Treatment of Acute Promyelocytic Leukemia with High White Cell Blood Counts
C. Kelaidi1, L. Adès2 and P. Fenaux2
1Department of
Hematology, G. Papanikolaou Hospital of Thessaloniki, Exochi
57010, Greece.
2Service d’Hématologie, Hôpital Avicenne - Université Paris 13, 125, rue de Stalingrad 93000 Bobigny, France.
2Service d’Hématologie, Hôpital Avicenne - Université Paris 13, 125, rue de Stalingrad 93000 Bobigny, France.
Correspondence
to: Department of Hematology, G. Papanikolaou Hospital of
Thessaloniki, Exochi 57010, Greece. E-mail: charikleia.kelaidi@gmail.com
Published: September 8, 2011
Received: July 16, 2011
Accepted: August 12, 2011
Mediterr J Hematol Infect Dis 2011, 3: e20110038, DOI 10.4084/MJHID.2011.038
This article is available from: http://www.mjhid.org/article/view/8840
This is an Open Access article
distributed under the terms of
the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Acute
promyelocytic leukemia (APL) with WBC above 10 G/L has long been
considered, even in the all-trans retinoic acid (ATRA) era, to carry a
relatively poor prognosis (compared to APL with WBC below 10
G/L), due to increased early mortality and relapse. However, early
deaths can to a large extent be avoided if specific measures are
rapidly instigated, including prompt referral to a specialized center,
immediate onset of ATRA and chemotherapy, treatment of coagulopathy
with adequate platelet transfusional support, and prevention and
management of differentiation syndrome. Strategies to reduce relapse
rate include chemotherapy reinforcement with cytarabine and/or arsenic
trioxide during consolidation, prolonged maintenance treatment,
especially with ATRA and low dose chemotherapy, and possibly, although
this is debated, intrathecal prophylaxis to prevent central nervous
system relapse. By applying those measures, outcomes of patients with
high risk APL have considerably improved, and have become in many
studies almost similar to those of standard risk APL patients.
Introduction
Acute promyelocytic leukemia (APL) is a distinct type of acute myeloid leukemia (AML) characterized by specific morphology (M3 in the FAB classification), frequent association of a coagulopathy, the t(15:17) translocation resulting in the fusion protein PML-RARα. The APL has a specific sensitivity to the differentiating properties of all-trans retinoic acid (ATRA) and the proapoptotic effect of arsenic trioxide (ATO), which have, in combination with anthracyline-based chemotherapy, considerably improved its prognosis in the last 20 years.[1-6]
WBC counts are generally low in APL, with only 20-25% and <5% of patients having WBC higher than 10 G/L and 50 G/L, respectively.[7-11] High WBC counts have always been associated with poorer prognosis in APL, even since the advent of ATRA, but recent results suggest that the outcome of those patients may have become almost similar to that of APL with lower WBC counts, with the optimal use of all therapeutic tools, including early massive transfusional support.
Characteristics and prognosis of APL with increased WBC counts
22.6%, 6.1% and 1.4% of 902 patients, included in combined analysis of APL 93 and APL 2000 trials of the European APL group, had WBC 10-50 G/L, 50-100 G/L and >100 G/L, respectively.[12] The 10 G/L and 50 G/L thresholds for WBC are used hereafter to define APL with high and very high WBC. APL with high WBC is more frequent in children and is often associated with the microgranular variant (M3v), with the short PML-RARα isoform (bcr3), with the FLT3 mutation and the CD56 expression which are all adverse prognostic factors in APL, although not independent from WBC counts.[13-18] APL with increased WBC is frequently associated with severe coagulopathy and, sometimes with organomegaly. The fibrinogen level is indeed <1.5 g/L in at least half the patients with increased WBC and is often accompanied by severe hemorrhagic manifestations, intracranial hemorrhage either at presentation or during the first days of remission induction obviously being the most severe. Before the ATRA era, i.e. in patients treated with anthracycline-based chemotherapy, early mortality in patients with increased WBC averaged 50-70%, but even in the early ATRA era, most studies found an increased early mortality (10-20%) in patients with high WBC counts.[7,19-21] They also found, by comparison with patients with WBC<10 G/L, an increased incidence of relapse (30%), including of central nervous system (CNS) relapse (Tables 1 and 2).[8,9,22-28] This increased relapse risk led to the design of Sanz score for APL relapse, discriminating between low and intermediate risk (hereafter referred to as standard risk) patients with WBC <10 G/L and platelet counts more and less than 40 G/L, respectively, and high risk patients with WBC counts greater than 10 G/L.[29] In that study, with an ATRA and anthracycline-based regimen, 3-year relapse-free survival was 100%, 90% and 75% for low, intermediate and high risk patients, respectively. Thus, preventing relapse is particularly relevant for high risk patients. In addition, considering that event-free survival (EFS) and OS are probably better after salvage treatment delivered at the molecular relapse stage (rather than at full blown hematologic relapse), monitoring of PML-RARα after CR achievement is particularly important for high risk patients.[30-32]
Management of APL with Increased WBC Counts
-1) Preventing early mortality: Hyperleukocytic APL is, even more than standard risk APL, a medical emergency. An undetermined percentage of patients with high and very high WBC die before treatment onset without being registered in clinical trials.[33] Early mortality rates as high as 42% have been reported in APL patients with high WBC, many of those deaths occurring before patients could reach a specialized hospital facility, let alone be included in a clinical trial, while in a report from Surveillance, Epidemiology, and End Results (SEER) an overall early mortality rate of 17% was reported in APL, which was presumably more in patients with high WBC counts.[34,35] The risk of early mortality being particularly high during the first days of treatment, specific measures must be urgently implemented to prevent those early fatalities. Resistance to ATRA-based induction regimens being very rare (<1/500 cases), induction remission failures actually reflect early mortality. Published trials of the modern era reporting on early mortality of high risk patients are listed in Table 1 although, as said above, they may sometimes underestimate the reality, some patients possibly dying before they can enter a clinical trial.
Bleeding, particularly in the CNS, is the leading cause of early and very early (<48 h) mortality. Presence of coagulopathy, high WBC and higher than normal creatinine levels have been identified as predictive factors of fatal hemorrhage.[36,37] Coagulopathy should be treated intensively with fresh frozen plasma and high dose prophylactic platelet transfusions in order to maintain platelet counts ≥50 G/L until normalization of bleeding parameters. In accordance with published guidelines, invasive procedures including placement of central venous catheters and leukapheresis are contra-indicated during induction remission.34 Any delay in diagnosis and treatment initiation in hyperleukocytic APL is unequivocally associated with a higher risk of early hemorrhagic death.[38] ATRA has a rapid impact on both fibrinolytic and procoagulant aspects of the coagulopathy, and should be initiated as soon as the diagnosis of APL is clinically or morphologically suspected. However, at least in hyperleukocytic forms, chemotherapy should be started concomitantly, in order to avoid a life threatening differentiation syndrome (see below). This concomitant use of ATRA and chemotherapy had led to reduction of early mortality in patients with very high WBC (>50 G/L), from at least 50% before the ATRA era to 18% in the APL93 trial.[8]
Differentiation syndrome (DS) with ATRA, with manifestations of unexplained respiratory failure, pulmonary infiltrates, fever, weight gain, pleuropericardial effusions, hypotension and renal failure, usually occurring concomitantly with a rise in WBC count, is another major cause of early mortality in high risk patients.[39-41] Severe DS, requiring mechanical ventilation or complicated with pericarditis or life threatening hemorrhage, is more frequent in patients with high presenting WBC counts. Early recognition and systematic prevention of DS with high-dose dexamethasone from the first day of treatment certainly contributed to reduce the number of fatal DS from 5.7% to 3.9%, among high risk patients included in two consecutive trials of the European APL group (APL 93 and APL 2000).[12]
Overall, improved DS management and transfusional support were likely the key factors of reduction of early mortality between our APL 93 and 2000 trials, from 10.4% to 7% in patients with high WBC counts, and 18% to 9% in patients with very high WBC counts. In APL 2000 trial, the early death rate was not dependent from WBC count.[12] Moreover, in our ongoing APL 2006 trial, using the same induction regimen of ATRA and anthracycline based chemotherapy (with substitution of idarubicin for daunorubicin), none of the 45 high risk patients included had early death.[42]
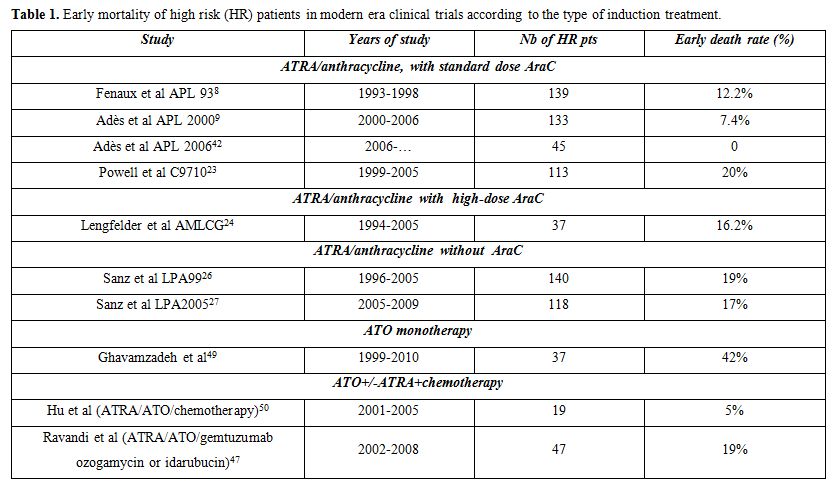
Table 1. Early mortality of high risk (HR) patients in modern era clinical trials according to the type of induction treatment.
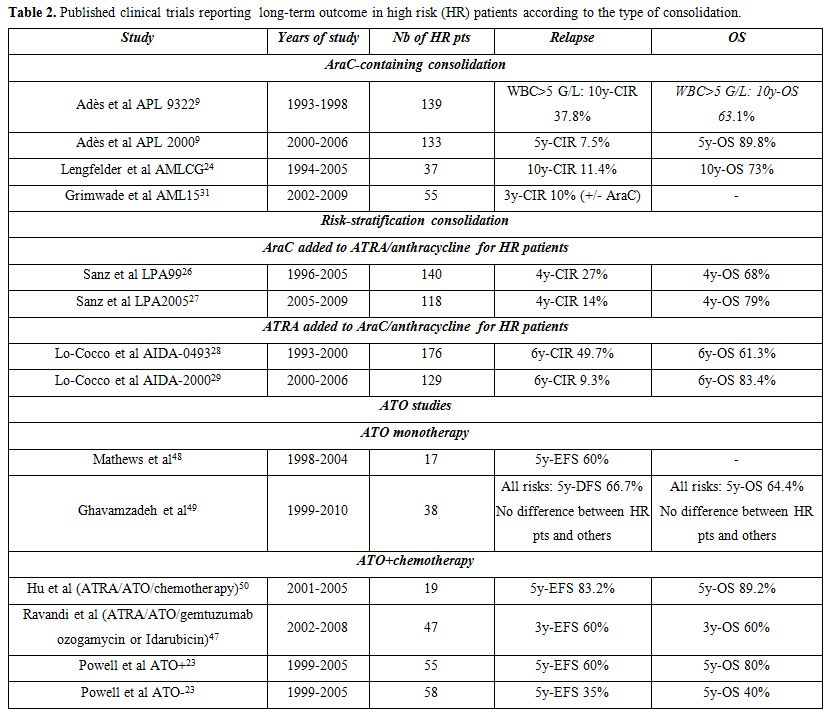
Table 2. Published clinical trials reporting long-term outcome in high risk (HR) patients according to the type of consolidation.
-2) Preventing relapse:
Presenting WBC >10 G/L remains the strongest prognostic factor for relapse, including for extramedullary relapse, especially in CNS. Several strategies can reduce the incidence of relapse in those patients, and attenuate the adverse prognostic character of hyperleukocytic APL, which was no more significant in our recent experience.
-3) APL with high WBC counts in specific age groups:
Conclusion
Improvement in outcomes of APL patients with high WBC counts was made possible by combining specific measures to prevent early mortality and relapse. Those results indicate that, with risk-tailored management, outcome of APL patients could become independent of WBC counts in the Acute promyelocytic leukemia. More investigation and efforts are still needed to reduce very early mortality not reflected by clinical trials results.
Acute promyelocytic leukemia (APL) is a distinct type of acute myeloid leukemia (AML) characterized by specific morphology (M3 in the FAB classification), frequent association of a coagulopathy, the t(15:17) translocation resulting in the fusion protein PML-RARα. The APL has a specific sensitivity to the differentiating properties of all-trans retinoic acid (ATRA) and the proapoptotic effect of arsenic trioxide (ATO), which have, in combination with anthracyline-based chemotherapy, considerably improved its prognosis in the last 20 years.[1-6]
WBC counts are generally low in APL, with only 20-25% and <5% of patients having WBC higher than 10 G/L and 50 G/L, respectively.[7-11] High WBC counts have always been associated with poorer prognosis in APL, even since the advent of ATRA, but recent results suggest that the outcome of those patients may have become almost similar to that of APL with lower WBC counts, with the optimal use of all therapeutic tools, including early massive transfusional support.
Characteristics and prognosis of APL with increased WBC counts
22.6%, 6.1% and 1.4% of 902 patients, included in combined analysis of APL 93 and APL 2000 trials of the European APL group, had WBC 10-50 G/L, 50-100 G/L and >100 G/L, respectively.[12] The 10 G/L and 50 G/L thresholds for WBC are used hereafter to define APL with high and very high WBC. APL with high WBC is more frequent in children and is often associated with the microgranular variant (M3v), with the short PML-RARα isoform (bcr3), with the FLT3 mutation and the CD56 expression which are all adverse prognostic factors in APL, although not independent from WBC counts.[13-18] APL with increased WBC is frequently associated with severe coagulopathy and, sometimes with organomegaly. The fibrinogen level is indeed <1.5 g/L in at least half the patients with increased WBC and is often accompanied by severe hemorrhagic manifestations, intracranial hemorrhage either at presentation or during the first days of remission induction obviously being the most severe. Before the ATRA era, i.e. in patients treated with anthracycline-based chemotherapy, early mortality in patients with increased WBC averaged 50-70%, but even in the early ATRA era, most studies found an increased early mortality (10-20%) in patients with high WBC counts.[7,19-21] They also found, by comparison with patients with WBC<10 G/L, an increased incidence of relapse (30%), including of central nervous system (CNS) relapse (Tables 1 and 2).[8,9,22-28] This increased relapse risk led to the design of Sanz score for APL relapse, discriminating between low and intermediate risk (hereafter referred to as standard risk) patients with WBC <10 G/L and platelet counts more and less than 40 G/L, respectively, and high risk patients with WBC counts greater than 10 G/L.[29] In that study, with an ATRA and anthracycline-based regimen, 3-year relapse-free survival was 100%, 90% and 75% for low, intermediate and high risk patients, respectively. Thus, preventing relapse is particularly relevant for high risk patients. In addition, considering that event-free survival (EFS) and OS are probably better after salvage treatment delivered at the molecular relapse stage (rather than at full blown hematologic relapse), monitoring of PML-RARα after CR achievement is particularly important for high risk patients.[30-32]
Management of APL with Increased WBC Counts
-1) Preventing early mortality: Hyperleukocytic APL is, even more than standard risk APL, a medical emergency. An undetermined percentage of patients with high and very high WBC die before treatment onset without being registered in clinical trials.[33] Early mortality rates as high as 42% have been reported in APL patients with high WBC, many of those deaths occurring before patients could reach a specialized hospital facility, let alone be included in a clinical trial, while in a report from Surveillance, Epidemiology, and End Results (SEER) an overall early mortality rate of 17% was reported in APL, which was presumably more in patients with high WBC counts.[34,35] The risk of early mortality being particularly high during the first days of treatment, specific measures must be urgently implemented to prevent those early fatalities. Resistance to ATRA-based induction regimens being very rare (<1/500 cases), induction remission failures actually reflect early mortality. Published trials of the modern era reporting on early mortality of high risk patients are listed in Table 1 although, as said above, they may sometimes underestimate the reality, some patients possibly dying before they can enter a clinical trial.
Bleeding, particularly in the CNS, is the leading cause of early and very early (<48 h) mortality. Presence of coagulopathy, high WBC and higher than normal creatinine levels have been identified as predictive factors of fatal hemorrhage.[36,37] Coagulopathy should be treated intensively with fresh frozen plasma and high dose prophylactic platelet transfusions in order to maintain platelet counts ≥50 G/L until normalization of bleeding parameters. In accordance with published guidelines, invasive procedures including placement of central venous catheters and leukapheresis are contra-indicated during induction remission.34 Any delay in diagnosis and treatment initiation in hyperleukocytic APL is unequivocally associated with a higher risk of early hemorrhagic death.[38] ATRA has a rapid impact on both fibrinolytic and procoagulant aspects of the coagulopathy, and should be initiated as soon as the diagnosis of APL is clinically or morphologically suspected. However, at least in hyperleukocytic forms, chemotherapy should be started concomitantly, in order to avoid a life threatening differentiation syndrome (see below). This concomitant use of ATRA and chemotherapy had led to reduction of early mortality in patients with very high WBC (>50 G/L), from at least 50% before the ATRA era to 18% in the APL93 trial.[8]
Differentiation syndrome (DS) with ATRA, with manifestations of unexplained respiratory failure, pulmonary infiltrates, fever, weight gain, pleuropericardial effusions, hypotension and renal failure, usually occurring concomitantly with a rise in WBC count, is another major cause of early mortality in high risk patients.[39-41] Severe DS, requiring mechanical ventilation or complicated with pericarditis or life threatening hemorrhage, is more frequent in patients with high presenting WBC counts. Early recognition and systematic prevention of DS with high-dose dexamethasone from the first day of treatment certainly contributed to reduce the number of fatal DS from 5.7% to 3.9%, among high risk patients included in two consecutive trials of the European APL group (APL 93 and APL 2000).[12]
Overall, improved DS management and transfusional support were likely the key factors of reduction of early mortality between our APL 93 and 2000 trials, from 10.4% to 7% in patients with high WBC counts, and 18% to 9% in patients with very high WBC counts. In APL 2000 trial, the early death rate was not dependent from WBC count.[12] Moreover, in our ongoing APL 2006 trial, using the same induction regimen of ATRA and anthracycline based chemotherapy (with substitution of idarubicin for daunorubicin), none of the 45 high risk patients included had early death.[42]

Table 1. Early mortality of high risk (HR) patients in modern era clinical trials according to the type of induction treatment.

Table 2. Published clinical trials reporting long-term outcome in high risk (HR) patients according to the type of consolidation.
-2) Preventing relapse:
Presenting WBC >10 G/L remains the strongest prognostic factor for relapse, including for extramedullary relapse, especially in CNS. Several strategies can reduce the incidence of relapse in those patients, and attenuate the adverse prognostic character of hyperleukocytic APL, which was no more significant in our recent experience.
- Cytarabine during
induction and consolidation treatment:
While anthracycline-based regimens without AraC have been preferred to
limit toxicity in low and intermediate risk APL patients, several lines
of evidence strongly support that cytarabine added to anthracycline
during remission induction and consolidation treatment may contribute
to reduce relapses in high risk patients.
Indeed, very few relapses occurred in our APL 2000 trial and in a German study using high dose cytarabine, added to anthracyclines, during consolidation, (Table 2).[9,24,43] In addition, a joint analysis of the European group trial APL 2000 and the Spanish PETHEMA trial LPA 99, which used no cytarabine during induction and consolidation, showed a significant advantage, in terms of EFS, CIR and OS in high risk patients included in APL 2000 trial.[44] Finally, risk-stratification in the subsequent PETHEMA-HOVON LPA 2005 trial, based on reinforcement of consolidation with cytarabine in high risk patients, reduced relapse rate by comparison with LPA99 trial.[26]
The dose of cytarabine could also be of importance. Increasing the total cytarabine dose from 8 to 20 g/m2 during the second consolidation cycle indeed possibly contributed to the lower incidence of relapse of patients with high and very high WBC count included in APL 2000, compared with APL 93 trial.[12] Moreover, long term analysis of our APL 2000 trial found that outcome of high risk patients was better than that of standard risk patients treated without cytarabine.[43] - Maintenance treatment: In our experience, maintenance treatment with continuous low dose 6MP+MTX and intermittent ATRA is particularly useful in high risk patients. Its impact on the long-term outcome was clearly demonstrated in the very long term analysis of the randomized APL 93 trial where 10-y CIR was 68.4%, 53.1%, 32.8% and 20.6% in patients with WBC >5 G/L who had received no maintenance, maintenance with only ATRA, only chemotherapy (6MP and MTX) and combined maintenance with ATRA+chemotherapy, respectively (P<0.001). The difference was less important for patients with WBC <5 G/L, suggesting that combined maintenance treatment mostly benefited high risk patients.[22] Moreover, a combined analysis of APL 93 trial (where not all patients received maintenance) and APL 2000 trial, where combined maintenance treatment was systematic, showed that, in patients with WBC count >10 G/L, receiving combined maintenance treatment was the strongest prognostic factor associated with reduction of CIR and increase in OS.[12] We also found discontinuation of this maintenance treatment after less than one year to be associated with an increased risk of relapse. Two-year combined maintenance with intermittent ATRA and low dose chemotherapy thus appears to further improve the outcome of hyperleukocytic APL. This treatment is associated with no excessive toxicity, provided co mplete blood count is regularly monitored to adjust doses and avoid excessive cytopenias.
- Arsenic trioxide:
Arsenic trioxide is the most potent single agent in APL, capable
of inducing complete responses, including molecular ones, and it
is particularly successful in the setting of molecular and hematologic
relapse.[32] A US intergroup trial clearly
demonstrated the benefit of adding two cycles of single agent ATO to a
classical first line ATRA and chemotherapy based regimen, particularly
in high risk patients.[24] In addition, 5-year EFS of
high risk patients who received ATO was not significantly different
from that of standard risk patients, indicating that ATO consolidation
may overcome the negative prognosis conferred by high risk disease.
Other studies using ATO during consolidation also generated promising
results (Table 2).[45-47]
In contrast, induction with ATO monotherapy is considered more
experimental, in particular due to the fact that high risk patients
have a major risk of developing severe DS, unless chemotherapy is
administered concomitantly.[48,49] Nevertheless, once
CR is achieved, durable responses have been reported with this approach.
Thus, although ATO may become frequently used in the front-line induction therapy of APL, published results caution against using it without chemotherapy in high risk patients. In its current randomized APL 2006 trial, the European APL group is investigating the impact of ATO versus that of AraC during consolidation in high risk patients.[42] - Prevention of CNS relapse: High WBC count is also a major
risk factor for extramedullary relapse, in particular CNS relapse,
although its incidence is small (cumulative incidence of 5% among high
risk patients).[51,52] Given the
published poor prognosis of extramedullary relapse,[51]
CNS prophylaxis with intrathecal chemotherapy may be considered
systematically in high risk patients. Agents that cross the blood-brain
barrier such as high dose cytarabine or arsenic trioxide (ATO) may also
reduce that risk. In APL 93 trial, using high dose cytarabine, 3 (0.9%)
patients in CR had CNS relapse as compared with 15 (2.5%) and 9 (2.1%)
patients in AIDA 0493 and AIDA 2000 trials, respectively, where the
dose of AraC was conventional.[22,27,28]
Moreover, systematic CNS prophylaxis with 5 triple intrathecal
injections, in addition to higher dose cytarabine, in patients with
high WBC count in APL 2000 trial was associated with the absence of CNS
or other extramedullary relapse. The German AMLCG study using higher
dose cytarabine also reported no CNS or other extramedullary relapse in
standard and high risk patients.24 Despite penetration in cerebrospinal
fluid, prolonged use of ATO did not seem efficacious in preventing CNS
relapse in the absence of intrathecal chemotherapy in one study, where
all 4 relapses (among 80 patients) involved the CNS.[50]
However, other authors consider the number of high risk patients to treat prophylactically too high given the overall low incidence of CNS relapse.[52] Also of note is that, since the advent of ATO to treat relapse, outcome of patients with CNS relapse has been better in our experience than with previously used salvage regimens.[53]
-3) APL with high WBC counts in specific age groups:
- Children: The frequency of high WBC counts in children is
relatively high and even more (50%) in children aged <12 years.[13-16]
In our experience, children aged <4 years have a higher risk of
relapse even with prolonged maintenance treatment and high dose AraC.[16]
For older children, EFS did not differ from that of adults after
adjustment for WBC counts, and OS was often better due to better
results of salvage treatment.14 High risk pediatric patients included
in Italian trials had a 10-year EFS of 59%, significantly worse than in
standard risk patients, while the Spanish group reported an overall
5-year CIR of 31% in children with high WBC counts. By contrast,
similar outcomes were reported in high and standard risk patients in a
Japanese study using cytarabine, added to ATRA and anthracyclines.[54]
ATO, in combination to ATRA and chemotherapy, may offer an interesting perspective for disease control in pediatric patients with high WBC counts, especially in those aged <4 years, associated in our experience to a higher relapse risk. No chronic arsenic toxicity was observed in a Chinese report on childhood APL, despite protracted intermittent administration of ATO used as a single agent.[55] - Elderly patients: Approximately 20% of APL patients are aged over 60 years, with a proportion of cases with WBC >10 G/L slightly lower than in younger adults (20% vs. 23% high risk patients among those aged ≥60 years vs.[19-59] years, respectively, in the PETHEMA studies).[56-58] Increased incidence of early deaths and deaths in CR in elderly APL patients in general renders particularly challenging the management of those patients, especially those with high risk features. Indeed, in both the GIMEMA and PETHEMA studies, one third of elderly patients died during induction remission.[56,57] Early deaths were due to hemorrhage and DS but also to cardiac complications.[56] Relapse rate of high risk elderly patients is similar to that of high risk younger patients.[58] Age above 50 years, in spite of absence of CIR difference across age groups, was, however, an adverse prognostic factor for EFS and OS in high and very high risk patients, in the combined analysis of APL 93 and APL 2000 trials.[12] Rather than age-adjusted chemotherapy dosage, incorporation of ATO in current regimens could counteract those adverse features with better disease control.
Conclusion
Improvement in outcomes of APL patients with high WBC counts was made possible by combining specific measures to prevent early mortality and relapse. Those results indicate that, with risk-tailored management, outcome of APL patients could become independent of WBC counts in the Acute promyelocytic leukemia. More investigation and efforts are still needed to reduce very early mortality not reflected by clinical trials results.
References
- Bennett JM, Catovsky D, Daniel MT, Flandrin
G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification
of the acute leukaemias: French-American-British (FAB) co-operative
group. Br J Haematol. 1976;33:451-8 http://dx.doi.org/10.1111/j.1365-2141.1976.tb03563.x
PMid:188440

- Larson RA, Kondo K, Vardiman JW, Butler AE,
Golomb HM, Rowley JD. Evidence for a 15;17 translocation in every
patient with acute promyelocytic leukemia. Am J Med. 1984;76:827-41
http://dx.doi.org/10.1016/0002-9343(84)90994-X

- de Thé H, Lavau C, Marchio A, Chomienne C,
Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the
t(15;17) translocation in acute promyelocytic leukemia encodes a
functionally altered RAR. Cell. 1991;66:675-84 http://dx.doi.org/10.1016/0092-8674(91)90113-D

- Kakizuka A, Miller WH Jr, Umesono K,
Warrell RP Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM.
Chromosomal translocation t(15;17) in human acute promyelocytic
leukemia fuses RAR alpha with a novel putative transcription factor,
PML. Cell. 1991; 66:663-74 http://dx.doi.org/10.1016/0092-8674(91)90112-C

- Huang ME, Ye YC, Chen SR, Chai JR, Lu JX,
Zhoa L, Gu LJ, Wang ZY. Use of all-trans retinoic acid in the treatment
of acute promyelocytic leukemia. Blood.1988;72:567-72 PMid:3165295

- Castaigne S, Chomienne C, Daniel MT,
Ballerini P, Berger R, Fenaux P, Degos L. All-trans retinoic acid as a
differentiation therapy for acute promyelocytic leukemia. I. Clinical
results. Blood. 1990;76:1704-9 PMid:2224119

- Fenaux P, Pollet J, Vandenbossche L, Morel
P, Zandecki M, Jouet JP, Bauters F. Treatment of acute promyelocytic
leukemia: A report on 70 cases. Leuk Lymphoma. 1991; 4:239-8 http://dx.doi.org/10.3109/10428199109068072

- Fenaux P, Chastang C, Chevret S, Sanz M,
Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C,
Makhoul PC, Travade P, Solary E, Fegueux N, Bordessoule D, Miguel JS,
Link H, Desablens B, Stamatoullas A, Deconinck E, Maloisel F, Castaigne
S, Preudhomme C, Degos L. A randomized comparison of all transretinoic
acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the
role of maintenance therapy in newly diagnosed acute promyelocytic
leukemia. The European APL Group. Blood. 1999; 94:1192-1200
PMid:10438706

- Adès L, Chevret S, Raffoux E, de Botton S,
Guerci A, Pigneux A, Stoppa AM, Lamy T, Rigal-Huguet F, Vekhoff A,
Meyer-Monard S, Maloisel F, Deconinck E, Ferrant A, Thomas X, Fegueux
N, Chomienne C, Dombret H, Degos L, Fenaux P; European Acute
Promyelocytic Leukemia Group. Is cytarabine useful in the treatment of
acute promyelocytic leukemia? Results of a randomized trial from the
European Acute Promyelocytic Leukemia Group. J Clin Oncol.
2006;24:5703-10 http://dx.doi.org/10.1200/JCO.2006.08.1596
PMid:17116939

- Asou N, Adachi K, Tamura J, Kanamaru A,
Kageyama S, Hiraoka A, Omoto E, Akiyama H, Tsubaki K, Saito K, Kuriyama
K, Oh H, Kitano K, Miyawaki S, Takeyama K, Yamada O, Nishikawa K,
Takahashi M, Matsuda S, Ohtake S, Suzushima H, Emi N, Ohno R. Analysis
of prognostic factors in newly diagnosed acute promyelocytic leukemia
treated with all-trans retinoic acid and chemotherapy. Japan Adult
Leukemia Study Group. J Clin Oncol. 1998;16:78-85 PMid:9440726

- Avvisati G, Petti MC, Lo-Coco F, Vegna ML,
Amadori S, Baccarani M, Cantore N, Di Bona E, Ferrara F, Fioritoni G,
Gallo E, Invernizzi R, Lazzarino M, Liso V, Mariani G, Ricciuti F,
Selleri C, Sica S, Veneri D, Mandelli F; GIMEMA (Gruppo Italiano
Malattie Ematologische dell'Adulto) Italian Cooperative Group.
Induction therapy with idarubicin alone significantly influences
event-free survival duration in patients with newly diagnosed
hypergranular acute promyelocytic leukemia: final results of the GIMEMA
randomized study LAP 0389 with 7 years of minimal follow-up. Blood.
2002;100: 3141-6 http://dx.doi.org/10.1182/blood-2002-02-0352
PMid:12384411

- Kelaidi C, Chevret S, De Botton S, Raffoux
E, Guerci A, Thomas X, Pigneux A, Lamy T, Rigal-Huguet F, Meyer-Monard
S, Chevallier P, Maloisel F, Deconinck E, Ferrant A, Fegueux N, Ifrah
N, Sanz M, Dombret H, Fenaux P, Adès L. Improved outcome of acute
promyelocytic leukemia with high WBC counts over the last 15 years: the
European APL Group experience. J Clin Oncol. 2009;27:2668-76 http://dx.doi.org/10.1200/JCO.2008.18.4119
PMid:19414681

- Testi AM, Biondi A, Lo Coco F, Moleti ML,
Giona F, Vignetti M, Menna G, Locatelli F, Pession A, Barisone E, De
Rossi G, Diverio D, Micalizzi C, Aricò M, Basso G, Foa R, Mandelli F.
GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute
promyelocytic leukemia (APL) in children. Blood. 2005;106:447-53 http://dx.doi.org/10.1182/blood-2004-05-1971
PMid:15677559

- de Botton S, Coiteux V, Chevret S, Rayon
C, Vilmer E, Sanz M, de La Serna J, Philippe N, Baruchel A, Leverger G,
Robert A, San Miguel J, Conde E, Sotto JJ, Bordessoule D, Fegueux N,
Fey M, Parry A, Chomienne C, Degos L, Fenaux P. Outcome of childhood
acute promyelocytic leukemia with all-trans-retinoic acid and
chemotherapy. J Clin Oncol. 2004;22:1404-12 http://dx.doi.org/10.1200/JCO.2004.09.008
PMid:15084614

- Ortega JJ, Madero L, Martín G, Verdeguer
A, García P, Parody R, Fuster J, Molines A, Novo A, Debén G, Rodríguez
A, Conde E, de la Serna J, Allegue MJ, Capote FJ, González JD, Bolufer
P, González M, Sanz MA; PETHEMA Group. Treatment with all-trans
retinoic acid and anthracycline monochemotherapy for children with
acute promyelocytic leukemia: a multicenter study by the PETHEMA Group.
J Clin Oncol. 2005;23:7632-40 http://dx.doi.org/10.1200/JCO.2005.01.3359
PMid:16234524

- Bally C, Fadlallah J, Leverger G, Bertrand Y, Robert A, Baruchel A, Guerci A, Recher C, Raffoux E, Thomas X, Leblanc T, Vey N, Dombret H, Sanz M, Fenaux P, Adès L. Outcome of APL in young children and adolescents in two consecutive trials of the European APL group. Blood (ASH Annual Meeting Abstracts), Nov 2010; 116: 224
- Callens C, Chevret S, Cayuela JM, Cassinat
B, Raffoux E, de Botton S, Thomas X, Guerci A, Fegueux N, Pigneux A,
Stoppa AM, Lamy T, Rigal-Huguet F, Vekhoff A, Meyer-Monard S, Ferrand
A, Sanz M, Chomienne C, Fenaux P, Dombret H; European APL Group.
Prognostic implication of FLT3 and Ras gene mutations in patients with
acute promyelocytic leukemia (APL): a retrospective study from the
European APL Group. Leukemia. 2005;19:1153-60 http://dx.doi.org/10.1038/sj.leu.2403790
PMid:15889156

- Montesinos P, Rayón C, Vellenga E, Brunet
S, González J, González M, Holowiecka A, Esteve J, Bergua J, González
JD, Rivas C, Tormo M, Rubio V, Bueno J, Manso F, Milone G, de la Serna
J, Pérez I, Pérez-Encinas M, Krsnik I, Ribera JM, Escoda L, Lowenberg
B, Sanz MA; PETHEMA; HOVON Groups. Clinical significance of CD56
expression in patients with acute promyelocytic leukemia treated with
all-trans retinoic acid and anthracycline-based regimens. Blood.
2011;117:1799-805 http://dx.doi.org/10.1182/blood-2010-04-277434
PMid:21148082

- Marty M, Ganem G, Fischer J, Flandrin G,
Berger R, Schaison G, Degos L, Boiron M. [Acute promyelocytic leukemia:
retrospective study of 119 patients treated with daunorubicin]. Nouv
Rev Fr Hematol. 1984;26:371-8 PMid:6597407

- Cunningham I, Gee TS, Reich LM, Kempin SJ,
Naval AN, Clarkson BD. Acute promyelocytic leukemia: treatment results
during a decade at Memorial Hospital. Blood. 1989;73:1116-22
PMid:2930837

- Sanz MA, Jarque I, Martín G, Lorenzo I,
Martínez J, Rafecas J, Pastor E, Sayas MJ, Sanz G, Gomis F. Acute
promyelocytic leukemia. Therapy results and prognostic factors. Cancer.
1988;61:7-13 http://dx.doi.org/10.1002/1097-0142(19880101)61:1<7::AID-CNCR2820610103>3.0.CO;2-6

- Adès L, Guerci A, Raffoux E, Sanz M,
Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S,
Tournilhac O, de Botton S, Ifrah N, Cahn JY, Solary E, Gardin C, Fegeux
N, Bordessoule D, Ferrant A, Meyer-Monard S, Vey N, Dombret H, Degos L,
Chevret S, Fenaux P; European APL Group. Very long-term outcome of
acute promyelocytic leukemia after treatment with all-trans retinoic
acid and chemotherapy: the European APL Group experience. Blood.
2010;115:1690-6 http://dx.doi.org/10.1182/blood-2009-07-233387
PMid:20018913

- Powell BL, Moser B, Stock W, Gallagher RE,
Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban
S, Appelbaum FR, Tallman MS, Larson RA. Arsenic trioxide improves
event-free and overall survival for adults with acute promyelocytic
leukemia: North American Leukemia Intergroup Study C9710. Blood.
2010;116:3751-7 http://dx.doi.org/10.1182/blood-2010-02-269621
PMid:20705755

- Lengfelder E, Haferlach C, Saussele S,
Haferlach T, Schultheis B, Schnittger S, Ludwig WD, Staib P, Aul C,
Grüneisen A, Kern W, Reichle A, Serve H, Berdel WE, Braess J,
Spiekermann K, Wörmann B, Sauerland MC, Heinecke A, Hiddemann W,
Hehlmann R, Büchner T; German AML Cooperative Group. High dose ara-C in
the treatment of newly diagnosed acute promyelocytic leukemia:
long-term results of the German AMLCG. Leukemia. 2009;23:2248-58 http://dx.doi.org/10.1038/leu.2009.183
PMid:19741727

- Sanz MA, Montesinos P, Vellenga E, Rayón
C, de la Serna J, Parody R, Bergua JM, León A, Negri S, González M,
Rivas C, Esteve J, Milone G, González JD, Amutio E, Brunet S,
García-Laraña J, Colomer D, Calasanz MJ, Chillón C, Barragán E, Bolufer
P, Lowenberg B. Risk-adapted treatment of acute promyelocytic leukemia
with all-trans retinoic acid and anthracycline monochemotherapy:
long-term outcome of the LPA 99 multicenter study by the PETHEMA Group.
Blood. 2008;112:3130-4 http://dx.doi.org/10.1182/blood-2008-05-159632
PMid:18664623

- Sanz MA, Montesinos P, Rayón C, Holowiecka
A, de la Serna J, Milone G, de Lisa E, Brunet S, Rubio V, Ribera JM,
Rivas C, Krsnik I, Bergua J, González J, Díaz-Mediavilla J, Rojas R,
Manso F, Ossenkoppele G, González JD, Lowenberg B; PETHEMA and HOVON
Groups. Risk-adapted treatment of acute promyelocytic leukemia based on
all-trans retinoic acid and anthracycline with addition of cytarabine
in consolidation therapy for high-risk patients: further improvements
in treatment outcome. Blood. 2010;115:5137-46 http://dx.doi.org/10.1182/blood-2010-01-266007
PMid:20393132

- Lo Coco F, Avvisati G, Vignetti M,
Fioritoni G, Liso V, Ferrara F, Cimino G, Gallo E, Rossi G Giustolisi
R, Rodeghiero F, Cantore N, Barbui T, Fazi P, Peta A, Bosi A, Madon E,
Biondi A, Masera F, Nobile F, Mirto S, Petti M, Mandelli F. Front-line
tretament of acute promyelocytic leukemia with AIDA induction followed
by risk-adapted consolidation: Results of the AIDA-2000 trial of the
Italian GIMEMA group. Blood. 2004; 104: 392a

- Lo-Coco F, Avvisati G, Vignetti M, Breccia
M, Gallo E, Rambaldi A, Paoloni F, Fioritoni G, Ferrara F, Specchia G,
Cimino G, Diverio D, Borlenghi E, Martinelli G, Di Raimondo F, Di Bona
E, Fazi P, Peta A, Bosi A, Carella AM, Fabbiano F, Pogliani EM, Petti
MC, Amadori S, Mandelli F; Italian GIMEMA Cooperative Group. Front-line
treatment of acute promyelocytic leukemia with AIDA induction followed
by risk-adapted consolidation for adults younger than 61 years: results
of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171-9 http://dx.doi.org/10.1182/blood-2010-03-276196
PMid:20644121

- Sanz MA, Lo Coco F, Martin G, Avvisati G,
Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González JD, Liso V,
Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F,
Colomer D, Petti MC, Ribera JM, Mandelli F. Definition of relapse risk
and role of nonanthracycline drugs for consolidation in patients with
acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA
cooperative groups. Blood. 2000; 96:1247-53 PMid:10942364

- Lo Coco F, Diverio D, Avvisati G, Petti
MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri
C, Martino B, Specchia G, Mandelli F. Therapy of molecular relapse in
acute promyelocytic leukemia. Blood. 1999;94:2225-9 PMid:10498592

- Grimwade D, Jovanovic JV, Hills RK, Nugent
EA, Patel Y, Flora R, Diverio D, Jones K, Aslett H, Batson E, Rennie K,
Angell R, Clark RE, Solomon E, Lo-Coco F, Wheatley K, Burnett AK.
Prospective minimal residual disease monitoring to predict relapse of
acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide
therapy. J Clin Oncol. 2009;27:3650-8 http://dx.doi.org/10.1200/JCO.2008.20.1533
PMid:19506161

- Sanz MA, Grimwade D, Tallman MS, Lowenberg
B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H,
Burnett AK, Lo-Coco F. Management of acute promyelocytic leukemia:
recommendations from an expert panel on behalf of the European
LeukemiaNet. Blood. 2009;113:1875-91 http://dx.doi.org/10.1182/blood-2008-04-150250
PMid:18812465

- Micol, JB, Raffoux E, Boissel N, Lengline E, Canet E, Leblanc T, Daniel MT, de Labarthe A, Maarek O, Cassinat B, Chomienne C, Ades L, Baruchel A, Degos L, Azoulay E, Dombret H. Do early events excluding patients with acute promyelocytic leukemia (APL) from trial enrollment modify treatment result evaluation? Real-life management of 100 patients referred to the University Hospital Saint-Louis between 2000 and 2010. Blood (ASH Annual Meeting Abstracts) 2010; 116: 473
- Lehmann S, Ravn A, Carlsson L, Antunovic
P, Deneberg S, Möllgård L, Rangert Derolf A, Stockelberg D, Tidefelt U,
Wahlin A, Wennström L, Höglund M, Juliusson G. Continuing high early
death rate in acute promyelocytic leukemia: a population-based report
from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011 Apr 19.

- Park JH, Qiao B, Panageas KS, Schymura MJ,
Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM, Tallman MS. Early
death rate in acute promyelocytic leukemia remains high despite
all-trans retinoic acid. Blood. 2011 Jun 8

- Di Bona E, Avvisati G, Castaman G, Luce
Vegna M, De Sanctis V, Rodeghiero F, Mandelli F. Early haemorrhagic
morbidity and mortality during remission induction with or without
all-trans retinoic acid in acute promyelocytic leukaemia. Br J
Haematol. 2000;108:689-95 http://dx.doi.org/10.1046/j.1365-2141.2000.01936.x
PMid:10792270

- de la Serna J, Montesinos P, Vellenga E,
Rayón C, Parody R, León A, Esteve J, Bergua JM, Milone G, Debén G,
Rivas C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S,
Amutio E, Brunet S, Lowenberg B, Sanz MA. Causes and prognostic factors
of remission induction failure in patients with acute promyelocytic
leukemia treated with all-trans retinoic acid and idarubicin. Blood.
2008;111:3395-402 http://dx.doi.org/10.1182/blood-2007-07-100669
PMid:18195095

- Breccia M, Latagliata R, Cannella L,
Minotti C, Meloni G, Lo-Coco F. Early hemorrhagic death before starting
therapy in acute promyelocytic leukemia: association with high WBC
count, late diagnosis and delayed treatment initiation. Haematologica.
2010;95:853-4 http://dx.doi.org/10.3324/haematol.2009.017962
PMid:20015875 PMCid:2864399

- Frankel SR, Eardley A, Lauwers G, Weiss M,
Warrell RP Jr. The "retinoic acid syndrome" in acute promyelocytic
leukemia. Ann Intern Med. 1992;117:292-6 PMid:1637024

- De Botton S, Dombret H, Miguel JS, Caillot
D, Zittoun R, Gardembas M, Stamatoulas A, Condé E, Guerci A, Gardin C,
Geiser K, Makhoul DC, Reman O, de la Serna J, Lefrere F, Chomienne C,
Chastang C, Degos L, Fenaux P. Incidence, clinical features, and
outcome of all trans-retinoic acid syndrome in 413 cases of newly
diagnosed acute promyelocytic leukemia: The European APL Group. Blood.
1998; 92:2712-8 PMid:9763554

- Montesinos P, Bergua JM, Vellenga E, Rayón
C, Parody R, de la Serna J, León A, Esteve J, Milone G, Debén G, Rivas
C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S, Amutio
E, Brunet S, Lowenberg B, Sanz MA. Differentiation syndrome in patients
with acute promyelocytic leukemia treated with all-trans retinoic acid
and anthracycline chemotherapy: characteristics, outcome, and
prognostic factors. Blood. 2009;113:775-83 http://dx.doi.org/10.1182/blood-2008-07-168617
PMid:18945964

- Adès L, Raffoux E, Chevret S, Pigneux A, Thomas X, Bordessoule B, Vey N, Guerci A, Lamy T, Recher C, Muller B, Tournilhac O, Pautas C, Cahn JY, Delaunay J, Deconinck E, Quesnel B, de Botton S, Stamatoullas A, Chomienne C, Dombret H, Degos L, Fenaux P. Arsenic trioxide in the consolidation treatment of newly diagnosed APL – First interim analysis of a randomized trial (APL 2006) by the French Belgian Swiss Group. Blood (ASH Annual Meeting Abstracts) 2010; 116: 224
- Ades L, Raffoux E, Chevret S, de Botton S, Guerci A, Pigneux A, Vey N, Lamy T, Huguet F, Muller B, Maloisel F, Deconinck E, Caillot D, Gratecos N, Sotto JJ, Ferrant A, Turlure P, Thomas X, Chevallier P, Ifrah N, Stamatoullas A, Chomienne C, Dombret H, Degos L, Fenaux P. Is AraC Required In the Treatment of Standard Risk APL? Long Term Results of a Randomized Trial (APL 2000) From the French Belgian Swiss APL Group. Blood (ASH Annual Meeting Abstracts), Nov 2010; 116: 11
- Adès L, Sanz MA, Chevret S, Montesinos P,
Chevallier P, Raffoux E, Vellenga E, Guerci A, Pigneux A, Huguet F,
Rayon C, Stoppa AM, de la Serna J, Cahn JY, Meyer-Monard S, Pabst T,
Thomas X, de Botton S, Parody R, Bergua J, Lamy T, Vekhoff A, Negri S,
Ifrah N, Dombret H, Ferrant A, Bron D, Degos L, Fenaux P. Treatment of
newly diagnosed acute promyelocytic leukemia (APL): a comparison of
French-Belgian-Swiss and PETHEMA results. Blood. 2008;111:1078-84
PMid:17975017

- Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu
YM, Shi JY, Zheng PZ, Yan H, Liu YF, Chen Y, Shen Y, Wu W, Tang W,
Waxman S, De Thé H, Wang ZY, Chen SJ, Chen Z. All-trans retinoic
acid/As2O3 combination yields a high quality remission and survival in
newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A.
2004;101:5328-35 http://dx.doi.org/10.1073/pnas.0400053101
PMid:15044693 PMCid:397380

- Gore SD, Gojo I, Sekeres MA, Morris L,
Devetten M, Jamieson K, Redner RL, Arceci R, Owoeye I, Dauses T,
Schachter-Tokarz E, Gallagher RE. Single cycle of arsenic
trioxide-based consolidation chemotherapy spares anthracycline exposure
in the primary management of acute promyelocytic leukemia. J Clin
Oncol. 2010;28:1047-53 http://dx.doi.org/10.1200/JCO.2009.25.5158
PMid:20085935 PMCid:2834430

- Ravandi F, Estey E, Jones D, Faderl S,
O'Brien S, Fiorentino J, Pierce S, Blamble D, Estrov Z, Wierda W,
Ferrajoli A, Verstovsek S, Garcia-Manero G, Cortes J, Kantarjian H.
Effective treatment of acute promyelocytic leukemia with
all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J
Clin Oncol. 2009;27:504-10 http://dx.doi.org/10.1200/JCO.2008.18.6130
PMid:19075265

- Mathews V, George B, Chendamarai E,
Lakshmi KM, Desire S, Balasubramanian P, Viswabandya A, Thirugnanam R,
Abraham A, Shaji RV, Srivastava A, Chandy M. Single-agent arsenic
trioxide in the treatment of newly diagnosed acute promyelocytic
leukemia: long-term follow-up data. J Clin Oncol. 2010;28:3866-71
http://dx.doi.org/10.1200/JCO.2010.28.5031 PMid:20644086

- Ghavamzadeh A, Alimoghaddam K, Rostami S,
Ghaffari SH, Jahani M, Iravani M Mousavi SA, Bahar B, Jalili M. Phase
II Study of Single-Agent Arsenic Trioxide for the Front-Line Therapy of
Acute Promyelocytic Leukemia. J Clin Oncol. 2011;29:2753-7 http://dx.doi.org/10.1200/JCO.2010.32.2107
PMid:21646615

- Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu
YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB,
Zelent A, Waxman S, Wang ZY, Chen SJ, Chen Z. Long-term efficacy and
safety of all-trans retinoic acid/arsenic trioxide-based therapy in
newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A.
2009;106:3342-7 http://dx.doi.org/10.1073/pnas.0813280106
PMid:19225113 PMCid:2651325

- de Botton S, Sanz M, Chevret S, Dombret H,
Martin G, Thomas X, Mediavilla JD, Recher C, Ades L, Quesnel B, Brault
P, Fey M, Wandt H, Machover D, Guerci A, Maloisel F, Stoppa AM, Rayon
C, Ribera JM, Chomienne C, Degos L, Fenaux P; European APL Group;
PETHEMA Group. Extramedullary relapse in acute promyelocytic leukemia
treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006;
20:35-41 http://dx.doi.org/10.1038/sj.leu.2404006
PMid:16307026

- Montesinos P, Díaz-Mediavilla J, Debén G,
Prates V, Tormo M, Rubio V, Pérez I, Fernández I, Viguria M, Rayón C,
González J, de la Serna J, Esteve J, Bergua JM, Rivas C, González M,
González JD, Negri S, Brunet S, Lowenberg B, Sanz MA. Central nervous
system involvement at first relapse in patients with acute
promyelocytic leukemia treated with all-trans retinoic acid and
anthracycline monochemotherapy without intrathecal prophylaxis.
Haematologica. 2009;94:1242-9 http://dx.doi.org/10.3324/haematol.2009.007872
PMid:19608685 PMCid:2738716

- Ferini GA, Raffoux E, Guerci A, Pigneux A, Huguet F, Vey N, Lamy T, Muller B, Chevallier P, Castaigne S, Turlure P, Ferrant A, Ifrah N, de Botton S, Dombret H, Ades L, Fenaux P. Central Nervous System (CNS) at First Relapse In APL. A Report on 2 Multicenter Trials. Blood (ASH Annual Meeting Abstracts), Nov 2010; 116: 473
- Imaizumi M, Tawa A, Hanada R, Tsuchida M,
Tabuchi K, Kigasawa H, Kobayashi R, Morimoto A, Nakayama H, Hamamoto K,
Kudo K, Yabe H, Horibe K, Tsuchiya S, Tsukimoto I. Prospective study of
a therapeutic regimen with all-trans retinoic acid and anthracyclines
in combination of cytarabine in children with acute promyelocytic
leukaemia: the Japanese childhood acute myeloid leukaemia cooperative
study. Br J Haematol. 2011;152:89-98 http://dx.doi.org/10.1111/j.1365-2141.2010.08332.x
PMid:20735397

- Zhou J, Zhang Y, Li J, Li X, Hou J, Zhao
Y, Liu X, Han X, Hu L, Wang S, Zhao Y, Zhang Y, Fan S, Lv C, Li L, Zhu
L. Single-agent arsenic trioxide in the treatment of children with
newly diagnosed acute promyelocytic leukemia. Blood. 2010;115:1697-702 http://dx.doi.org/10.1182/blood-2009-07-230805
PMid:20029047

- Mandelli F, Latagliata R, Avvisati G, Fazi
P, Rodeghiero F, Leoni F, Gobbi M, Nobile F, Gallo E, Fanin R, Amadori
S, Vignetti M, Fioritoni G, Ferrara F, Peta A, Giustolisi R, Broccia G,
Petti MC, Lo-Coco F; Italian GIMEMA Cooperative Group. Treatment of
elderly patients (> or =60 years) with newly diagnosed acute
promyelocytic leukemia. Results of the Italian multicenter group GIMEMA
with ATRA and idarubicin (AIDA) protocols. Leukemia. 2003;17:1085-90 http://dx.doi.org/10.1038/sj.leu.2402932
PMid:12764372

- Sanz MA, Vellenga E, Rayón C,
Díaz-Mediavilla J, Rivas C, Amutio E, Arias J, Debén G, Novo A, Bergua
J, de la Serna J, Bueno J, Negri S, Beltrán de Heredia JM, Martín G.
All-trans retinoic acid and anthracycline monochemotherapy for the
treatment of elderly patients with acute promyelocytic leukemia. Blood.
2004;104:3490-3 http://dx.doi.org/10.1182/blood-2004-04-1642
PMid:15292063

- Ades L, Chevret S, De Botton S, Thomas X,
Dombret H, Beve B, Sanz M, Guerci A, Miguel JS, Dela Serna J, Garo C,
Stoppa AM, Reman O, Stamatoulas A, Fey M, Cahn JY, Sotto JJ, Bourhis
JH, Parry A, Chomienne C, Degos L, Fenaux P; European APL Group.
Outcome of acute promyelocytic leukemia treated with all trans retinoic
acid and chemotherapy in elderly patients: the European group
experience. Leukemia. 2005;19:230-3
http://dx.doi.org/10.1038/sj.leu.2403597 PMid:15565164
