Cerebral Venous Thrombosis in the Mediterranean Area in Children
Menascu S1, Lotan A2,3 , Ben Zeev B1, Nowak- Gottl U4 and Kenet G3
1Department
of Pediatric Neurology, The Edmond and Lily Safra Children’s hospital,
Sheba Medical Center, Tel Hashomer and the Sackler Medical
School, Tel Aviv University, Israel
2Department of Pediatrics, E Wolfson Medical Center, Holon and the Sackler Medical School, Tel Aviv University, Israel
3Thrombosis Unit, The national Hemophilia center, Sheba Medical Center, Tel Hashomer and the Sackler Medical School, Tel Aviv University, Israel
4Universitätsklinikum Schleswig-Holstein, Rechtsfähige Anstalt des öffentlichen Rechts der Christian-Albrechts-Universität zu Kiel und der Universität zu Lübeck, Germany
2Department of Pediatrics, E Wolfson Medical Center, Holon and the Sackler Medical School, Tel Aviv University, Israel
3Thrombosis Unit, The national Hemophilia center, Sheba Medical Center, Tel Hashomer and the Sackler Medical School, Tel Aviv University, Israel
4Universitätsklinikum Schleswig-Holstein, Rechtsfähige Anstalt des öffentlichen Rechts der Christian-Albrechts-Universität zu Kiel und der Universität zu Lübeck, Germany
Correspondence
to: G
Kenet, MD. Director, Thrombosis Unit, The National Hemophilia Center,
Sheba Medical Center, Tel Hashomer 52621, Israel.
Phone:+972-3-5302120, Fax:+972-3-5351806. E-mail: Gili.kenet@sheba.health.gov.il
Published: July 8, 2011
Received: April 20, 2011
Accepted: June 12 , 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011029, DOI 10.4084/MJHID.2011.029
This article is available from: http://www.mjhid.org/article/view/8458
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Cerebral
Venous Sinus (sinovenous) Thrombosis (CSVT) is a serious and rare
disorder, increasingly recognized and diagnosed in pediatric
patients. The etiology and pathophisiology has not yet been completely
clarified, and unlike adults with CSVT, management in children and
neonates remains controversial. However, morbidity and mortality are
significant, highlighting the continued need for high-quality studies
within this field. The following review will highlight aspects of CSVT
in the mediteranian area in children.
Epidemiology
The incidence of childhood CSVT varies between 0.4 and 0.7 per 100,000 children per year.[1-3] More than 40% of childhood CSVT occurs within the neonatal period, with an incidence of 2.6 per 100,000 children per year in one series.[1] Notably, these reported figures are probably underestimates of the true rate of CSVT occurrence, since neonates often present with nonfocal neurologic signs and symptoms, potentialy leading to missed diagnosis.[2] Old imaging techniques, the variable anatomy of sinovenous channels and rapid recanalization are all factors which may contribute to underdiagnosis.[4] The annual incidence of sinus vein thrombosis among children in the mediteranian area is not reported. Some case- series, cohort and case controlled multicenter studies are available from Lebanon, Turkey, France, Italy, Portugal and Israel.[5-10] CSVT incidence evaluated among children and neonates hospitalized in a tertiary medical center in Israel was as high as 2/10,000,[11] potentially due to selection bias, in a referral center with high clinical awareness and high index of suspicion that led towards early diagnosis and treatment of CSVT. The largest European CSVT cohort consisted of 396 children , prospectively studied and followed in order to asses the risk factors associated with thrombosis recurrence.[12]
Anatomy and Pathophysiology
Cerebral vascular malformations may involve the venous circulation, with “developmental venous anomaly” ( DVA) being the most frequent. This term, proposed by Lasjaunias et al.,[13] is now widely used as a synonym for venous angioma, cerebral venous malformation, or cerebral venous medullary malformation. DVAs are encountered with an incidence of up to 2.6% in a series of 4,069 brain autopsies.[14] They represent an anomalous venous disposition due to the absence of normal pial or subependymal veins. They may drain into both the superficial and deep venous systems, and a stenosis of the collecting vein of DVAs is commonly observed, typically at the point where the vein crosses the dura to drain into a dural venous sinus. The most frequently involved sites of CSVT are the superior sagittal sinus (90% of all cases in most studies) and transverse sinuses of the superficial venous system and the straight sinus of the deep system.[15] After CSVT, venous pressure escalates in the occluded vessels and secondary hemorrhagic infarction develops due to brain tissue hypertension, localized venous congestion, vasogenic edema, and increased capillary hydrostatic pressure in excess of cerebral arteriolar blood flow. Approximately 65% of neonates with CSVT have brain parenchymal lesions, frequently detected in the frontal and parietal lobes.[16]
Clinical Presentation and Symptoms
It is apparent that the clinical manifestations of CSVT are non-specific and may be subtle. Clinical scenarios occur at all ages and the clinician should consider this diagnosis in a wide range of acute neurological presentations in childhood. These include seizures, coma, stroke, headache and increased intracranial pressure.[17] Common illnesses, eg: ear infections, meningitis, anaemia, diabetes and head injury, may lead to CSVT evolution, various clinical settings including sepsis, dehydration, renal failure, trauma, cancer and haematological disorders may precipitate.[18] Although presentation with pseudotumour cerebri has been well documented, there are few data on the prevalence of CSVT in otherwise unexplained hydrocephalus or in convulsive and non-convulsive seizures and status epilepticus.[19] It has been suggested that toddlers frequently present with seizures and focal signs, mainly hemiparesis, whereas older children present with headache and changes in mental status and seizures may be less common.[20] In a study of neonatal sinus vein thrombosis (52 neonates with a median gestational age of 39 weeks), symptoms developed at a median postnatal age of 1.5 days (range: 0 to 28 days) and consisted mainly of seizures (29 of 52).[21] Other presenting findings in neonates were apnea (17.3%), agitation (5.8%), sepsis like(3.8%) and decreased consciousness in up to 2%.[22] The majority of young children present acutely with seizures, focal signs and symptoms of raised intracranial pressure, such as headache and decreased level of consciousness. Subacute presentation, with chronic headache, vomiting, lethargy, anorexia or drowsiness for 3 weeks or more, may also occur.[23]
Diagnosis and Imaging Studies
The clinical spectrum of CVST closely mimics that of idiopathic intracranial hypertension. Ultrasonography, which is the preliminary screening test for hemorrhage in the neonatal period, may detect intraventricular bleeding, centrally located venous infarcts or hemorrhages, and ventricular dilation and leukomalacia secondary to CSVT infarction and ischemia. However, ultrasound has poor sensitivity for cerebral infarction and should not be used for the primary detection of CSVT and the extent of ensuing injury.[24,25] Cranial computed tomography (CT) can be performed rapidly in neonates with minimum sedation but misses CSVT in 15% of cases. The classic features that indicate CSVT include the “dense triangle” or the “cord sign,” which describe the increased density over the thrombosed venous sinus in a plain CT, or the“empty triangle”.[26] A communicating hydrocephalus may be associated with sagittal sinus thrombosis caused by the impaired CSF absorption over the arachnoid granulations that line the sagittal sinus. A CT venogram using the multislice technique is more sensitive and specific for the diagnosis of CSVT, but the consequent radiation exposure is a definite concern in neonates. MRI is free of radiation risk and permits earlier and more accurate detection of CSVT.[27] Diffusion-weighted imaging is a sensitive technique for detecting areas of infarction. Parenchymal changes can be seen within minutes of injury, which allows for early identification and intervention and Magnetic resonance venography allows for noninvasive assessment of the venous system without exposure to radiograph radiation. Using time-offlight techniques, the cerebral sinuses can be imaged even without the use of contrast. Currently, contrast-enhanced magnetic resonance venography, which is less susceptible to alterations in flow, is the imaging technique of choice for the detection of CSVT in the neonatal population.[28] Attached are 2 representative MR figures of CSVT in pediatric patients (Figures 1,2).
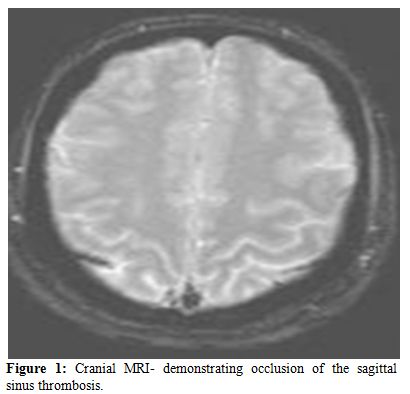
Figure 1. Cranial MRI- demonstrating occlusion of the sagittal sinus thrombosis.
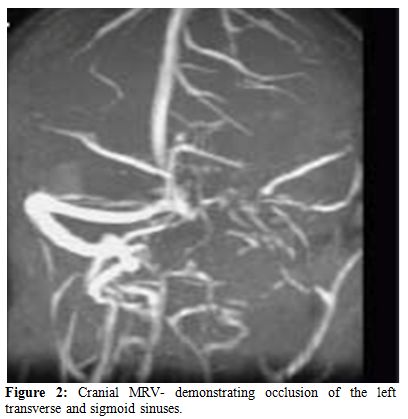
Figure 2. Cranial MRV- demonstrating occlusion of the left transverse and sigmoid sinuses.
Etiology and Risk Factors
The etiology and pathophysiology of CSVT in the pediatric population is still poorly understood, and the role of thrombophilic risk factors remains to be elucidated.[1-5,10-12,29-30] Trauma, infections (especially sinusitis, mastoiditis and other conditions affecting the head and neck area) , collagen vascular disorders, hemoglobinopathies, metabolic and inflamatory bowel diseases have been suggested as potential risk factors for CSVT occurrence in small cohort and case study series.[31-38] Several case-control studies have dealth with the role of prothrombotic risk factors in the pathphysiology of CSVT.[1-5,10-12,39-44] In a recent meta- analysis of pediatric case- controlled studies thrombophilia certainly increased the risk of stroke or CSVT in patients aged 0-18 years old, especially when combination of thrombophilic risk factors was diagnosed.45 A statistically significant association with first event occurrence was demonstrated for each thrombophilia trait evaluated, with no difference found between arterial ischemic stroke and CSVT. Summary ORs (fixed-effects model) were as follows: antithrombin deficiency, 7.06 (95% CI, 2.44 to 22.42); protein C deficiency, 8.76 (95% CI, 4.53 to 16.96); protein S deficiency, 3.20 (95% CI, 1.22 to 8.40), factor V G1691A, 3.26 (95% CI, 2.59 to 4.10); factor II G20210A, 2.43 (95% CI, 1.67 to 3.51); MTHFR C677T (AIS), 1.58 (95% CI, 1.20 to 2.08); antiphospholipid antibodies (AIS), 6.95 (95% CI, 3.67 to 13.14); elevated lipoprotein(a), 6.27 (95% CI, 4.52 to 8.69), and combined thrombophilias, 11.86 (95% CI, 5.93 to 23.73). Genetic thrombophilia, and especially presence of prothrombin G20210A mutation significantly increase the risk of recurrent thromboembolism in CSVT patients older than 2 years of age,[12] in situations of future acquired hypercoagulability.
Outcome
Over all neonates have a more favorable outcome as compared to older children.[46] Nevertheless, in the majority of studies the authors did not adequately describe outcomes in neonates compared with those of children with CSVT. The mean age of follow-up varied across the studies, and disabilities were variably classified.[47] Curently, there is a substantial differences in reporting the correct neurodevelopment outcome of neonates and children with CSVT, mainly due to lack of standardized assesment protocols. Neurologic deficits including epilepsy, motor impairments, and a range of cognitive impairments were reported for 10% to 80% of all neonates on follow up. Wasay et al.[48] reported a higher mortality rate from CSVT in neonates, significantly associated with coma and seizures occuring at presentation. Other predictors of poor neurologic outcome were coagulation abnormalities, multiple sinus thrombosis, seizures at presentation, and venous infarction.[49-50] Notably, CSVT prognosis was found to be better when compared to arterial ischemic stroke.[51-52] Nearly 50% of CSVT patients do not have infarcts and may suffer fewer motor deficits or no permanent damage.[53]
Therapy
There are no evidence based recommendations for the therapy of thrombosis in children due to the lack of randomized controlled trials.[54] The aim of antithrombotic therapy after CSVT is vascular patency, mitigation of thrombus growth and the avoidance of a new thrombosis in the phase of secondary prophylaxis after the acute treatment period. Therapy outcome in children differs. This is in part due to the absence of controlled trials with comparable end points. Although not treating CSVT may significantly increase the risk of thrombus propagation that is associated with new venous infarctions and worse clinical outcomes, safety issues should be considered.
Whereas in adults anticoagulant therapy of CSVT is recommended,[55] such therapy is currently less obvious for children and neonates.[56] A recent multi-center Portuguese study reported no influence of anticoagulation upon CSVT outcome.[57] However, in a single center pediatric CSVT cohort, Mahendranath et. al describe a rate of 6% treatment related hemorrhage and a 31% rate of thrombus propagation in untreated patients.[58]
Notably, prospective follow up of the European pediatric CSVT cohort reflected the need for secondary anticoagulant prophylaxis in future high risk situations, especially in children with thrombophilia and lack of thrombus recanalization following acute phase therapy.[12] Pediatric hematology expert groups recommend tailored therapy regimens based upon patient’s condition, combinations of risk factors and presumed recurrence risk.[59-61]
Thrombolytic therapy is generally limited to severe cases with a high risk of mortality.[62-64]
Generally, disease severity would be associated with antithrombotic treatment, especially in non- neonates. Markers of severity may include presentation with diffuse signs/ massive extended thrombosis or altered level of consciousness. Children with congenital or acquired heart disease require heparinization. The presence of thrombophilia and lack of transient risk factors (namely: idiopathic CSVT) determines the need for prolonged anticoagulant therapy.
The incidence of childhood CSVT varies between 0.4 and 0.7 per 100,000 children per year.[1-3] More than 40% of childhood CSVT occurs within the neonatal period, with an incidence of 2.6 per 100,000 children per year in one series.[1] Notably, these reported figures are probably underestimates of the true rate of CSVT occurrence, since neonates often present with nonfocal neurologic signs and symptoms, potentialy leading to missed diagnosis.[2] Old imaging techniques, the variable anatomy of sinovenous channels and rapid recanalization are all factors which may contribute to underdiagnosis.[4] The annual incidence of sinus vein thrombosis among children in the mediteranian area is not reported. Some case- series, cohort and case controlled multicenter studies are available from Lebanon, Turkey, France, Italy, Portugal and Israel.[5-10] CSVT incidence evaluated among children and neonates hospitalized in a tertiary medical center in Israel was as high as 2/10,000,[11] potentially due to selection bias, in a referral center with high clinical awareness and high index of suspicion that led towards early diagnosis and treatment of CSVT. The largest European CSVT cohort consisted of 396 children , prospectively studied and followed in order to asses the risk factors associated with thrombosis recurrence.[12]
Anatomy and Pathophysiology
Cerebral vascular malformations may involve the venous circulation, with “developmental venous anomaly” ( DVA) being the most frequent. This term, proposed by Lasjaunias et al.,[13] is now widely used as a synonym for venous angioma, cerebral venous malformation, or cerebral venous medullary malformation. DVAs are encountered with an incidence of up to 2.6% in a series of 4,069 brain autopsies.[14] They represent an anomalous venous disposition due to the absence of normal pial or subependymal veins. They may drain into both the superficial and deep venous systems, and a stenosis of the collecting vein of DVAs is commonly observed, typically at the point where the vein crosses the dura to drain into a dural venous sinus. The most frequently involved sites of CSVT are the superior sagittal sinus (90% of all cases in most studies) and transverse sinuses of the superficial venous system and the straight sinus of the deep system.[15] After CSVT, venous pressure escalates in the occluded vessels and secondary hemorrhagic infarction develops due to brain tissue hypertension, localized venous congestion, vasogenic edema, and increased capillary hydrostatic pressure in excess of cerebral arteriolar blood flow. Approximately 65% of neonates with CSVT have brain parenchymal lesions, frequently detected in the frontal and parietal lobes.[16]
Clinical Presentation and Symptoms
It is apparent that the clinical manifestations of CSVT are non-specific and may be subtle. Clinical scenarios occur at all ages and the clinician should consider this diagnosis in a wide range of acute neurological presentations in childhood. These include seizures, coma, stroke, headache and increased intracranial pressure.[17] Common illnesses, eg: ear infections, meningitis, anaemia, diabetes and head injury, may lead to CSVT evolution, various clinical settings including sepsis, dehydration, renal failure, trauma, cancer and haematological disorders may precipitate.[18] Although presentation with pseudotumour cerebri has been well documented, there are few data on the prevalence of CSVT in otherwise unexplained hydrocephalus or in convulsive and non-convulsive seizures and status epilepticus.[19] It has been suggested that toddlers frequently present with seizures and focal signs, mainly hemiparesis, whereas older children present with headache and changes in mental status and seizures may be less common.[20] In a study of neonatal sinus vein thrombosis (52 neonates with a median gestational age of 39 weeks), symptoms developed at a median postnatal age of 1.5 days (range: 0 to 28 days) and consisted mainly of seizures (29 of 52).[21] Other presenting findings in neonates were apnea (17.3%), agitation (5.8%), sepsis like(3.8%) and decreased consciousness in up to 2%.[22] The majority of young children present acutely with seizures, focal signs and symptoms of raised intracranial pressure, such as headache and decreased level of consciousness. Subacute presentation, with chronic headache, vomiting, lethargy, anorexia or drowsiness for 3 weeks or more, may also occur.[23]
Diagnosis and Imaging Studies
The clinical spectrum of CVST closely mimics that of idiopathic intracranial hypertension. Ultrasonography, which is the preliminary screening test for hemorrhage in the neonatal period, may detect intraventricular bleeding, centrally located venous infarcts or hemorrhages, and ventricular dilation and leukomalacia secondary to CSVT infarction and ischemia. However, ultrasound has poor sensitivity for cerebral infarction and should not be used for the primary detection of CSVT and the extent of ensuing injury.[24,25] Cranial computed tomography (CT) can be performed rapidly in neonates with minimum sedation but misses CSVT in 15% of cases. The classic features that indicate CSVT include the “dense triangle” or the “cord sign,” which describe the increased density over the thrombosed venous sinus in a plain CT, or the“empty triangle”.[26] A communicating hydrocephalus may be associated with sagittal sinus thrombosis caused by the impaired CSF absorption over the arachnoid granulations that line the sagittal sinus. A CT venogram using the multislice technique is more sensitive and specific for the diagnosis of CSVT, but the consequent radiation exposure is a definite concern in neonates. MRI is free of radiation risk and permits earlier and more accurate detection of CSVT.[27] Diffusion-weighted imaging is a sensitive technique for detecting areas of infarction. Parenchymal changes can be seen within minutes of injury, which allows for early identification and intervention and Magnetic resonance venography allows for noninvasive assessment of the venous system without exposure to radiograph radiation. Using time-offlight techniques, the cerebral sinuses can be imaged even without the use of contrast. Currently, contrast-enhanced magnetic resonance venography, which is less susceptible to alterations in flow, is the imaging technique of choice for the detection of CSVT in the neonatal population.[28] Attached are 2 representative MR figures of CSVT in pediatric patients (Figures 1,2).

Figure 1. Cranial MRI- demonstrating occlusion of the sagittal sinus thrombosis.

Figure 2. Cranial MRV- demonstrating occlusion of the left transverse and sigmoid sinuses.
Etiology and Risk Factors
The etiology and pathophysiology of CSVT in the pediatric population is still poorly understood, and the role of thrombophilic risk factors remains to be elucidated.[1-5,10-12,29-30] Trauma, infections (especially sinusitis, mastoiditis and other conditions affecting the head and neck area) , collagen vascular disorders, hemoglobinopathies, metabolic and inflamatory bowel diseases have been suggested as potential risk factors for CSVT occurrence in small cohort and case study series.[31-38] Several case-control studies have dealth with the role of prothrombotic risk factors in the pathphysiology of CSVT.[1-5,10-12,39-44] In a recent meta- analysis of pediatric case- controlled studies thrombophilia certainly increased the risk of stroke or CSVT in patients aged 0-18 years old, especially when combination of thrombophilic risk factors was diagnosed.45 A statistically significant association with first event occurrence was demonstrated for each thrombophilia trait evaluated, with no difference found between arterial ischemic stroke and CSVT. Summary ORs (fixed-effects model) were as follows: antithrombin deficiency, 7.06 (95% CI, 2.44 to 22.42); protein C deficiency, 8.76 (95% CI, 4.53 to 16.96); protein S deficiency, 3.20 (95% CI, 1.22 to 8.40), factor V G1691A, 3.26 (95% CI, 2.59 to 4.10); factor II G20210A, 2.43 (95% CI, 1.67 to 3.51); MTHFR C677T (AIS), 1.58 (95% CI, 1.20 to 2.08); antiphospholipid antibodies (AIS), 6.95 (95% CI, 3.67 to 13.14); elevated lipoprotein(a), 6.27 (95% CI, 4.52 to 8.69), and combined thrombophilias, 11.86 (95% CI, 5.93 to 23.73). Genetic thrombophilia, and especially presence of prothrombin G20210A mutation significantly increase the risk of recurrent thromboembolism in CSVT patients older than 2 years of age,[12] in situations of future acquired hypercoagulability.
Outcome
Over all neonates have a more favorable outcome as compared to older children.[46] Nevertheless, in the majority of studies the authors did not adequately describe outcomes in neonates compared with those of children with CSVT. The mean age of follow-up varied across the studies, and disabilities were variably classified.[47] Curently, there is a substantial differences in reporting the correct neurodevelopment outcome of neonates and children with CSVT, mainly due to lack of standardized assesment protocols. Neurologic deficits including epilepsy, motor impairments, and a range of cognitive impairments were reported for 10% to 80% of all neonates on follow up. Wasay et al.[48] reported a higher mortality rate from CSVT in neonates, significantly associated with coma and seizures occuring at presentation. Other predictors of poor neurologic outcome were coagulation abnormalities, multiple sinus thrombosis, seizures at presentation, and venous infarction.[49-50] Notably, CSVT prognosis was found to be better when compared to arterial ischemic stroke.[51-52] Nearly 50% of CSVT patients do not have infarcts and may suffer fewer motor deficits or no permanent damage.[53]
Therapy
There are no evidence based recommendations for the therapy of thrombosis in children due to the lack of randomized controlled trials.[54] The aim of antithrombotic therapy after CSVT is vascular patency, mitigation of thrombus growth and the avoidance of a new thrombosis in the phase of secondary prophylaxis after the acute treatment period. Therapy outcome in children differs. This is in part due to the absence of controlled trials with comparable end points. Although not treating CSVT may significantly increase the risk of thrombus propagation that is associated with new venous infarctions and worse clinical outcomes, safety issues should be considered.
Whereas in adults anticoagulant therapy of CSVT is recommended,[55] such therapy is currently less obvious for children and neonates.[56] A recent multi-center Portuguese study reported no influence of anticoagulation upon CSVT outcome.[57] However, in a single center pediatric CSVT cohort, Mahendranath et. al describe a rate of 6% treatment related hemorrhage and a 31% rate of thrombus propagation in untreated patients.[58]
Notably, prospective follow up of the European pediatric CSVT cohort reflected the need for secondary anticoagulant prophylaxis in future high risk situations, especially in children with thrombophilia and lack of thrombus recanalization following acute phase therapy.[12] Pediatric hematology expert groups recommend tailored therapy regimens based upon patient’s condition, combinations of risk factors and presumed recurrence risk.[59-61]
Thrombolytic therapy is generally limited to severe cases with a high risk of mortality.[62-64]
Generally, disease severity would be associated with antithrombotic treatment, especially in non- neonates. Markers of severity may include presentation with diffuse signs/ massive extended thrombosis or altered level of consciousness. Children with congenital or acquired heart disease require heparinization. The presence of thrombophilia and lack of transient risk factors (namely: idiopathic CSVT) determines the need for prolonged anticoagulant therapy.
References
- Heller C., Heinecke A., Junker R. Cerebral
venous thrombosis in children: a multifactorial origin. Circulation.
2003;108:1362–1367. doi:10.1161/01.CIR.0000087598.05977.45
PMid:12939214

- deVeber G., Andrew M. The Canadian
Paediatric Ischemic Stroke Study group. The epidemiology and outcome of
sinovenous thrombosis in pediatric patients. N Engl J Med.
2001;345:417–423. doi:10.1056/NEJM200108093450604 PMid:11496852

- Lynch J.K., Nelson K.B. Epidemiology of
perinatal stroke. Curr Opin Pediatr. 2001;13:499–505.
doi:10.1097/00008480-200112000-00002 PMid:11753097

- Dlamini N, Billinghurst L, Kirkham FJ.
Cerebral venous sinus thrombosis in children. Neurosurg Clin N Am
2010;21: 511-27. doi:10.1016/j.nec.2010.03.006 PMid:20561500
PMCid:2892748

- Otrock ZK, Taher AT, Shamseddeen WA,
Mahfouz RA. Thrombophilic risk factors among 16 Lebanese patients with
cerebral venous and sinus thrombosis. J Thromb Thrombolysis.
2008;26:41-3. doi:10.1007/s11239-007-0093-x PMid:17823778

- Teksam M, Moharir M, Deveber G, Shroff M.
Frequency and topographic distribution of brain lesions in pediatric
cerebral venous thrombosis AJNR Am J Neuroradiol. 2008;29:1961-5.
doi:10.3174/ajnr.A1246 PMid:18687742

- Barbosa M, Mahadevan J, Weon YC, Yoshida Y,
Ozanne A, Rodesch G, Alvarez H, Lasjaunias P. Dural Sinus Malformations
(DSM) with Giant Lakes, in Neonates and Infants. Review of 30
Consecutive Cases. Interv Neuroradiol. 2003;9:407-24. PMid:20591322

- Buccino G, Cossu G, De Fanti A, Manotti C,
Izzi GC, Mancia D. Cerebral venous sinus thrombosis in childhood:
clinical aspects and neurological and cognitive long-term outcome in
three cases. Neurol Sci. 2004;25:296-300. doi:10.1007/s10072-004-0357-6
PMid:15624088

- Vieira JP, Luis C, Monteiro JP, Temudo T,
Campos MM, Quintas S, Nunes S. Cerebral sinovenous thrombosis in
children: clinical presentation and extension, localization and
recanalization of thrombosis. Eur J Paediatr Neurol. 2010;14:80-5.
doi:10.1016/j.ejpn.2008.12.004 PMid:19201633

- Kenet G, Waldman D, Lubetsky A, Kornbrut N
et al. Paediatric cerebral sinus vein thrombosis. A multi-center,
case-controlled study. Thromb Haemost. 2004; 92:713-8. PMid:15467900

- Waldman D, Manashku S, Strauss T,
Goldstein G, Ben-Zeev B, Kenet G. Thrombophilia and thrombosis in
children--lessons from cerebral sinus vein thrombosis registry of a
tertiary center. Harefuah. 2010;149:270-3, 337. PMid:20929064

- Kenet G., Kirkham F., Niederstadt T. et
al. Risk factors for recurrent venous thromboembolism in the European
collaborative paediatric database on cerebral venous thrombosis: a
multicentre cohort study. Lancet Neurol. 2007;6:595–603
doi:10.1016/S1474-4422(07)70131-X

- Lasjaunias P, Burrows P, Planet C .
Developmental venous anomalies (DVA): the so-called venous angioma.
Neurosurg Rev. 1986;9:233–42. doi:10.1007/BF01743138 PMid:3550523

- Lai PH, Chen PC, Pan HB, Yang CF. Venous
infarction from a venous angioma occurring after thrombosis of a
drainage vein. AJR Am J Roentgenol 1999;172:1698–99. PMid:10350326

- Sarwar M, McCormick WF. Intracerebral
venous angioma. Case report and review. Arch Neurol. 1978;35:323–25.
PMid:646686

- San Millan Ruiz D, Delavelle J, Yilmaz H,
Gailloud P, Piovan E, Bertramello A, Pizzini F, Rufenacht DA.
Parenchymal abnormalities associated with developmental venous
anomalies. Neuroradiology. 2007;49:987–95.
doi:10.1007/s00234-007-0279-0 PMid:17703296

- Fitzgerald KC, Williams LS, Garg BP,
Carvalho KS, Golomb MR. Cerebral sinovenousthrombosis in the neonate.
Arch Neurol. 2006;63:405–9. doi:10.1001/archneur.63.3.405 PMid:16533968

- Barron TF, Gusnard DA, Zimmerman RA,
Clancy RR. Cerebral venous thrombosis in neonates and children. Pediatr
Neurol. 1992;8:112–16. doi:10.1016/0887-8994(92)90030-3

- Wu YW, Miller SP, Chin K, et al. Multiple
risk factors in neonatal sinovenous thrombosis.Neurology.
2002;59:438–40. PMid:12177381

- Carvalho KS, Bodensteiner JB, Connolly PJ,
Garg BP. Cerebral venous thrombosis in children. J Child Neurol.
2001;16:574 –80. PMid:11510928

- Hunt RW, Badawi N, Laing S, Lam A.
Preeclampsia: a predisposing factor for neonatal neonatal venous sinous
thrombosis? Pediatr Neurol. 2001;25:242–6.
doi:10.1016/S0887-8994(01)00291-0

- Newton TH, Gooding CA. Compression of
superior sagittal sinus by neonatal calvarial molding. Radiology.
1975;115:635–40. PMid:1129476

- Heller C, Heinecke A, Junker R, et al;
Childhood Stroke Study Group. Cerebral venous thrombosis in children: a
multifactorial origin. Circulation. 2003;108:1362–7.
doi:10.1161/01.CIR.0000087598.05977.45 PMid:12939214

- Kersbergen KJ, de Vries LS, van Straaten
HL, Benders MJ, Nievelstein RA, Groenendaal F. Anticoagulation therapy
and imaging in neonates with a unilateral thalamic hemorrhage due to
cerebral sinovenous thrombosis. Stroke. 2009;40:2754–60.
doi:10.1161/STROKEAHA.109.554790 PMid:19542053

- Lequin MH, Dudink J, Tong KA, Obenaus A.
Magnetic resonance imaging in neonatal stroke. Semin Fetal Neonatal
Med. 2009;14:299–310. doi:10.1016/j.siny.2009.07.005 PMid:19632909

- Justich E, Lammer J, Fritsch G, Beitzke A,
Walter GF. CT diagnosis of thrombosis of dural sinuses in childhood.
Eur J Radiol. 1984;4:294–5. PMid:6519062

- Nwosu ME, Williams LS, Edwards-Brown M,
Eckert GJ, Golomb MR. Neonatal sinovenous thrombosis: presentation and
association with imaging. Pediatr Neurol. 2008;39:155–61.
doi:10.1016/j.pediatrneurol.2008.06.001 PMid:18725059

- Widjaja E, Shroff M, Blaser S, Laughlin S,
Raybaud C. 2D time-of-flight MR venography in neonates: anatomy and
pitfalls. AJNR Am J Neuroradiol. 2006;27:1913–18. PMid:17032865

- Uthman I, Khalil I, Sawaya R, Taher A.
Lupus anticoagulant, factor V Leiden, and methylenetetrahydrofolate
reductase gene mutation in a lupus patient with cerebral venous
thrombosis. Clin Rheumatol. 2004;23:362-3.

- Akbalik M, Duru F, Fisgin T, Tasdemir HA,
Incesu L, Albayrak D, Ozyurek E. Cerebral thrombosis associated with
heterozygous factor V Leiden mutation and high lipoprotein(a) level in
a girl with factor XIII deficiency. Blood Coagul Fibrinolysis.
2007;18:371-4. doi:10.1097/MBC.0b013e3280d5a7be

- Stiefel D, Eich G, Sacher P. Posttraumatic
dural sinus thrombosis in children. Eur J Pediatr Surg. 2000;10:41–4.
doi:10.1055/s-2008-1072321 PMid:10770246

- Matsushige T, Nakaoka M, Kiya K. Cerebral
sinovenous thrombosis after closed head injury. J Trauma.
2009;66:1599–604. doi:10.1097/TA.0b013e3181a3a8e6 PMid:19509620

- Krishnan A, Karnad DR, Limaye U. Cerebral
venous and dural sinus thrombosis in severe falciparum malaria. J
Infect. 2004;48:86–90. doi:10.1016/S0163-4453(03)00130-0

- Prasad R, Singh R, Joshi B. Lateral sinus
thrombosis in neurocysticercosis. Trop Doct. 2005;35:182–3.]
doi:10.1258/0049475054620914 PMid:16105354

- Standridge S., de los Reyes E.
Inflammatory bowel disease and cerebrovascular arterial and venous
thromboembolic events in 4 pediatric patients: a case series and review
of the literature. J Child Neurol. 2008;23:59–66.
doi:10.1177/0883073807308706 PMid:18184942

- Uziel Y, Laxer RM, Blaser S. Cerebral vein
thrombosis in childhood systemic lupus erythematosus. J Pediatr.
1995;126:722–7. doi:10.1016/S0022-3476(95)70399-3

- Yoshimura S, Ago T, Kitazono T. Cerebral
sinus thrombosis in a patient with Cushing's syndrome. J Neurol
Neurosurg Psychiatr. 2005;76:1182–3. doi:10.1136/jnnp.2004.057315
PMid:50411

- Siegert CE, Smelt AH, de Bruin TW.
Superior sagittal sinus thrombosis and thyrotoxicosis. Possible
association in two cases. Stroke. 1995;26:496–7.
doi:10.1161/01.STR.26.3.496 PMid:7886732

- Bonduel M, Sciuccati G, Hepner M. Factor V
Leiden and prothrombin gene G20210A mutation in children with cerebral
thromboembolism. Am J Hematol. 2003;73:81–6. doi:10.1002/ajh.10326
PMid:12749008

- Cakmak S, Derex L, Berruyer M. Cerebral
venous thrombosis: clinical outcome and systematic screening of
prothrombotic factors. Neurology. 2003;60:1175–8. PMid:12682328

- Johnson MC, Parkerson N, Ward S. Pediatric
sinovenous thrombosis. J Pediatr Hematol Oncol. 2003;25:312–5.
doi:10.1097/00043426-200304000-00009 PMid:12679646

- Vorstman E, Keeling D, Leonard J. Sagittal
sinus thrombosis in a teenager: homocystinuria associated with
reversible antithrombin deficiency. Dev Med Child Neurol. 2002;44:498.
doi:10.1111/j.1469-8749.2002.tb00314.x

- Martinelli I, Battaglioli T, Pedotti P.
Hyperhomocysteinemia in cerebral vein thrombosis. Blood.
2003;102:1363–6. doi:10.1182/blood-2003-02-0443 PMid:12714502

- Cantu C, Alonso E, Jara A.
Hyperhomocysteinemia, low folate and vitamin B12 concentrations, and
methylene tetrahydrofolate reductase mutation in cerebral venous
thrombosis. Stroke. 2004;35:1790–4.
doi:10.1161/01.STR.0000132570.24618.78 PMid:15192249

- Kenet G, Lütkhoff LK, Albisetti M et al
Impact of thrombophilia on risk of arterial ischemic stroke or cerebral
sinovenous thrombosis in neonates and children: a systematic review and
meta-analysis of observational studies. Circulation 2010;121:1838-47.
doi:10.1161/CIRCULATIONAHA.109.913673 PMid:20385928

- deVeber GA, MacGregor D, Curtis R, Mayank
S. Neurologic outcome in survivors of childhood arterial ischemic
stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–24.
doi:10.1177/088307380001500508 PMid:10830198

- Sיbire G, Tabarki B, Saunders DE, et al.
Cerebra venous sinus thrombosis in children: risk factors,
presentation, diagnosis and outcome. Brain. 2005;128:477–89.

- Wasay M, Dai AI, Ansari M, Shaikh Z, Roach
ES. Cerebral venous sinus thrombosis in children: a multicenter cohort
from the United States. J Child Neurol. 2008;23:26–31.
doi:10.1177/0883073807307976 PMid:18184940

- Johnson MC, Parkerson N, Ward S, de
Alarcon PA. Pediatric sinovenous thrombosis. J Pediatr Hematol Oncol.
2003;25:312–5. doi:10.1097/00043426-200304000-00009 PMid:12679646

- Jacobs K, Moulin T, Bogousslavsky J,
Woimant F, Dehaene I, Tatu L. The stroke syndrome of cortical vein
thrombosis. Neurology. 1996;47:376–82. PMid:8757007

- De Schryver EL, Blom I, Braun KP, Kappelle
LJ, Rinkel GJ, Peters AC, et al. Long-term prognosis of cerebral venous
sinus thrombosis in childhood. Dev Med Child Neurol. 2004;46:514–9.
doi:10.1111/j.1469-8749.2004.tb01008.x

- de Bruijn SF, de Haan RJ, Stam J for the
Cerebral Venous Sinus Thrombosis Study. Clinical features and
prognostic factors of cerebral venous sinus thrombosis in a prospective
series of 59 patients. J Neurol Neurosurg Psychiatr. 2001;70:105–8.
doi:10.1136/jnnp.70.1.105 PMid:50411

- Huisman TA, Holzmann D, Martin E, Willi
UV. Cerebral venous thrombosis in childhood. Eur Radiol 2001;11:1760–5.
doi:10.1007/s003300100822 PMid:11511899

- Kersbergen KC, de Vries LS, van Straaten
HLM. Anticoagulation therapy and imaging in neonates with a unilateral
thalamic hemorrhage due to cerebral sinovenous thrombosis. Stroke.
2009;40:2754–60. doi:10.1161/STROKEAHA.109.554790 PMid:19542053

- Stam J, de Bruijn SF, deVeber G.
Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst
Rev. 2002;4:CD002005. PMid:12519565

- deVeber G., Chan A., Monagle P.
Anticoagulation therapy in pediatric patients with sinovenous
thrombosis: a cohort study. Arch Neurol. 1998;55:1533–7.
doi:10.1001/archneur.55.12.1533 PMid:9865797

- Viera JP, Luis C,Monteiro JP et al.
Cerebral sinovenous thrombosis in children: clinical presentation and
extention,localization and recanalization of thrombosis. European
Journal of Pediatric Neurology. 2010;14:80-5.
doi:10.1016/j.ejpn.2008.12.004 PMid:19201633

- Mahendranath Moharir D, Shroff M, Stephens
D et al. Anticoagulants in Pediatric Cerebral Sinovenous Thrombosis A
Safety and Outcome Study. Ann Neurol 2010;67:590-9. PMid:20437556

- Royal College of Physicians Paediatric Stroke Working Group. Stroke in Childhood: clinical guidelines for diagnosis, management and rehabilitation. Royal College of Physicians, London, November, 2004. Available at: http://www.rcplondon.ac.uk/pubs/books/childstroke/childstroke_guidelines.pdf
- Roach E.S., Golomb M.R., Adams R.
Management of stroke in infants and children: a scientific statement
from a Special Writing Group of the American Heart Association Stroke
Council and the Council on Cardiovascular Disease in the Young. Stroke.
2008;39:2644–91. doi:10.1161/STROKEAHA.108.189696 PMid:18635845

- Monagle P., Chalmers E., Chan A.
Antithrombotic therapy in neonates and children: American College of
Chest Physicians evidence-based clinical practice guidelines (8th
edition) Chest. 2008;133:887S–968S. doi:10.1378/chest.08-0762
PMid:18574281

- Griesemer DA, Theodorou AA, Berg RA. Local
fibrinolysis in cerebral venous thrombosis. Pediatr Neurol.
1994;10:78–80. doi:10.1016/0887-8994(94)90075-2

- Wasay M, Bakshi R, Kojan S. Nonrandomized
comparison of local urokinase thrombolysis versus systemic heparin
anticoagulation for superior sagittal sinus thrombosis. Stroke.
2001;32:2310–7. doi:10.1161/hs1001.096192 PMid:11588319

- Ciccone A, Canhao P, Falcao F.
Thrombolysis for cerebral vein and dural sinus thrombosis. Cochrane
Database Syst Rev. 2004;1 CD003693. PMid:14974030
