Jameel Al-Ghazaly¹,², Waled Al-Dubai³, Munasser Abdullah4 and Leila Al-Gharasi²
1 Department of Medicine, Faculty of Medicine and Health Sciences, Sana'a University, Sana'a, Yemen
2 Department of Medicine, Hematology Unit, Al-Jomhori Teaching Hospital, Sana'a, Yemen
3 Department of Biochemistry and cytogenetics, Faculty of Medicine and Health Sciences, Sana'a University, Sana'a, Yemen
4 Al-Amana Specialized Laboratories, Sana'a, Yemen
Corresponding
author: Jameel Al-Ghazaly, Consultant Hematologist and Associate
Professor, Sana'a University, Head of Hematology Unit, Al-Jomhori
Teaching Hospital, Sana'a, Yemen. Tel: 00967-738168457. E-mail:
jameel_alghazaly@yahoo.com
Published: September 1, 2017
Received: June 26, 2017
Accepted: August 3, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017056 DOI
10.4084/MJHID.2017.056
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objectives:
Delay in the diagnosis of visceral leishmaniasis (VL) particularly in
non-endemic areas is associated with higher mortality. In our
experience, we found that marked bone marrow eosinopenia was a very
frequent accompaniment of VL and might be a useful clue for the
diagnosis, which indicates the opportunity for further morphological
assessment. The aim of this study was to describe the hematological
characteristics including peripheral blood and bone marrow findings of
Yemeni adults and children with VL.
Methods:
We conducted a descriptive analytic study to evaluate systematically
peripheral blood and bone marrow findings of Yemeni adults and children
with VL. Peripheral blood and bone marrow aspiration of patients with
bone marrow aspirate confirmed VL were examined. Forty-seven patients
with the main age (±SD) of 17.34±11.37 years (Range: 1-60) were
included in the study. Fifty-one non-VL subjects with splenomegaly and
pancytopenia or bicytopenia served as control group.
Results:
All patients with VL had anemia, 41 (87%) leukopenia, 42 (89%)
neutropenia, 44 (94%) thrombocytopenia, 42 (89%) eosinopenia, 34 (72%)
pancytopenia and 13 (28%) had bicytopenia. In bone marrow examination
40 (85%) showed hypercellularity, 44 (94%) eosinopenia, 24 (51%)
dyserythropoiesis, 22 (47%) lymphocytosis, 8 (17%) plasmacytosis, 27
(57%) decreased iron stores and 20 (43%) showed decreased sideroblasts.
Comparison of VL patients with the control group showed significantly
more frequent peripheral blood eosinopenia and lymphopenia and marrow
eosinopenia. There was no significant difference between adults and
children in any of the hematological features.
Conclusion:
Anemia, leukopenia, neutropenia, thrombocytopenia, eosinopenia,
pancytopenia and marked bone marrow eosinopenia were the most common
findings. The finding of marked bone marrow eosinopenia is a
significant clue for the diagnosis of visceral leishmaniasis in
patients who present with splenomegaly associated with cytopenias. This
finding is particularly valuable in non-endemic areas.
|
Introduction
The
hematological features of visceral leishmaniasis (VL) have evoked
particular interest because of their high frequency and severity and
because they cause significant mortality and morbidity.[1,2,3]
There are frequent reports of the hematological manifestations which
describe mainly their relative frequencies in different regions of the
world.[3,4] Common non-specific hematological features of VL include anemia, leukopenia, thrombocytopenia, and pancytopenia.[3,5,6]
Such hematological features are also frequently encountered in patients
with hematological malignancies such as acute leukemia, lymphomas, and
myelodysplastic syndrome as well as various infectious diseases.[7]
There are also frequent reports of VL presenting as an autoimmune
disease mimicking autoimmune hepatitis, primary biliary cirrhosis,
rheumatoid arthritis and systemic lupus erythematosus.[8,9] Higher rates of morbidity and mortality are consequences of the delay in diagnosis.[10,11,12]
VL is also reported to be one of the most common causes of fever of
unknown origin causing troublesome diagnosis in a European low-income
country.[13] In addition to the non-specificity of
clinical and general laboratory features of VL, the confirmatory
laboratory tests with the exception of identification of the parasites
in Giemsa stained tissue aspirates, are usually interpreted in the
light of clinical and epidemiological data which are not helpful in
non-endemic areas.[14,15] On the other hand, the
sensitivity of bone marrow aspirates, which is comparatively a safer
procedure compared to splenic aspirates for identification of the
parasite, was found to be proportional to the amount of time spent
searching for the amastigotes (65.5 percent and 95.4 percent at 5
minutes and one hour respectively).[16] Finding a
collection of hematological features will help to demand a diligent
search to confirm the diagnosis particularly in non-endemic areas. Only
a few reports have looked at hematological manifestations as helpful
clues for the diagnosis, which included mainly bone marrow cytological
features.[17,18] In Yemen, Leishmania IgG ELISA is
rarely available in some centres and experience showed it to be
unreliable because of the high frequency of false positive and false
negative results when compared to identification of the parasites in
Giemsa stained tissue aspirates. Such findings were also addressed by
WHO expert group who reported that a significant proportion of people
living in endemic areas with no history of VL is positive for
antileishmanial antibodies owing to asymptomatic infections.[15]
The experts also recommend that in areas of low endemicity more
accurate diagnostic algorithms are required that would include
parasitology in blood and bone marrow. The role of serology in the
diagnosis of VL has been reviewed.[19,20] PCR for the
diagnosis of leishmania is not available in Yemen, and only one report
has been recently published in which PCR was used for research purposes
with the collaboration of University of Malaya and reported the first
Molecular characterization of VL in Yemen.[21]
Therefore, identification of the parasites in Giemsa stained bone
marrow aspirate smears remains the only reliable diagnostic method for
the diagnosis of VL in Yemen.[3,22]
In our experience, we observed that in addition to known hematological
features, the presence of marked bone marrow eosinopenia constitutes a
crucial clue to the presence of visceral leishmaniasis in challenging
cases. Such evidence prompted a careful review of the smears searching
for amastigotes, which were identified -although sometimes with a
little and scanty distribution- in all suspected cases showing these
two features. The aim of this study is to evaluate systematically the
hematological characteristics of Yemeni adults and children with VL
including objective documentation of the frequency and degree
peripheral and bone marrow eosinopenia to find clues that may help to
arrive at the diagnosis early. This procedure by avoiding delay in
specific treatment will decrease morbidity and mortality. A full
epidemiological study of VL in Yemen is not available. The causative
organisms are Leishmania donovani complex (anthroponotic VL) and
Leishmania infantum complex (zoonotic VL). The pattern of VL in Yemen
derives from the few studies published. The disease seems to be endemic
in the country, particularly in Hajjah, Taiz and Amran governorates of
the Northern part of the country and Lahj and Abyan governorates of the
south of the country.[3,22]
Materials and Methods
The
study is a descriptive analytic study conducted in Sana'a, which is the
capital city of Yemen, at the hematology unit of Al-Jomhori teaching
hospital which is a referral tertiary teaching hospital. The hematology
unit deals with all types of hematological diseases including
hematological malignancies which are referred from all over the
country. The study included 47 patients with VL who were
prospectively evaluated and managed at our center between October 2010
and October 2014. Their diagnosis was confirmed by identification of
amastigotes in Giemsa stained bone marrow smears. Complete blood count
(CBC) was done for each patient using an automated cell counter (Sysmex
Automated machine, Sysmex Corporation, Kobe, Japan), The white blood
cell count (WBC) differential and red blood cell (RBC) morphology were
confirmed manually by a well-trained laboratory hematologist and
adjusted accordingly. One peripheral blood Giemsa stained smear and
three bone marrow aspiration Giemsa stained smears were examined by the
consultant laboratory and clinical hematologist. Informed consent was
obtained from the patients or responsible persons and the study was
approved by the Ethical Committee of the Faculty of Medicine and Health
Sciences of Sana’a University.
The control group included 51
subjects, randomly selected from the records of 207 non-VL patients, 60
years old or younger (considering that the maximum age for patients
with VL was 60 years old), who presented with fever, splenomegaly and
pancytopenia or bicytopenia during the period of the study between
October 2010 and October 2014. Their presenting CBC and WBC
differential were taken before any treatment. They were performed by
the same machine and in the same way as for all patients including
patients with VL. The bone marrow examination was carried out at
initial presentation as part of the evaluation of their splenomegaly
and cytopenia. Their marrow aspiration Giemsa stained smears were
reviewed to determine the eosinophil series percentage and to compare
the results with those of patients with VL. They were examined by the
same laboratory and clinical hematologist who reviewed the bone marrow
smears of patients with VL.
Definitions.
Bone marrow eosinopenia: eosinophil series count of less than 0.3% of
total marrow myeloid cells calculated as the average number in at least
20 cellular fields examined i.e. at least 20x200= 4000 cells were
counted [Normal range of eosinophils on aspirated bone marrow: 0.3-4.0%
and the normal mean: 2.2%].[23]
Dyserythropoiesis:
Presence of dysplastic changes of erythropoiesis including
megaloblastic features, binuclear and polynuclear normoblasts and other
dyserythropoietic features (e.g. internuclear bridges, nuclear budding)
with a frequency of > 5 per 100 erythroid cells
Hypercellular marrow: a cellularity > 50% in adults and > 80% in children.
Increased lymphocytes (marrow): Lymphocytes > 5% of total non-erythroid cells in adults and > 10% in children.
Increased plasma cells (marrow): Plasma cells > 5% of total non-erythroid cells in both adults and children.
Hemophagocytosis:
Presence in the bone marrow of macrophages which phagocytize blood and
bone marrow cells including red cells, erythroblasts, other leukocytes
and or platelets.
Evaluation of iron stores: decreased marrow iron
stores: less than one iron-positive cell, on the average for each x 40
field or absent iron-positive cells; increased marrow iron stores: more
than two iron-positive cells for each x 40 field. Decreased marrow
sideroblasts: sideroblasts less than 3% of total erythroblasts in the
marrow.[24]
Statistical analysis.
The data were collected, tabulated and compiled in a computer database.
SPSS version 21 was used to analyze data. Frequencies and percentages
were used to describe categorical data. Unpaired Independent Samples T
test was used to evaluate the comparison between adults and children
regarding the means of Hb, PCV, MCV, MCH, WBC, platelet, neutrophil,
lymphocyte, monocyte and eosinophil counts. Chi squared test was used
to compare the degree of abnormal peripheral blood counts and also the
peripheral blood and bone marrow morphological data between adults and
children. Unpaired Independent Samples T test was used to evaluate the
comparison between Patients with VL and control subjects regarding the
means of Hb, WBC, platelet, neutrophil, lymphocyte, monocyte, and
eosinophil counts. Chi squared test was used to compare the degree of
abnormal peripheral blood counts and bone marrow eosinopenia between VL
patients and controls.
Results
Forty-seven
(32 males and 15 females) patients with the main age (±SD) of
17.34±11.37 years (Range: 1-60) were included in the study. Of these
patients, 28 (59.6%) were adults aged 16-60 years with a mean age (±SD)
of 24.3 years ±9.2 and 19 (40.4%) patients were children aged 1-15
years with a mean age (±SD) of 7.1 years ±4.7.
Table 1 shows the mean values of the peripheral blood counts of adults and children with VL and table 2 shows the type and degree of abnormal peripheral blood counts.
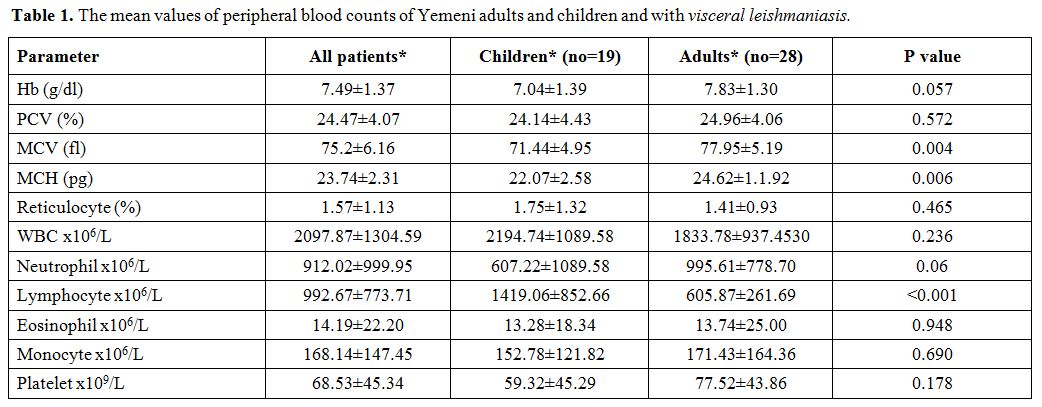 |
Table 1.
The mean values of peripheral blood counts of Yemeni adults and children and with visceral leishmaniasis. |
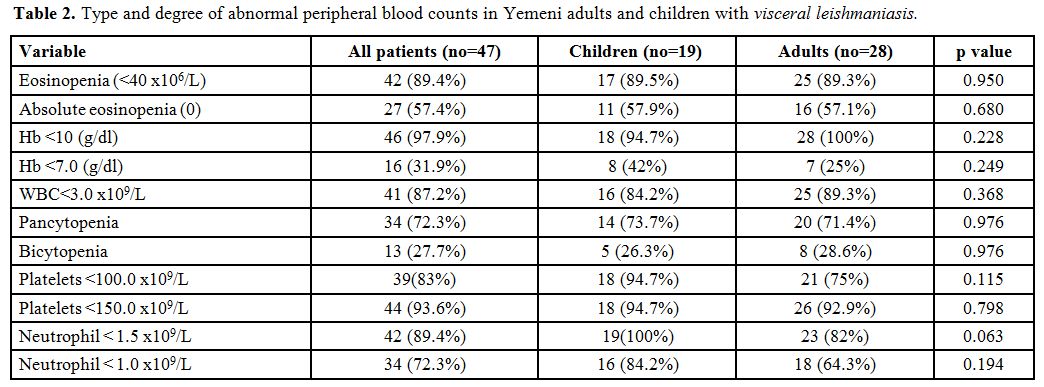 |
Table 2. Type and degree of abnormal peripheral blood counts in Yemeni adults and children with visceral leishmaniasis. |
All
patients had moderate to severe anemia (Hb range: 4.6-10.4 g/dl)
including 16 (32%) patients who had severe anemia; only one patient had
Hb > 10 g/dl (10.4 g/dl).
Forty-one (87%) patients had
leukopenia, and 42 (89.4%) patients had neutropenia including 34
(72.3%) patients who had significant neutropenia.
Thrombocytopenia
was present in 44 (93.6%) patients including 39 (83%) patients who had
significant thrombocytopenia. Eosinopenia was present in 42 (89.4%)
patients including 27 (57.4%) patients who had absolute eosinopenia.
All patients had either pancytopenia or bicytopenia: 34 (72.3%) and 13 (27.7%) respectively.
The
red blood cell morphological characteristics of Yemeni adults and
children with visceral leishmaniasis showed that anisocytosis,
anisochromia, and microcytic RBCs were the most common red blood cell
morphological findings which were present in 30 (63.8%), 25 (53.2%) and
23 (49%) respectively. Ten (21%) patients had poikilocytosis, and five
(10%) had tear drop red blood cells.
Table 3
shows the bone marrow morphological characteristics of Yemeni adults
and children with visceral leishmaniasis. Regarding bone marrow
morphological findings, marked bone marrow eosinopenia was the most
common finding which was seen in 44 (93.6%) patients. Forty (85%)
patients had hypercellular marrow, and 24 (51%) patients had
dyserythropoiesis. Decreased iron stores were present in 27 (57.4%)
patients, and 20 (42.6%) had a reduced number of sideroblasts. Only
three (6.4 %) patients had increased iron stores. Hemophagocytosis was
recognized in two (4.3) patients, and bone marrow plasmacytosis was
seen in eight (17%) patients (Figures 1,2,3).
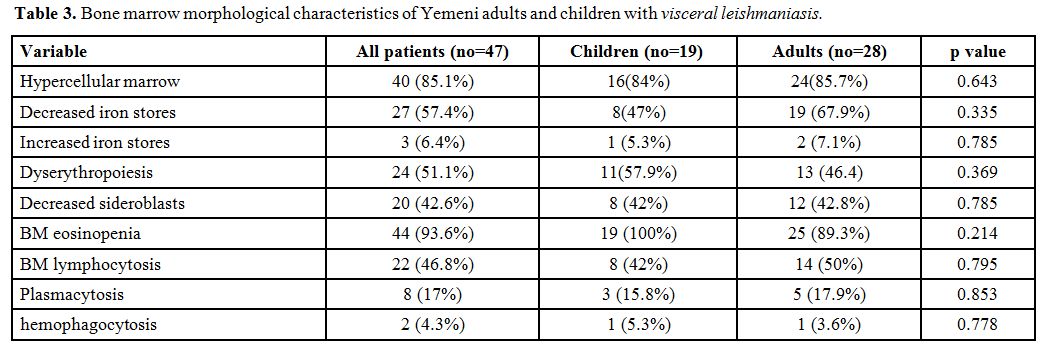 |
Table 3.
Bone marrow morphological characteristics of Yemeni adults and children with visceral leishmaniasis. |
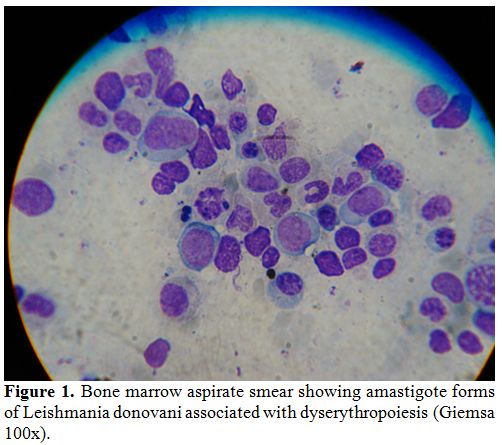 |
Figure 1. Bone marrow aspirate smear
showing amastigote forms of Leishmania donovani associated with
dyserythropoiesis (Giemsa 100x). |
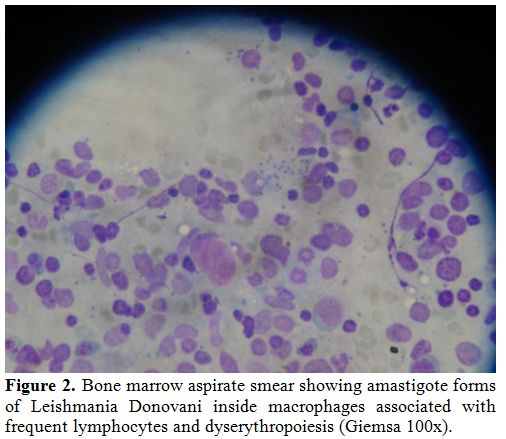 |
Figure 2. Bone marrow aspirate smear
showing amastigote forms of Leishmania Donovani inside macrophages
associated with frequent lymphocytes and dyserythropoiesis (Giemsa
100x). |
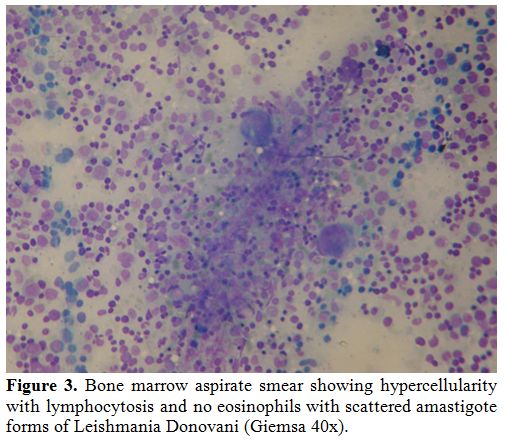 |
Figure 3. Bone marrow aspirate smear
showing amastigote forms of Leishmania Donovani inside macrophages
associated with frequent lymphocytes and dyserythropoiesis (Giemsa
100x). |
The
control group included 51 subjects (30 males and 21 females) with the
main age (±SD) of 20.71±11.92 years (Range: 0.5-60). The mean values of
the peripheral blood counts of control subjects was 7.80±1.75 (g/dl)
for Hb, 2703.92±826.55 (x106/L) for WBC, 1069.76±626.50 (x106/L) for neutrophils, 1347.76±630.26 (x106/L) for lymphocytes, 88.10±108.84 (x106/L) for eosinophils, 201.06±197.72 (x106/L) for monocytes and 74.65±62.68 (x109/L)
for platelets. Comparison of the mean values of peripheral blood counts
between patients with VL and control subjects showed no significant
difference in any of the above mean values except that patients had
significantly lower eosinophil counts (p value 0.000) and lower
lymphocyte count (p value 0.014). Table 4
shows comparison of abnormal peripheral blood counts and bone marrow
eosinopenia between Yemeni patients with VL and control subjects.
Patients had significantly more peripheral blood eosinopenia and
lymphopenia and bone marrow eosinopenia compared to control
subjects.
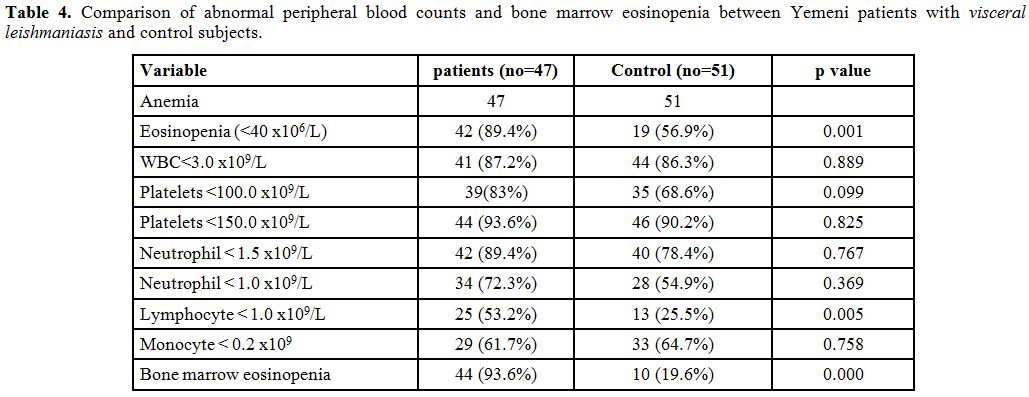 |
Table 4. Comparison of abnormal peripheral blood counts and bone marrow eosinopenia between Yemeni patients with visceral leishmaniasis and control subjects. |
Discussion
Our
study showed that anemia, leukopenia, neutropenia, thrombocytopenia,
eosinopenia, and pancytopenia were the most common peripheral blood
findings in patients with VL and that hypercellularity, eosinopenia,
dyserythropoiesis, lymphocytosis and decreased marrow iron were the
most common bone marrow findings.
Most hematological features
including anemia, leukopenia, thrombocytopenia, and pancytopenia are
non-specific. Such features are frequent in patients with other
infectious diseases, some hematological disorders and some autoimmune
collagenous diseases.[7,25-28]
Diagnosis of VL is straight forward in endemic areas where the disease
is suspected and aided by confirmatory laboratory tests including
reliable serological tests.[14] However, in
non-endemic areas, the differential diagnosis includes a broad spectrum
of diseases as mentioned above and serological diagnosis is not
reliable.[15] Finding amastigotes in tissue smears is
the most reliable diagnostic test. Splenic aspiration is a risky
procedure and is not a usual in non-endemic areas. Bone marrow
aspiration remains the safest procedure. However, the sensitivity of
such method depends on the time spent examining the smears.[16]
Paying careful oriented attention and adequate time searching for the
parasites increases the sensitivity to around 100%. However, such a
time which may take few hours cannot be paid for all patients
presenting with the above hematological features because of the high
incidence of the diseases presenting with such features. Finding
additional clues may limit the number of cases highly suspected of
being VL, which need careful, time-consuming study.
The peripheral
blood features of our patients which included anemia, thrombocytopenia,
leukopenia, bicytopenia, and pancytopenia are not different from those
reported from other studies in Asia, Africa, and Mediterranean region
or South America.[4,29]
Hypercellular marrow and dyserythropoiesis were also common in our
patient group which is similar to that reported in other studies.[30,31] Bone marrow lymphocytosis was also frequent among our patients similar to other studies.[6]
Our patients also had significant peripheral blood lymphocytopenia. The
presence of peripheral blood lymphopenia in association with bone
marrow lymphocytosis has been explained by the notion that lymphocytes
migrate to the affected lymphoid tissues to build an inflammatory
response and that bone marrow lymphocytosis is a compensatory response
that provides lymphocytes to organs affected by the parasite.[32,33]
Only one child and one adult of our patients had bone marrow features
of hemophagocytosis. Hemophagocytosis was reported to be a rare
occurrence in patients with visceral leishmaniasis causing diagnostic
dilemma and unusual presentation.[11,34]
Our patients showed decreased marrow iron which is consistent with
their common finding of microcytic red blood cells. This picture is due
to the malnutrition these patients usually have as a consequence of
anorexia and is also explained by the fact that the disease affects the
poor population predominantly.[25,35] Severe anemia, malnutrition and long duration of illness were shown to be associated with an increased risk of death.[25,36]
These issues should be addressed in evaluating and managing patients
with VL. The marked bone marrow eosinopenia associated with marked
peripheral blood eosinopenia are the characteristics which were most
common in our patients with VL and usually are not reported both
together in other simulating illnesses. Bone marrow eosinopenia in VL
has not been signaled in humans so far. However, it has been reported
in symptomatic canine VL as opposed to asymptomatic canine VL and was
found to be correlated with peripheral eosinopenia and it has been
regarded together with peripheral blood lymphocytopenia as a biomarker
of severe disease.[37] Eosinophilic hypoplasia in
symptomatic canine VL has been explained by bone marrow dysfunction,
which may have contributed to the severe eosinopenia.[37,38]
On the other hand, Eosinophil infiltration in the lymph-nodes of mice
infected by Leishmania major was found to be influenced by sex and
parasitic load and that it reflects ineffective inflammation.[39]
The previous studies in humans on hematological manifestations of VL
did not evaluate bone marrow eosinopenia as a feature or as a clue to
the diagnosis of the disease. A single study reported three of 18 bone
marrow aspirates who had prominent marrow eosinophils.[40]
However, the authors themselves of the study did not find any report of
similar observation in the searched medical literature. We also didn't
find other reports of this finding of marrow eosinophilia. Therefore,
secondary causes of eosinophilia could not be excluded in these cases.
Furthermore, the percentage of those patients with eosinophilia was too
small to regard it a significant finding: 3/18 [16%].[40]
Our study also showed that there was no significant difference between
adults and children regarding peripheral blood and bone marrow
eosinopenia or concerning other hematological features.
Conclusions
Based
on the above findings we conclude that in the proper clinical setting
associated with peripheral blood cytopenias, the finding of marked bone
marrow eosinopenia is a critical clue for the diagnosis of symptomatic
VL demanding careful, lengthy search for the parasites in bone marrow
aspirate smears. This finding is particularly valuable in non-endemic
areas.
References
- Pace D (2014) Leishmaniasis. J Infect 69 S10-18. https://doi.org/10.1016/j.jinf.2014.07.016 PMid:25238669

- Ready PD. Epidemiolgy of Visceral Leishmaniasis. Clin Epidemiol 2014; 6:147-154 https://doi.org/10.2147/CLEP.S44267 PMid:24833919 PMCid:PMC4014360

- Abdul
Hamid G, Gobah GA. Clinical and hematological manifestations of
visceral leishmaniasis in Yemeni children. Turk J Hematol 2009; 26:
25–28.

- Sarkari
B, Naraki T, Ghatee MA , Abdolahi KS, Davami MH. Visceral Leishmaniasis
in Southwestern Iran: A Retrospective Clinico-Hematological Analysis of
380 Consecutive Hospitalized Cases (1999-2014). PLOS ONE 2016;11(3):
e0150406. https://doi.org/10.1371/journal.pone.0150406

- Agrawal
Y, Sinha AK, Upadhyaya P, Kafle SU, Rijal S, Khanal B. Hematological
profile in visceral leishmaniasis. Int J Infect Microbiol
2013;2(2):39-44 https://doi.org/10.3126/ijim.v2i2.8320

- Varma N, Naseem S. Hematologic Changes in Visceral Leishmaniasis/Kala Azar. Indian J Hematol Blood Transfus 2010; 26: 78-78 https://doi.org/10.1007/s12288-010-0027-1 PMid:21886387 PMCid:PMC3002089

- Jain
A, Naniwadekar M. An etiological reappraisal of pancytopenia - largest
series reported to date from a single tertiary care teaching hospital.
BMC Hematol 2013;13:10. https://doi.org/10.1186/2052-1839-13-10

- Tunccan
OG, Tufan A, Telli G, Akyürek N, Pamukçuoglu M, Yilmaz Get al. Visceral
Leishmaniasis Mimicking Autoimmune Hepatitis, Primary Biliary
Cirrhosis, and Systemic Lupus Erythematosus Overlap. Korean J Parasitol
2012; 50 (2): 133-136 https://doi.org/10.3347/kjp.2012.50.2.133 PMid:22711924 PMCid:PMC3375451

- Cakar
M, Cinar M, Yilmaz S, Sayin S, Ozgur G, Pay S. A case of leishmaniasis
with a lupus-like presentation. Seminar Arthritis Rheum.
2015;45(1):e3-4. https://doi.org/10.1016/j.semarthrit.2015.04.001 PMid:25953712

- Prasad
R, Muthusami S, Pandey N, Tilak V, Shukla J, Mishra OP. Unusual
presentations of Visceral leishmaniasis. Indian J Pediatr 2009;76:
843–845. https://doi.org/10.1007/s12098-009-0148-4 PMid:19475352

- Celik
U, Alabaz D, Alhan E, Bayram I, Celik T. Diagnostic dilemma in an
adolescent boy: hemophagocytic syndrome in association with kala azar.
Am J Med Sci 2007;334:139–141. https://doi.org/10.1097/MAJ.0b013e31812e97f4 PMid:17700207

- Driemeier
M, de Oliveira PA, Druzian AF, Lopes Brum GF, Pontes ER, Dorval ME,
Paniago AM. Late diagnosis: a factor associated with death from
visceral leishmaniasis in elderly patients. Pathog Glob Health
2015;109(6): 283-9. https://doi.org/10.1179/2047773215Y.0000000029 PMid:26257311 PMCid:PMC4727583

- Bosilkovski
M, Dimozva M, Stevanovic M, Cvetkovska VS, Duganovska M. Fever of
unknown origin--diagnostic methods in a European developing country.
Voinosanit Pregl. 2016;73(6):553-8. https://doi.org/10.2298/VSP140827050B

- Sakkas
H, Gartzonika C, Levidiotou S. Laboratory diagnosis of human visceral
leishmaniasis. J Vector Borne Dis
2016;53(1):8-16. PMid:27004573

- Report
of a meeting of the WHO Expert Committee on the control of
Leishmaniasis, Geneve, 22-26 March 2010. Available at:
whqlibdoc.who.int, accessed: April, 2, 2017

- da
Silva MR, Stewart JM, Costa CH. Sensitivity of bone marrow aspirates in
the diagnosis of visceral leishmaniasis. AM J Trop Med Hyg
2005;72(6):811-4 PMid:15964968

- Bhatia
P, Haldar D, Varma N, Marwaha RK, Varma S. A Case Series Highlighting
the Relative Frequencies of common/uncommon and Atypical/Unusual
Hematological Findings on bone marrow examination in cases of Visceral
Leishmaniasis. Mediterr J Hematol Infect Dis 2011; 3: e2011035. https://doi.org/10.4084/mjhid.2011.035 PMid:22084650 PMCid:PMC3212968

- Chaufal
SS, Pant P, Chachra U, Singh P, Thapliyal N, Rawat V. Role of
Haematological Changes in Predicting Occurrence of Leishmaniasis- A
Study in Kumaon Region of Uttarakhand. J Clin Diag Res
2016;10(5):FC39-34. https://doi.org/10.7860/JCDR/2016/15438.7885

- Pagliano
P., Ascione T., Di Flumeri G., Boccia G., De Caro F. Visceral
leishmaniasis in immunocompromised: diagnostic and therapeutic approach
and evaluation of the recently released IDSA guidelines. Infez. Med
2016; 24(4): 265-271 PMid:28011960

- Franceschini
E, Puzzolante C, Menozzi M, Rossi L, Bedini A, Orlando G, Gennari W,
Meacc Mi, Rugna G, Carra E, Codeluppi M, Mussini C. Clinical and
Microbiological Characteristics of Visceral Leishmaniasis Outbreak in a
Northern Italian Nonendemic Area: A Retrospective Observational Study.
BioMed Research International Volume 2016 (2016), Article ID 6481028, 7
pages

- Mahdy
MAK, Al-Mekhlafi AM, Abdul-Ghani R, Saif-Ali R, Al-Mekhlafi HM,
Al-Eryani SM, Lim YAL, Mahmud R. First Molecular Characterization of
Leishmania Species causing Visceral Leishmaniasis among Children in
Yemen. PLOS ONE 2016;11(3): e0151265
 .
.
- Al-Ghazaly
J., Al-Dubai W. The clinical and biochemical characteristics of Yemeni
adults and children with visceral leishmaniasis and the differences
between them: a prospective cross-sectional study before and after
treatment. Trop Doct 2016;46(4): 224-231. https://doi.org/10.1177/0049475515622862 PMid:26746626

- Bates
I, Burthern J. Bone marrow biopsy In: Dacie and Lewis Practical
Haematology, Bain B, Bates I, Laffan M, Lewis SM (eds), Churchill
Livingstone Elsevier, 11th edition 2012. p.130 .
- Ryan
DH. Examination of the marrow In: Williams Hematology, Kaushansky K,
Lichtman M, Beutler E, Kipps TJ, Seligsohn U, Prchal JT (eds), McGraw
Hill Medical, 8th edition 2011. p. 33.
- Collin
S, Davidson R, Ritmeijer K, Keus K, Melaku Y, Kipngetich S, Davies C.
Conflict and kala-azar: determinants of adverse outcomes of kala-azar
among patients in southern Sudan. Clin. Infect. Dis 2004; 38 (5):
612–19. https://doi.org/10.1086/381203 PMid:14986243

- Kopterides P, Halikias S, Tsavaris N. Visceral leishmaniasis masquerading as myelodysplasia. Am J Hematol 2003;74:198–199 https://doi.org/10.1002/ajh.10408 PMid:14587050

- Arlet
JB, Capron L, Pouchot J. Visceral leishmaniasis mimicking systemic
lupus erythematosus. J Clin Rheumatol 2010; 16: 203-204. https://doi.org/10.1097/RHU.0b013e3181dfd26f PMid:20511988

- Pagliano,
P., Costantini, S., Gradoni, L., Faella, F.S., Spasiano, A.,
Mascarella, G., Prossomariti, L., Fusco, U., Ricchi, P. Case report:
Distinguishing visceral leishmaniasis from intolerance to pegylated
interferon-a in a thalassemic splenectomized patient treated for
chronic hepatitis C. American Journal of Tropical Medicine and Hygiene
2008; 79(1): 9-11 PMid:18606757

- Chakrabarti
S, Sarkar S, Goswami BK, Sarkar N, Das S. Clinico-hematological profile
of visceral leishmaniasis among immunocompetent patients. Southeast
Asian J Trop Med Public Health. 2013;44(2):143-9. PMid:23691621

- Bain BJ. Dyserythropoiesis in visceral leishmaniasis. American J Hematol 2010;85(10):781 https://doi.org/10.1002/ajh.21787 PMid:20652969

- Temiz
F, Gurbuz BB, Leblebisatan G, Ozkan A, Canoz PY, Harmanogullari S,
Gezer H, Tumogor G, Turgut M. An association of leishmaniasis and
dyserythropoiesis in children. Indian J Hematol Blood Transfus 2014; 30
(1):19-21 https://doi.org/10.1007/s12288-012-0189-0 PMid:24554815 PMCid:PMC3921338

- Bourdoiseau
G, Bonnefont C, Magnol JP, Saint-Andre I, Chabanne L (1997) Lymphocyte
subset abnormalities in canine leishmaniasis. Vet ImmunolImmunopathol
1997; 56: 345–351 https://doi.org/10.1016/S0165-2427(96)05768-6

- Reis
AB, Teixeira-Carvalho A, Giunchetti RC, Guerra LL, Carvalho MG, et al.
Phenotypic features of circulating leucocytes as immunological markers
for clinical status and bone marrow parasite density in dogs naturally
infected by Leishmania chagasi. Clin Exp Immunol 2006;146: 303–311 https://doi.org/10.1111/j.1365-2249.2006.03206.x PMid:17034583 PMCid:PMC1942052

- Scalzone
M, Ruggiero A, Mastsngelo S, Trombatore G, Ridola V, Maurii P, Riccardi
R. Hemophagocytic lymphohistiocytosis and visceral leishmaniasis in
children: case report and systematic review of literature. J Infect Dev
Ctries 2016;10(1):103-8 https://doi.org/10.3855/jidc.6385 PMid:26829545

- Jeronimo
SMB, de Queiroz Sousa A, Pearson RD. Leishmaniasis In: Tropical
infectious diseases: principles, pathogens and practice, Guerrant, RL,
Walker, DH, Weller, PF (Eds), Churchill Livingstone Elsevier,
Edinburgh, Scotland 2006. p. 1095-1113.
- Houweling
TA, Karim-Kos HE, Kulik Mc, Stolk WA, Haagsama JA, Lenk EJ, Richardus
JH, de Vlas SJ. Socioeconomic Inequalities in Neglected Tropical
Diseases: A Systematic Review. J Clin Exp Hepatol 2016;6(2):146-8. https://doi.org/10.1371/journal.pntd.0004546

- Nicolato
RC, Abreu RT, Roatt BM, Aguiar-Soares RDO, Reis LES, Carvalho MG,
Carneiro CM, Giunchetti RC et al. Clinical Forms of Canine Visceral
Leishmaniasis in Naturally Leishmania infantum–Infected Dogs and
Related Myelogram and Hemogram Changes. PLOS ONE 2013;8(12): e82947. https://doi.org/10.1371/journal.pone.0082947 PMid:24376612 PMCid:PMC3871677

- Tryphonas
L, Zawidzka Z, Bernard MA, Janzen EA. Visceral leishmaniasis in a dog:
clinical, hematological and pathological observations. Can J Comp Med
1997;41: 1–12.

- Salpnickova
M, Volkova V, Cepickova M, Kobets T, Sima M, Svobodova M et al.
Gene-specific sex effects on eosinophil infiltration in leishmaniasis
Biology of Sex Differences 2016; 7:59.

- Dhingra
KK, Gupta P, Saroha V, Setia N,Khurana N, Singh T. Morphological
findings in bone marrow biopsy and aspirate smears of visceral Kala
Azar: A review. Indian J Pathol Microbiol 2010; 53 (1): 96-100. https://doi.org/10.4103/0377-4929.59193 PMid:20090232

[TOP]





























 .
. 














