
Other aspects of treating invasive candidiasis
Once the initial therapy for candidemia is started, several clinical issues remain open. First, the efficacy of the treatment should be assessed by the documentation of blood cultures returning sterile. Indeed, the date of the first negative blood culture is important, because the recommended length of treatment is 14 days after the last positive blood culture and resolution of symptoms attributable to candidemia.
Second, the antifungal chosen initially can be changed on the basis of species identification or susceptibility testing. Thus, for stable patients with C. albicans or other azole-susceptible strains, fluconazole probably remains the drug of choice. Fluconazole might be preferred over echinocandins for treating C parapsilosis, as caspofungin MICs for C. parapsilosis are higher than those for other Candida species.[41,78] However, in a recent analysis of data from five clinical trials, that included 71 cases of infection due to C. parapsilosis, the success rate was comparable with other non-albicans species.[79]
Third, patients who improve clinically and who cleared Candida from the bloodstream, might be suitable for step-down oral therapy to complete the course of 14 days. The available oral antifungals are fluconazole, itraconazole, voriconazole and posaconazole. Fluconazole is an obvious choice for susceptible species, while voriconazole can be indicated as step-down therapy for C. krusei or voriconazole-susceptible C. glabrata and in ocular or cerebral infections, because of excellent tissue concentration.
Additionally, ophthalmologic fundus examination is indicated in all patients to exclude endocular infection, while endocarditis should be excluded in case of persistently positive blood cultures, known valve pathology or any other sign or symptom suggestive of endocardial involvement. As described elsewhere, in both these complicated cases the duration of treatment should be much longer (more than 4 weeks and up to lifelong suppressive therapy).[41]
Finally, intravenous catheter removal is strongly recommended for patients with candidemia. Indeed all guidelines, both on the management of candidiasis and on the management of catheter-related bloodstream infections, state clearly that catheters should be removed, even though one should admit that all statements indicate grade II or III of scientific validity of recommendation, in absence of data from properly randomised, controlled trials.[41,80,81] However, the issue might still be controvertial since a recent study, based on a multivariate analysis of 842 adults included in candidemia trials, did not find any benefit of early central venous catheter removal (i.e. within 24 or 48 hours after initiation of antifungal therapy) on survival.[81]
Conclusions
Candida is one of the most common causes of nosocomial bloodstream infection. Non-neutropenic patients now constitute a large but heterogeneous population of patients at risk of IC, which includes subjects admitted to adult or neonatal ICU, undergoing abdominal surgery and those with cancer or numerous medical comorbidities 8e.g. diabetes). Morbidity and mortality associated with candidemia are significant and the epidemiology of species have been shifting towards non-albicans strains. Even though numerous risk factors for invasive Candida infection have been reported and several antifungals are widely available, the optimal management of candidemia remains a challenge. Prophylaxis might be beneficial in population with incidence > 10%, while novel diagnostic techniques should be further studied to enable pre-emptive treatment in populations with lower incidence rates.
Candida is a yeast responsible for the majority of fungal infections in humans. This fungus causes pathologies of different severity, ranging from mucocutaneous infections to invasive disease that can involve any organ. The incidence of invasive candidiasis (IC), particularly candidemia, has increased significantly in recent years and Candida spp. is now the fourth most common pathogen isolated in blood cultures in the US.[1] In Europe it ranks among the ten most frequently isolated pathogens.[2,3] Candidemia is a life-threatening infection with high morbidity and mortality.[4-7] Even in the most recent studies, crude mortality rates reached 50-60% in critically ill patients,[8-10] although attributable mortality can be substantially lower.
Immunocompromised patients, such as those affected by solid tumours or haematological malignancies are at high risk for developing Candida infection. However, the widespread use of fluconazole prophylaxis in haematological and stem cell transplant settings might be responsible for a decreased incidence of invasive Candida infections in these populations.[11] On the contrary, patients with multiple severe comorbidities, undergoing gastrointestinal surgery or admitted to ICU constitute now the largest population at risk for developing candidemia.[12] In fact, IC can affect up to about 10% of all critically ill subjects.[13,14] Fungal infections are being increasingly diagnosed in these patients, because advances in medical science now allow patients in desperate underlying conditions to survive. However, this is not obtained without a price, such as the development of infectious complications. Therefore, the population of subjects vulnerable to a range of infections is increasing and this trend will likely continue.
From a clinical point of view, Candida causes bloodstream infections, sometimes with endophtalmitis, followed by peritonitis and other abdominal infection and endocarditis. A matter of debate can be how often a blood culture positive for Candida represents the external sign of a deep-seated infection, or it is simply a bloodstream infection without localisation. Most of the patients included in studies on epidemiology or treatment of invasive candidiasis had candidemia (approximately 68-90%), with or without localisation, while peritonitis was the second most common disease (approximately 7-30% of subjects).[9,15,16] In a recent French study, isolated candidemia, IC with candidemia and IC without candidemia accounted each for 1/3 of all episodes of IC.[9] Additionally, Candida accounted for approximately 3% of all surgery-related peritoneal infections, both community-acquired and nosocomial.[9]
On the of main points regarding invasive Candida infection is the fact that delaying antifungal treatment significantly increases mortality.[17-20] Even 12-24 hours delay can result in twofold increase in crude mortality rate in candidemia.[21,22] However, nosocomial fungal infections have one of the highest rates of inappropriate therapy, that consists mostly of omission of including an antifungal in the initial empirical therapy and use the of inadequate doses, all of which have been associated with increased mortality.[12,21-23] Additionally, the estimated cost of each episode of IC in hospitalised adults is tremendous.[24,25] Thus, high awareness of this infection, early diagnosis and appropriate prompt therapy remain the cornerstone of treatment.
During the last decade several new antifungal drugs have been developed and obtained approval for treatment of Candida infections. Therefore, treating a candidemia has become a difficult exercise, because of the need to make the appropriate choice at the appropriate time. In the following lines we will try to discuss epidemiology, risk factors, diagnosis and management of IC in non-haematological patients.
Epidemiology of invasive candidiasis
The epidemiology of Candida infections, both on a worldwide scale, and more importantly on the local level, has significant implications for the management of these infections.
During the past two decades, most hospitals have reported a progressive shift in the species of Candida. In the past, almost all the isolates responsible for bloodstream infections were C. albicans, whereas in recent years a growing proportion of episodes of candidemia have been caused by Candida species other than albicans.[26-31] Although, C. albicans remains the predominant strain in most countries,[9,32,33] non-albicans species are increasingly common and in some adult ICUs they were responsible for over 50% of candidemias.[29,34] The most common non-albicans species are C. parapsilosis and C. glabrata, followed by C. tropicalis and C. krusei.[9,29,35-37] Rare species reported to cause candidemia include C. lusitaniae, C. guilliermondii, and C. rugosa.[12,35]
Numerous studies have tried to find reasons for this shift and several risk factors have been associated with the emergence of non-albicans species.[30,38,39] It is likely that the widespread use of fluconazole can predispose patients to the development of infections due to species that are intrinsically resistant to azoles or have developed resistance during treatment. Indeed, the previous use of fluconazole has been found to be a risk factor for the presence of non-albicans fungemia in many studies,[29,30,40] even though others did not find the same association.[28] In particular, risk factors for candidemia due to C. parapsilosis include the presence of in-dwelling devices, hyperalimentation and neonatal age.[35] The specific risk factors associated with IC and with different Candida species are outlined in table 1.
The overall rise in the incidence of non-albicans strains is alarming, since there are important differences among species. Specifically, the main difference between C. albicans and C. krusei or C. glabrata is the resistance to the most frequently used antifungal, i.e. fluconazole.[41] Therefore, species identification and the knowledge of local epidemiology of Candida strains causing candidemia is of utmost importance for guiding appropriate empirical therapy. In vitro susceptibility testing of clinical isolates of Candida might prove valuable for guiding therapy in patients who have received prior antifungal treatment or who are not responding to first line therapy, especially if performed by experience microbiologists.

Table 1. Risk factors associated with invasive candidiasis in general and candidemia due to different Candida species
Risk factors for invasive candidiasis and predictive scores
The predominant source of invasive Candida infections is endogenous, from superficial mucosal and cutaneous colonisation to haematogenous dissemination,42 although cases of exogenous transmission due to contaminated materials or transmission from healthcare workers to patients and from patients to patients have been described.[43-46] The suppression of the normal bacterial flora of the gastrointestinal tract by broad spectrum antibiotic therapy allows the yeast to proliferate and long-term and high density colonisation has been shown to predispose to candidemia.[47,48] Numerous other conditions, frequent in hospitalised patients, such as steroid treatment and poor control of blood glucose concentrations (diabetes) have been described. In addition, parenteral nutrition, intravascular catheters or ischemia and reperfusion, may damage the integrity of the skin or gastrointestinal mucosa, with traslocation and bloodstream invasion. In particular, as much as one third of patients with recurrent gastrointestinal perforations, anastomotic leaks or necrotising pancreatitis develop IC (table 1).[49,50]
The effort to identify patients who are at high risk of developing IC has been made in order to reduce mortality by offering them prophylaxis, empirical or pre-emptive treatment. Once risk factors have been reported, they were combined to create reliable risk prediction scores.
Candida colonisation index (CI), reported in 1994, was studied in a surgical population with the aim of predicting patients who would develop IC,[48] and was used as a base for pre-emptive therapy.[51] Although it is highly predictive for IC, its routine use has been limited by workload required and consequent costs.
In 2006, Leon and colleagues described their Candida Score (CS) system, that was helpful to select patients who could benefit from early antifungal therapy (those with CS > 2.5 were almost 8 times more likely to develop IC than those with CS < 2.5).[52] Subsequently, the same group validated their CS in a prospective multicenter trial that included 1107 patients admitted for at least 7 days to ICU.[53] CS was calculated as follows: 1 point for the presence of parenteral nutrition, surgery or multifocal Candida colonization, 2 points for severe sepsis. In patients with Candida Score <3, the incidence of IC was 2.3%, thus allowing to withhold empirical antifungal treatment. On the contrary, one of four patients with a CS of 5 developed IC.[53]
Another clinical risk prediction score was developed by Ostrosky-Zeichner and colleagues. In this study, systemic antibiotic treatment or central venous catheter, combined with two or more of additional five parameters (parenteral nutrition, dialysis, major surgery, pancreatitis, treatment with steroids or other immunosuppressive agents), were able to identify patients with candidemia, with positive and negative predictive values of 10% and 97%, respectively.[54]
Finally, Dupont and colleagues studied a predictive score for peritoneal Candida infection in an ICU population and found that the presence of 3 out of 4 factors (female gender, upper gastrointestinal tract origin of peritonitis, intraoperative cardiovascular failure and previous antibiotic therapy) had positive and negative predictive values of 67% and 72%, respectively.[55]
Diagnosis of candidemia
Blood cultures remain the mainstay for the diagnosis of candidemia, although sensitivity is not optimal and the time from the blood sample collection to the microbiological response of a growing yeast is long. Furthermore, at least 24-48 hours are required for species identification and susceptibility testing. Traditional cultures from sterile sites other than the bloodstream (e.g. peritoneum), remain useful for the diagnosis IC, but more sensitive and more rapid diagnostic methods are needed.
In recent years, non-invasive markers have been investigated, which include serological markers (mannan, antimannan and (1,3)-beta-D-glucan) and polymerase chain reaction. Although the mannan and antimannan commercially available ELISA tests have been marketed for almost 10 years, the only data derive from a single-centre studies that differ significantly in terms of sensitivity and specificity.[56-59] The (1,3)-beta-D-glucan test has been marketed more recently in Europe and in the US. Despite promising results in various cohorts, no large prospective study able to evaluate sensitivity, specificity, and especially cost-effectiveness, has been performed.[60,61] The main problems of the routine use of (1,3)-beta-D-glucan are its high cost and high rate of false positive results. Indeed, (1,3)-beta-D-glucan is ubiquitous in nature contamination can be caused by concomitant bacterial bloodstream infections, presence of surgical gauzes, use of glucan-containing membranes for haemofiltration and use of albumin or immunoglobulins.[62] For example, in a study that focused on the validation of the Candida Score, (1,3)-beta-D-glucan testing was performed in a subgroup of 240 patients with Candida species colonisation or invasive fungal infection.[53] For a cut-off of 75 pg/ml, good sensitivity of 77.8% was reported, but the specificity was low (52.7%). In particular, among patients with a positive result, only 12% developed documented invasive candidiasis. However, a positive (1,3)-beta-D-glucan result is one of microbiological criteria defining a probable invasive fungal infection according to 2008 definitions of invasive fungal disease published by the European Organization for Research and Treatment of Cancer and the Infectious Diseases Mycoses Study Group (EORTC/MSG).[63]
Finally, two new rapid methods are available for species identification and they include matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and fluorescence in-situ hybridization (FISH).
Management of candidemia in non-haematological setting
Different management strategies cen be used for managing suspected or documented IC, including prophylaxis, empirical or pre-emptive therapy and treatment of a culture-proven infection. Based on the incidence of IC, prophylaxis may be judged appropriate in patients with high risk of IC (incidence > 10%). In settings with lower incidence rate, patients might benefit from pre-emptive strategies based on predictive scores. Obviously, the knowledge of local epidemiology helps to define the most appropriate antifungal therapy, based on the most frequent species and susceptibility patterns of Candida isolated in a single centre.
Prophylaxis, defined as administration of an antifungal to a patient with no evidence of infection, has been evaluated in surgical and critically ill patients in several studies and metaanalyses.[33,64-73] Fluconazole prophylaxis reduced by approximately 50% the incidence of IC, and seemed associated with improved outcome.[70-72] Naturally, antifungal prophylaxis is efficacious and cost-effective in populations with high prevalence of IC, when the number of patients that need to receive the prophylactic treatment in order to prevent one episode of IC (number needed to treat) is low. On the other hand, the disadvantages of fluconazole prophylaxis include overtreatment, possible toxicity and profound influence on local epidemiology with the emergence of azole-resistant isolates.[74] Therefore, antifungal prophylaxis might be indicated only for patients or procedures in which the rate of IC is higher than 10%, as compared to the normal rates of 1-2%.[41,54,75] In such populations, the number needed to treat is less than 20, as compared with over 100 in an average population of ICU patients with the incidence of IC of 2%.
Empirical treatment is defined as the administration of antifungals in the presence of persistent or refractory fever in subjects who are at high risk of developing a fungal infection. This strategy has been developed almost 3 decades ago for neutropenic cancer patients, when it became evident that the lack of sensitivity of microbiological and clinical findings was likely resulting in delayed diagnosis and increased morbidity and mortality.[76] Although the first studies on empirical therapy had numerous methodological flaws, this fever-driven strategy is being used in different clinical settings and various antifungals are recommended for empirical treatment of invasive candidiasis, both in neutropenic and non-neutropenic patients.[41] However, in ICU or surgery patients, there are several causes of protracted fever and, probably for this reason, in a randomised multicenter study in critically ill patients, the empirical therapy with fluconazole was not more beneficial than placebo.[77]
With the availability of diagnostic tools such as radiological imaging, invasive diagnostic procedures, improved cultures techniques and serological markers, it became evident that a diagnosis-driven approach was possible and should be pursued. Pre-emptive treatment is characterised by starting antifungal therapy when one or more microbiological or clinical markers result positive. Microbiological markers include multiple colonisation, positivity of mannan, (1,3)-beta-D-glucan or molecular testing.[60,62] However, there is a certain degree of confusion between prophylaxis, empirical and pre-emptive treatment in patients with high risk of IC, as defined by high Candida colonisation index. In fact, the IDSA guidelines recommend a pre-emptive approach (although they continue to call it empirical treatment) based on clinical assessment of risk factors, serologic markers, and/or culture data from nonsterile sites, rather than fever.[41]
Despite all the advances in diagnostic tools, it is to be remembered that repeated blood cultures, both from CVC and peripheral line, remain the cornerstone of diagnosis of candidemia, and that any positive blood culture for Candida must be taken seriously and needs appropriate treatment.
Broad spectrum antifungals are recommended for the first line treatment while species identification is pending, but when species is known, a de-escalation can be recommended.[41] The initial choice of antifungals depends on patient’s clinical condition and the risk of azole-resistant strain, due to previous azole exposure or local epidemiology.[41] For patients in severe or moderately severe clinical conditions (e.g. hemodynamically unstable, or with suspected concomitant organ involvement), echinocandins are the first choice because of their cidal activity against Candida and excellent toxicity profile.[41] Liposomal amphotericin B - another fungicidal agent indicated for first line treatment in critically ill patients, is more expensive and probably associated with a higher toxicity.
Other aspects of treating invasive candidiasis
Once the initial therapy for candidemia is started, several clinical issues remain open. First, the efficacy of the treatment should be assessed by the documentation of blood cultures returning sterile. Indeed, the date of the first negative blood culture is important, because the recommended length of treatment is 14 days after the last positive blood culture and resolution of symptoms attributable to candidemia.
Second, the antifungal chosen initially can be changed on the basis of species identification or susceptibility testing. Thus, for stable patients with C. albicans or other azole-susceptible strains, fluconazole probably remains the drug of choice. Fluconazole might be preferred over echinocandins for treating C parapsilosis, as caspofungin MICs for C. parapsilosis are higher than those for other Candida species.[41,78] However, in a recent analysis of data from five clinical trials, that included 71 cases of infection due to C. parapsilosis, the success rate was comparable with other non-albicans species.[79]
Third, patients who improve clinically and who cleared Candida from the bloodstream, might be suitable for step-down oral therapy to complete the course of 14 days. The available oral antifungals are fluconazole, itraconazole, voriconazole and posaconazole. Fluconazole is an obvious choice for susceptible species, while voriconazole can be indicated as step-down therapy for C. krusei or voriconazole-susceptible C. glabrata and in ocular or cerebral infections, because of excellent tissue concentration.
Additionally, ophthalmologic fundus examination is indicated in all patients to exclude endocular infection, while endocarditis should be excluded in case of persistently positive blood cultures, known valve pathology or any other sign or symptom suggestive of endocardial involvement. As described elsewhere, in both these complicated cases the duration of treatment should be much longer (more than 4 weeks and up to lifelong suppressive therapy).[41]
Finally, intravenous catheter removal is strongly recommended for patients with candidemia. Indeed all guidelines, both on the management of candidiasis and on the management of catheter-related bloodstream infections, state clearly that catheters should be removed, even though one should admit that all statements indicate grade II or III of scientific validity of recommendation, in absence of data from properly randomised, controlled trials.[41,80,81] However, the issue might still be controvertial since a recent study, based on a multivariate analysis of 842 adults included in candidemia trials, did not find any benefit of early central venous catheter removal (i.e. within 24 or 48 hours after initiation of antifungal therapy) on survival.[81]
Conclusions
Candida is one of the most common causes of nosocomial bloodstream infection. Non-neutropenic patients now constitute a large but heterogeneous population of patients at risk of IC, which includes subjects admitted to adult or neonatal ICU, undergoing abdominal surgery and those with cancer or numerous medical comorbidities 8e.g. diabetes). Morbidity and mortality associated with candidemia are significant and the epidemiology of species have been shifting towards non-albicans strains. Even though numerous risk factors for invasive Candida infection have been reported and several antifungals are widely available, the optimal management of candidemia remains a challenge. Prophylaxis might be beneficial in population with incidence > 10%, while novel diagnostic techniques should be further studied to enable pre-emptive treatment in populations with lower incidence rates.
References
- Wisplinghoff H, Bischoff T, Tallent SM,
Seifert H, Wenzel RP, Edmond MB: Nosocomial bloodstream infections in
US hospitals: analysis of 24,179 cases from a prospective nationwide
surveillance study. Clin Infect Dis 2004, 39:309-317. doi:10.1086/421946 PMid:15306996

- Bouza E, Munoz P: Epidemiology of
candidemia in intensive care units. Int J Antimicrob Agents 2008, 32
Suppl 2:S87-91. doi:10.1016/S0924-8579(08)70006-2

- Bouza E, Perez-Molina J, Munoz P: Report of ESGNI01 and ESGNI02 studies. Bloodstream infections in Europe. Clin Microbiol Infect 1999, 5(Suppl 2):2S1−12.
- Blumberg HM, Jarvis WR, Soucie JM, Edwards
JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L,
Wiblin RT, Wenzel RP: Risk factors for candidal bloodstream infections
in surgical intensive care unit patients: the NEMIS prospective
multicenter study. The National Epidemiology of Mycosis Survey. Clin
Infect Dis 2001, 33:177-186. doi:10.1086/321811 PMid:11418877

- Jarvis WR: Epidemiology of nosocomial
fungal infections, with emphasis on Candida species. Clin Infect Dis
1995, 20:1526-1530. PMid:7548503

- Wey SB, Mori M, Pfaller MA, Woolson RF,
Wenzel RP: Risk factors for hospital-acquired candidemia. A matched
case-control study. Arch Intern Med 1989, 149:2349-2353. doi:10.1001/archinte.149.10.2349
PMid:2802900

- Richards MJ, Edwards JR, Culver DH, Gaynes
RP: Nosocomial infections in combined medical-surgical intensive care
units in the United States. Infect Control Hosp Epidemiol 2000,
21:510-515. doi:10.1086/501795 PMid:10968716

- Bougnoux ME, Kac G, Aegerter P, d'Enfert C,
Fagon JY: Candidemia and candiduria in critically ill patients admitted
to intensive care units in France: incidence, molecular diversity,
management and outcome. Intensive Care Med 2008, 34:292-299. doi:10.1007/s00134-007-0865-y
PMid:17909746

- Leroy O, Gangneux JP, Montravers P, Mira
JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B,
Lortholary O: Epidemiology, management, and risk factors for death of
invasive Candida infections in critical care: a multicenter,
prospective, observational study in France (2005-2006). Crit Care Med
2009, 37:1612-1618. doi:10.1097/CCM.0b013e31819efac0
PMid:19325476

- Marriott DJ, Playford EG, Chen S, Slavin
M, Nguyen Q, Ellis D, Sorrell TC: Determinants of mortality in
non-neutropenic ICU patients with candidaemia. Crit Care 2009, 13:R115.

- Kontoyiannis DP, Marr KA, Park BJ,
Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown
JM, et al: Prospective surveillance for invasive fungal infections in
hematopoietic stem cell transplant recipients, 2001-2006: overview of
the Transplant-Associated Infection Surveillance Network (TRANSNET)
Database. Clin Infect Dis 2010, 50:1091-1100. doi:10.1086/651263
PMid:20218877

- Horn DL, Neofytos D, Anaissie EJ, Fishman
JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM:
Epidemiology and outcomes of candidemia in 2019 patients: data from the
prospective antifungal therapy alliance registry. Clin Infect Dis 2009,
48:1695-1703. doi:10.1086/599039 PMid:19441981

- Eggimann P, Garbino J, Pittet D:
Epidemiology of Candida species infections in critically ill
non-immunosuppressed patients. Lancet Infect Dis 2003, 3:685-702. doi:10.1016/S1473-3099(03)00801-6

- Magnason S, Kristinsson KG, Stefansson T,
Erlendsdottir H, Jonsdottir K, Kristjansson M, Jonmundsson E,
Baldursdottir L, Sigvaldason H, Gudmundsson S: Risk factors and outcome
in ICU-acquired infections. Acta Anaesthesiol Scand 2008, 52:1238-1245.
doi:10.1111/j.1399-6576.2008.01763.x
PMid:18823463

- Kuse ER, Chetchotisakd P, da Cunha CA,
Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A,
Ramasubramanian V, Demeyer I, et al: Micafungin versus liposomal
amphotericin B for candidaemia and invasive candidosis: a phase III
randomised double-blind trial. Lancet 2007, 369:1519-1527.
doi:10.1016/S0140-6736(07)60605-9

- Pappas PG, Rotstein CM, Betts RF, Nucci M,
Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL,
Ostrosky-Zeichner L, et al: Micafungin versus caspofungin for treatment
of candidemia and other forms of invasive candidiasis. Clin Infect Dis
2007, 45:883-893. doi:10.1086/520980
PMid:17806055

- Fraser VJ, Jones M, Dunkel J, Storfer S,
Medoff G, Dunagan WC: Candidemia in a tertiary care hospital:
epidemiology, risk factors, and predictors of mortality. Clin Infect
Dis 1992, 15:414-421. PMid:1520786

- Nguyen MH, Peacock JE, Jr., Tanner DC,
Morris AJ, Nguyen ML, Snydman DR, Wagener MM, Yu VL: Therapeutic
approaches in patients with candidemia. Evaluation in a multicenter,
prospective, observational study. Arch Intern Med 1995, 155:2429-2435. doi:10.1001/archinte.155.22.2429
PMid:7503601

- Nucci M, Colombo AL, Silveira F, Richtmann
R, Salomao R, Branchini ML, Spector N: Risk factors for death in
patients with candidemia. Infect Control Hosp Epidemiol 1998,
19:846-850. doi:10.1086/647743 PMid:9831941

- Blot SI, Vandewoude KH, Hoste EA, Colardyn
FA: Effects of nosocomial candidemia on outcomes of critically ill
patients. Am J Med 2002, 113:480-485. doi:10.1016/S0002-9343(02)01248-2

- Garey KW, Rege M, Pai MP, Mingo DE, Suda
KJ, Turpin RS, Bearden DT: Time to initiation of fluconazole therapy
impacts mortality in patients with candidemia: a multi-institutional
study. Clin Infect Dis 2006, 43:25-31. doi:10.1086/504810
PMid:16758414

- Morrell M, Fraser VJ, Kollef MH: Delaying
the empiric treatment of candida bloodstream infection until positive
blood culture results are obtained: a potential risk factor for
hospital mortality. Antimicrob Agents Chemother 2005, 49:3640-3645. doi:10.1128/AAC.49.9.3640-3645.2005
PMid:16127033 PMCid:1195428

- Parkins MD, Sabuda DM, Elsayed S, Laupland
KB: Adequacy of empirical antifungal therapy and effect on outcome
among patients with invasive Candida species infections. J Antimicrob
Chemother 2007, 60:613-618. doi:10.1093/jac/dkm212 PMid:17576697

- Cuenca-Estrella M, Rodriguez D, Almirante
B, Morgan J, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez
M, et al: In vitro susceptibilities of bloodstream isolates of Candida
species to six antifungal agents: results from a population-based
active surveillance programme, Barcelona, Spain, 2002-2003. J
Antimicrob Chemother 2005, 55:194-199. doi:10.1093/jac/dkh548
PMid:15618284

- Fridkin SK: The changing face of fungal
infections in health care settings. Clin Infect Dis 2005, 41:1455-1460.
doi:10.1086/497138 PMid:16231257

- Diekema DJ, Messer SA, Brueggemann AB,
Coffman SL, Doern GV, Herwaldt LA, Pfaller MA: Epidemiology of
candidemia: 3-year results from the emerging infections and the
epidemiology of Iowa organisms study. J Clin Microbiol 2002,
40:1298-1302. doi:10.1128/JCM.40.4.1298-1302.2002
PMid:11923348 PMCid:140380

- Passos XS, Costa CR, Araujo CR, Nascimento
ES, e Souza LK, Fernandes Ode F, Sales WS, Silva Mdo R: Species
distribution and antifungal susceptibility patterns of Candida spp.
bloodstream isolates from a Brazilian tertiary care hospital.
Mycopathologia 2007, 163:145-151. doi:10.1007/s11046-007-0094-5
PMid:17334813

- Shorr AF, Lazarus DR, Sherner JH, Jackson
WL, Morrel M, Fraser VJ, Kollef MH: Do clinical features allow for
accurate prediction of fungal pathogenesis in bloodstream infections?
Potential implications of the increasing prevalence of non-albicans
candidemia. Crit Care Med 2007, 35:1077-1083. doi:10.1097/01.CCM.0000259379.97694.00
PMid:17312565

- Bassetti M, Righi E, Costa A, Fasce R,
Molinari MP, Rosso R, Pallavicini FB, Viscoli C: Epidemiological trends
in nosocomial candidemia in intensive care. BMC Infect Dis 2006,
6:21. doi:10.1186/1471-2334-6-21
PMid:16472387 PMCid:1379648

- Chow JK, Golan Y, Ruthazer R, Karchmer AW,
Carmeli Y, Lichtenberg D, Chawla V, Young J, Hadley S: Factors
associated with candidemia caused by non-albicans Candida species
versus Candida albicans in the intensive care unit. Clin Infect Dis
2008, 46:1206-1213. doi:10.1086/529435
PMid:18444857

- Hachem R, Hanna H, Kontoyiannis D, Jiang
Y, Raad I: The changing epidemiology of invasive candidiasis: Candida
glabrata and Candida krusei as the leading causes of candidemia in
hematologic malignancy. Cancer 2008, 112:2493-2499. doi:10.1002/cncr.23466
PMid:18412153

- Tortorano AM, Peman J, Bernhardt H,
Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K,
Seaton S, Grillot R: Epidemiology of candidaemia in Europe: results of
28-month European Confederation of Medical Mycology (ECMM)
hospital-based surveillance study. Eur J Clin Microbiol Infect Dis
2004, 23:317-322. doi:10.1007/s10096-004-1103-y
PMid:6756909

- Calandra T, Marchetti O: Clinical trials
of antifungal prophylaxis among patients undergoing surgery. Clin
Infect Dis 2004, 39 Suppl 4:S185-192. doi:10.1086/421955
PMid:15546116

- Pereira GH, Muller PR, Szeszs MW, Levin
AS, Melhem MS: Five-year evaluation of bloodstream yeast infections in
a tertiary hospital: the predominance of non-C. albicans Candida
species. Med Mycol 2010.

- Krcmery V, Barnes AJ: Non-albicans Candida
spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp
Infect 2002, 50:243-260. doi:10.1053/jhin.2001.1151
PMid:12014897

- Ruan SY, Lee LN, Jerng JS, Yu CJ, Hsueh
PR: Candida glabrata fungaemia in intensive care units. Clin Microbiol
Infect 2008, 14:136-140. doi:10.1111/j.1469-0691.2007.01892.x
PMid:18042196

- Trick WE, Fridkin SK, Edwards JR, Hajjeh
RA, Gaynes RP: Secular trend of hospital-acquired candidemia among
intensive care unit patients in the United States during 1989-1999.
Clin Infect Dis 2002, 35:627-630. doi:10.1086/342300 PMid:12173140

- Dimopoulos G, Ntziora F, Rachiotis G,
Armaganidis A, Falagas ME: Candida albicans versus non-albicans
intensive care unit-acquired bloodstream infections: differences in
risk factors and outcome. Anesth Analg 2008, 106:523-529, table of
contents.doi:10.1213/ane.0b013e3181607262

- Cohen Y, Karoubi P, Adrie C, Gauzit R,
Marsepoil T, Zarka D, Clec'h C: Early prediction of Candida glabrata
fungemia in nonneutropenic critically ill patients. Crit Care Med 2010,
38:826-830. doi:10.1097/CCM.0b013e3181cc4734
PMid:20042858

- Viscoli C, Girmenia C, Marinus A, Collette
L, Martino P, Vandercam B, Doyen C, Lebeau B, Spence D, Krcmery V, et
al: Candidemia in cancer patients: a prospective, multicenter
surveillance study by the Invasive Fungal Infection Group (IFIG) of the
European Organization for Research and Treatment of Cancer (EORTC).
Clin Infect Dis 1999, 28:1071-1079.doi:10.1086/514731 PMid:10452637

- Pappas PG, Kauffman CA, Andes D, Benjamin
DK, Jr., Calandra TF, Edwards JE, Jr., Filler SG, Fisher JF, Kullberg
BJ, Ostrosky-Zeichner L, et al: Clinical practice guidelines for the
management of candidiasis: 2009 update by the Infectious Diseases
Society of America. Clin Infect Dis 2009, 48:503-535. doi:10.1086/596757 PMid:19191635

- Pfaller MA: Nosocomial candidiasis:
emerging species, reservoirs, and modes of transmission. Clin Infect
Dis 1996, 22 Suppl 2:S89-94.

- Asmundsdottir LR, Erlendsdottir H,
Haraldsson G, Guo H, Xu J, Gottfredsson M: Molecular epidemiology of
candidemia: evidence of clusters of smoldering nosocomial infections.
Clin Infect Dis 2008, 47:e17-24.

- Bliss JM, Basavegowda KP, Watson WJ,
Sheikh AU, Ryan RM: Vertical and horizontal transmission of Candida
albicans in very low birth weight infants using DNA fingerprinting
techniques. Pediatr Infect Dis J 2008, 27:231-235. doi:10.1097/INF.0b013e31815bb69d
PMid:18277930

- Hernandez-Castro R, Arroyo-Escalante S,
Carrillo-Casas EM, Moncada-Barron D, Alvarez-Verona E,
Hernandez-Delgado L, Torres-Narvaez P, Lavalle-Villalobos A: Outbreak
of Candida parapsilosis in a neonatal intensive care unit: a health
care workers source. Eur J Pediatr 2010, 169:783-787. doi:10.1007/s00431-009-1109-7
PMid:19957192

- Vazquez JA, Sanchez V, Dmuchowski C,
Dembry LM, Sobel JD, Zervos MJ: Nosocomial acquisition of Candida
albicans: an epidemiologic study. J Infect Dis 1993, 168:195-201.
PMid:8515108

- Richet HM, Andremont A, Tancrede C, Pico
JL, Jarvis WR: Risk factors for candidemia in patients with acute
lymphocytic leukemia. Rev Infect Dis 1991, 13:211-215. PMid:2041951

- Pittet D, Monod M, Suter PM, Frenk E,
Auckenthaler R: Candida colonization and subsequent infections in
critically ill surgical patients. Ann Surg 1994, 220:751-758. doi:10.1097/00000658-199412000-00008
PMid:7986142 PMCid:1234477

- De Waele JJ, Vogelaers D, Blot S, Colardyn
F: Fungal infections in patients with severe acute pancreatitis and the
use of prophylactic therapy. Clin Infect Dis 2003, 37:208-213. doi:10.1086/375603PMid:12856213

- Calandra T, Bille J, Schneider R, Mosimann
F, Francioli P: Clinical significance of Candida isolated from
peritoneum in surgical patients. Lancet 1989, 2:1437-1440. doi:10.1016/S0140-6736(89)92043-6

- Piarroux R, Grenouillet F, Balvay P, Tran
V, Blasco G, Millon L, Boillot A: Assessment of preemptive treatment to
prevent severe candidiasis in critically ill surgical patients. Crit
Care Med 2004, 32:2443-2449. doi:10.1097/01.CCM.0000147726.62304.7F
PMid:15599149

- Leon C, Ruiz-Santana S, Saavedra P,
Almirante B, Nolla-Salas J, Alvarez-Lerma F, Garnacho-Montero J, Leon
MA: A bedside scoring system ("Candida score") for early antifungal
treatment in nonneutropenic critically ill patients with Candida
colonization. Crit Care Med 2006, 34:730-737. doi:10.1097/01.CCM.0000202208.37364.7D
PMid:16505659

- Leon C, Ruiz-Santana S, Saavedra P, Galvan
B, Blanco A, Castro C, Balasini C, Utande-Vazquez A, Gonzalez de Molina
FJ, Blasco-Navalproto MA, et al: Usefulness of the "Candida score" for
discriminating between Candida colonization and invasive candidiasis in
non-neutropenic critically ill patients: a prospective multicenter
study. Crit Care Med 2009, 37:1624-1633. doi:10.1097/CCM.0b013e31819daa14
PMid:19325481

- Ostrosky-Zeichner L, Sable C, Sobel J,
Alexander BD, Donowitz G, Kan V, Kauffman CA, Kett D, Larsen RA,
Morrison V, et al: Multicenter retrospective development and validation
of a clinical prediction rule for nosocomial invasive candidiasis in
the intensive care setting. Eur J Clin Microbiol Infect Dis 2007,
26:271-276.doi:10.1007/s10096-007-0270-z
PMid:6756909

- Dupont H, Bourichon A, Paugam-Burtz C,
Mantz J, Desmonts JM: Can yeast isolation in peritoneal fluid be
predicted in intensive care unit patients with peritonitis? Crit Care
Med 2003, 31:752-757. doi:10.1097/01.CCM.0000053525.49267.77
PMid:12626979

- Ellis M, Al-Ramadi B, Bernsen R,
Kristensen J, Alizadeh H, Hedstrom U: Prospective evaluation of mannan
and anti-mannan antibodies for diagnosis of invasive Candida infections
in patients with neutropenic fever. J Med Microbiol 2009, 58:606-615. doi:10.1099/jmm.0.006452-0
PMid:19369522

- Prella M, Bille J, Pugnale M, Duvoisin B,
Cavassini M, Calandra T, Marchetti O: Early diagnosis of invasive
candidiasis with mannan antigenemia and antimannan antibodies. Diagn
Microbiol Infect Dis 2005, 51:95-101. doi:10.1016/j.diagmicrobio.2004.08.015
PMid:15698714

- Sendid B, Tabouret M, Poirot JL, Mathieu
D, Fruit J, Poulain D: New enzyme immunoassays for sensitive detection
of circulating Candida albicans mannan and antimannan antibodies:
useful combined test for diagnosis of systemic candidiasis. J Clin
Microbiol 1999, 37:1510-1517. PMid:10203514
PMCid:84817

- Mikulska M, Calandra T, Sanguinetti M,
Poulain D, Viscoli C, The 3rd European Conference on Infections in
Leukemia Group: Mannan antigen and anti-mannan antibodies in the
diagnosis of invasive candidiasis: recommendations from the European
Conference on Infections in Leukemia. Crit Care 2010, 14.

- Ostrosky-Zeichner L, Alexander BD, Kett
DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R,
Tamura H, et al: Multicenter clinical evaluation of the (1-->3)
beta-D-glucan assay as an aid to diagnosis of fungal infections in
humans. Clin Infect Dis 2005, 41:654-659. doi:10.1086/432470 PMid:16080087

- Alam FF, Mustafa AS, Khan ZU: Comparative
evaluation of (1, 3)-beta-D-glucan, mannan and anti-mannan antibodies,
and Candida species-specific snPCR in patients with candidemia. BMC
Infect Dis 2007, 7:103.
doi:10.1186/1471-2334-7-103
PMid:17784947 PMCid:2075513

- Presterl E, Parschalk B, Bauer E, Lassnigg
A, Hajdu S, Graninger W: Invasive fungal infections and
(1,3)-beta-D-glucan serum concentrations in long-term intensive care
patients. Int J Infect Dis 2009, 13:707-712. doi:10.1016/j.ijid.2008.10.013
PMid:19157947

- De Pauw B, Walsh TJ, Donnelly JP, Stevens
DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O,
Kauffman CA, et al: Revised definitions of invasive fungal disease from
the European Organization for Research and Treatment of Cancer/Invasive
Fungal Infections Cooperative Group and the National Institute of
Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG)
Consensus Group. Clin Infect Dis 2008, 46:1813-1821. doi:10.1086/588660
PMid:18462102 PMCid:2671227

- Jacobs S, Price Evans DA, Tariq M, Al Omar
NF: Fluconazole improves survival in septic shock: a randomized
double-blind prospective study. Crit Care Med 2003, 31:1938-1946. doi:10.1097/01.CCM.0000074724.71242.88
PMid:12847386

- Sandven P, Qvist H, Skovlund E, Giercksky
KE: Significance of Candida recovered from intraoperative specimens in
patients with intra-abdominal perforations. Crit Care Med 2002,
30:541-547. doi:10.1097/00003246-200203000-00008
PMid:11990912

- He YM, Lv XS, Ai ZL, Liu ZS, Qian Q, Sun
Q, Chen JW, Lei DX, Jiang CQ, Yuan YF: Prevention and therapy of fungal
infection in severe acute pancreatitis: A prospective clinical study.
World J Gastroenterol 2003, 9:2619-2621. PMid:14606111

- Garbino J, Lew DP, Romand JA, Hugonnet S,
Auckenthaler R, Pittet D: Prevention of severe Candida infections in
nonneutropenic, high-risk, critically ill patients: a randomized,
double-blind, placebo-controlled trial in patients treated by selective
digestive decontamination. Intensive Care Med 2002, 28:1708-1717. doi:10.1007/s00134-002-1540-y
PMid:12447512

- Eggimann P, Francioli P, Bille J,
Schneider R, Wu MM, Chapuis G, Chiolero R, Pannatier A, Schilling J,
Geroulanos S, et al: Fluconazole prophylaxis prevents intra-abdominal
candidiasis in high-risk surgical patients. Crit Care Med 1999,
27:1066-1072. doi:10.1097/00003246-199906000-00019PMid:10397206

- Lipsett PA: Clinical trials of antifungal
prophylaxis among patients in surgical intensive care units: concepts
and considerations. Clin Infect Dis 2004, 39 Suppl 4:S193-199. doi:10.1086/421956
PMid:15546117

- Cruciani M, de Lalla F, Mengoli C:
Prophylaxis of Candida infections in adult trauma and surgical
intensive care patients: a systematic review and meta-analysis.
Intensive Care Med 2005, 31:1479-1487. doi:10.1007/s00134-005-2794-y
PMid:16172847

- Shorr AF, Chung K, Jackson WL, Waterman
PE, Kollef MH: Fluconazole prophylaxis in critically ill surgical
patients: a meta-analysis. Crit Care Med 2005, 33:1928-1935; quiz 1936.

- Playford EG, Webster AC, Sorrell TC, Craig
JC: Antifungal agents for preventing fungal infections in
non-neutropenic critically ill and surgical patients: systematic review
and meta-analysis of randomized clinical trials. J Antimicrob Chemother
2006, 57:628-638. doi:10.1093/jac/dki491 PMid:16459344

- Pelz RK, Hendrix CW, Swoboda SM,
Diener-West M, Merz WG, Hammond J, Lipsett PA: Double-blind
placebo-controlled trial of fluconazole to prevent candidal infections
in critically ill surgical patients. Ann Surg 2001, 233:542-548. doi:10.1097/00000658-200104000-00010
PMid:11303137 PMCid:1421284

- Bassetti M, Ansaldi F, Nicolini L,
Malfatto E, Molinari MP, Mussap M, Rebesco B, Bobbio Pallavicini F,
Icardi G, Viscoli C: Incidence of candidaemia and relationship with
fluconazole use in an intensive care unit. J Antimicrob Chemother 2009,
64:625-629. doi:10.1093/jac/dkp251
PMid:19622536

- Ostrosky-Zeichner L: Prophylaxis and
treatment of invasive candidiasis in the intensive care setting. Eur J
Clin Microbiol Infect Dis 2004, 23:739-744. doi:10.1007/s10096-004-1215-4
PMid:6756909

- Pizzo PA, Robichaud KJ, Gill FA, Witebsky
FG: Empiric antibiotic and antifungal therapy for cancer patients with
prolonged fever and granulocytopenia. Am J Med 1982, 72:101-111. doi:10.1016/0002-9343(82)90594-0

- Schuster MG, Edwards JE, Jr., Sobel JD,
Darouiche RO, Karchmer AW, Hadley S, Slotman G, Panzer H, Biswas P, Rex
JH: Empirical fluconazole versus placebo for intensive care unit
patients: a randomized trial. Ann Intern Med 2008, 149:83-90.
PMid:18626047

- Trofa D, Gacser A, Nosanchuk JD: Candida
parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 2008,
21:606-625. doi:10.1128/CMR.00013-08
PMid:18854483 PMCid:2570155

- Colombo AL, Ngai AL, Bourque M, Bradshaw
SK, Strohmaier KM, Taylor AF, Lupinacci RJ, Kartsonis NA: Caspofungin
use in patients with invasive candidiasis caused by common non-albicans
Candida species: review of the caspofungin database. Antimicrob Agents
Chemother 2010, 54:1864-1871. doi:10.1128/AAC.00911-09
PMid:20231388 PMCid:2863639

- Mermel LA, Allon M, Bouza E, Craven DE,
Flynn P, O'Grady NP, Raad, II, Rijnders BJ, Sherertz RJ, Warren DK:
Clinical practice guidelines for the diagnosis and management of
intravascular catheter-related infection: 2009 Update by the Infectious
Diseases Society of America. Clin Infect Dis 2009, 49:1-45. doi:10.1086/599376
PMid:19489710

- Nucci M, Anaissie E, Betts RF, Dupont BF,
Wu C, Buell DN, Kovanda L, Lortholary O: Early removal of central
venous catheter in patients with candidemia does not improve outcome:
analysis of 842 patients from 2 randomized clinical trials. Clin Infect
Dis 2010, 51:295-303. doi:10.1086/653935 PMid:20578829
