Possible Clinical Failure of Artemether-Lumefantrine in an Italian Traveler with Uncomplicated Falciparum Malaria
Ernestina C. Repetto1, Antonio Traverso1 and Claudio G. Giacomazzi2
1 Infectious
Diseases Unit, Regional Hospital U. Parini, Aosta, Italy.
2 Microbiology Laboratory, Regional Hospital U. Parini, Aosta, Italy.
2 Microbiology Laboratory, Regional Hospital U. Parini, Aosta, Italy.
Correspondence
to: Ernestina C. Repetto M.D. Tel +39.3291109328; Fax
+39.0165.543248. E-mail. erniesby@gmail.com
Published: October 1, 2011
Received: July 18, 2011
Accepted: September 17, 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011041, DOI 10.4084/MJHID.2011.041
This article is available from: http://www.mjhid.org/article/view/8851
This is an Open Access article
distributed under the terms of
the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Artemisinin-combination
therapies (ACTs) are recommended for the treatment of uncomplicated
malaria in endemic areas with multidrug resistant Plasmodium
falciparum. We report a case of possible artemether-lumefantrine
clinical failure in an Italian traveler with uncomplicated P.
falciparum malaria imported from Democratic Republic of Congo.
Introduction
Many studies have reported artemisinin-combination therapies (ACTs) to be the best antimalarial drugs available, due to their efficacy and potential to lower the emergence of resistance.[1,2] ACTs are recommended by the World Health Organization (WHO) for the treatment of uncomplicated malaria in endemic areas with multidrug resistant Plasmodium falciparum.[3]
Case
A 32-year old Caucasian man, two days after coming back from one month travel business in Democratic Republic of Congo, was admitted to the Infectious Diseases Unit because of high fever, headache, nausea and epigastric pain started 48 hours before. He had not taken any prophylaxis for malaria. Upon admission patient's vital parameters were: body temperature 37,1°C, pulse rate 112/minute, blood pressure 120/60 mm Hg, peripheral oxygen saturation 99%. Blood test results are summarized in Table 1. Peripheral blood smear was positive for Plasmodium falciparum with 0,2% parasitemia. Chest X-Ray and electrocardiogram were normal. The combination drug Riamet® (arthemeter-lumefantrine 20/120 mg), bought in Switzerland, was started following manufacturer advice: 6 dose regimen of 4 tablets at 0, 6, 18, 30, 42, 54 hours after meals. Within 48 hours the patient was afebrile and blood smear was negative for Plasmodium. The patient was discharged after 72 hours in good clinical condition. After 14 days he was readmitted to the Infectious Diseases unit with high fever (39.6 °C) and headache, started 48 hours before. Laboratory tests showed mild leukocytosis and anemia: hepatic enzymes were substantially similar to his previous values.
Laboratory tests are shown in Table 1. Blood cultures were done using the BacT/ALERT system (bioMérieux®, Marcy l’Etoile, France) and blood smear was performed and resulted positive for Plasmodium falciparum with 2% parasitemia. The combination of oral quinine solfate 600 mg three times per day and doxycicline 100 mg twice per day was started and continued for 7 days. He was discharged after 48 hours and completed the combination treatment without adverse events. Blood cultures for bacteria and fungi were negative. A control of biochemical tests and blood smear done after 2 days showed resolution of anemia and negativity of parasitemia. The patient did not report other febrile episodes.
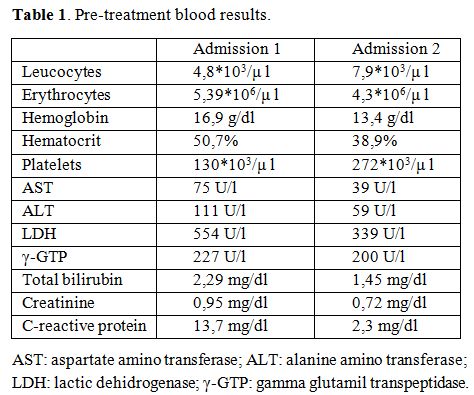
Table 1. Pre-treatment blood results.
The association of arthemeter-lumefantrine is one of the most popular ACTs and is currently used in many countries: it is an effective blood schizonticidal drug, it is well tolerated and fast acting.[4] Arthemeter rapidly reduces the parasite mass and resolves the symptoms while lumefantrine eliminates residual parasites. The efficacy of the combination mostly depends in the number of parasites remaining after arthemeter has been eliminated from the body and the duration of which lumefantrine plasma concentration exceeds the minimum inhibitor concentration against the parasites. No problems with absorption of arthemeter has been reported while co-administration with fatty food ensures maximum absorption of lumefantrine component[5] and the wide variation in the pharmacokinetics of lumefantrine among individuals is known to influence the efficacy of artemether-lumefantrine combination.
We report a case of possible clinical failure of artemether-lumefantrine in an Italian traveler with imported uncomplicated Plasmodium falciparum malaria. To our knowledge one previous case of treatment failure of artemether-lumefantrine in Plasmodium falciparum malaria imported from Sierra Leone has been published so far.[6] The authors hypothesized that this was due to low plasma concentration of lumefantrine component for its assumption without meals.
The combination drug artemether-lumefantrine is highly effective in Africa and published data indicated a good efficacy in Democratic Republic of Congo.[7-8] Nonetheless falciparum recrudescence after ACTs were recently increasingly reported[8-10] and high failure rates were seen in Cambodia (13,5% in 2006), where the emergence of lumefantrine resistance could not be excluded and could be explained by the cross resistance between mefloquine and lumefantrine.
According to WHO classification of treatment outcome in high-transmission areas (2009) our patient must be considered a late clinical failure. Our laboratory could not perform either an in vitro antimalarial susceptibility test or to measure blood antimalarial levels so the association therapy with oral quinine and doxycicline was chosen in the suspicion of inefficacy of the combination artemether-lumefantrine. Two hypothesis were made: the first was that the patient’s second episode was a re-infection rather than a recrudescence, being infected twice over 14 days before his return. As the association of artemether-lumefantrine is ineffective against exo-erytrocytic cycle, the first course treatment could have cured first malaria episode but could have been partially effective for the incubating Plasmodium. The second hypothesis was a recrudescence due to low plasma concentration of lumefantrine for an unrecognized patient’s defect in intestinal absorption rather than an incorrect drug assumption (in fact the patient properly took each dose after meal).
With increasing number of travelers into malaria endemic areas more attention should be paid in advising the patients to consult health practitioners if fever reappears after a full course of antimalarial treatment, to early diagnose recrudescences or re-infections.
Conflict Of Interest Statement.
The authors have declared that no competing interests exist.
Many studies have reported artemisinin-combination therapies (ACTs) to be the best antimalarial drugs available, due to their efficacy and potential to lower the emergence of resistance.[1,2] ACTs are recommended by the World Health Organization (WHO) for the treatment of uncomplicated malaria in endemic areas with multidrug resistant Plasmodium falciparum.[3]
Case
A 32-year old Caucasian man, two days after coming back from one month travel business in Democratic Republic of Congo, was admitted to the Infectious Diseases Unit because of high fever, headache, nausea and epigastric pain started 48 hours before. He had not taken any prophylaxis for malaria. Upon admission patient's vital parameters were: body temperature 37,1°C, pulse rate 112/minute, blood pressure 120/60 mm Hg, peripheral oxygen saturation 99%. Blood test results are summarized in Table 1. Peripheral blood smear was positive for Plasmodium falciparum with 0,2% parasitemia. Chest X-Ray and electrocardiogram were normal. The combination drug Riamet® (arthemeter-lumefantrine 20/120 mg), bought in Switzerland, was started following manufacturer advice: 6 dose regimen of 4 tablets at 0, 6, 18, 30, 42, 54 hours after meals. Within 48 hours the patient was afebrile and blood smear was negative for Plasmodium. The patient was discharged after 72 hours in good clinical condition. After 14 days he was readmitted to the Infectious Diseases unit with high fever (39.6 °C) and headache, started 48 hours before. Laboratory tests showed mild leukocytosis and anemia: hepatic enzymes were substantially similar to his previous values.
Laboratory tests are shown in Table 1. Blood cultures were done using the BacT/ALERT system (bioMérieux®, Marcy l’Etoile, France) and blood smear was performed and resulted positive for Plasmodium falciparum with 2% parasitemia. The combination of oral quinine solfate 600 mg three times per day and doxycicline 100 mg twice per day was started and continued for 7 days. He was discharged after 48 hours and completed the combination treatment without adverse events. Blood cultures for bacteria and fungi were negative. A control of biochemical tests and blood smear done after 2 days showed resolution of anemia and negativity of parasitemia. The patient did not report other febrile episodes.

Table 1. Pre-treatment blood results.
The association of arthemeter-lumefantrine is one of the most popular ACTs and is currently used in many countries: it is an effective blood schizonticidal drug, it is well tolerated and fast acting.[4] Arthemeter rapidly reduces the parasite mass and resolves the symptoms while lumefantrine eliminates residual parasites. The efficacy of the combination mostly depends in the number of parasites remaining after arthemeter has been eliminated from the body and the duration of which lumefantrine plasma concentration exceeds the minimum inhibitor concentration against the parasites. No problems with absorption of arthemeter has been reported while co-administration with fatty food ensures maximum absorption of lumefantrine component[5] and the wide variation in the pharmacokinetics of lumefantrine among individuals is known to influence the efficacy of artemether-lumefantrine combination.
We report a case of possible clinical failure of artemether-lumefantrine in an Italian traveler with imported uncomplicated Plasmodium falciparum malaria. To our knowledge one previous case of treatment failure of artemether-lumefantrine in Plasmodium falciparum malaria imported from Sierra Leone has been published so far.[6] The authors hypothesized that this was due to low plasma concentration of lumefantrine component for its assumption without meals.
The combination drug artemether-lumefantrine is highly effective in Africa and published data indicated a good efficacy in Democratic Republic of Congo.[7-8] Nonetheless falciparum recrudescence after ACTs were recently increasingly reported[8-10] and high failure rates were seen in Cambodia (13,5% in 2006), where the emergence of lumefantrine resistance could not be excluded and could be explained by the cross resistance between mefloquine and lumefantrine.
According to WHO classification of treatment outcome in high-transmission areas (2009) our patient must be considered a late clinical failure. Our laboratory could not perform either an in vitro antimalarial susceptibility test or to measure blood antimalarial levels so the association therapy with oral quinine and doxycicline was chosen in the suspicion of inefficacy of the combination artemether-lumefantrine. Two hypothesis were made: the first was that the patient’s second episode was a re-infection rather than a recrudescence, being infected twice over 14 days before his return. As the association of artemether-lumefantrine is ineffective against exo-erytrocytic cycle, the first course treatment could have cured first malaria episode but could have been partially effective for the incubating Plasmodium. The second hypothesis was a recrudescence due to low plasma concentration of lumefantrine for an unrecognized patient’s defect in intestinal absorption rather than an incorrect drug assumption (in fact the patient properly took each dose after meal).
With increasing number of travelers into malaria endemic areas more attention should be paid in advising the patients to consult health practitioners if fever reappears after a full course of antimalarial treatment, to early diagnose recrudescences or re-infections.
Conflict Of Interest Statement.
The authors have declared that no competing interests exist.
References
- Adjuik M, Babiker A, Garner P, Olliaro P,
Taylor W, White N; International Artemisinin Study Group. Artesunate
combinations for treatment of malaria: meta-analysis. Lancet. 2004 Jan
3;363(9402):9-17. http://dx.doi.org/10.1016/S0140-6736(03)15162-8

- Mutabingwa TK. Artemisinin-based
combination therapies (ACTs): best hope for malaria treatment but
inaccessible to the needy! Acta Trop. 2005 Sep;95(3):305-15. http://dx.doi.org/10.1016/j.actatropica.2005.06.009
PMid:16098946

- World Health Organization 2005: malaria control policies and strategies. World Malaria Report 2005. p14-17.
- van Agtmael M, Bouchaud O, Malvy D, Delmont
J, Danis M, Barette S, Gras C, Bernard J, Touze JE, Gathmann I, Mull R.
The comparative efficacy and tolerability of CGP 56697 (artemether +
lumefantrine) versus halofantrine in the treatment of uncomplicated
falciparum malaria in travellers returning from the Tropics to The
Netherlands and France. Int J Antimicrob Agents. 1999 Jul;12(2):159-69.
http://dx.doi.org/10.1016/S0924-8579(99)00070-9

- Ashley EA, Stepniewska K, Lindegårdh N,
Annerberg A, Kham A, Brockman A, Singhasivanon P, White NJ, Nosten F.
How much fat is necessary to optimize lumefantrine oral
bioavailability? Trop Med Int Health. 2007 Feb;12(2):195-200. http://dx.doi.org/10.1111/j.1365-3156.2006.01784.x

- Mizuno Y, Kato Y, Kudo K, Kano S. First
case of treatment failure of artemether- lumefantrine in a Japanese
traveler with imported falciparum malaria Jpn J Infect Dis. 2009
Mar;62(2):139-41. PMid:19305055

- Tshefu AK, Gaye O, Kayentao K, Thompson R,
Bhatt KM, Sesay SS, Bostos DG, Tjitra E, Bedu-Addo G, Borghini-Fuhrer
I, Duparc S, Shin CS, Fleckenstein L; Pyronaridine-artsunate Study
Team. Efficacy and safety of a fixed-dose oral combination of
pyronaridine-artesunate compared with artemether-lumefantrine in
children and adults with uncomplicated Plasmodium falciparum malaria: a
randomised non-inferiority trial. Lancet. 2010 Apr
24;375(9724):1457-67. http://dx.doi.org/10.1016/S0140-6736(10)60322-4

- World Health Organization 2010: global report on antimalarial drug efficacy and drug resistance: 2000-2010. p36-40.
- Smithuis F, Kyaw MK, Phe O, Win T, Aung PP,
Oo AP, Naing AL, Nyo MY, Myint NZ, Imwong M, Ashley E, Lee SJ, White
NJ. Effectiveness of five artemisinin combination regimens with or
without primaquine in uncomplicated falciparum malaria: an open-label
randomised trial. Lancet Infect Dis. 2010 Oct;10(10):673-81. http://dx.doi.org/10.1016/S1473-3099(10)70187-0

- Yavo W, Faye B, Kuete T, Djohan V, Oga SA,
Kassi RR, Diatta M, Ama MV, Tine R, Ndiaye JL, Evi JB, Same-Ekobo A,
Faye O, Kone M. Multicentric assessment of the efficacy and
tolerability of dihydroartemisinin-piperaquine compared to
artemether-lumefantrine in the treatment of uncomplicated Plasmodium
falciparum malaria in sub-Saharan Africa. Malar J. 2011 Jul
20;10(1):198. http://dx.doi.org/10.1186/1475-2875-10-198
PMid:21774826 PMCid:3164625
