Review Articles
X. Papanikolaou, B.Barlogie and S.Z. Usmani
E-mail: susmani@uams.edu
Published: October 24, 2011
Received: September 20, 2011
Accepted: October 14, 2011
Mediterr J Hematol Infect Dis 2011, 3(1): e2011047, DOI 10.4084/MJHID.2011.047
This article is available from: http://www.mjhid.org/article/view/9207
Abstract
Therapy related myeloid
malignancies are an increasingly recognized
treatment complication in patients undergoing therapy for multiple
myeloma. The main predisposing factors are the alkylating agents,
topoisomerase II inhibitors and radiotherapy, but recently questions
have been raised regarding the immunomodulatory agent lenalidomide.
Little is known about the new antimyeloma agents in the context of
therapy related myeloid malignancies. The duration of treatment and the
time from diagnosis are the main contributing factors in alkylating
induced myeloid malignancies which occur 5-10 years after treatment,
chromosome 5 and 7 abnormalities being the characteristic finding. High
dose therapy (HDT) does not seem to be a major contributing factor per
se in multiple myeloma. In a number of large published series, all the
factors related with therapy-induced myelodysplasia were defined prior
to HDT. Topoisomerase II inhibitors induce mainly acute leukemias which
invariably correlate with dysregulation of the MLL gene. Radiotherapy
causes therapy related myelodysplasia if applied in bone marrow
producing areas, especially if combined with chemotherapy. Therapy
related myeloid malignancies generally herald a poor prognosis.
Karyotypic abnormalities seem to be the main prognostic factor. In all
cases the risk for therapy related myeloid malignancies drops sharply
by 10 years after the treatment.
Introduction
The problem of therapy related myelodysplastic syndromes (t-MDS) and
acute myeloid leukemia (t-AML) in the context of cytotoxic chemotherapy
is perhaps as old as the cytotoxic chemotherapy itself[1]
and it is part of the more general problem of the second malignancies
after cytotoxic chemotherapy.[2]
It is a well known fact that as the overall survival (OS) for a
malignant disease increases due to the treatment done, so the late
effects of this treatment become much more evident with the advent of
time.[3]
Multiple Myeloma (MM) is the second most prevalent hematological
malignancy in the Western World[4]
the last 15 years. Since the beginning of the era of chemotherapeutical
agents in the 70’s where the rate of complete remission (CR) for MM was
below 3%, with the incorporation of tandem autologous hematopoietic
cell –supported high dose therapy (HDT)[5,6] and the
newer agents as thalidomide, lenalidomide and bortezomib, the rate of
CR has increased to over 80% under the Total Therapy TT3 protocol,[7] making the MM median OS well over the past three year
landmark. In fact 10-year survivals of over 30% have been observed.[7]
It is thus a natural consequence that the problem of t-MDS and t-AML
becomes significant, requiring more attention form a biological
perspective and likely requires special therpuetic considerations.
Epidemiology
It is rather appropriate that if one considers and
examines the epidemiological data of t-MDS in MM to firstly acknowledge
the fact that MDS and MM can co-exist de novo. Both the morphological[8] and the cytogenetic[9]
evidence of this fact have been well described , with the cytogenetic
anomalies seen in ~4% of the total MM patient population and
having a distinctly different prognosis from the rest of the MM
subtypes.[9] In a series of 648 MM patients that were
enrolled in two non-HDT British Medical Research Council trials,[10]
the 5-year actuarial prevalence and the 8-year prevalence of
t-MDS were 3% and 10% respectively (FAB morphological criteria were
used for t-MDS and t-AML diagnosis). This series brought to the
forefront, the issue of MM-therapy related myeloid neoplasms, a fact
that was preciously well known in the context of other hematologic
malignancies. The Arkansas group reported on the cytogenetically
defined MDS of more than 3000 MM patients that underwent HDT[11]
and reported a prevalence of cytogenetically defined MDS of 3%. Most of
the cytogenetic abnormalities (68%) were transient and clinical t-MDS
and t-AML developed in 26 patients. It is therefore evident that there
is a discrepancy of the reported prevalence and incidence of t-MDS in
the various big series of the MM patients. Given the available
knowledge on the main causes of t-MDS, one has to evaluate the
incidence of MDS in the context of the therapeutic regimen given.[12]
Conventional Chemotherapy and
t-MDS in MM
The causative relationship
of alkylating therapy in MM and t-MDS has been acknowledged as early as
in the 1970s.[13,14] The widespread use of alkylating
agents in various hematological and non hematological malignancies has
resulted in valuable knowledge of the characteristics of the alkylating
induced t-MDS. It is occurring mainly as a late event of the
chemotherapy with a characteristic latency of 5-10 years.[15]
Patients will present with t-MDS and evidence of bone marrow failure
with at least one cytopenias while a minority will present as t-AML or
t-myeloproliferative / myelodysplastic syndrome.[16]
This category is commonly associated with unbalanced loss of genetic
material, often involving chromosomes 5 and/or 7, although that is not
universal.[16] The decades of therapeutic
experience has also contributed the knowledge that it is the amount of
time and cumulative dosing of these agents and not the intensity of the
therapy that contributes to the development of t-MDS. This fact is well
established in many malignancies[17] and is also
evident in the MM population.[10]
Also well established is the knowledge that all alkylating agents are
not the same in their leukaemogenic potential. Melphalan and BCNU are
considered more leukaemogenic than cyclophosphamide in general[18] and this fact has also been established also in MM
patients treated with these drugs.[10] The
combination of alkylating agents and radiotherapy increases the
incidence of t-MDS.[16]
The second category of t-MDS related to the conventional chemotherapy
is related to the topoisomerase II inhibitors, namely adriamycin,
etoposide, chemotherapeutics that interact through DNA topoisomerase
II. This category of chemotherapeutics has long been successfully used
in the treatment of MM. The t-MDS/AML that they produce has a latency
period of 1-5 years, usually does not present as a t-MDS but as an
overt t-AML from the beginning and is often associated with balanced
chromosomal translocation.[16,17] The amount of
cumulative dosage is equivocal and in the setting of the therapy of
other hematological malignancies several regimen-related factors, as
the schedule and concurrent use of asparaginase and G-SCF, are
important in determining the relative risk.[18,19]
Especially etoposide has strictly been associated with translocations
of the MLL gene on chromosome band 11q23. MLL is a critical
transcription regulator and the fact that there are over 40 partner
genes in reciprocal translocations found in MDS/AML, suggests that it
holds a crucial role in the pathogenesis of t-MDS/AML and MDS/AML in
general.
In practice however most MM patients have received polychemotherapy of
the above substances/modalities either concurrently or subsequently.
The boundaries of the chromosomal, clinical and laboratory
characteristics of the resulting t-MDS/AML characteristics regarding
the causal chemotherapeutic are not always sharp.[11]
t-MDS/AML and HDT in MM. HDT has become the standard of care in the
management of younger patients with symptomatic or progressive MM.[20,21] Tandem autotransplantation has doubled survival
in relationship to standard-dose therapy.[22]
Sizable series have reported on the development of t-MDS/AML in the
context of HDT for Hodgkin, non-Hodgkin lymphoma as well as MM.[11,23,24]
There is a clear tendency for attribution of t-MDS/AML, at least in the
Hodgkin and non-Hodgkin lymphomas, in HDT. Since standard dose regimens
precede autologous peripheral blood stem cell (PBSC) collection and
HDT, it is unclear whether the t-MDS is associated with HDT or the
preceding chemotherapy. Primary HDT after non stem cell damaging
vincristine – adriamycin- dexamethasone (VAD) therapy resulted in an
incidence of t-MDS at 0% at 4.7 years.[25] In the
biggest HDT MM series reported till now11 multivariate analysis showed
that the t-MDS/AML development was correlated with age -15% in 10 years
for the older patients (>65 years), poor (<2.5x 106/kg) PBSC
collection, time interval between the preceding chemotherapy and HDT
reflecting longer pre transplant chemotherapeutic exposure and low
platelet recovery 3 months after the first transplantation
(<150x109/kg). The type of the HDT regimen was not significant in
terms of subsequent t-MDS/AML development. From the aforementioned it
appears that HDT is likely a contributing factor in t-MDS/AML, along
with a host of other important ones. The later is supported from the
fact that studies in lymphoma patients that applied fluorescence in
situ hybridization (FISH) analysis for the detection of MDS lesions in
interphase cells, found that such abnormalities were already present in
PBSCs prior to HDT and were similar or identical to those subsequently
detected after HDT.[26] Thus the question of the main
contributing factor remains still open and could very well be that the
main contribution of HDT to t-MDS/AML in MM is improvement in overall
survival and patient longevity.
Newer Therapies and t-MDS in MM
Very little is known about the
contribution or not to t-MDS of the newer MM therapies. There were not
differences in the incidence of t-MDS between the thalidomide and
control arm in the Arkansas Total Therapy 2 trial.[11]
The recent reports on the association of lenalidomide with myeloid
malignancies have born mixed results. The IFM 2005-02 study[27] and CALGB 100104 study[28]
reported increased incidence of second primary malignancies, including
myeloid malignanices, in the order of 5.5%-6.5%. In the MM-015 study,
Palumbo et al[29] reported a 0.7% incidence of
t-AML/t-MDS in MM transplant ineligible patients with use of
lenalidomide combined with melphalan/prednisone and receiving
additional lenalidomide maintenance, compared with those receiving
melphalan/prednisone alone. This has given rise to the debate of
optimal duration of maintenance with lenalidomide, as it clearly has
shown progression free survival benefit in MM. To date, there have no
reports regarding bortezomib in t-MDS/t-AML development in MM or
lymphoma patients.
Therapeutic Modalities and
Future Directions
It is crucial for anyone
to realize that preventing is far better than treating! Present and
future efforts have to be -at least partially- directed towards the
maximum effective anti-MM therapy with the lowest t-MDS potential. For
conventional chemotherapy cumulative experience favors the short
exposure to alkylating agents without intensity of treatment being a
worrying factor in terms of t-MDS development. Radiotherapy perhaps
should better be avoided upfront and concurrently with chemotherapy at
least in bone marrow producing regions. There are enough data to
support its leukaemogenic potential but not enough data to support its
superiority in MM treatment at least compared with other therapeutic
modalities. Bortezomib and thalidomide seem rather safe agents in MM
regarding t-MDS.[30] The role of lenalidomide in
t-MDS in the context of maintenance treatment in MM seems rather
controversial. There is a clear need for more series with the maximum
amount of uniformity for the rest of the MM treatment for someone to
draw more definite conclusions.
Drug or xenobiotic metabolizing enzymes (DME) play central roles in the
metabolism, biotransformation, and detoxification of xenobiotics and
foreign compounds. They generally protect from potential harmful
insults from the environment and also influence the metabolism of drugs
(Table 1). Polymorphisms of
these genes have been associated with the development of t-MDS/AML
relative to the previous cytotoxic therapy. Although some of the
reports are conflicting, the hall concept appears to be a very
promising sector of pharmacogenomics and the individualization of
cytotoxic therapy in general.[31]
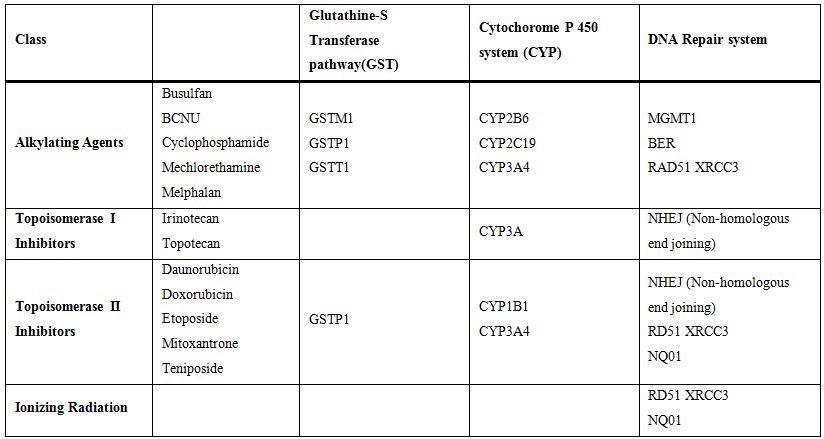
Table 1. Role of gene polymorphism in t-MDS/t-AML development
The prognosis of t-MDS/AML is generally considered poor. An overall 5-year survival of less than 10% is commonly reported.[15] It is strongly associated with the underlying karyotypic abnormality, something that recently has been recognized in de novo MDS also, as it is portrayed in the revised IPSS that showed in the last International MDS Symposium (ISMDS 2011, Edinburgh May [18-21]). Cases with abnormalities of chromosome 5 and/or 7 and a complex caryotype have a particular poor prognosis with a median survival of less than one year regardless of the number of myeloblasts present in bone marrow biopsy at initial MDS diagnosis.[32,33] Perhaps for these patients, an allogeneic transplantation should be strongly considered upfront. For not eligible patients autologous transplantation with PBSC collected early in the course of the patient could serve as an alternative. In the cases of 5q- chromosomal abnormalities lenalidomide has proved a valuable drug in releaving the accompanying anemia and in some cases inducing cytogenetic remission.[34] The drug can be given also to non 5q- MDS with a amount of myeloblasts <10% with good results as long as the Gene Expression Profile of the MDS resembles the one of 5q- syndrome.[34] Hypo ethylating agents azacytidine and decitabine although have promising results in de novo high IPSS MDS, have not been tested enough in t-MDS/AML and the results in cases with 7 monosomy and complex caryotype are rather disappointing. Perhaps their use is better suited for cases of t-MDS with a number of myeloblasts>10% and karyotypic abnormalities that represent balanced translocations. This group can also benefit from the traditional chemotherapy approach at least for induction and/or salvage chemotherapy in terms of RAEB II MDS or t-AML. Of notice is the fact that the rare antracycline related Acute Promyelocytic Leukemias herald the exact same prognosis with the de novo ones,[35] a fact that highlights the importance of the underlying karyotypic abnormality in the prognostic and therapeutical evaluation of t-MDS. Supportive care (erythropoietin agents, transfusion policy, iron chelating therapy) is the same as with the de novo MDS.
Conclusion
t-MDS represents a real and emerging problem in MM treatment. As the
median MM OS survival universally increases it will possibly establish
further its presence in the MM course. Although the diagnostic,
prognostic and therapeutic capabilities of t-MDS and MDS in general are
continuously expanding, one has to remember that “to prevent is always
better than curing’’ meaning that a good amount of present and future
efforts has to be concentrated in the recognition and improvement of
the MM therapy with the best anti myeloma effect and the fewer t-MDS
complications.
References
- Rowley
J, Golomb H, Vardiman J. Nonrandom chromosome abnormalities in acute
leukemia and dysmyelopoietic syndromes in patients with previously
treated malignant disease. Blood 1981; 58:759-767. PMid:7272506

- Obedian E, Fischer DB, Haffty BG. Second
Malignancies After Treatment of Early-Stage Breast Cancer: Lumpectomy
and Radiation Therapy Versus Mastectomy JCO 2000;18:2406-2412

- Doria R, Holford T, Farber LR, et al.
Second solid malignancies after combined modality therapy for Hodgkin's
disease. JCO 1995:13;2016-2022

- Ries LAG, Melbert D, Krapcho M et al. SEER Cancer Statistics Review, 1973-1999, Bethesda, MD: National Cancer Institute, 2002.
- Barlogie B, Jagannath S, Vesole D, et al.
Superiority of tandem autologous transplantation over standard therapy
for previously untreated multiple myeloma. Blood. 1997;89:789-793.
PMid:9028309

- Attal M, Harousseau JL, Facon T, et al.
Single versus double autologous stem-cell transplantation for multiple
myeloma. N Engl J Med. 2003; 349:2495-2502. http://dx.doi.org/10.1056/NEJMoa032290
PMid:14695409

- Barlogie B, Tricot G, van Rhee F, et al.
Long-term outcome results of the first tandem autotransplant trial for
multiple myeloma. Br J Haematol. 2006; 135:1365-2141. http://dx.doi.org/10.1111/j.1365-2141.2006.06271.x
PMid:16939489

- Mufti GJ, Hamblin TJ, Clein GP, et al.
Coexistent myelodysplasia and plasma cell neoplasia. Br J Haematol.
1983;54:91 http://dx.doi.org/10.1111/j.1365-2141.1983.tb02070.x
PMid:6849839

- Jacobson J, Barlogie B, Shaughnessy JD, et
al. MDS-type abnormalities within myeloma signature karyotype (MM-MDS):
only 13% 1-year survival despite tandem transplants. Br J Haematol
2003;122:430–440 http://dx.doi.org/10.1046/j.1365-2141.2003.04455.x
PMid:12877670

- Cuzickl J, Erskine S, Edelman D et al. A
comparison of the incidence of the myelodysplastic syndrome and acute
myeloid leukaemia following melphalan and cyclophosphamide treatment
for myelomatosis A report to the Medical Research Council's working
party on leukaemia in adults. Br J Cancer 1987;55:523-529 PMid:3300761
PMCid:2001731

- Barlogie B, Tricot G, Haessler J, et al.
Cytogenetically defined myelodysplasia after melphalan-based
autotransplantation for multiple myeloma linked to poor hematopoietic
stem-cell mobilization: the Arkansas experience in more than 3000
patients treated since 1989. Blood 2008;111: 94-100 http://dx.doi.org/10.1182/blood-2007-06-097444
PMid:17895401 PMCid:2200826

- Kantarjian HM, Keating MJ, Walters RS et
al. Therapy-related leukemia and myelodysplastic syndrome: clinical,
cytogenetic, and prognostic features. J Clin Oncol 1986;4:1748-1757
PMid:3783201

- Kyle RA, Pierre RV, Bayrd ED. Multiple
myeloma and acute myelomonocytic leukaemia. N Engl J Med
1970;283:1121-5. http://dx.doi.org/10.1056/NEJM197011192832101
PMid:5273282

- Karchmer R, Amare M, Larsen W et al.
Alkylating agents as leukemogenesis in multiple myeloma. Cancer
1974;33: 1103-1107 http://dx.doi.org/10.1002/1097-0142(197404)33:4<1103::AID-CNCR2820330432>3.0.CO;2-S

- Pedersen-Bjergaard J, Andersen MK,
Christiansen DH, et al. Genetic pathways in therapy-related
myelodysplasia and acute myeloid leukemia. Blood 2002;99:1909-12 http://dx.doi.org/10.1182/blood.V99.6.1909
PMid:11877259

- Larson RA. Therapy related myeloid
neoplasms in WHO Classification of Tumours of Hematopoietic and
Lymphoid Tissues. Haematologica 2008;94:454-459 http://dx.doi.org/10.3324/haematol.2008.005157
PMid:19336749 PMCid:2663607

- Smith SM, Le Beau MM, Huo D, et al.
Clinical-cytogenetic associations in 306 patients with therapy related
myelodysplasia and myeloid leukemia: the University of Chicago series.
Blood 2003;102:43-52. http://dx.doi.org/10.1182/blood-2002-11-3343
PMid:12623843

- Josting A, Wiedenmann S, Franklin J, t al.
Secondary myeloid leukemia and myelodysplastic syndromes in patients
treated for Hodgkin's disease: a report from the German Hodgkin’s
Lymphoma Study Group. J Clin Oncol 2003; 21:3440-6. http://dx.doi.org/10.1200/JCO.2003.07.160
PMid:12668650

- Armitage JO, Carbone PP, Connors JM,et al.
Treatment-Related myelodysplasia and acute leukemia in non-Hodgkin’s
lymphoma patients. J Clin Oncol 2003; 21:897-906 http://dx.doi.org/10.1200/JCO.2003.07.113
PMid:12610191

- Attal M, Harousseau JL, Stoppa AM, et al.
A prospective, randomized trial of autologous bone marrow
transplantation and chemotherapy in multiple myeloma: Intergroupe
Francais du Myelome. N Engl J Med. 1996;335:91-97.
http://dx.doi.org/10.1056/NEJM199607113350204 PMid:8649495

- Child JA, Morgan GJ, Davies FE, et al.
High-dose chemotherapy with hematopoietic stem-cell rescue for multiple
myeloma. N Engl J Med. 2003; 348:1857-1883 http://dx.doi.org/10.1056/NEJMoa022340
PMid:12736280

- Desikan R, Barlogie B, Sawyer J, et al.
Results of high-dose therapy for 1000 patients with multiple myeloma:
durable complete remissions and superior survival in the absence of
chromosome13 abnormalities. Blood. 2000;95:4008-4010 PMid:10845942

- van Leeuwen FE, Chorus AMJ, van den Belt-
Dusebout AW, et al. Leukemia risk following Hodgkin’s disease: relation
to cumulative dose of alkylating agents, treatment with teniposide
combinations, number of episodes of chemotherapy and bone marrow
damage. J Clin Oncol. 1994; 12:1063-1073. PMid:8164031

- Armitage J, Carbone P, Connors J, et al.
Treatment- related myelodysplasia and acute leukemiain non-Hodgkin’s
lymphoma patients. J Clin Oncol. 2003;21:897-906. http://dx.doi.org/10.1200/JCO.2003.07.113
PMid:12610191

- Govindarajan R, Jagannath S, Flick JT, et
al. Preceding standard therapy is the likely cause of MDS after
autotransplants for multiple myeloma. Br J Haematol. 1996;95:349-353. http://dx.doi.org/10.1046/j.1365-2141.1996.d01-1891.x
PMid:8904891

- Abruzzese E, Radford JE, Miller JS, et al.
Detection of abnormal pretransplant clones in progenitor cells of
patients who developed myelodysplasia after autologous transplantation.
Blood. 1999; 94:1814-1819. PMid:10477708

- Attal M, Lauwers VC, AMrit G, et al. Maintenance Treatment with Lenalidomide After Transplantation for MYELOMA : Final Analysis of the IFM 2005-02. Blood 2010;116:310
- McCarthy PL, Owzar K, Anderson KC, et al. Phase III Intergroup Study of Lenalidomide Versus Placebo Maintenance Therapy Following Single Autologous Hematopoietic Stem Cell Transplantation (AHSCT) for Multiple Myeloma: CALGB 100104. Blood 2010;116: 37
- Palumbo A, Delforge M, Catalano J, et al. Incidence of second primary malignancy (SPM) in melphalan-prednisone-lenalidomide combination followed by lenalidomide maintenance (MPR-R) in newly diagnosed multiple myeloma patients (pts) age 65 or older. J Clin Oncol 2011;29:8007
- Ladetto M, Pagliano G, Ferrero S, et al.
Major Tumor Shrinking and Persistent Molecular Remissions After
Consolidation With Bortezomib, Thalidomide, and Dexamethasone in
Patients With Autografted Myeloma. J Clin Oncol 2010; 28:2077-2084 http://dx.doi.org/10.1200/JCO.2009.23.7172
PMid:20308672

- Leone G, Pagano L, Ben-Yehuda D, et al.
Therapy-related leukemia and myelodysplasia: susceptibility and
incidence. Haematologica 2007; 92:1389-1398. http://dx.doi.org/10.3324/haematol.11034
PMid:17768113

- Singh ZN, Huo D, Anastasi J, et al.
Therapy-related myelodysplastic syndrome: morphologic
sub-classification may not be clinically relevant. Am J Clin Pathol
2007;127:197-205 http://dx.doi.org/10.1309/NQ3PMV4U8YV39JWJ
PMid:17210514

- Michiels JJ, McKenna RW, Arthur DC, et al.
Therapy-related acute myeloid leukemia and myelodysplastic syndrome: a
clinical and morphological study of 65 cases. Blood 1985;65:1364-1372
PMid:3857944

- Ebert BL, Galili N, Tamayo P, et al. An
erythroid differentiation signature predicts response to lenalidomide
in myelodysplastic syndrome. PLoS Med 2008; 5:e35 http://dx.doi.org/10.1371/journal.pmed.0050035
PMid:18271621 PMCid:2235894

- Beaumont M, Sanz M, Carli PM, et al.
Therapy-Related Acute Promyelocytic Leukemia. J Clin Oncol
2003;21:2123-2137 http://dx.doi.org/10.1200/JCO.2003.09.072
PMid:12775738
