Alessia Castellino1, Stefano Guidi2, Chiara Maria Dellacasa3, Antonella Gozzini2, Irene Donnini2, Chiara Nozzoli2, Sara Manetta3, Semra Aydin1, Luisa Giaccone3, Moreno Festuccia3, Lucia Brunello3, Enrico Maffini3, Benedetto Bruno3, Ezio David4 and Alessandro Busca3.
1 A.O.U. Città della Salute e della Scienza di Torino, Dipartimento di Oncologia, Ematologia, Torino, Italy.
2 SODc Terapie Cellulari e Medicina Trasfusionale, AOU Careggi, Firenze.
3
A.O.U. Città della Salute e della Scienza di Torino, Dipartimento di
Oncologia, SSD Trapianto allogenico di cellule staminali, Torino, Italy.
4 S.C. Anatomia Patologica 1, A.O.U. Città della Salute e della Scienza di Torino.
Corresponding
author: Castellino Alessia MD, A.O.U.
Città della Salute e della Scienza di Torino, Dipartimento di
Oncologia, Ematologia, Torino, Italy. Corso Bramante 88, 10126 Torino.
Tel: +39 0116335359 FAX: +39 011 6335759. E-mail:
acastellino@cittadellasalute.to.it
Published: January 1, 2018
Received: August 2, 2017
Accepted: November 5, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018001 DOI
10.4084/MJHID.2018.001
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Hepatic
Veno-Occlusive Disease (VOD) is a potentially severe complication of
hematopoietic stem cell transplantation (HSCT). Here we report two
patients receiving an allogeneic HSCT who developed late onset VOD with
atypical clinical features. The two patients presented with only few
risk factors, namely, advanced acute leukemia, a myeloablative
busulphan-containing regimen and received grafts from an unrelated
donor. The first patient did not experience painful hepatomegaly and
weight gain and both patients showed only a mild elevation in total
serum bilirubin level. Most importantly, the two patients developed
clinical signs beyond day 21 post-HSCT. Hepatic transjugular biopsy
confirmed the diagnosis of VOD. Intravenous defibrotide was promptly
started leading to a marked clinical improvement. Based on our
experience, liver biopsy may represent a useful diagnostic tool when
the clinical features of VOD are ambiguous. Early therapeutic
intervention with defibrotide represents a crucial issue for the
successful outcome of patients with VOD.
|
Introduction
Veno-occlusive
disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is
a potentially life-threatening complication of hematopoietic stem cell
transplantation (HSCT).[1] The diagnosis of VOD is
primarily based on clinical criteria defined almost 20 years ago,
including the triad of painful hepatomegaly, jaundice and fluid
retention.[2-4] This observation could at least
partially explain the highly variable incidence of VOD reported in the
literature, ranging from 8% to 14%. VOD usually develops within 20-30
days after HSCT. However, few cases of late-onset VOD have been
reported.[5] According to this observation, the
European Group for Bone marrow Transplantation (EBMT) recently endorsed
the revised diagnostic criteria for VOD/SOS, which now include either a
classical form of VOD and a late-onset variant (Table 1).[6]
Here we describe two HSCT recipients who developed late-onset VOD with atypical clinical features.
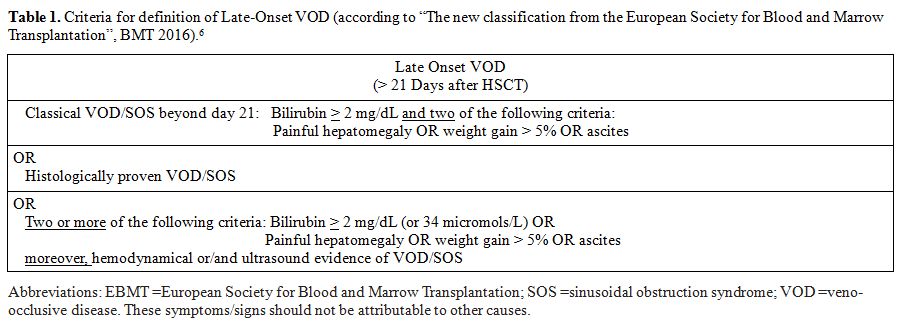 |
Table
1. Criteria for definition of Late-Onset VOD (according to “The new
classification from the European Society for Blood and Marrow
Transplantation”, BMT 2016).[6] |
Case 1
A
55-year-old male was diagnosed with acute myeloid leukemia in May 2015.
He failed to achieve the complete remission (CR) after two induction
chemotherapy courses with high dose cytarabine, idarubicin and
etoposide and salvage treatment with fludarabine and idarubicin. The
presence of a matched unrelated donor in the International Marrow Donor
Registries prompted us to proceed with an allogeneic HSCT following a
“sequential” conditioning regimen. The patient was initially treated
with mitoxantrone (6 mg/sqm/day), etoposide (80 mg/sqm/day) and
cytarabine (1 g/sqm/day for four days), followed, 10 days later, by a
conditioning which included i.v. Busulphan (3.2 mg/kg/day) and
Fludarabine (50 mg/mq/day) for four days and the infusion of mobilized
donor peripheral blood stem cells (PBSC). Graft-vs.-Host disease (GVHD)
prophylaxis consisted of anti-thymocyte globulin, cyclosporine and a
short course of methotrexate. An absolute neutrophil count higher than
0.5x109/L and a platelet count higher
than 20.000/mcl were achieved on day +13. On day +33, the patient
suddenly showed abdominal distension with ascites, increase in liver
enzymes (AST 391 U/l, ALT 245 U/l) and in total bilirubin (1.2 mg/dL)
and signs of liver and renal insufficiency (INR 1.43; aPTT 65.5’’;
serum creatinine value 3.04 mg/dL). The patient did not present
either painful hepatomegaly or weight gain >2%, or signs of
intestinal or cutaneous acute GVHD. Viral hepatitis was ruled out by
microbiological testing. Ultrasonography showed normal liver
parenchyma, regular biliary tract, moderate splenomegaly (15 cm) with
ascites and right pleural effusion. Doppler exam ruled out portal vein
thrombosis but showed increased portal vein diameter (10 mm) suggestive
of portal hypertension. Paracentesis was performed and showed presence
of transudate fluid (serum albumin =3 mg/dL, ascites albumin 1.5 mg/dL,
serum-ascites albumin ratio=2).
Given that clinical symptoms and
laboratory tests did not allow to discriminate between VOD, acute GVHD,
toxicity or infective causes, a hepatic transjugular biopsy was
performed. Histology studies showed the expansion of the hepatic
sinusoid spaces, with gaps in the sinusoidal barrier which were highly
suggestive of hepatic VOD in the light of the involvement of zone 3,
zone 2 and partially zone 1 of the hepatic acinus (Figure 1).
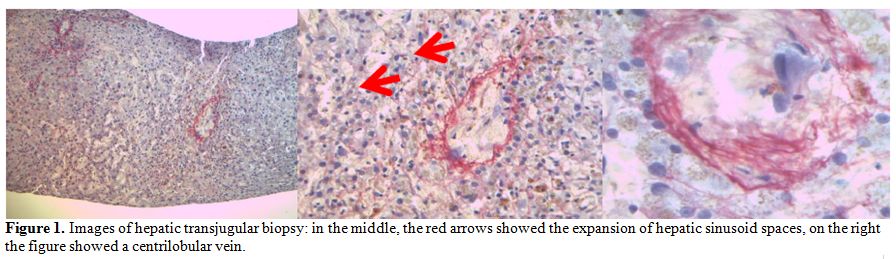 |
Figure 1. Images of hepatic transjugular
biopsy: in the middle, the red arrows showed the expansion of hepatic
sinusoid spaces, on the right the figure showed a centrilobular vein |
Intravenous
defibrotide was started at the dose of 6.25 mg/kg QID on day +37 for 21
days, along with ancillary therapy including albumin replacement and
low dose diuretics; nonsteroids have been administered. Two-three days
after the beginning of defibrotide, the patient showed a marked
clinical improvement with gradual improvement and normalization of
liver and renal function tests (Figure 2).
One week after the beginning of defibrotide, the patient developed
hemorrhagic cystitis, treated with 2 bladder instillations of
hyaluronic acid which led to progressive improvement and complete
resolution upon regular discontinuation of defibrotide after 21 days of
treatment. Hemorrhagic cystitis did not require an earlier
discontinuation of defibrotide. During the treatment course, platelet
count remained low between 10.000 to 20.000/mmc even with transfusion
support. The patient was discharged on day +76 in complete remission of
his underlying disease on low doses of cyclosporine.
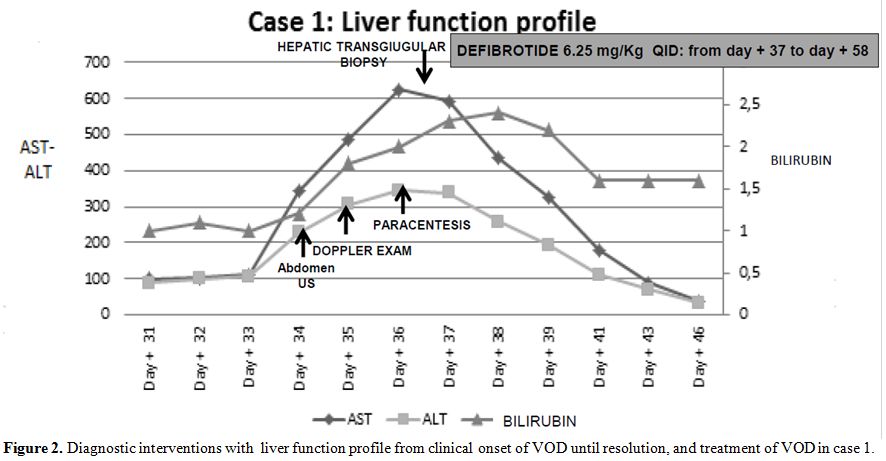 |
Figure 2. Diagnostic interventions with
liver function profile from clinical onset of VOD until resolution, and
treatment of VOD in case 1. |
Case 2
A
46-year old male was diagnosed with acute myeloid leukemia - normal
karyotype, FLT3, and NPM1 wild-type - in May 2015. The patient was
treated with idarubicin plus etoposide and cytarabine with no
hematologic response. Complete remission was subsequently obtained with
a course of high-dose cytarabine, followed consolidation with 2
additional courses of high-dose cytarabine. An unrelated marrow donor
search was started and a partially (one-antigen mismatched) HLA-matched
donor was identified. The patient received mobilized donor HSCT after a
conditioning regimen with Thiotepa (5 mg/kg/day for 2 days), Busulphan
(3,2 mg/kg/day for 3 days) and Fludarabine (50 mg/kg/day for 3 days).
GvHD prophylaxis included cyclosporine, short course methotrexate, and
anti-thymocyte globulin ATG (2,5 mg/kg/day for 3 days). Neutrophil and
platelet engraftment occurred on day +14 and +12 respectively. The
patient experienced a transient skin rash suggestive of grade I acute
GVHD on day +19, and 3 episodes of CMV reactivation on days +27, +43
and +82 successfully treated with preemptive valganciclovir and
immunoglobulins.
The patient was readmitted because of severe
anemia and thrombocytopenia (Hb 5,8 gr/dl; platelet 5000/ul) and
complaints of right abdominal pain with melena on day +89.
Significant weight gain (+7 kg) along with abdominal distension and
anasarca were observed on day +91. Laboratory exams showed total
bilirubin 3,30 mg/dl, AST/ALT 140/164 UI, GGT 725 Ul, INR 1,7; aPTT
41,3”, serum creatinine 2,0 mg/dl; platelet count was 20.000/mmc. An
abdominal CT scan revealed ascites and hepatic veins compression.
Transjugular measurement of the hepatic venous pressure gradient showed
severe sinusoidal portal hypertension with a significant
transhepatic/caval gradient diagnostic for severe VOD. Transjugular
liver biopsy showed sinusoidal dilation and bleeding with erythrocytes
in the Disse space, and significant iron overload. Histology studies
were consistent with severe VOD.
Intravenous defibrotide was promptly started at the dose of 6.25 mg/kg QID for 22 days.
Ancillary
therapy included plasma and red blood cells transfusions, no steroid
have been administered. A complete and sustained response was achieved.
The patient was discharged on day +121. The patient is currently alive,
188 days after transplant, with normal liver function, no evidence
of GvHD, or any other relevant clinical complication. A bone
marrow aspirate showed complete remission of his underlying disease.
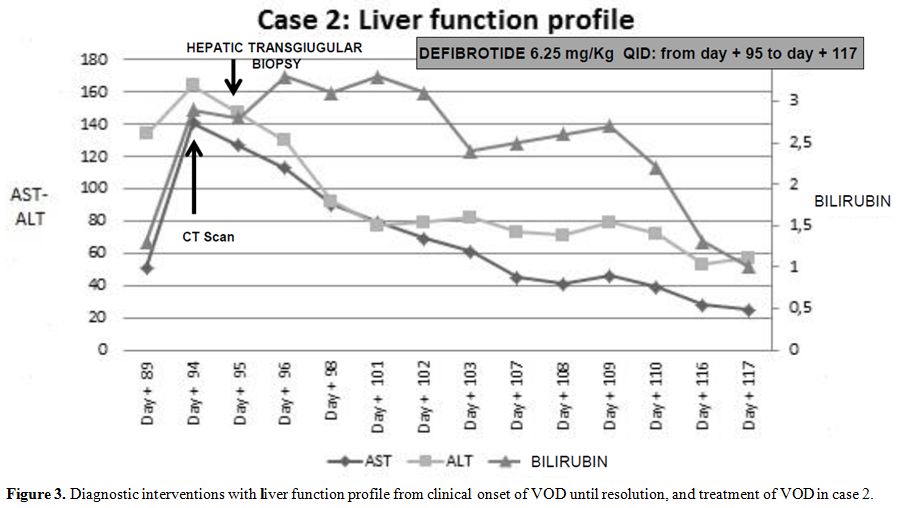 |
Figure 3. Diagnostic interventions with
liver function profile from clinical onset of VOD until resolution, and
treatment of VOD in case 2. |
Discussion
Recognition
of potential risk factors for VOD is a key point for early diagnosis
and prompt therapeutic intervention. Recently, the EBMT group has
categorized these risk factors as transplant-, hepatic-, patient- and
disease-related.[7-9] Interestingly, our patients
presented with only a few risk factors, namely, advanced acute
leukemia, a myeloablative busulphan-containing conditioning and an
unrelated donor. Moreover, both patients did not show the typical
clinical VOD features described by the Seattle[2-3] and Baltimore[4]
criteria. In particular, the first patient did not experience either
painful hepatomegaly or weight gain, and only a mild elevation in total
serum bilirubin level was observed after the development of ascites,
while the second patient showed only mild hyper-bilirubinemia
concurrent with painful hepatomegaly and significant weight gain. Most
importantly, both patients developed clinical signs beyond day 21
post-HSCT (on days +33 and +89 respectively). In this respect, it
should be emphasized that the EBMT consensus6 has now recognized
the existence of a “late onset” VOD, defined with less stringent
diagnostic criteria and where hyper-bilirubinemia should no longer be
mandatory for diagnosis. Overall, in these two patients, the short time
between the onset of clinical symptoms and the final diagnosis, and the
higher than 5 fold increase in transaminases combine to diagnose a
severe form of VOD by the new EBMT criteria (Table 1)
Both the British guidelines[10]
and the EBMT recommendations indicate that liver biopsy should be
reserved for those patients in whom the diagnosis of VOD is unclear,
and there is an urgent need to rule out other possible causes of liver
dysfunction. In our experience, a transjugular liver biopsy was safe
despite severe thrombocytopenia and, most importantly, was conclusive
for the diagnosis of VOD ruling out drug toxicities, viral infections,
sepsis or GVHD.[11-13] In keeping with our findings, Kis et al. reported only 1.8% of major complications during 166 transjugular liver biopsies.[14]
Defibrotide
is the only agent approved for the treatment of VOD in Europe.
Defibrotide has been shown to have antithrombotic and anti-inflammatory
properties and may promote revascularization inducing endothelial cell
proliferation and angiogenesis.[15] In our patients,
the combination of clinical features and histology studies prompted us
to start defibrotide only a few days after the onset of symptoms. Our
experience strengthens the observation reported by Richardson et al.,[16]
that the timely administration of defibrotide may represent a crucial
issue for the successful outcome of patients with VOD. Sixty % of
patients were alive when defibrotide was started within 2 days from the
onset of symptoms as compared with 14% when treatment was delayed and
started after 7 days. Similar results were reported by Corbacioglu et
al.[17] Our patients began defibrotide treatment
within 7 days from the onset of symptoms but within 1 day from the
histological diagnosis. They did not fall exactly in the early
treatment category. Nevertheless, defibrotide has been initiated within
7 days, representing the crucial threshold to achieve a good outcome.
Phase 2 and 3 studies[18-22] demonstrated that
defibrotide was generally well tolerated with manageable toxicity.
Hemorrhagic complications were reported as the most frequent adverse
event. Hemorrhagic cystitis, which occurred in one of our patients, is
a common complication in HSCT recipients. Though other causes may have
been involved, we could not rule out that it may have been related to
defibrotide treatment given its prompt resolution upon drug
discontinuation.[22]
Conclusions
VOD
should be considered in the differential diagnosis of HSCT recipients
who present with unexplained liver injuries, ascites and/or MOF. Liver
biopsy may represent a useful diagnostic tool when the clinical
criteria for VOD are not entirely fulfilled. Early therapeutic
intervention with defibrotide may improve the clinical outcomes of
these patients.
References
- Carreras E, et al. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol 2000;64: 281-291. https://doi.org/10.1034/j.1600-0609.2000.9r200.x PMid:10863974

- McDonald,
G.B., Hinds, M.S., Fisher, L.D., et al. Veno-occlusive disease of the
liver and multiorgan failure after bone marrow transplantation: a
cohort study of 355 patients. Annals of Internal Medicine 1993; 118,
255-267. https://doi.org/10.7326/0003-4819-118-4-199302150-00003 PMid:8420443

- Jones,
R.J., Lee, K.S., Beschorner, W.E., et al. Venoocclusive disease of the
liver following bone marrow transplantation. Transplantation, 1987;44,
778-783. https://doi.org/10.1097/00007890-198712000-00011 PMid:3321587

- Lee,
J.L., Gooley, T., Bensinger, W., et al. Veno-occlusive disease of the
liver after busulfan, melphalan, and thiotepa conditioning therapy:
incidence, risk factors, and outcome. Biology of Blood and Marrow
Transplantation, 1999;5, 306-315. https://doi.org/10.1016/S1083-8791(99)70006-6

- Coppell
JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A et al.
Hepatic veno-occlusive disease following stem cell transplantation:
incidence, clinical course, and outcome. Biol Blood Marrow Transplant
2010;16: 157-168. https://doi.org/10.1016/j.bbmt.2009.08.024 PMid:19766729 PMCid:PMC3018714

- Mohty
M, Malard F, Abecassis M, et al. Revised diagnosis and severity
criteria for sinusoidal obstruction syndrome/veno-occlusive disease in
adult patients: a new classification from the European Society for
Blood and Marrow Transplantation. BMT 2016, 1-7.

- Bearman
SI, et al. The syndrome of hepatic veno-occlusive disease after marrow
transplantation. Blood 1995; 85:3005-20. PMid:7756636

- Carreras
E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic
veno-occlusive disease after blood or marrow transplantation: A
prospective cohort study of the European Group for Blood and Marrow
Transplantation: European Group for Blood and Marrow Transplantation
Chronic Leukemia Working Party. Blood 1998;92:3599-604
PMid:9808553

- Cesaro,
S., Pillon, M., Talenti, E., et al. A prospective survey on incidence,
risk factors and therapy of hepatic veno-occlusive disease in children
after hematopoietic stem cell transplantation. Haematologica, 2005;90,
1396-1404. PMid:16219577

- Dignan
FL, Wynn RF, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and
management of veno-occlusive disease (sinusoidal obstruction syndrome)
following haematopoietic stem cell transplantation. BJH 2013, 163,
444-457. https://doi.org/10.1111/bjh.12558 PMid:24102514

- DeLeve,
L.D., Shulman, H.M. & McDonald, G.B. (2002) Toxic injury to hepatic
sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease).
Seminars in Liver Disease, 22, 27. https://doi.org/10.1055/s-2002-23204 PMid:11928077

- Shulman,
H.M., Gown, A.M. & Nugent, D.J. (1987) Hepatic veno-occlusive
disease after bone marrow transplantation. Immunohistochemical
identi?cation of the material within occluded central venules. The
American Journal of Pathology, 127, 549-558 PMid:2438942
PMCid:PMC1899766

- Shulman,
H.M., Fisher, L.B., Schoch, H.G., Henne, K.W. & McDonald, G.B.
(1994) Veno-occlusive disease of the liver after marrow
transplantation: histological correlates of clinical signs and
symptoms. Hepatology, 19, 1171-1181. https://doi.org/10.1002/hep.1840190515 PMid:8175139

- Kis
b, Pamarthi V, Fan C-M et al. Safety and Utility of Transjugular Liver
Biopsy in Hematopoietic Stem Cell Transplant Recipients. J Vasc Interv
Radiol 2013; 24:85-89 https://doi.org/10.1016/j.jvir.2012.09.011 PMid:23200125

- Richardson,
P.G., Elias, A.D., Krishnan, A., et al. Treatment of severe
veno-occlusive disease with de?brotide: compassionate use results in
response without signi?cant toxicity in a high-risk population. Blood,
1998;92, 737-744. PMid:9680339

- Richardson
PG, Smith AR, Triplett BM, et al. Early Initiation of Defibrotide in
Patients with Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction
Syndrome Following Hematopoietic Stem Cell Transplantation Improves Day
+100 Survival. ASH 2015. Poster Abs.
- Corbacioglu
S, Greil J, Peters C., et al. Defibrotide in the treatment of children
with veno-occlusive disease (VOD): a retrospective multicentre study
demonstrates therapeutic efficacy upon early intervention. BMT 2004;
Jan;33(2):189-95. https://doi.org/10.1038/sj.bmt.1704329

- Bearman,
S.I., Anderson, G.L., Mori, M., Hinds, M.S., Shulman, H.M. &
McDonald, G.B. (1993) Venoocclusive disease of the liver: development
of a model for predicting fatal outcome after marrow transplantation.
Journal of Clinical Oncology, 11, 1729-1736. https://doi.org/10.1200/JCO.1993.11.9.1729 PMid:8355040

- Chopra
R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C, et al. Defibrotide
for the treatment of hepatic veno-occlusive disease: Results of the
European compassionate-use study. Br J Haematol 2000;111:1122-9. https://doi.org/10.1046/j.1365-2141.2000.02475.x PMid:11167751

- Richardson
PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al.
Multi-institutional use of defibrotide in 88 patients after stem cell
transplantation with severe veno-occlusive disease and multisystem
organ failure: response without significant toxicity in a high-risk
population and factors predictive of outcome. Blood 2002;100:4337-43. https://doi.org/10.1182/blood-2002-04-1216 PMid:12393437

- Richardson
PG, Soiffer R, Antin J, Jin Z, Kurtzberg J, Martin P, et al.
Defibrotide (DF) for the treatment of severe veno-occlusive disease
(sVOD) and multi-organ failure (MOF) post SCT: Final results of a
multi-center, randomized, dose-finding trial. Blood 2006;108:178.

- Richardson
PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the
treatment of severe veno-occlusive disease and multi-organ failure.
Blood. 2016;127(13):1656-65. https://doi.org/10.1182/blood-2015-10-676924 PMid:26825712 PMCid:PMC4817309

[TOP]


























