Review Articles
Dennis A. Eichenauer1,2 and Andreas Engert1,2
2German Hodgkin Study Group (GHSG), Cologne, Germany.
Published: October 24, 2011
Received: September 5, 2011
Accepted: September 29, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011046, DOI 10.4084/MJHID.2011.046
This article is available from: http://www.mjhid.org/article/view/9133
Abstract
Hodgkin lymphoma (HL) is a
malignancy of the lymphatic system with an
incidence of 2-3/100.000/year in developed countries. With modern
multi-agent chemotherapy protocols optionally combined with
radiotherapy (RT), 80% to 90% of HL patients achieve long-term
remission and can be considered cured. However, current standard
approaches bear a considerable risk for the development of
treatment-related late effects. Thus, one major focus of current
clinical research in HL is reducing the incidence of these late effects
that include heart failure, infertility, chronic fatigue and
therapy-related myelodysplastic syndrome/acute myeloid leukemia
(t-MDS/t-AML). In previous analyses, t-MDS/t-AML after treatment for HL
was associated with a poor prognosis. Nearly all patients died rapidly
after diagnosis. However, more recent analyses indicated an improved
outcome among patients with t-MDS/t-AML who are eligible for modern
anti-leukemic treatment and allogeneic stem cell transplantation
(aSCT). This article gives an overview of recent reports on the
incidence and the treatment of t-MDS/t-AML after HL therapy and
describes the efforts currently made to reduce the risk to develop this
severe late effect.
Introduction
Hodgkin
lymphoma (HL) is a malignancy of the lymphatic system with an incidence
of 2-3/100.000/year in Europe and North America.[1]
The disease generally
occurs in all age groups but young adults are most often affected.[2] As
a result of substantial treatment improvements in the past decades
including the introduction of highly effective multi-agent chemotherapy
protocols and the optimization of radiation fields and doses, HL has
become one of the best curable adult malignancies. Irrespective of the
initial stage, 80% to 90% of patients achieve long-term remission (Figure 1).[3-5]
This has led to a steadily growing number of HL survivors. Since these
survivors often suffer from treatment-related late effects such as
heart failure, infertility, chronic fatigue and secondary malignancies,
reducing the frequency of long-term sequelae without compromising
treatment efficacy has become one of the major challenges of current
clinical research in HL.[6-9] Therapy-related
myelodysplastic
syndrome/acute myeloid leukemia (t-MDS/t-AML) represents one of the
most severe late effects after HL treatment and has been associated
with a particularly poor prognosis. Nearly all patients died rapidly
after diagnosis.[10]
However, incidence and prognosis of t-MDS/t-AML after HL treatment may
change in the coming years. Response-adapted treatment strategies that
are currently being evaluated in clinical trials will potentially lead
to a decrease of cumulative chemotherapy and radiation doses in many HL
patients. Thus, the risk to develop t-MDS/t-AML will be reduced.
Improvements in the treatment of t-MDS/t-AML mainly due to the
increased availability and the more efficient use of allogeneic stem
cell transplantation (aSCT) will probably have a positive impact on the
prognosis of the mostly young patients diagnosed with t-MDS/t-AML after
HL treatment.
This review aims at giving an overview of relevant analyses on the
development, incidence, clinical course and treatment of t-MDS/t-AML
after HL. Current strategies to reduce the risk of t-MDS/t-AML are also
discussed.
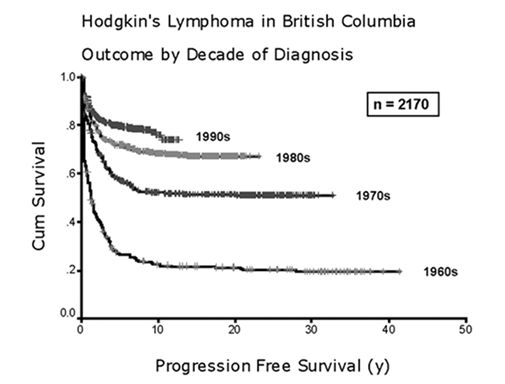
Figure 1. Progression-free survival among Hodgkin lymphoma patients treated in British Columbia during the indicated decades (adopted from Connors, Hematology Am Soc Hematol Educ Program, 2003)
Leukemogenic drugs in HL treatment
ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) and BEACOPP
(bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine,
procarbazine, prednisone) are the chemotherapy protocols most widely
used for the first-line treatment of adult patients with HL.[11-12] Both
regimens include alkylating agents, namely dacarbazine in the ABVD
protocol and cyclophosphamide and procarbazine in the BEACOPP schema.
Alkylating agents are known to be leukemogenic. Secondary leukemias
induced by this drug class mostly occur three to eight years after
exposition and are often preceded by a pre-leukemic phase that is
characterized by myelodysplasia. Chromosome aberrations are common in
t-MDS/t-AML induced by alkylating agents. Losses of chromosome 5 or
chromosome 7 as well as deletions of the long arm of the same
chromosomes are most frequently observed.[13]
Topoisomerase-II-inhibitors represent another drug class with
leukemogenic potential used in the treatment of HL. Particularly
etoposide is frequently applied. Besides first-line protocols such as
BEACOPP it is also contained in high-dose regimens used in the salvage
setting, BEAM (BCNU, etoposide, ara-c, melphalan) for instance.[12,14] In
comparison with alkylating agents, secondary leukemias induced by
topoisomerase-II-inhibitors are characterized by a more rapid
development after exposure. Thus, the median latency period between
treatment with topoisomerase-II-inhibitors and diagnosis of secondary
leukemia is about two years; a pre-leukemic phase with myelodysplastic
alterations in the bone marrow is usually not observed. However,
similar to alkylating agents, chromosomal aberrations are also often
found in secondary leukemias after treatment with
topoisomerase-II-inhibitors. Translocations at the myeloid-lymphoid
leukemia (MLL) gene locus 11q23 represent the most frequent
aberration.[13]
Incidence, clinical course and
treatment of t-MDS/t-AML in HL patients
The
two most recent reports on t-MDS/t-AML in HL patients come from the
German Hodgkin Study Group (GHSG). Josting and colleagues screened
5.411 patients treated within GHSG clinical trial protocols between
1981 and 1998 for the development of t-MDS/t-AML. Results were
published in 2003. At a median follow-up of 55 months, 46 patients had
developed t-MDS/t-AML; the cumulative risk to develop t-MDS/t-AML was
1%. In the majority of cases, HL treatment had consisted of ABVD or
ABVD-based chemotherapy protocols mostly combined with consolidating
radiotherapy (RT); some patients had received BEACOPP. The median time
interval between HL treatment and the diagnosis of t-MDS/t-AML was 12.5
months. An evaluation of cytogenetic changes was performed in 15
patients. All of them had chromosomal abnormalities. Aberrations
affecting chromosome 5 or chromosome 7 and the presence of an
MLL-rearrangement were most often observed. Clinical outcome of
patients with t-MDS/t-AML included in this analysis was poor with a
median overall survival (OS) of only four months for the whole patient
group and ten months for the nine patients who underwent aSCT. At 24
months, freedom from treatment failure and OS rates were 2% and 8%,
respectively (Figure 2).[10]
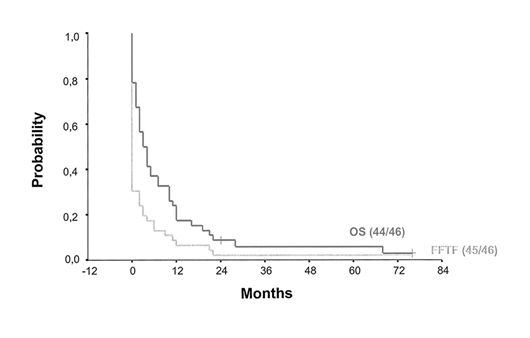
Figure 2. Freedom from treatment failure and overall survival among patients with t-MDS/t-AML treated for Hodgkin lymphoma (adopted from Josting et al., J Clin Oncol, 2003)
An update analysis including patients treated according to GHSG trial protocols between 1993 and 2009 was presented in abstract form in 2010. In contrast to the report from Josting and colleagues, an increased portion of patients included in this analysis had received the intensive BEACOPPescalated protocol representing the current standard of care for younger patients with advanced HL within the GHSG. A total of 11891 patients were screened for the occurrence of secondary myeloid neoplasms. Therapy-related MDS/AML had been diagnosed in 99 of them. However, 13 patients were excluded from final analysis due to a concurrent event prior to the diagnosis of t-MDS/t-AML so that 86 patients were eventually taken into account. Since the intensity of HL treatment appears to have a significant impact on the risk to develop t-MDS/t-AML, patients were divided into three subgroups. Patients from the first group had received no BEACOPPescalated - containing chemotherapy, patients from the second group had received less than four cycles of BEACOPPescalated and patients from the third group had received four of more cycles of BEACOPPescalated. As a result, the risk for the development of t-MDS/t-AML was significantly increased for patients treated with four or more cycles of escalated BEACOPP while the risk for patients from the other groups was comparable (1.5% vs 0.5% vs 0.3%). In comparison with the report from Josting and co-workers, a higher portion of patients included in the update analysis had received aSCT for the treatment of t-MDS/t-AML. These mostly young patients who had been eligible for intensive induction and/or conditioning protocols prior to aSCT showed an improved outcome. However, overall outcome after diagnosis of t-MDS/t-AML was still poor with a median survival of 7.2 months for the whole patient group.[15]
The improved treatment results reported for selected patients eligible for aSCT are in line with a recent analysis from Kayser and colleagues. The outcome of 200 patients with t-AML who were previously treated for different solid and hematologic malignancies was compared with the treatment results of 2653 patients with de novo AML. Relapse-free survival and OS were inferior in patients with t-AML with 4-year rates of 24.5% and 25.5%, respectively, compared to 37.9% and 39.5%, respectively, for patients with de novo AML. However, 40 of the 200 patients with t-AML included in the analysis received aSCT in first complete remission and had a 4-year OS rate of 42.6%. This is still inferior when compared with patients with de novo AML undergoing aSCT who had a 4-year OS rate of 58.0% but significantly better than reported in older analyses on t-AML.[16]
A similar analysis was performed by Litzow and co-workers. The outcome of 545 patients with t-AML and 323 patients with t-MDS who underwent allogeneic bone marrow or stem cell transplantation between 1990 and 2004 was analyzed. Disease-free survival and OS rates were 32% and 37%, respectively, at one year and 21% and 22%, respectively, at four years. At first sight, these results appear inferior in comparison with the data reported by Kayser and colleagues. However, the analyses are difficult to compare since Litzow and co-workers also included patients who received an allograft in the early 1990īs. Results achieved with aSCT at that time are not comparable with those observed today.[17]
In conclusion, the prognosis of patients with t-MDS/t-AML has apparently improved within the past years. The main reason for this improvement consists in the increased availability and the optimized use of aSCT.
Prevention of late effects including t-MDS/t-AML
With current standard approaches consisting of multi-agent chemotherapy optionally combined with RT, patients diagnosed with HL achieve long-term remission and can be considered cured in more than 80% of cases.3-5 Since remission rates can hardly be improved, the reduction of acute and long-term side effects including secondary hematologic malignancies such as t-MDS/t-AML has become increasingly important in recent years.
Ongoing clinical trials aim at reducing the risk to develop late sequelae including t-MDS/t-AML without compromising treatment efficacy. Thus, response-adapted treatment strategies are being evaluated. Positron emission tomography (PET) is currently considered the most promising tool to distinguish between patients who are sufficiently treated with less aggressive approaches and patients who require standard or even intensified protocols.
Within the GHSG HD15 trial, patients with advanced HL were initially randomized to receive either eight cycles of BEACOPPescalated, six cycles of BEACOPPescalated or eight cycles of BEACOPP-14. Then, a PET scan was performed in patients with residual lymphoma larger than 2.5 cm. Localized RT was confined to those patients with PET-positive residual disease. As a result, the negative predictive value of PET defined as the proportion of PET-negative patients without progression, relapse or RT within 12 months was 94.6%. Thus, it appears possible to restrict consolidating RT to patients with larger PET-positive residual lymphoma after intensive chemotherapy with escalated BEACOPP.[18]
In the ongoing GHSG follow-up trial, HD18 (NCT0051554), all patients receive two cycles of escalated BEACOPP before an interim PET is conducted. Then, patients with a negative PET are randomized between the standard treatment consisting of six further cycles of BEACOPPescalated and a reduced treatment consisting of only two further cycles of BEACOPPescalated while patients with insufficient metabolic response are randomized between the standard treatment and an intensified protocol consisting of six further cycles of escalated BEACOPP supplemented by the anti-CD20 antibody rituximab in the last five cycles.
In a trial conducted by an Italian Group (NCT00795613), treatment for patients with advanced HL is also stratified according to early interim PET. All patients initially receive two cycles of ABVD. Then, a PET scan is performed. Patients without detection of active disease continue treatment with ABVD while patients with PET-positive residual lymphoma switch to the more intensive BEACOPPescalated protocol for the rest of treatment.
In addition to the trials mentioned, further studies investigating response-adapted strategies with the aim to reduce treatment intensity in patients with good initial response on the one hand and intensify treatment in high-risk patients on the other hand are currently recruiting patients.
Another possible way to reduce the incidence of t-MDS/t-AML may consist in choosing the HL treatment according to the patientīs predisposition to develop therapy-related secondary malignancies. However, no susceptibility factors such as certain single nucleotide polymorphisms (SNP) predicting the risk of the individual patient to develop t-MDS/t-AML have been identified to date. Analyses of larger patient series addressing this issue appear necessary but are pending.
Summary
Within the past decades, HL has turned from an incurable disease to one
of the adult malignancies with best cure rates. Thus, treatment
efficacy can hardly be improved and the prevention of acute and
long-term toxicity including lung and heart failure, temporary or
permanent infertility and secondary malignancies including t-MDS/t-AML
has become one of the major challenges.
Currently ongoing trials aim at reducing the cumulative treatment
toxicity by using response-adapted strategies. Low-risk and high-risk
patients are being distinguished according to the result of an interim
PET. Once valid results from these trials will be available, treatment
stratification based on early interim PET might become standard of care
in HL therapy. Thus, cumulative chemotherapy and radiation doses could
decrease in a relevant portion of patients.
Patients who are diagnosed with t-MDS/t-AML after HL treatment still
have a poor prognosis although substantial therapeutic improvements
were made in the past decade. These improvements are mainly due to the
increased availability and the optimized use of aSCT. About 20% to 40%
of patients with t-MDS/t-AML achieve long-term remission when
treated with aSCT.[16-17]
Conclusion
The major goals in connection with t-MDS/t-AML after treatment for HL
consist in 1) the establishment of risk-adapted treatment strategies
for HL and 2) the further optimization of treatment for patients
diagnosed with t-MDS/t-AML.
References
- Engert A, Eichenauer DA,
Dreyling M. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v168-71. http://dx.doi.org/10.1093/annonc/mdq181
PMid:20555072

- Morton LM, Wang SS, Devesa SS, Hartge P,
Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype
in the United States, 1992-2001. Blood 2006;107:265-76. http://dx.doi.org/10.1182/blood-2005-06-2508
PMid:16150940 PMCid:1895348

- Engert A, Plutschow A, Eich HT, et al.
Reduced treatment intensity in patients with early-stage Hodgkin's
lymphoma. N Engl J Med 2010;363:640-52. http://dx.doi.org/10.1056/NEJMoa1000067
PMid:20818855

- Engert A, Borchmann P, Pluetschow A, et al. Dose-Escalation with BEACOPP Escalated Is Superior to ABVD In the Combined-Modality Treatment of Early Unfavorable Hodgkin Lymphoma: Final Analysis of the German Hodgkin Study Group (GHSG) HD14 Trial. ASH Annual Meeting Abstracts 2010;116:765-.
- Engert A, Diehl V, Franklin J, et al.
Escalated-dose BEACOPP in the treatment of patients with advanced-stage
Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin
Oncol 2009;27:4548-54. http://dx.doi.org/10.1200/JCO.2008.19.8820
PMid:19704068

- Behringer K, Josting A, Schiller P, et al.
Solid tumors in patients treated for Hodgkin's disease: a report from
the German Hodgkin Lymphoma Study Group. Ann Oncol 2004;15:1079-85.PMid:15205202

- Aleman BM, van den Belt-Dusebout AW, De
Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin
lymphoma. Blood 2007;109:1878-86. http://dx.doi.org/10.1182/blood-2006-07-034405
PMid:17119114

- Behringer K, Breuer K, Reineke T, et al.
Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at
treatment, stage of disease, chemotherapy regimen, and the use of oral
contraceptives during therapy: a report from the German Hodgkin's
Lymphoma Study Group. J Clin Oncol 2005;23:7555-64. http://dx.doi.org/10.1200/JCO.2005.08.138
PMid:16234521

- Ruffer JU, Flechtner H, Tralls P, et al.
Fatigue in long-term survivors of Hodgkin's lymphoma; a report from the
German Hodgkin Lymphoma Study Group (GHSG). Eur J Cancer
2003;39:2179-86. http://dx.doi.org/10.1016/S0959-8049(03)00545-8

- Josting A, Wiedenmann S, Franklin J, et
al. Secondary myeloid leukemia and myelodysplastic syndromes in
patients treated for Hodgkin's disease: a report from the German
Hodgkin's Lymphoma Study Group. J Clin Oncol 2003;21:3440-6. http://dx.doi.org/10.1200/JCO.2003.07.160
PMid:12668650

- Bonadonna G, Zucali R, Monfardini S, De
Lena M, Uslenghi C. Combination chemotherapy of Hodgkin's disease with
adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus
MOPP. Cancer 1975;36:252-9. http://dx.doi.org/10.1002/1097-0142(197507)36:1<252::AID-CNCR2820360128>3.0.CO;2-7

- Diehl V, Franklin J, Hasenclever D, et al.
BEACOPP, a new dose-escalated and accelerated regimen, is at least as
effective as COPP/ABVD in patients with advanced-stage Hodgkin's
lymphoma: interim report from a trial of the German Hodgkin's Lymphoma
Study Group. J Clin Oncol 1998;16:3810-21. PMid:9850026

- Leone G, Voso MT, Sica S, Morosetti R,
Pagano L. Therapy related leukemias: susceptibility, prevention and
treatment. Leuk Lymphoma 2001;41:255-76. http://dx.doi.org/10.3109/10428190109057981

- Chopra R, Linch DC, McMillan AK, et al.
Mini-BEAM followed by BEAM and ABMT for very poor risk Hodgkin's
disease. Br J Haematol 1992;81:197-202. http://dx.doi.org/10.1111/j.1365-2141.1992.tb08207.x
PMid:1643017

- Eichenauer DA, Haverkamp H, Behringer K, et al. Secondary MDS/AML In Hodgkin Lymphoma Patients Treated within German Hodgkin Study Group (GHSG) Clinical Trials After Introduction of the BEACOPP Protocol. ASH Annual Meeting Abstracts 2010;116:2682.
- Kayser S, Dohner K, Krauter J, et al. The
impact of therapy-related acute myeloid leukemia (AML) on outcome in
2853 adult patients with newly diagnosed AML. Blood 2011;117:2137-45. http://dx.doi.org/10.1182/blood-2010-08-301713
PMid:21127174

- Litzow MR, Tarima S, Perez WS, et al.
Allogeneic transplantation for therapy-related myelodysplastic syndrome
and acute myeloid leukemia. Blood 2010;115:1850-7. http://dx.doi.org/10.1182/blood-2009-10-249128
PMid:20032503 PMCid:2832815

- Engert A, Kobe C, Markova J, et al. Assessment of Residual Bulky Tumor Using FDG-PET In Patients with Advanced-Stage Hodgkin Lymphoma After Completion of Chemotherapy: Final Report of the GHSG HD15 Trial. ASH Annual Meeting Abstracts 2010;116:764.