Review Articles
Epidemiology of
Invasive Fungal Infections in the Mediterranean Area Ulrike Binder and Cornelia Lass-Flörl
Division of Hygiene and
Medical Microbiology, Medical University Innsbruck, Austria.
Correspondence
to: Cornelia Lass-Flörl, Department of Hygiene, Microbiology and
Social Medicine, Division of Hygiene and Medical Microbiology, Medical
University Innsbruck, Fritz Pregl Str. 3/3, A-6020 Innsbruck, Tirol,
Austria. Tel.: +43(0)512 9003 70700; fax: +43(0)512 9003 73700.
E-mail: cornelia-lass-floerl@i-med.ac.at
Published: March 31, 2011
Received: March 03, 2011
Accepted: March 29, 2011
Medit J Hemat Infect Dis 2011, 3: e20110016, DOI 10.4084/MJHID.2011.016
This article is available from: http://www.mjhid.org/article/view/8123
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Abstract
Although Candida species
remain the relevant cause of IFI, other fungi (especially moulds) have
become increasingly prevalent. In particular, Aspergillus species are
the leading cause of mould infections but also Glomeromycota (formerly
Zygomycetes) and Fusarium species are increasing in frequency, and are
associated with high mortality rates. Many of these emerging infections
occur as breakthrough infections in patients treated with new
antifungal drugs. The causative pathogens, incidence rate and severity
are dependent on the underlying condition, as well as on the geographic
location of the patient population. France and Italy show the highest
incident rates of Fusarium infections in Europe, following the US,
where numbers are still increasing. Scedosporium prolificans, which
primarily is found in soil in Spain and Australia, is most frequently
isolated from blood cultures in a Spanish hospital. Geotrichum
capitatum represents another species predominantly found in Europe with
especially high rates in Mediterranean countries. The increasing
resistance to antifungal drugs especially of these new emerging
pathogens is a severe problem for managing these IFIs.
Introduction
Invasive fungal infections (IFIs) are an increasingly important clinical dilemma, engendering high rates of morbidity and mortality, particularly in immunocompromised populations. As a result of growing numbers of patients with a variety of risk factors (e.g. transplantation, chemotherapy, HIV infection, use of corticosteroids or new immunosuppressive agents), the incidence of IFIs has increased substantially in recent years.[1,2,3,4,5,6,7] For example, aggressive new therapies for transplant recipients and patients with hematologic malignancies have led to more profound immunosuppression of longer duration.[7]
In addition, advances in medical care are extending the survival of critically ill patients, rendering them more vulnerable to IFIs. [1,2,8,9] The incidence and severity of IFIs as well as the causative pathogens are dependent on various risk factors concerning the patient such as the underlying condition, the state of immunosuppression, but also the geographic location of the patient.[2,6,10]
A wide variety of pathogens can be associated with IFIs. Historically, Candida species have by far been the most common infective organisms among fungi. However, the epidemiology has changed dramatically in recent years: IFIs caused by moulds – predominantly Aspergillus species - have increased substantially and newly emerging and rare fungal pathogens such as Glomeromycota (e.g. Rhizopus and Mucor species), hyaline moulds (e.g. Fusarium species) and other opportunistic species (e.g. Scedosporium species) are increasingly being reported.[7,8,11,12] This article will predominantly review the most common causatives of IFIs, concentrating on the changing epidemiology of fungal infections and focusing on surveys carried out in the Mediterranean area.
Yeasts and Yeast-Like Pathogens
Candida species: Candida infections are the most frequent cause of IFIs worldwide, with a case rate of 72.8 per 1,000,000 per year [13] and can result in a wide range of clinical symptoms, from mucocutaneous overgrowth to blood stream infections and metastatic infections.[8,14,15] More than 100 Candida species have been found to be pathogenic with their frequency varying according to the geographic setting.[16,17,18,19]
The burden of invasive candidiasis remains substantial; after a decline in mortality throughout the early to mid 1990s, mortality rates have leveled off in recent years.[13] In the United States, Candida species are the fourth most common cause of nosocomial blood stream infection.[13] Candida (C.) albicans remains by far the most common species causing invasive candidiasis worldwide (62% in 2003) although the frequency of candidiasis caused by other species including C. tropicalis, C. parapsilosis, C. glabrata, and C. krusei has been increasing steadily over the last 10 years.[13] Two studies in Italy and Spain show the distribution of Candida species in the Mediterranean area which was shown to be generally similar to reports from other European countries.[20,21,22,23]
An Italian study [21] revealed, that C. albicans (61 % of all isolates) was followed by C. parapsilosis, C. glabrata and C.tropicalis, which is similar to reports from other European countries, with the only difference that here C. glabrata was shown to be the 3rd most common species, while it is the 2nd most common in Switzerland,[24] the UK25 and the US.[26] Interestingly, in a Spanish study, carried out in Barcelona, C. glabrata was shown to be only the 4th most common species, with C. tropicalis being the 3rd and C. parapsilosis the 2nd most common following C. albicans. [27] The same Spanish study [27] revealed, that the overall incidence of bloodstream infections caused by Candida in Barcelona is lower (4.3 cases per 100 000 population) than in the US (6-10 per 100 000 population).[3,28,29] Nevertheless, the number of incidence in Spain correlated well with reports from Northern European countries.[30,31] Candida bloodstream infections are in general very high among neonates and infants.[20,27,32] With 38.8 cases per 100 000 population the number of incidence in Barcelona/Spain is within the range of numbers obtained from studies in the US.26 However, C. parapsilosis was the most common species isolated from neonates in Spain (67% of all cases)20, whereas in the US C. albicans was the most common species and the proportion of C. parapsilosis infections was significantly lower (27-45%) than in Spain.[20,28,29]
Since the 1990s, fluconazole has been widely used for both treatment and prophylaxis of immunosuppressed patients resulting in decreasing rates of Candida bloodstream infections worldwide. The downside of this application was that C. glabrata, being less susceptible to fluconazole,[3,33] as well as other non-albicans infections are emerging, such as C. krusei which is fluconazole resistant. [34] In a nationwide surveillance study in Spain the frequency of antifungal resistance was determined next to species distribution and incident rates. This study revealed that 7 % of all isolates exhibited decreased susceptibility to fluconazole with a linear correlation to voriconazole resistance. Furthermore, MICs for voriconazole where increased in patients that received fluconazole before, than in those without previous exposure to fluconazole.[35] Another Spanish study investigated the susceptibility to voriconazole of more than 4000 clinical Candida isolates according to EUCAST testing, and revealed that among C. albicans, C. parapsilosis and C. tropicalis resistance to voriconazole was uncommon (with a maximum of 11%), but higher MICs were obtained for C. glabrata and C. krusei.[36] The antifungal susceptibility of the C. parapsilosis, which recently was found to consist of three different species, namely C. parapsilosis sensu strict, C. metapsilosis and C. parapsilosis, was shown to be low for echinocandins.[37,38] A Portuguese study testing 175 clinical and environmental isolates of the C. parapsilosis group showed that the majority (91.4 %) of all isolates are C. parapsilosis sensu stricto, and of those most isolates were susceptible to fluconazole. All of the isolates C. metapsilosis and C. parapsilosis were susceptible to azoles and amphotericinB, while a high number was non-susceptible to echinocandins.[38] The 10 year ARTEMIS DISK global antifungal surveillance study, where 256 882 isolates of Candida sp. were collected from 142 sites in 42 countries and tested against fluconazole, showed that the frequency of azole resistance varied considerably by geographic region.[39] Higher rates of resistance to both fluconazole and voriconazole were found in isolates from North America. Not only for C. glabrata and C. krusei decreased susceptibility was shown, but also for C. guilliermondii, C. inconspicua, C. rugosa and others, demonstrating that 13 out of the 31 species found exhibited increased resistance to fluconazole. As described before, cross- resistance between fluconazole and voriconazole is evident and seems to be more pronounced in some species of Candida than in others.[39]
Cryptococcus species
The genus Cryptococcus includes encapsulated yeasts that lack a mycelium.[40,41,42] Infection is usually initiated in the pulmonary tract with later possible dissemination, usually to the CNS, causing meningitis.[43,44] Involvement of parenchyma of the brain and meningitis occurs in between 40 and 86% of patients.[44] Cryptococcosis usually occurs in patients with impaired immunity.[44] The concern about Cryptococcus sp. has dramatically increased as it still remains one of the most common life threatening fungal infections in HIV- patients, where the risk of a Cryptococcus infection is between 2.9 – 13.3%. In non-HIV infected individuals, incidence rates of 0.2–0.9% have been reported in the United States.[44] Patients with AIDS have a much higher risk of infection (2.9–13.3%). In non – AIDS patients, but those with hematologic malignancies, administration of steroids and diabetes mellitus were the most frequent risk factors (6 and 4 out of 17 patients, respectively), as demonstrated in a retrospective study conducted in Italy between 1993 and 2002.[45]
In SOT recipients, an incidence of 2.8% has been reported.[44] Risk factors for mortality are pre-existing renal failure and liver failure in transplant recipients.[44]
In humans two Cryptococcus species can provoke disease: C. neoformans and C. gattii, which include 5 different serotypes altogether. Two varieties of C. neoformans (C. neoformans var. neoformans and C. neoformans var. grubii) representing serotypes D, A and AD (a hybrid from of both A and D), respectively, have been isolated.[42,46,47] C. gattii was previously listed as a further variety of C. neoformans, but is now known to be a distinct species. C. gattii includes serotypes B and C, both commonly seen as true pathogens provoking disease also in immunocompetent persons.[41,46,48]
C. neoformans has a worldwide distribution and has been isolated from a variety of environmental sources, mainly from bird excreta, where the microorganism can survive for a long time due to protection from sun and high temperatures. Its capsule even makes it resistant to natural drying of the vector matter. The distribution of C. gattii was thought to be restricted to tropical and subtropical environments, often associated with Eucalyptus trees and the koala bear.[49] Yet, in recent years, an outbreak of C. gattii infections has been reported from Vancouver Island/Canada, where more than 66 human cases with at least 4 fatalities have been reported in otherwise healthy persons, all due to infections with serotype B.50 In recent years, some other species such as C. laurentii and C. albidus were isolated from cryptococcosis patients.[51,52]
High mortality rates of 30-40%41 are mainly due to the difficulty in killing the pathogen. Without treatment, the invasive infection is fatal, which makes rapid diagnosis and treatment inevitable. A combination of amphothericin B and flucytosin, followed by fluconazole maintenance therapy is the therapeutic option in most cases,[53] although C. gattii was found to show resistance to amphotericin B and fluconazole.[54] Furthermore, a trend of increasing fluconazole resistance of C. neoformans isolates from the Asia-Pacific, Africa/Middle East, and Latin America regions but not among isolates from Europe or Northern America has been described in a 10 year antifungal surveillance study.[55]
Trichosporon species
Systemic trichosporonosis is a relatively uncommon but frequently fatal opportunistic fungal infection in immunocompromised individuals.[56] The taxonomy of the yeasts that cause trichosporonosis has been extensively revised.[56,57] It is now widely accepted that the previously named Trichosporon (T). beigelii actually consists of six species: T. asahii, T. asteroides, T. cutaneum, T. inkin, T. mucoides, and T. ovoides. Geotrichum capitatum, originally considered a species of Trichosporon and now reclassified, is also a common cause of trichosporonosis.[56] While any immunocompromised patient can develop invasive trichosporonosis, the risk is highest for those with hematologic malignancies.[58,59] Incidence rates of 0.4 and 0.5%, respectively, for infections due to Trichosporon sp. and G. capitatum have been reported in patients with leukemia.[59] One of the largest multicenter retrospective studies on invasive trichosporonosis, carried out in Italy, [56] revealed that acute myeloid leukemia was the most frequent underlying hematologic disease for trichosporonosis. A total of 17 of the 52 patients with hematological malignancies were diagnosed with infections caused by Trichosporon sp., while the majority of infections (35 out of 52) was attributed to G. capitatum. Furthermore, the study showed that the frequency of Trichosporon sp. infections is similar on all continents, while G. capitatum is predominantly a European pathogen, with high rates especially countries of the Mediterranean area.[56] Several Trichosporon sp. were shown to be multidrug resistant.[56] Echinocandins have poor activity against Trichosporon sp. as demonstrated by high MICs[58 ]and breakthrough cases in immunocompromised patients treated with caspofungin [59,60,61] or micafungin [61] have been reported.
Moulds
Aspergillus species: Aspergillus species are opportunistic moulds that can cause both allergic and invasive syndromes.[62] More than 300 Aspergillus species are known today of which only a small number cause opportunistic infections.[62] The most common species causing aspergillosis is Aspergillus (A.) fumigatus, accounting for approximately 90% of Aspergillus infections.[63] Depending on regional distinctions A. flavus, A. nidulans and A. terreus are frequently reported as well, and there is evidence that these non-fumigatus pathogens are increasingly common etiologic agents.[63,64,65] There are differences in the clinical presentations produced by these different species.
For example, A. flavus produces a disproportionate number of infections in the paranasal sinus, while A. nidulans is a common culprit in chronic granulomatous disease.[63] Although A. terreus remains uncommon, infection caused by this pathogen is associated with high mortality rates because of its resistance to amphotericin B.[64] A study including three European countries, namely Austria, Denmark and Spain, revealed that A. terreus seems to be endemic for Tirol, Austria as it was exclusively found in hospital samples from Austria.[66] In Spain/Madrid A. niger was the most isolated non-fumigatus species. Furthermore, it was shown that azole resistance of Aspergilli is significantly increasing, especially in the UK (Manchester) and the Netherlands (Nijmegen). The Dutch study, involving almost 2000 A. fumigatus isolates collected over a 14-year period in the Netherlands, of which 32 isolates exhibited increased resistance to all azoles tested, showed that 30 of the 32 strains had the same “dominant resistance mechanism”. They all exhibited a single amino acid change in the cyp51A gene (encoding the target enzyme cytochrome P450 sterol 14-α-demethylase) and an alteration in the promoter region of this gene. Six isolates out of 317 from other European countries also exhibited resistance to itraconazole. In a study by Pfaller et al. 1789 Aspergillus isolates from centers all over the world between 2001 and 2009 were evaluated for their susceptibility to triazoles (voriconazole, posaconazole, itraconazole). For each of the three triazoles tested, decreased susceptibility was observed and varied according to the species. 49 isolates exhibited MICs higher than 4 µg/ml for itraconazole, of which some were shown to be cross resistant to posaconazole and voriconazole.[68] There exist clinical reports on primary invasive Aspergillus infections due to resistant isolates involving various manifestations, e.g. in the lung, the brain, in bones.[69,70,71,72,73] Furthermore, cases have been shown, where itraconazole treatment is lacking clinical efficacy in patients with aspergilloma.[71,74] In Austria the occurrence of azole resistance among clinical A. fumigatus is 0% while in Spain it is 2%. Reasons for this increase in resistance are not clear yet, nevertheless there exists some evidence that it is due to excessive use of azoles in agriculture.[75,76]
Invasive aspergillosis has remained the predominate cause of invasive mould infections over the last 10–15 years.[77] Reasons for this include a continued increase in high-risk populations such as solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients, HIV-infected individuals, and those receiving intensified chemotherapy regimens.[2,63,78,79] Invasive aspergillosis is associated with a high rate of mortality, however, there is some evidence that survival rates have increased in recent years among those undergoing HSCT, primarily because of the use of non-myeloablative conditioning regimens, the use of peripheral blood stem cells, prompt diagnosis, and the use of effective antifungal therapy.[6]
An Italian study on invasive aspergillosis in AML-patients (SEIFEM-2008 registry study)80 showed that there is a clear downward trend in the aspergillosis-attributable mortality rate. In various consecutive multicenter studies Pagano et al. showed a decrease from 48% (1987-1998), to 38.5% (1999-2003) and 27% (2004-2007).[5,80,81,82] It is important to note, that according to the latest study,80 about two-thirds of the patients developed invasive aspergillosis despite standard antifungal prophylaxis based on fluconazole and itraconazole, which points out that the use of systemic prophylaxis needs to be further discussed. Independently from whatever prophylaxis was applied, A.fumigatus was the most causative species of aspergillosis in the Italian study.
Fusarium species
Fusarium sp. can be found in soil, plants and air. Clinical manifestations are diverse and depend largely on the immune status of the patient. Often, Fusarium sp. affects the skin (70-90%), lungs and sinuses (70-80%). Fusariosis is a life-threatening and increasingly important mycosis in immunocompromised hosts.[82,83,84] Risk factors for such infections are skin lesions, burns, use of corticosteroids, prolonged neutropenia and hematological malignancy.[12,84,85,86] Fusarium sp. are angiotropic and angioinvasive moulds that produce hemorrhagic infarction and low tissue perfusion, resulting in tissue necrosis.[83] More than 50 species of Fusarium have been identified but only a few are pathogenic in humans.83 These include F. solani (causes 50% of cases), F. oxysporum, F. moniliforme, F. verticillioides, F. dimerum, and F. proliferatum.[87]
In terms of global occurrence, fusariosis is most common in the United States (50–80% of all cases), followed by France, Italy, and Brazil.[83,87,88] In the SEIFEM-2004 survey, Fusarium species were responsible for 0.1% of infections, the majority in AML patients (0.3%).[81] In another Italian study, including 14 haematological centers, Fusarium infection was documented in 6 out of 351 patients (1.7%),88 with aplastic anaemia and AML as the underlying diseases (3 cases each). While the incidence in Italy remains stable, it increased in some US centers.[84] Because the clinical presentation of fusariosis may be non-specific, differentiating it from invasive aspergillosis can be challenging.[83] More than 90% of cases of fusariosis have been reported in neutropenic patients with hematologic malignancies.[88] Incidence rates of 0.06% (acute leukemia), 0.2% (autologous bone marrow transplant [BMT]), and 1.2% (allogeneic BMT) have been reported.82 In patients with hematologic malignancies, persistent neutropenia (hazard ratio [HR] = 5.43) and use of corticosteroids (HR = 2.18) were the most important predictors of mortality. Ideal treatment of fusariosis is still unclear. Azoles and polyenes seem to be most effective. Nevertheless, Fusarium sp. exhibit high resistance to antifungal drugs.[81,84,89]
Scedosporium species
Scedosporium sp. are ubiquitously distributed worldwide, commonly found in soil, sewage or polluted water. S. apiospermum (also known by its teleomorphic name Pseudoallescheria boydii) and S. prolificans have the greatest impact in human infections.[12,90] These two species differ in their epidemiological niches, morphology and antifungal sensitivity and can cause infections in both immunocompetent and immunosuppressed populations.[85,90] S. apiospermum has a worldwide distribution usually in association with water, and is therefore often reported as a cause of pneumonia and disseminated infection in near-drowning victims.[91] On the other hand, S. prolificans is found in soil, mainly in Spain and Australia.[92] In a Spanish survey, conducted between 1990 and 1999, S. prolificans was the most frequent filamentous fungi isolated from blood cultures,[93] comprising 5.2% of all of the filamentous fungi isolated in the respective hospital (San Sebastion/Spain).[93]
Mycetoma, a disfiguring, but non-life-threatening infection of the skin and subcutaneous tissue, is one type of disease caused by S. apiospermum, frequently developed through thorn punctures, wood splinters or preexisting trauma.[11,12,85] Pseudallescheriasis or scedosporiosis is mainly found in immunocompromised patients with hematological malignancies or in organ transplant recipients. For 11 % of cases in SOT recipients fungemia with Scedosporium sp. was reported.[11] Interestingly, neutropenia was not a variable in connection with Scedosporium infection in SOT patients. Also S. prolificans was found to cause deep invasive disseminated infections associated with high mortality rates. Dissemination throughout the body might be easier for this organism due to its ability to produce conidia in tissue.[12] Both pathogenic species of Scedosporium are highly resistant to amphotericin B and echinocandins, with S. prolificans being highly resistant to almost all of the currently available antifungal drugs. Voriconazole seems to have the strongest effect on both, S. apiospermum and S. prolificans, although most data exist from in vitro studies, where MICs for S. prolificans are at a level that would not be achieved in human compartiments and not be beneficial for the patient. As an approach, synergistic killing was investigated with a combination of voriconazole and terbinafine, which might be worthwhile to try.[12,85,94,95,96,97] Hence, mortality rates have been reported to be as high as 65-75 % for S. apiospermum and even higher (85-100%) for S. prolificans.
Glomeromycota (formerly Zygomycetes)
Infections with species of the Glomeromycota (medically referred to as zygomycosis or mucormycosis) play an increasingly important role in immunocompromised patients. Two orders of the Glomeromycota are clinically relevant: Mucorales and Entomophthorales.[98,99,100] Members of the Mucorales are distributed worldwide while Entomophthorales are generally limited to the tropics and subtropics.[101,102] Species provoking human disease mostly belong to the group of Mucorales, which is characterized by a rapidly evolving course, tissue destruction, and invasion of blood vessels.[101,103] The most common species causing mucormycosis are Rhizopus (R.) arrhizus (R. oryzae), R. microsporus var. rhizopodiformis, and R. pusillus. Other causative species include Absidia corymbifera, Mucor species, and Cunninghamella bertholletiae.[101] Mycoses caused by Entomophthorales are more indolent and chronically progressive.[101,103]
Commonly infections affect the paranasal sinus (39%), the lungs (24%), and the skin (19%) with the primary site of infection depending on the patient population.[7,104] Disseminated disease is reported in approximately one-fourth of patients,[104] resulting in high mortality rates (96%).[7]A case-control observational study found that prolonged neutropenie rather than a low neutrophil count is more common in patients with zygomycosis.[104] Frequent underlying risk factors are diabetes mellitus, particularly enhanced by ketoacidosis, hematological malignances and bone marrow or solid organs transplantation.[85,101,104] Diabetes still remains the most common risk factor with 36% to 88% among mucormycosis-cases having diabetes as a predisposing condition.[101] However, the cases of mucormycosis in patients with hematological malignancies or those who have received hematopoetic stem cell or SOTs is dramatically increasing in the past two decades.[85] Invasive mucormycosis is now considered to be the 2nd most frequent mould infection in patients with hematological malignances, with reported cumulative incidence ranging from 0.1 – 2.5 % in different series.104 An Italian study reports that 45 (11.5%) out of 391 patients with hematological malignancies had infections with a representative of the Mucorales.[105] In France the incidence rate within this patient group increased of 24 % per year from 1997 to 2006.[106] The so far largest and geographically most diverse study on epidemiology of zygomycosis in Europe, including 15 countries and 230 cases in total, once more pointed out that the most frequent underlying condition for zygomycosis is hematological malignancy (44% of all cases), whereas diabetes is only present in 17 % of all cases.[99] This is controversial to a study by,[103] reporting that diabetes account in 36 % of all cases to glomeromycota-infection. One possible explanation for this contrast might be the high increase of immunocompromised hosts in the recent decade.[99] The presence of available free iron predisposes to zygomycosis.[103] The application of the iron chelator deferoxamine allows the fungus to utilize deferoxamine-bound iron by recognizing it as a siderophore and enable it to acquire the – for the fungus inevitable – iron via siderophore-specific mechanism/high affinity non-reductive mechanism (sufficient levels of iron increases the ability proliferation and tissue penetration for the fungus). Other chelators (i.e., deferasirox) do not allow iron utilization and may decrease the risk of infection.[107,108] Antifungal prophylaxis with voriconazole also appears to be associated with an increased risk of developing zygomycosis.[85] For successful eradication of these pathogens a multifactorial treatment strategy is needed. This includes reducing the predisposing factors of the patient, surgical debridement and application of antifungal therapy. Amphotericin B, especially new lipid formulations, is still the agent of choice, and data exist that suggest a combinational therapy with posaconazole as promising.[85,109,110,111,112,113]
Conclusion
With invasive mould infections becoming increasingly important, including those caused by rare, unusual pathogens, the epidemiology of IFIs is shifting in Europe. In some populations mould infections have already overtaken candidiasis, which was once the predominant type of IFIs. Reasons for this shift are multifactorial, but the augmented use of fluconazole as prophylaxis may account, at least in part, for this phenomenon, especially regarding infections with previously rare pathogens that occur as breakthrough infections. The management of IFIs is challenging – complicated by the difficulty in diagnosis and increasing resistance of the pathogens to available antifungal drugs.
Invasive fungal infections (IFIs) are an increasingly important clinical dilemma, engendering high rates of morbidity and mortality, particularly in immunocompromised populations. As a result of growing numbers of patients with a variety of risk factors (e.g. transplantation, chemotherapy, HIV infection, use of corticosteroids or new immunosuppressive agents), the incidence of IFIs has increased substantially in recent years.[1,2,3,4,5,6,7] For example, aggressive new therapies for transplant recipients and patients with hematologic malignancies have led to more profound immunosuppression of longer duration.[7]
In addition, advances in medical care are extending the survival of critically ill patients, rendering them more vulnerable to IFIs. [1,2,8,9] The incidence and severity of IFIs as well as the causative pathogens are dependent on various risk factors concerning the patient such as the underlying condition, the state of immunosuppression, but also the geographic location of the patient.[2,6,10]
A wide variety of pathogens can be associated with IFIs. Historically, Candida species have by far been the most common infective organisms among fungi. However, the epidemiology has changed dramatically in recent years: IFIs caused by moulds – predominantly Aspergillus species - have increased substantially and newly emerging and rare fungal pathogens such as Glomeromycota (e.g. Rhizopus and Mucor species), hyaline moulds (e.g. Fusarium species) and other opportunistic species (e.g. Scedosporium species) are increasingly being reported.[7,8,11,12] This article will predominantly review the most common causatives of IFIs, concentrating on the changing epidemiology of fungal infections and focusing on surveys carried out in the Mediterranean area.
Yeasts and Yeast-Like Pathogens
Candida species: Candida infections are the most frequent cause of IFIs worldwide, with a case rate of 72.8 per 1,000,000 per year [13] and can result in a wide range of clinical symptoms, from mucocutaneous overgrowth to blood stream infections and metastatic infections.[8,14,15] More than 100 Candida species have been found to be pathogenic with their frequency varying according to the geographic setting.[16,17,18,19]
The burden of invasive candidiasis remains substantial; after a decline in mortality throughout the early to mid 1990s, mortality rates have leveled off in recent years.[13] In the United States, Candida species are the fourth most common cause of nosocomial blood stream infection.[13] Candida (C.) albicans remains by far the most common species causing invasive candidiasis worldwide (62% in 2003) although the frequency of candidiasis caused by other species including C. tropicalis, C. parapsilosis, C. glabrata, and C. krusei has been increasing steadily over the last 10 years.[13] Two studies in Italy and Spain show the distribution of Candida species in the Mediterranean area which was shown to be generally similar to reports from other European countries.[20,21,22,23]
An Italian study [21] revealed, that C. albicans (61 % of all isolates) was followed by C. parapsilosis, C. glabrata and C.tropicalis, which is similar to reports from other European countries, with the only difference that here C. glabrata was shown to be the 3rd most common species, while it is the 2nd most common in Switzerland,[24] the UK25 and the US.[26] Interestingly, in a Spanish study, carried out in Barcelona, C. glabrata was shown to be only the 4th most common species, with C. tropicalis being the 3rd and C. parapsilosis the 2nd most common following C. albicans. [27] The same Spanish study [27] revealed, that the overall incidence of bloodstream infections caused by Candida in Barcelona is lower (4.3 cases per 100 000 population) than in the US (6-10 per 100 000 population).[3,28,29] Nevertheless, the number of incidence in Spain correlated well with reports from Northern European countries.[30,31] Candida bloodstream infections are in general very high among neonates and infants.[20,27,32] With 38.8 cases per 100 000 population the number of incidence in Barcelona/Spain is within the range of numbers obtained from studies in the US.26 However, C. parapsilosis was the most common species isolated from neonates in Spain (67% of all cases)20, whereas in the US C. albicans was the most common species and the proportion of C. parapsilosis infections was significantly lower (27-45%) than in Spain.[20,28,29]
Since the 1990s, fluconazole has been widely used for both treatment and prophylaxis of immunosuppressed patients resulting in decreasing rates of Candida bloodstream infections worldwide. The downside of this application was that C. glabrata, being less susceptible to fluconazole,[3,33] as well as other non-albicans infections are emerging, such as C. krusei which is fluconazole resistant. [34] In a nationwide surveillance study in Spain the frequency of antifungal resistance was determined next to species distribution and incident rates. This study revealed that 7 % of all isolates exhibited decreased susceptibility to fluconazole with a linear correlation to voriconazole resistance. Furthermore, MICs for voriconazole where increased in patients that received fluconazole before, than in those without previous exposure to fluconazole.[35] Another Spanish study investigated the susceptibility to voriconazole of more than 4000 clinical Candida isolates according to EUCAST testing, and revealed that among C. albicans, C. parapsilosis and C. tropicalis resistance to voriconazole was uncommon (with a maximum of 11%), but higher MICs were obtained for C. glabrata and C. krusei.[36] The antifungal susceptibility of the C. parapsilosis, which recently was found to consist of three different species, namely C. parapsilosis sensu strict, C. metapsilosis and C. parapsilosis, was shown to be low for echinocandins.[37,38] A Portuguese study testing 175 clinical and environmental isolates of the C. parapsilosis group showed that the majority (91.4 %) of all isolates are C. parapsilosis sensu stricto, and of those most isolates were susceptible to fluconazole. All of the isolates C. metapsilosis and C. parapsilosis were susceptible to azoles and amphotericinB, while a high number was non-susceptible to echinocandins.[38] The 10 year ARTEMIS DISK global antifungal surveillance study, where 256 882 isolates of Candida sp. were collected from 142 sites in 42 countries and tested against fluconazole, showed that the frequency of azole resistance varied considerably by geographic region.[39] Higher rates of resistance to both fluconazole and voriconazole were found in isolates from North America. Not only for C. glabrata and C. krusei decreased susceptibility was shown, but also for C. guilliermondii, C. inconspicua, C. rugosa and others, demonstrating that 13 out of the 31 species found exhibited increased resistance to fluconazole. As described before, cross- resistance between fluconazole and voriconazole is evident and seems to be more pronounced in some species of Candida than in others.[39]
Cryptococcus species
The genus Cryptococcus includes encapsulated yeasts that lack a mycelium.[40,41,42] Infection is usually initiated in the pulmonary tract with later possible dissemination, usually to the CNS, causing meningitis.[43,44] Involvement of parenchyma of the brain and meningitis occurs in between 40 and 86% of patients.[44] Cryptococcosis usually occurs in patients with impaired immunity.[44] The concern about Cryptococcus sp. has dramatically increased as it still remains one of the most common life threatening fungal infections in HIV- patients, where the risk of a Cryptococcus infection is between 2.9 – 13.3%. In non-HIV infected individuals, incidence rates of 0.2–0.9% have been reported in the United States.[44] Patients with AIDS have a much higher risk of infection (2.9–13.3%). In non – AIDS patients, but those with hematologic malignancies, administration of steroids and diabetes mellitus were the most frequent risk factors (6 and 4 out of 17 patients, respectively), as demonstrated in a retrospective study conducted in Italy between 1993 and 2002.[45]
In SOT recipients, an incidence of 2.8% has been reported.[44] Risk factors for mortality are pre-existing renal failure and liver failure in transplant recipients.[44]
In humans two Cryptococcus species can provoke disease: C. neoformans and C. gattii, which include 5 different serotypes altogether. Two varieties of C. neoformans (C. neoformans var. neoformans and C. neoformans var. grubii) representing serotypes D, A and AD (a hybrid from of both A and D), respectively, have been isolated.[42,46,47] C. gattii was previously listed as a further variety of C. neoformans, but is now known to be a distinct species. C. gattii includes serotypes B and C, both commonly seen as true pathogens provoking disease also in immunocompetent persons.[41,46,48]
C. neoformans has a worldwide distribution and has been isolated from a variety of environmental sources, mainly from bird excreta, where the microorganism can survive for a long time due to protection from sun and high temperatures. Its capsule even makes it resistant to natural drying of the vector matter. The distribution of C. gattii was thought to be restricted to tropical and subtropical environments, often associated with Eucalyptus trees and the koala bear.[49] Yet, in recent years, an outbreak of C. gattii infections has been reported from Vancouver Island/Canada, where more than 66 human cases with at least 4 fatalities have been reported in otherwise healthy persons, all due to infections with serotype B.50 In recent years, some other species such as C. laurentii and C. albidus were isolated from cryptococcosis patients.[51,52]
High mortality rates of 30-40%41 are mainly due to the difficulty in killing the pathogen. Without treatment, the invasive infection is fatal, which makes rapid diagnosis and treatment inevitable. A combination of amphothericin B and flucytosin, followed by fluconazole maintenance therapy is the therapeutic option in most cases,[53] although C. gattii was found to show resistance to amphotericin B and fluconazole.[54] Furthermore, a trend of increasing fluconazole resistance of C. neoformans isolates from the Asia-Pacific, Africa/Middle East, and Latin America regions but not among isolates from Europe or Northern America has been described in a 10 year antifungal surveillance study.[55]
Trichosporon species
Systemic trichosporonosis is a relatively uncommon but frequently fatal opportunistic fungal infection in immunocompromised individuals.[56] The taxonomy of the yeasts that cause trichosporonosis has been extensively revised.[56,57] It is now widely accepted that the previously named Trichosporon (T). beigelii actually consists of six species: T. asahii, T. asteroides, T. cutaneum, T. inkin, T. mucoides, and T. ovoides. Geotrichum capitatum, originally considered a species of Trichosporon and now reclassified, is also a common cause of trichosporonosis.[56] While any immunocompromised patient can develop invasive trichosporonosis, the risk is highest for those with hematologic malignancies.[58,59] Incidence rates of 0.4 and 0.5%, respectively, for infections due to Trichosporon sp. and G. capitatum have been reported in patients with leukemia.[59] One of the largest multicenter retrospective studies on invasive trichosporonosis, carried out in Italy, [56] revealed that acute myeloid leukemia was the most frequent underlying hematologic disease for trichosporonosis. A total of 17 of the 52 patients with hematological malignancies were diagnosed with infections caused by Trichosporon sp., while the majority of infections (35 out of 52) was attributed to G. capitatum. Furthermore, the study showed that the frequency of Trichosporon sp. infections is similar on all continents, while G. capitatum is predominantly a European pathogen, with high rates especially countries of the Mediterranean area.[56] Several Trichosporon sp. were shown to be multidrug resistant.[56] Echinocandins have poor activity against Trichosporon sp. as demonstrated by high MICs[58 ]and breakthrough cases in immunocompromised patients treated with caspofungin [59,60,61] or micafungin [61] have been reported.
Moulds
Aspergillus species: Aspergillus species are opportunistic moulds that can cause both allergic and invasive syndromes.[62] More than 300 Aspergillus species are known today of which only a small number cause opportunistic infections.[62] The most common species causing aspergillosis is Aspergillus (A.) fumigatus, accounting for approximately 90% of Aspergillus infections.[63] Depending on regional distinctions A. flavus, A. nidulans and A. terreus are frequently reported as well, and there is evidence that these non-fumigatus pathogens are increasingly common etiologic agents.[63,64,65] There are differences in the clinical presentations produced by these different species.
For example, A. flavus produces a disproportionate number of infections in the paranasal sinus, while A. nidulans is a common culprit in chronic granulomatous disease.[63] Although A. terreus remains uncommon, infection caused by this pathogen is associated with high mortality rates because of its resistance to amphotericin B.[64] A study including three European countries, namely Austria, Denmark and Spain, revealed that A. terreus seems to be endemic for Tirol, Austria as it was exclusively found in hospital samples from Austria.[66] In Spain/Madrid A. niger was the most isolated non-fumigatus species. Furthermore, it was shown that azole resistance of Aspergilli is significantly increasing, especially in the UK (Manchester) and the Netherlands (Nijmegen). The Dutch study, involving almost 2000 A. fumigatus isolates collected over a 14-year period in the Netherlands, of which 32 isolates exhibited increased resistance to all azoles tested, showed that 30 of the 32 strains had the same “dominant resistance mechanism”. They all exhibited a single amino acid change in the cyp51A gene (encoding the target enzyme cytochrome P450 sterol 14-α-demethylase) and an alteration in the promoter region of this gene. Six isolates out of 317 from other European countries also exhibited resistance to itraconazole. In a study by Pfaller et al. 1789 Aspergillus isolates from centers all over the world between 2001 and 2009 were evaluated for their susceptibility to triazoles (voriconazole, posaconazole, itraconazole). For each of the three triazoles tested, decreased susceptibility was observed and varied according to the species. 49 isolates exhibited MICs higher than 4 µg/ml for itraconazole, of which some were shown to be cross resistant to posaconazole and voriconazole.[68] There exist clinical reports on primary invasive Aspergillus infections due to resistant isolates involving various manifestations, e.g. in the lung, the brain, in bones.[69,70,71,72,73] Furthermore, cases have been shown, where itraconazole treatment is lacking clinical efficacy in patients with aspergilloma.[71,74] In Austria the occurrence of azole resistance among clinical A. fumigatus is 0% while in Spain it is 2%. Reasons for this increase in resistance are not clear yet, nevertheless there exists some evidence that it is due to excessive use of azoles in agriculture.[75,76]
Invasive aspergillosis has remained the predominate cause of invasive mould infections over the last 10–15 years.[77] Reasons for this include a continued increase in high-risk populations such as solid organ transplant (SOT) and hematopoietic stem cell transplant (HSCT) recipients, HIV-infected individuals, and those receiving intensified chemotherapy regimens.[2,63,78,79] Invasive aspergillosis is associated with a high rate of mortality, however, there is some evidence that survival rates have increased in recent years among those undergoing HSCT, primarily because of the use of non-myeloablative conditioning regimens, the use of peripheral blood stem cells, prompt diagnosis, and the use of effective antifungal therapy.[6]
An Italian study on invasive aspergillosis in AML-patients (SEIFEM-2008 registry study)80 showed that there is a clear downward trend in the aspergillosis-attributable mortality rate. In various consecutive multicenter studies Pagano et al. showed a decrease from 48% (1987-1998), to 38.5% (1999-2003) and 27% (2004-2007).[5,80,81,82] It is important to note, that according to the latest study,80 about two-thirds of the patients developed invasive aspergillosis despite standard antifungal prophylaxis based on fluconazole and itraconazole, which points out that the use of systemic prophylaxis needs to be further discussed. Independently from whatever prophylaxis was applied, A.fumigatus was the most causative species of aspergillosis in the Italian study.
Fusarium species
Fusarium sp. can be found in soil, plants and air. Clinical manifestations are diverse and depend largely on the immune status of the patient. Often, Fusarium sp. affects the skin (70-90%), lungs and sinuses (70-80%). Fusariosis is a life-threatening and increasingly important mycosis in immunocompromised hosts.[82,83,84] Risk factors for such infections are skin lesions, burns, use of corticosteroids, prolonged neutropenia and hematological malignancy.[12,84,85,86] Fusarium sp. are angiotropic and angioinvasive moulds that produce hemorrhagic infarction and low tissue perfusion, resulting in tissue necrosis.[83] More than 50 species of Fusarium have been identified but only a few are pathogenic in humans.83 These include F. solani (causes 50% of cases), F. oxysporum, F. moniliforme, F. verticillioides, F. dimerum, and F. proliferatum.[87]
In terms of global occurrence, fusariosis is most common in the United States (50–80% of all cases), followed by France, Italy, and Brazil.[83,87,88] In the SEIFEM-2004 survey, Fusarium species were responsible for 0.1% of infections, the majority in AML patients (0.3%).[81] In another Italian study, including 14 haematological centers, Fusarium infection was documented in 6 out of 351 patients (1.7%),88 with aplastic anaemia and AML as the underlying diseases (3 cases each). While the incidence in Italy remains stable, it increased in some US centers.[84] Because the clinical presentation of fusariosis may be non-specific, differentiating it from invasive aspergillosis can be challenging.[83] More than 90% of cases of fusariosis have been reported in neutropenic patients with hematologic malignancies.[88] Incidence rates of 0.06% (acute leukemia), 0.2% (autologous bone marrow transplant [BMT]), and 1.2% (allogeneic BMT) have been reported.82 In patients with hematologic malignancies, persistent neutropenia (hazard ratio [HR] = 5.43) and use of corticosteroids (HR = 2.18) were the most important predictors of mortality. Ideal treatment of fusariosis is still unclear. Azoles and polyenes seem to be most effective. Nevertheless, Fusarium sp. exhibit high resistance to antifungal drugs.[81,84,89]
Scedosporium species
Scedosporium sp. are ubiquitously distributed worldwide, commonly found in soil, sewage or polluted water. S. apiospermum (also known by its teleomorphic name Pseudoallescheria boydii) and S. prolificans have the greatest impact in human infections.[12,90] These two species differ in their epidemiological niches, morphology and antifungal sensitivity and can cause infections in both immunocompetent and immunosuppressed populations.[85,90] S. apiospermum has a worldwide distribution usually in association with water, and is therefore often reported as a cause of pneumonia and disseminated infection in near-drowning victims.[91] On the other hand, S. prolificans is found in soil, mainly in Spain and Australia.[92] In a Spanish survey, conducted between 1990 and 1999, S. prolificans was the most frequent filamentous fungi isolated from blood cultures,[93] comprising 5.2% of all of the filamentous fungi isolated in the respective hospital (San Sebastion/Spain).[93]
Mycetoma, a disfiguring, but non-life-threatening infection of the skin and subcutaneous tissue, is one type of disease caused by S. apiospermum, frequently developed through thorn punctures, wood splinters or preexisting trauma.[11,12,85] Pseudallescheriasis or scedosporiosis is mainly found in immunocompromised patients with hematological malignancies or in organ transplant recipients. For 11 % of cases in SOT recipients fungemia with Scedosporium sp. was reported.[11] Interestingly, neutropenia was not a variable in connection with Scedosporium infection in SOT patients. Also S. prolificans was found to cause deep invasive disseminated infections associated with high mortality rates. Dissemination throughout the body might be easier for this organism due to its ability to produce conidia in tissue.[12] Both pathogenic species of Scedosporium are highly resistant to amphotericin B and echinocandins, with S. prolificans being highly resistant to almost all of the currently available antifungal drugs. Voriconazole seems to have the strongest effect on both, S. apiospermum and S. prolificans, although most data exist from in vitro studies, where MICs for S. prolificans are at a level that would not be achieved in human compartiments and not be beneficial for the patient. As an approach, synergistic killing was investigated with a combination of voriconazole and terbinafine, which might be worthwhile to try.[12,85,94,95,96,97] Hence, mortality rates have been reported to be as high as 65-75 % for S. apiospermum and even higher (85-100%) for S. prolificans.
Glomeromycota (formerly Zygomycetes)
Infections with species of the Glomeromycota (medically referred to as zygomycosis or mucormycosis) play an increasingly important role in immunocompromised patients. Two orders of the Glomeromycota are clinically relevant: Mucorales and Entomophthorales.[98,99,100] Members of the Mucorales are distributed worldwide while Entomophthorales are generally limited to the tropics and subtropics.[101,102] Species provoking human disease mostly belong to the group of Mucorales, which is characterized by a rapidly evolving course, tissue destruction, and invasion of blood vessels.[101,103] The most common species causing mucormycosis are Rhizopus (R.) arrhizus (R. oryzae), R. microsporus var. rhizopodiformis, and R. pusillus. Other causative species include Absidia corymbifera, Mucor species, and Cunninghamella bertholletiae.[101] Mycoses caused by Entomophthorales are more indolent and chronically progressive.[101,103]
Commonly infections affect the paranasal sinus (39%), the lungs (24%), and the skin (19%) with the primary site of infection depending on the patient population.[7,104] Disseminated disease is reported in approximately one-fourth of patients,[104] resulting in high mortality rates (96%).[7]A case-control observational study found that prolonged neutropenie rather than a low neutrophil count is more common in patients with zygomycosis.[104] Frequent underlying risk factors are diabetes mellitus, particularly enhanced by ketoacidosis, hematological malignances and bone marrow or solid organs transplantation.[85,101,104] Diabetes still remains the most common risk factor with 36% to 88% among mucormycosis-cases having diabetes as a predisposing condition.[101] However, the cases of mucormycosis in patients with hematological malignancies or those who have received hematopoetic stem cell or SOTs is dramatically increasing in the past two decades.[85] Invasive mucormycosis is now considered to be the 2nd most frequent mould infection in patients with hematological malignances, with reported cumulative incidence ranging from 0.1 – 2.5 % in different series.104 An Italian study reports that 45 (11.5%) out of 391 patients with hematological malignancies had infections with a representative of the Mucorales.[105] In France the incidence rate within this patient group increased of 24 % per year from 1997 to 2006.[106] The so far largest and geographically most diverse study on epidemiology of zygomycosis in Europe, including 15 countries and 230 cases in total, once more pointed out that the most frequent underlying condition for zygomycosis is hematological malignancy (44% of all cases), whereas diabetes is only present in 17 % of all cases.[99] This is controversial to a study by,[103] reporting that diabetes account in 36 % of all cases to glomeromycota-infection. One possible explanation for this contrast might be the high increase of immunocompromised hosts in the recent decade.[99] The presence of available free iron predisposes to zygomycosis.[103] The application of the iron chelator deferoxamine allows the fungus to utilize deferoxamine-bound iron by recognizing it as a siderophore and enable it to acquire the – for the fungus inevitable – iron via siderophore-specific mechanism/high affinity non-reductive mechanism (sufficient levels of iron increases the ability proliferation and tissue penetration for the fungus). Other chelators (i.e., deferasirox) do not allow iron utilization and may decrease the risk of infection.[107,108] Antifungal prophylaxis with voriconazole also appears to be associated with an increased risk of developing zygomycosis.[85] For successful eradication of these pathogens a multifactorial treatment strategy is needed. This includes reducing the predisposing factors of the patient, surgical debridement and application of antifungal therapy. Amphotericin B, especially new lipid formulations, is still the agent of choice, and data exist that suggest a combinational therapy with posaconazole as promising.[85,109,110,111,112,113]

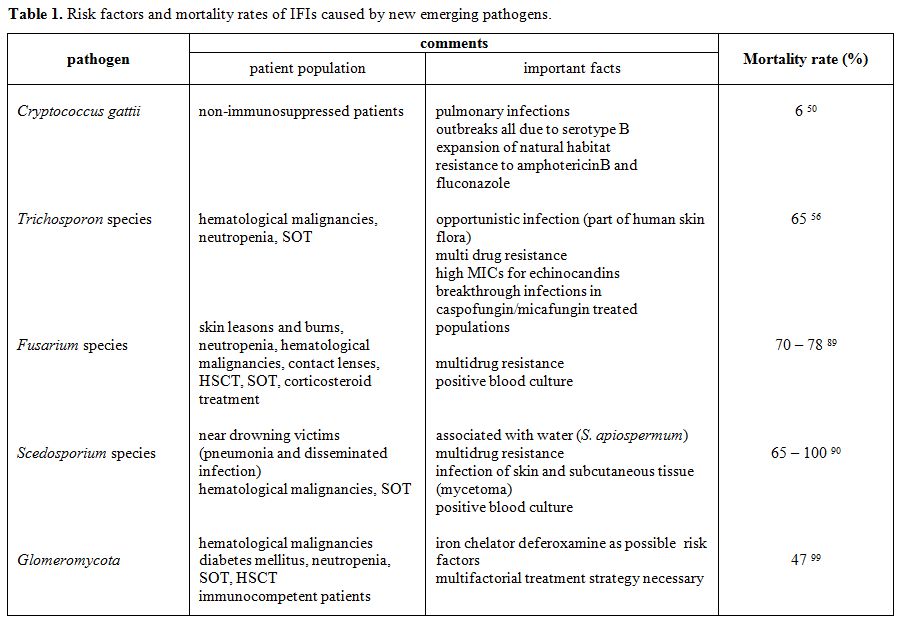
Table
1. Risk factors and mortality rates of IFIs caused by
new emerging pathogens.
Conclusion
With invasive mould infections becoming increasingly important, including those caused by rare, unusual pathogens, the epidemiology of IFIs is shifting in Europe. In some populations mould infections have already overtaken candidiasis, which was once the predominant type of IFIs. Reasons for this shift are multifactorial, but the augmented use of fluconazole as prophylaxis may account, at least in part, for this phenomenon, especially regarding infections with previously rare pathogens that occur as breakthrough infections. The management of IFIs is challenging – complicated by the difficulty in diagnosis and increasing resistance of the pathogens to available antifungal drugs.
References
- Holzheimer RG, Dralle H (2002) Management
of mycoses in surgical patients -- review of the literature. Eur J Med
Res 7: 200-226. PMid:12069912

- Mahfouz T, Anaissie E (2003) Prevention
of fungal infections in the immunocompromised host. Curr Opin Investig
Drugs 4: 974-990. PMid:14508882

- Marr KA, Seidel K, White TC, Bowden RA
(2000) Candidemia in allogeneic blood and marrow transplant recipients:
evolution of risk factors after the adoption of prophylactic
fluconazole. J Infect Dis 181: 309-316. doi:10.1086/315193 PMid:10608780

- Muhlemann K, Wenger C, Zenhausern R,
Tauber MG (2005) Risk factors for invasive aspergillosis in neutropenic
patients with hematologic malignancies. Leukemia 19: 545-550.
PMid:15729382

- Pagano L, Girmenia C, Mele L, Ricci P,
Tosti ME, et al. (2001) Infections caused by filamentous fungi in
patients with hematologic malignancies. A report of 391 cases by GIMEMA
Infection Program. Haematologica 86: 862-870.PMid:11522544

- Perkhofer S, Lass-Florl C, Hell M, Russ
G, Krause R, et al. (2010) The Nationwide Austrian Aspergillus
Registry: a prospective data collection on epidemiology, therapy and
outcome of invasive mould infections in immunocompromised and/or
immunosuppressed patients. Int J Antimicrob Agents 36: 531-536. doi:10.1016/j.ijantimicag.2010.08.010
PMid:20947312

- Richardson M, Lass-Florl C (2008)
Changing epidemiology of systemic fungal infections. Clin Microbiol
Infect 14 Suppl 4: 5-24. doi:10.1111/j.1469-0691.2008.01978.x
PMid:18430126

- Lass-Florl C (2009) The changing face of
epidemiology of invasive fungal disease in Europe. Mycoses 52: 197-205.doi:10.1111/j.1439-0507.2009.01691.x

- Peres-Bota D, Rodriguez-Villalobos H,
Dimopoulos G, Melot C, Vincent JL (2004) Potential risk factors for
infection with Candida spp. in critically ill patients. Clin Microbiol
Infect 10: 550-555. doi:10.1111/j.1469-0691.2004.00873.x
PMid:15191384

- Galgiani JN (1999) Coccidioidomycosis: a
regional disease of national importance. Rethinking approaches for
control. Ann Intern Med 130: 293-300. PMid:10068388

- Kubak BM, Huprikar SS (2009) Emerging
& rare fungal infections in solid organ transplant recipients. Am J
Transplant 9 Suppl 4: S208-226. doi:10.1111/j.1600-6143.2009.02913.x
PMid:20070683

- Varkey JB, Perfect JR (2008) Rare and
emerging fungal pulmonary infections. Semin Respir Crit Care Med 29:
121-131.doi:10.1055/s-2008-1063851
PMid:18365994

- Pfaller MA, Diekema DJ (2007)
Epidemiology of invasive candidiasis: a persistent public health
problem. Clin Microbiol Rev 20: 133-163. doi:10.1128/CMR.00029-06
PMid:17223626 PMCid:1797637

- Eggimann P, Garbino J, Pittet D (2003)
Management of Candida species infections in critically ill patients.
Lancet Infect Dis 3: 772-785. doi:10.1016/S1473-3099(03)00831-4

- Eggimann P, Garbino J, Pittet D (2003)
Epidemiology of Candida species infections in critically ill
non-immunosuppressed patients. Lancet Infect Dis 3: 685-702. doi:10.1016/S1473-3099(03)00801-6

- Bodey G, Bueltmann B, Duguid W, Gibbs D,
Hanak H, et al. (1992) Fungal infections in cancer patients: an
international autopsy survey. Eur J Clin Microbiol Infect Dis 11:
99-109.doi:10.1007/BF01967060 PMid:6756909

- Lass-Florl C, Resch G, Nachbaur D, Mayr
A, Gastl G, et al. (2007) The value of computed tomography-guided
percutaneous lung biopsy for diagnosis of invasive fungal infection in
immunocompromised patients. Clin Infect Dis 45: e101-104.doi:10.1086/521245 PMid:17806041

- Post MJ, Lass-Floerl C, Gastl G,
Nachbaur D (2007) Invasive fungal infections in allogeneic and
autologous stem cell transplant recipients: a single-center study of
166 transplanted patients. Transpl Infect Dis 9: 189-195.doi:10.1111/j.1399-3062.2007.00219.x
PMid:17511828

- Singh N (2001) Trends in the
epidemiology of opportunistic fungal infections: predisposing factors
and the impact of antimicrobial use practices. Clin Infect Dis 33:
1692-1696.doi:10.1086/323895 PMid:11641825

- Almirante B, Rodriguez D, Park BJ,
Cuenca-Estrella M, Planes AM, et al. (2005) Epidemiology and predictors
of mortality in cases of Candida bloodstream infection: results from
population-based surveillance, barcelona, Spain, from 2002 to 2003. J
Clin Microbiol 43: 1829-1835. doi:10.1128/JCM.43.4.1829-1835.2005
PMid:15815004 PMCid:1081396

- Bassetti M, Trecarichi EM, Righi E,
Sanguinetti M, Bisio F, et al. (2007) Incidence, risk factors, and
predictors of outcome of candidemia. Survey in 2 Italian university
hospitals. Diagn Microbiol Infect Dis 58: 325-331. doi:10.1016/j.diagmicrobio.2007.01.005
PMid:17350205

- Pfaller MA, Hazen KC, Messer SA, Boyken
L, Tendolkar S, et al. (2004) Comparison of results of fluconazole disk
diffusion testing for Candida species with results from a central
reference laboratory in the ARTEMIS global antifungal surveillance
program. J Clin Microbiol 42: 3607-3612.doi:10.1128/JCM.42.8.3607-3612.2004
PMid:15297505 PMCid:497595

- Tortorano AM, Biraghi E, Astolfi A, Ossi
C, Tejada M, et al. (2002) European Confederation of Medical Mycology
(ECMM) prospective survey of candidaemia: report from one Italian
region. J Hosp Infect 51: 297-304. doi:10.1053/jhin.2002.1261
PMid:12183145

- Marchetti O, Bille J, Fluckiger U,
Eggimann P, Ruef C, et al. (2004) Epidemiology of candidemia in Swiss
tertiary care hospitals: secular trends, 1991-2000. Clin Infect Dis 38:
311-320. doi:10.1086/380637 PMid:14727199

- Kibbler CC, Seaton S, Barnes RA,
Gransden WR, Holliman RE, et al. (2003) Management and outcome of
bloodstream infections due to Candida species in England and Wales. J
Hosp Infect 54: 18-24. doi:10.1016/S0195-6701(03)00085-9

- Diekema DJ, Messer SA, Brueggemann AB,
Coffman SL, Doern GV, et al. (2002) Epidemiology of candidemia: 3-year
results from the emerging infections and the epidemiology of Iowa
organisms study. J Clin Microbiol 40: 1298-1302. doi:10.1128/JCM.40.4.1298-1302.2002
PMid:11923348 PMCid:140380

- Almirante B, Rodriguez D,
Cuenca-Estrella M, Almela M, Sanchez F, et al. (2006) Epidemiology,
risk factors, and prognosis of Candida parapsilosis bloodstream
infections: case-control population-based surveillance study of
patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 44:
1681-1685. doi:10.1128/JCM.44.5.1681-1685.2006
PMid:16672393 PMCid:1479182

- Hajjeh RA, Sofair AN, Harrison LH, Lyon
GM, Arthington-Skaggs BA, et al. (2004) Incidence of bloodstream
infections due to Candida species and in vitro susceptibilities of
isolates collected from 1998 to 2000 in a population-based active
surveillance program. J Clin Microbiol 42: 1519-1527.doi:10.1128/JCM.42.4.1519-1527.2004
PMid:15070998 PMCid:387610

- Kao AS, Brandt ME, Pruitt WR, Conn LA,
Perkins BA, et al. (1999) The epidemiology of candidemia in two United
States cities: results of a population-based active surveillance. Clin
Infect Dis 29: 1164-1170. doi:10.1086/313450
PMid:10524958

- Asmundsdottir LR, Erlendsdottir H,
Gottfredsson M (2002) Increasing incidence of candidemia: results from
a 20-year nationwide study in Iceland. J Clin Microbiol 40: 3489-3492.doi:10.1128/JCM.40.9.3489-3492.2002
PMid:12202600 PMCid:130691

- Poikonen E, Lyytikainen O, Anttila VJ,
Ruutu P (2003) Candidemia in Finland, 1995-1999. Emerg Infect Dis 9:
985-990. PMid:12967498 PMCid:3020607

- Lockhart SR, Messer SA, Pfaller MA,
Diekema DJ (2008) Geographic distribution and antifungal susceptibility
of the newly described species Candida orthopsilosis and Candida
metapsilosis in comparison to the closely related species Candida
parapsilosis. J Clin Microbiol 46: 2659-2664. doi:10.1128/JCM.00803-08
PMid:18562582 PMCid:2519489

- Martino R, Subira M (2002) Invasive
fungal infections in hematology: new trends. Ann Hematol 81: 233-243.doi:10.1007/s00277-002-0466-3
PMid:12029531

- Laverdiere M, Rotstein C, Bow EJ,
Roberts RS, Ioannou S, et al. (2000) Impact of fluconazole prophylaxis
on fungal colonization and infection rates in neutropenic patients. The
Canadian Fluconazole Study. J Antimicrob Chemother 46: 1001-1008.doi:10.1093/jac/46.6.1001 PMid:11102422

- Cisterna R, Ezpeleta G, Telleria O,
Guinea J, Regueiro B, et al. (2010) Nationwide sentinel surveillance of
bloodstream Candida infections in 40 tertiary care hospitals in Spain.
J Clin Microbiol 48: 4200-4206. doi:10.1128/JCM.00920-10
PMid:20826636 PMCid:3020865

- Cuenca-Estrella M, Gomez-Lopez A, Cuesta
I, Zaragoza O, Mellado E, et al. (2011) Frequency of Resistance In
Vitro to Voriconazole among Spanish Clinical Isolates of Candida spp.
According to Breakpoints by the Antifungal Subcommittee of the European
Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents
Chemother.

- Canton E, Espinel-Ingroff A, Peman J,
del Castillo L (2010) In vitro fungicidal activities of echinocandins
against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis
evaluated by time-kill studies. Antimicrob Agents Chemother 54:
2194-2197.doi:10.1128/AAC.01538-09
PMid:20145083 PMCid:2863676

- Silva AP, Miranda IM, Lisboa C, Pina-Vaz
C, Rodrigues AG (2009) Prevalence, distribution, and antifungal
susceptibility profiles of Candida parapsilosis, C. orthopsilosis, and
C. metapsilosis in a tertiary care hospital. J Clin Microbiol 47:
2392-2397. doi:10.1128/JCM.02379-08
PMid:19494078 PMCid:2725652

- Pfaller MA, Diekema DJ, Gibbs DL, Newell
VA, Ellis D, et al. (2010) Results from the ARTEMIS DISK Global
Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of
susceptibilities of Candida Species to fluconazole and voriconazole as
determined by CLSI standardized disk diffusion. J Clin Microbiol 48:
1366-1377. doi:10.1128/JCM.02117-09
PMid:20164282 PMCid:2849609

- Halliday CL, Bui T, Krockenberger M,
Malik R, Ellis DH, et al. (1999) Presence of alpha and a mating types
in environmental and clinical collections of Cryptococcus neoformans
var. gattii strains from Australia. J Clin Microbiol 37:
2920-2926.PMid:10449476 PMCid:85414

- Iatta R, Napoli C, Borghi E, Montagna MT
(2009) Rare mycoses of the oral cavity: a literature epidemiologic
review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 647-655.doi:10.1016/j.tripleo.2009.07.010
PMid:19836721

- Perfect JR, Casadevall A (2002)
Cryptococcosis. Infect Dis Clin North Am 16: 837-874, v-vi. doi:10.1016/S0891-5520(02)00036-3

- Husain S, Wagener MM, Singh N (2001)
Cryptococcus neoformans infection in organ transplant recipients:
variables influencing clinical characteristics and outcome. Emerg
Infect Dis 7: 375-381. PMid:11384512 PMCid:2631789

- Wu G, Vilchez RA, Eidelman B, Fung J,
Kormos R, et al. (2002) Cryptococcal meningitis: an analysis among
5,521 consecutive organ transplant recipients. Transpl Infect Dis 4:
183-188.doi:10.1034/j.1399-3062.2002.t01-1-02005.x
PMid:12535260

- Pagano L, Fianchi L, Caramatti C,
D'Antonio D, Melillo L, et al. (2004) Cryptococcosis in patients with
hematologic malignancies. A report from GIMEMA-infection. Haematologica
89: 852-856. PMid:15257938

- Kwon-Chung KJ, Varma A (2006) Do major
species concepts support one, two or more species within Cryptococcus
neoformans? FEMS Yeast Res 6: 574-587.doi:10.1111/j.1567-1364.2006.00088.x
PMid:16696653

- Lucas S, da Luz Martins M, Flores O,
Meyer W, Spencer-Martins I, et al. (2010) Differentiation of
Cryptococcus neoformans varieties and Cryptococcus gattii using
CAP59-based loop-mediated isothermal DNA amplification. Clin Microbiol
Infect 16: 711-714. doi:10.1111/j.1469-0691.2009.02919.x
PMid:19694768

- Franzot SP, Salkin IF, Casadevall A
(1999) Cryptococcus neoformans var. grubii: separate varietal status
for Cryptococcus neoformans serotype A isolates. J Clin Microbiol 37:
838-840.PMid:9986871 PMCid:84578

- Vilcins I, Krockenberger M, Agus H,
Carter D (2002) Environmental sampling for Cryptococcus neoformans var.
gattii from the Blue Mountains National Park, Sydney, Australia. Med
Mycol 40: 53-60. PMid:11860013

- Fraser JA, Subaran RL, Nichols CB,
Heitman J (2003) Recapitulation of the sexual cycle of the primary
fungal pathogen Cryptococcus neoformans var. gattii: implications for
an outbreak on Vancouver Island, Canada. Eukaryot Cell 2: 1036-1045. doi:10.1128/EC.2.5.1036-1045.2003
PMid:14555486 PMCid:219376

- Dorneanu O, Filip O, Miftode E, Radu I,
Nicolau C, et al. (2008) [Cryptococcus meningitis, five years of
experience and literature review]. Rev Med Chir Soc Med Nat Iasi 112:
100-103.PMid:18677910

- Vlchkova-Lashkoska M, Kamberova S,
Starova A, Goleva-Mishevska L, Tsatsa-Biljanovska N, et al. (2004)
Cutaneous cryptococcus Laurentii infection in a human immunodeficiency
virus-negative subject. J Eur Acad Dermatol Venereol 18: 99-100. doi:10.1111/j.1468-3083.2004.00434.x
PMid:14678544

- Saag MS, Graybill RJ, Larsen RA, Pappas
PG, Perfect JR, et al. (2000) Practice guidelines for the management of
cryptococcal disease. Infectious Diseases Society of America. Clin
Infect Dis 30: 710-718. doi:10.1086/313757 PMid:10770733

- Khyriem AB, Sujatha S, Parija SC (2004)
Chronic meningitis in an immunocompetent adult caused by Cryptococcus
neoformans var gatti. Indian J Med Microbiol 22: 275.PMid:17642758

- Pfaller MA, Diekema DJ, Gibbs DL, Newell
VA, Bijie H, et al. (2009) Results from the ARTEMIS DISK Global
Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of
susceptibilities of noncandidal yeast species to fluconazole and
voriconazole determined by CLSI standardized disk diffusion testing. J
Clin Microbiol 47: 117-123. doi:10.1128/JCM.01747-08
PMid:19005141 PMCid:2620874

- Girmenia C, Pagano L, Martino B,
D'Antonio D, Fanci R, et al. (2005) Invasive infections caused by
Trichosporon species and Geotrichum capitatum in patients with
hematological malignancies: a retrospective multicenter study from
Italy and review of the literature. J Clin Microbiol 43: 1818-1828.doi:10.1128/JCM.43.4.1818-1828.2005
PMid:15815003 PMCid:1081342

- Gueho E, de Hoog GS, Smith MT (1992)
Neotypification of the genus Trichosporon. Antonie Van Leeuwenhoek 61:
285-288.PMid:1497333

- Espinel-Ingroff A (2003) In vitro
antifungal activities of anidulafungin and micafungin, licensed agents
and the investigational triazole posaconazole as determined by NCCLS
methods for 12,052 fungal isolates: review of the literature. Rev
Iberoam Micol 20: 121-136. PMid:15456349

- Goodman D, Pamer E, Jakubowski A, Morris
C, Sepkowitz K (2002) Breakthrough trichosporonosis in a bone marrow
transplant recipient receiving caspofungin acetate. Clin Infect Dis 35:
E35-36. doi:10.1086/341305 PMid:12115115

- Akagi T, Yamaguti K, Kawamura T,
Nakumura T, Kubo K, et al. (2006) Breakthrough trichosporonosis in
patients with acute myeloid leukemia receiving micafungin. Leuk
Lymphoma 47: 1182-1183. doi:10.1080/10428190500272499
PMid:16840220

- Matsue K, Uryu H, Koseki M, Asada N,
Takeuchi M (2006) Breakthrough trichosporonosis in patients with
hematologic malignancies receiving micafungin. Clin Infect Dis 42:
753-757.doi:10.1086/500323 PMid:16477548

- Denning DW (1998) Invasive
aspergillosis. Clin Infect Dis 26: 781-803; quiz 804-785.

- Marr KA, Patterson T, Denning D (2002)
Aspergillosis. Pathogenesis, clinical manifestations, and therapy.
Infect Dis Clin North Am 16: 875-894, vi. doi:10.1016/S0891-5520(02)00035-1

- Lass-Florl C, Griff K, Mayr A, Petzer A,
Gastl G, et al. (2005) Epidemiology and outcome of infections due to
Aspergillus terreus: 10-year single centre experience. Br J Haematol
131: 201-207. doi:10.1111/j.1365-2141.2005.05763.x
PMid:16197450

- Marr KA, Carter RA, Boeckh M, Martin P,
Corey L (2002) Invasive aspergillosis in allogeneic stem cell
transplant recipients: changes in epidemiology and risk factors. Blood
100: 4358-4366. doi:10.1182/blood-2002-05-1496
PMid:12393425

- Mortensen KL, Mellado E, Lass-Florl C,
Rodriguez-Tudela JL, Johansen HK, et al. (2010) Environmental study of
azole-resistant Aspergillus fumigatus and other aspergilli in Austria,
Denmark, and Spain. Antimicrob Agents Chemother 54: 4545-4549. doi:10.1128/AAC.00692-10 PMid:20805399

- Snelders E, van der Lee HA, Kuijpers J,
Rijs AJ, Varga J, et al. (2008) Emergence of azole resistance in
Aspergillus fumigatus and spread of a single resistance mechanism. PLoS
Med 5: e219.doi:10.1371/journal.pmed.0050219
PMid:18998768 PMCid:2581623

- Pfaller M, Boyken L, Hollis R, Kroeger
J, Messer S, et al. (2011) Use of epidemiological cutoff values to
examine 9-year trends in susceptibility of Aspergillus species to the
triazoles. J Clin Microbiol 49: 586-590. doi:10.1128/JCM.02136-10 PMid:21123534

- Hodiamont CJ, Dolman KM, Ten Berge IJ,
Melchers WJ, Verweij PE, et al. (2009) Multiple-azole-resistant
Aspergillus fumigatus osteomyelitis in a patient with chronic
granulomatous disease successfully treated with long-term oral
posaconazole and surgery. Med Mycol 47: 217-220. doi:10.1080/13693780802545600
PMid:19101840

- Howard SJ, Cerar D, Anderson MJ,
Albarrag A, Fisher MC, et al. (2009) Frequency and evolution of Azole
resistance in Aspergillus fumigatus associated with treatment failure.
Emerg Infect Dis 15: 1068-1076. doi:10.3201/eid1507.090043
PMid:19624922 PMCid:2744247

- Howard SJ, Pasqualotto AC, Denning DW
(2010) Azole resistance in allergic bronchopulmonary aspergillosis and
Aspergillus bronchitis. Clin Microbiol Infect 16: 683-688.doi:10.1111/j.1469-0691.2009.02911.x
PMid:19673966

- van der Linden JW, Jansen RR, Bresters
D, Visser CE, Geerlings SE, et al. (2009) Azole-resistant central
nervous system aspergillosis. Clin Infect Dis 48: 1111-1113.doi:10.1086/597465 PMid:19272019

- Verweij PE, Mellado E, Melchers WJ
(2007) Multiple-triazole-resistant aspergillosis. N Engl J Med 356:
1481-1483.doi:10.1056/NEJMc061720 PMid:17409336

- Chen J, Li H, Li R, Bu D, Wan Z (2005)
Mutations in the cyp51A gene and susceptibility to itraconazole in
Aspergillus fumigatus serially isolated from a patient with lung
aspergilloma. J Antimicrob Chemother 55: 31-37. doi:10.1093/jac/dkh507 PMid:15563516

- Snelders E, Huis In 't Veld RA, Rijs AJ,
Kema GH, Melchers WJ, et al. (2009) Possible environmental origin of
resistance of Aspergillus fumigatus to medical triazoles. Appl Environ
Microbiol 75: 4053-4057. doi:10.1128/AEM.00231-09
PMid:19376899 PMCid:2698372

- Verweij PE, Howard SJ, Melchers WJ,
Denning DW (2009) Azole-resistance in Aspergillus: proposed
nomenclature and breakpoints. Drug Resist Updat 12: 141-147.doi:10.1016/j.drup.2009.09.002
PMid:19879181

- Alangaden GJ (2011) Nosocomial fungal
infections: epidemiology, infection control, and prevention. Infect Dis
Clin North Am 25: 201-225. doi:10.1016/j.idc.2010.11.003
PMid:21316001

- Singh N, Paterson DL (2005) Aspergillus
infections in transplant recipients. Clin Microbiol Rev 18: 44-69.doi:10.1128/CMR.18.1.44-69.2005
PMid:15653818 PMCid:544171

- Upton A, Kirby KA, Carpenter P, Boeckh
M, Marr KA (2007) Invasive aspergillosis following hematopoietic cell
transplantation: outcomes and prognostic factors associated with
mortality. Clin Infect Dis 44: 531-540.doi:10.1086/510592
PMid:17243056

- Pagano L, Caira M, Candoni A, Offidani
M, Martino B, et al. (2010) Invasive aspergillosis in patients with
acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 95:
644-650. doi:10.3324/haematol.2009.012054
PMid:19850903 PMCid:2857195

- Pagano L, Caira M, Candoni A, Offidani
M, Fianchi L, et al. (2006) The epidemiology of fungal infections in
patients with hematologic malignancies: the SEIFEM-2004 study.
Haematologica 91: 1068-1075. PMid:16885047

- Pagano L, Caira M, Nosari A, Van Lint
MT, Candoni A, et al. (2007) Fungal infections in recipients of
hematopoietic stem cell transplants: results of the SEIFEM B-2004
study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie
Maligne. Clin Infect Dis 45: 1161-1170. doi:10.1086/522189 PMid:17918077

- Lionakis MS, Kontoyiannis DP (2004)
Fusarium infections in critically ill patients. Semin Respir Crit Care
Med 25: 159-169.PMid:16088459

- Nucci M, Anaissie EJ, Queiroz-Telles F,
Martins CA, Trabasso P, et al. (2003) Outcome predictors of 84 patients
with hematologic malignancies and Fusarium infection. Cancer 98:
315-319. doi:10.1002/cncr.11510 PMid:12872351

- Malani AN, Kauffman CA (2007) Changing
epidemiology of rare mould infections: implications for therapy. Drugs
67: 1803-1812. doi:10.2165/00003495-200767130-00001
PMid:17722951

- Nucci M (2003) Emerging moulds:
Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr
Opin Infect Dis 16: 607-612. doi:10.1097/00001432-200312000-00015
PMid:14624113

- Torres HA, Raad, II, Kontoyiannis DP
(2003) Infections caused by Fusarium species. J Chemother 15 Suppl 2:
28-35.PMid:14708964

- Girmenia C, Pagano L, Corvatta L, Mele
L, del Favero A, et al. (2000) The epidemiology of fusariosis in
patients with haematological diseases. Gimema Infection Programme. Br J
Haematol 111: 272-276. doi:10.1046/j.1365-2141.2000.02312.x
PMid:11091211

- Boutati EI, Anaissie EJ (1997) Fusarium,
a significant emerging pathogen in patients with hematologic
malignancy: ten years' experience at a cancer center and implications
for management. Blood 90: 999-1008. PMid:9242529

- Meletiadis J, Meis JF, Mouton JW,
Rodriquez-Tudela JL, Donnelly JP, et al. (2002) In vitro activities of
new and conventional antifungal agents against clinical Scedosporium
isolates. Antimicrob Agents Chemother 46: 62-68.doi:10.1128/AAC.46.1.62-68.2002
PMid:11751112 PMCid:126988

- Dworzack DL, Clark RB, Borkowski WJ,
Jr., Smith DL, Dykstra M, et al. (1989) Pseudallescheria boydii brain
abscess: association with near-drowning and efficacy of high-dose,
prolonged miconazole therapy in patients with multiple abscesses.
Medicine (Baltimore) 68: 218-224.

- Berenguer J, Rodriguez-Tudela JL,
Richard C, Alvarez M, Sanz MA, et al. (1997) Deep infections caused by
Scedosporium prolificans. A report on 16 cases in Spain and a review of
the literature. Scedosporium Prolificans Spanish Study Group. Medicine
(Baltimore) 76: 256-265.doi:10.1097/00005792-199707000-00004
PMid:9279332

- Idigoras P, Perez-Trallero E, Pineiro L,
Larruskain J, Lopez-Lopategui MC, et al. (2001) Disseminated infection
and colonization by Scedosporium prolificans: a review of 18 cases,
1990-1999. Clin Infect Dis 32: E158-165.doi:10.1086/320521 PMid:11340550

- Gilgado F, Serena C, Cano J, Gene J,
Guarro J (2006) Antifungal susceptibilities of the species of the
Pseudallescheria boydii complex. Antimicrob Agents Chemother 50:
4211-4213.doi:10.1128/AAC.00981-06
PMid:17015631 PMCid:1694014

- Meletiadis J, Mouton JW, Meis JF,
Verweij PE (2003) In vitro drug interaction modeling of combinations of
azoles with terbinafine against clinical Scedosporium prolificans
isolates. Antimicrob Agents Chemother 47: 106-117.doi:10.1128/AAC.47.1.106-117.2003
PMid:12499177 PMCid:149034

- Meletiadis J, te Dorsthorst DT, Verweij
PE (2003) Use of turbidimetric growth curves for early determination of
antifungal drug resistance of filamentous fungi. J Clin Microbiol 41:
4718-4725. doi:10.1128/JCM.41.10.4718-4725.2003
PMid:14532210 PMCid:254297

- Howden BP, Slavin MA, Schwarer AP, Mijch
AM (2003) Successful control of disseminated Scedosporium prolificans
infection with a combination of voriconazole and terbinafine. Eur J
Clin Microbiol Infect Dis 22: 111-113.PMid:12627286

- Hibbett DS, Binder M, Bischoff JF,
Blackwell M, Cannon PF, et al. (2007) A higher-level phylogenetic
classification of the Fungi. Mycol Res 111: 509-547. doi:10.1016/j.mycres.2007.03.004
PMid:17572334

- Skiada A, Pagano L, Groll A, Zimmerli S,
Dupont B, et al. (2011) Zygomycosis in Europe: Analysis of 230 cases
accrued by the registry of the European Confederation of Medical
Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007.
Clin Microbiol Infect.

- White MM, James TY, O'Donnell K, Cafaro
MJ, Tanabe Y, et al. (2006) Phylogeny of the Zygomycota based on
nuclear ribosomal sequence data. Mycologia 98: 872-884.doi:10.3852/mycologia.98.6.872
PMid:17486964

- Chayakulkeeree M, Ghannoum MA, Perfect
JR (2006) Zygomycosis: the re-emerging fungal infection. Eur J Clin
Microbiol Infect Dis 25: 215-229. doi:10.1007/s10096-006-0107-1
PMid:6756909

- Gonzalez CE, Rinaldi MG, Sugar AM
(2002) Zygomycosis. Infect Dis Clin North Am 16: 895-914, vi.doi:10.1016/S0891-5520(02)00037-5

- Roden MM, Zaoutis TE, Buchanan WL,
Knudsen TA, Sarkisova TA, et al. (2005) Epidemiology and outcome of
zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:
634-653. doi:10.1086/432579 PMid:16080086

- Kontoyiannis DP, Lionakis MS, Lewis RE,
Chamilos G, Healy M, et al. (2005) Zygomycosis in a tertiary-care
cancer center in the era of Aspergillus-active antifungal therapy: a
case-control observational study of 27 recent cases. J Infect Dis 191:
1350-1360. doi:10.1086/428780
PMid:15776383

- Pagano L, Offidani M, Fianchi L, Nosari
A, Candoni A, et al. (2004) Mucormycosis in hematologic patients.
Haematologica 89: 207-214. PMid:15003897

- Bitar D, Van Cauteren D, Lanternier F,
Dannaoui E, Che D, et al. (2009) Increasing incidence of zygomycosis
(mucormycosis), France, 1997-2006. Emerg Infect Dis 15: 1395-1401. doi:10.3201/eid1509.090334
PMid:19788806 PMCid:2819884

- Boelaert JR, van Roost GF, Vergauwe PL,
Verbanck JJ, de Vroey C, et al. (1988) The role of desferrioxamine in
dialysis-associated mucormycosis: report of three cases and review of
the literature. Clin Nephrol 29: 261-266. PMid:3293856

- Daly AL, Velazquez LA, Bradley SF,
Kauffman CA (1989) Mucormycosis: association with deferoxamine therapy.
Am J Med 87: 468-471. doi:10.1016/S0002-9343(89)80836-8

- Herbrecht R, Auvrignon A, Andres E,
Guillemain R, Suc A, et al. (2001) Efficacy of amphotericin B lipid
complex in the treatment of invasive fungal infections in
immunosuppressed paediatric patients. Eur J Clin Microbiol Infect Dis
20: 77-82. doi:10.1007/s100960000437

- Herbrecht R, Letscher-Bru V, Bowden RA,
Kusne S, Anaissie EJ, et al. (2001) Treatment of 21 cases of invasive
mucormycosis with amphotericin B colloidal dispersion. Eur J Clin
Microbiol Infect Dis 20: 460-466. doi:10.1007/s100960100528

- Kofteridis DP, Karabekios S,
Panagiotides JG, Bizakis J, Kyrmizakis D, et al. (2003) Successful
treatment of rhinocerebral mucormycosis with liposomal amphotericin B
and surgery in two diabetic patients with renal dysfunction. J
Chemother 15: 282-286. PMid:12868556

- Sun QN, Fothergill AW, McCarthy DI,
Rinaldi MG, Graybill JR (2002) In vitro activities of posaconazole,
itraconazole, voriconazole, amphotericin B, and fluconazole against 37
clinical isolates of zygomycetes. Antimicrob Agents Chemother 46:
1581-1582. doi:10.1128/AAC.46.5.1581-1582.2002
PMid:11959605 PMCid:127128

- Sun QN, Najvar LK, Bocanegra R,
Loebenberg D, Graybill JR (2002) In vivo activity of posaconazole
against Mucor spp. in an immunosuppressed-mouse model. Antimicrob
Agents Chemother 46: 2310-2312. doi:10.1128/AAC.46.7.2310-2312.2002
PMid:12069997 PMCid:127310
