Iron Chelation Therapy in Thalassaemia Syndromes
Paolo
Cianciulli
Department of Tropical Medicine, Gastroenterology and Liver Disease, Ain Shams University, Cairo, Egypt
Correspondence
to: Paolo Cianciulli, MD, Day Hospital
Talassemia, Ospedale S.Eugenio, P.le dell’Umanesimo 10, 00143 Roma. Tel +39 06 5100 2560, Fax + 39
06 5100 2506. E-mail: p.cianciulli@libero.it
Published: December 29, 2009
Received: December 27, 2009
Accepted:December 29, 2009
Medit J Hemat Infect Dis 2009, 1(1): e2009034; DOI 10.4084/MJHID.2009.034
This article is available from: http://www.mjhid.org/article/view/5291
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Abstract
Transfusional
hemosiderosis is a frequent complication in patients with transfusion
dependent chronic diseases such as thalassemias and severe type
of sickle cell diseases. As there are no physiological mechanisms to
excrete the iron contained in transfused red cells (1 unit of blood
contains approximately 200 mg of iron) the excess of iron is stored in
various organs. Cardiomyopathy is the most severe complication covering
more than 70% of the causes of death of thalassemic patients. Although
the current reference standard iron chelator deferoxamine (DFO) has
been used clinically for over four decades, its effectiveness is
limited by a demanding therapeutic regimen that leads to poor
compliance. Despite poor compliance, because of the inconvenience of
subcutaneous infusion, DFO improved considerably the survival and
quality of life of patients with thalassemia. Deferiprone since 1998
and Deferasirox since 2005 were licensed for clinical use. The oral
chelators have a better compliance because of oral use, a comparable
efficacy to DFO in iron excretion and probably a better penetration to
myocardial cells. Considerable increase in iron excretion was
documented with combination therapy of DFO and Deferiprone. The proper
use of the three chelators will improve the prevention and treatment of
iron overload, it will reduce complications, and improve survival
and quality of life of transfused patients.
Introduction
Iron overload occurs when the intake of iron is increased over a prolonged period of time and is commonly seen in patients with hereditary or refractory anemias (e.g. b-thalassaemia major, sickle cell anemia and myelodysplastic syndromes) who receive frequent blood transfusions. The iron excess is initially stored in the reticuloendotelial system, which has a capacity of about 10-15 g, and then in all parechymas [1], resulting in life-threatening complications, namely cardiopathy, liver and endocrine dysfunction and reduced patient’s survival [2,3,4]. Iron excess also increases cell concentration of iron-binding proteins such as ferritin and haemosiderin complexes in lysosomes [5]. Non-transferrin-bound-iron (NTBI), iron in low molecular weight forms, may initiate free radicals reactions6. Patients with b-thalassaemia require regular blood transfusion in order to have a normal life. Correct management inhibits bone marrow hyperactivity and delays the appearance of hypersplenism. Their mean annual intake is 165 or 140 mg of pure red cells/Kg (for non splenectomized and splenectomized patients, respectively), which corresponds to 0,49-0,44 mg/Kg/day [7]. Prior to the introduction of chelating therapy, most patients did not reach the second decade of life, mainly owing to heart disease [8].
Desferrioxamine:
The hexadentate chelator desferrioxamine B (DFO) was identified as the first effective biologically active Fe chelator. Released in the 1960s as the first clinically approved chelator for the treatment of iron overload, DFO has significantly improved the life expectancy and the quality of life of patients with iron overload [9] who previously would not have survived after their teen years. Moreover, it had been the gold standard in iron chelation therapy. The ability of a chelating agent to penetrate cells depends on its molecular size and affinity for lipids. For its large molecular size (molecular weight 656 Daltons) and low affinity for lipid, DFO is poorly absorbed from the gastrointestinal tract [10]. It is not orally active, undergoes rapid renal elimination. Thus, it must be administrated only parenterally. Its plasma half-life is short ( ~20 minutes). It binds iron very strongly with a ratio of 1:1. The usual dose of DFO is 20 to 60 mg/Kg/die. It is subcutaneously administered via a battery-operated portable pump over a period of 8-12 hours overnight, for 5 to 7 nights per week. This has resulted in a large proportion of patients (~ 33%) failing to comply with this regimen [11].
Deferiprone:
Deferiprone is a synthetic, bidentate iron chelator. Three molecules of deferiprone are required to bind one atom of iron. Deferiprone also binds other metals including zinc; zinc deficiency has been reported in a small number of patients with iron overload receiving long-term deferiprone [12]. It is orally administered at 75 mg/Kg/day in three divided doses. Compliance rates with deferiprone are generally higher than those associated with subcutaneous infusions of desferrioxamine [13]. The molecule undergoes extensive liver metabolism and > 85% of the administered dose is recovered in the urine as a non-chelating O-glicuronide conjugate. It is excreted predominantly via the renal system as the parent compound, its conjugate and as an iron-bound complex. The urinary iron excretion obtained with a dose of 75 mg/Kg is comparable with that obtained with 40-50 mg/Kg DFO infusion, while iron excretion in the stool is negligible. Deferiprone was introduced in Europe in 2000 as second-line therapy for b-thalassemia patients with DFO–related adverse events or contraindications to DFO. In general, the above indicated oral daily dose is required for the treatment of iron overload condition, although some investigators have used up to 100 mg/Kg/day. The most frequent deferiprone-related adverse reactions are gastrointestinal adverse reactions (nausea, abdominal pain, vomiting) and arthralgia, while the most serious adverse event is agranulocytosis [12]. In a multi-centre study designed to evaluate the incidence of agranulocytosis (neutrophil count of < 0.5 x 109/L ) in patients treated with deferiprone, 1 patient out of 187 (0,5%) was affected by this condition and 9 patients (4,5%) had moderate neutropenia (two consecutive neutrophil counts of 1.5 x 10 9 /L ) within the first year of treatment [14]. Over the following three years, there were no cases of agranulocytosis observed and seven new cases of moderate neutropenia were reported [15]. In an Italian study conducted in 532 patients with b-thalassemia treated for a total of 1154 patients-years, the rates of agranulocytosis and neutropenia were 0.43 and 2.08 respectively, per 100 patients-years [16]. An idiosyncrasic pathogenesis can be hypothesized [17]. Generally it is more frequent during the first year of deferiprone treatment. Agranulocitosys is sometimes reversible when the drug is stopped but sometimes can be necessary to quickly begin therapy with GCS-F. For elevated incidence of relapse, it is not suitable to submit the patient to the same therapy. An interesting advantage of deferiprone over DFO seem to be its ability to reduce cardiac iron levels in patients with b-thalassemia, wich is probably due to the small size of the molecule (molecular weight 139 versus 656 Dalton for DFO) and to its lipophilic properties[14,15,18]. Anderson et al. demonstrated that patients with b-thalassemia treated with deferiprone (n=15) presented a significantly (p=0.02) elevated myocardial T2* value, an RMI variable with inverse correlation to tissue iron levels, compared with that of mached control patients ( n=30) treated with DFO, and that more than half (67%) of the patients treated with DFO were not protected against cardiac siderosis. In contrast, almost three quartes (73%) of deferiprone recipients were protected against cardiac siderosis [19]. Furthermore in such patients who were randomized to open-label deferiprone at mean dose of 92 mg/Kg (n=29) or who continued to receive subcutaneous DFO at standard dose (n=32) , improvements from baseline in myocardial T2* values at 12 months (primary endpoint) were significant for both groups (p< 0.001 for both groups), but the between-group difference was significantly in favor to deferiprone (27% versus 13% improvement; p< 0.0023). In the same study, in patients treated with deferiprone, left ventricular ejection fraction improved by 3.1% ( absolute units), compared with 0.32% improvement in DFO-treated patients (p=0.003 versus DFO)20. Recently, some authors in a multicenter, prospective, long-term (7 years and 4 months) randomized trial on 265 enrolled thalassemic patients, have shown an improving of survival in patients with deferiprone treatment (alone or in sequential or in association with DFO) versus DFO21. Recently El-Beshlawy et al. in a multi-centre 24 week study period on 100 young thalassemic patients £ 10 years old showed that the a new liquid formulation had a lower incidence of gastrointestinal adverse reactions that previously reported with the tablet formulation (13% vs 42%), no important changes about neutropenia or agranulocytosis were observed [22]. Hoffbrand’s metanalysis18 demonstrated that treatment with deferiprone leads to a negative iron balance in some but not in all patients. Factors that may contribute to inter-individual response variation are the degree of iron overload, therapy duration, dosage, and compliance. One explanation for individual variation in response to the drug is that deferiprone is inactivated by glucuronidation, which may be very rapid in some individual [12].
Combined therapy:
Deferiprone can be associated to DFO when the chelation response is unsatisfactory or under conditions of serious cardiac siderosis with or without heart failure, in conditions with high risk of morbility or mortality. The efficacy of the combination of a low molecular weight chelating agent that is able to penetrate cells efficiently, with a high molecular weight chelating agent that is able to form a stable association with iron and thus achieve a satisfactory urinary iron excretion, has been shown in several clinical studies [23-25].Combination therapy leads a reduction of plasma ferritin levels in patients in which monotherapy with deferiprone failed to produce a satisfactory outcome [26] and shows an additive effect on the urinary excretion of iron [27]. Tanner et al demonstrated that, compared with DFO monotherapy, combination therapy significantly improved myocardial T2* values (primary endpoint), plasma ferritin levels, LVEF, endothelial function [23]. This approach has also proven effective in the acute phase treatment of heart failure caused by iron overload [29,30]. Recently, the beneficial effect of combination therapy on the pancreatic endocrine damage secondary to hemosiderosis has been described [30]. The mortality due to cardiac damage is strongly reduced by means of T2* cardiovascular magnetic resonance and combined therapy with deferiprone and desferrioxamine [31].
Deferasirox:
Deferasirox (ICL670) is a new iron chelator orally bioavailable. Well as Thalassemia Major and Intermedia, it has been used in many others chronic anemias transfusions dependent such as Sickle Cell Disease (SCD), Diamond Blackfan anemia, Myelodysplastic Syndromes (MDS) and other rare anemias. In 2005, Food and Drug Administation (FDA)[32], and afterward the European Medicine Agency (EMEA)[33] approved its use in Thalassemia Major patients (aged ≥ 6 years), transfused with ≥ 7ml/kg/month of packed red blood cells. Deferasirox therapy is allowed also in the following groups of multitransfused iron overloaded patients when treatment with DFO is contraindicated or inadeguate: Thalassemia Major patients transfused with <7ml/kg/month of packed red blood cells, Thalassemia Major patients aged 2-5 years, patients affected by other anemias. Actually, Deferasirox is available in 90 nations [34].
Deferasirox, a N-substituted bis-hydroxyphenyl-triazoles compound, belongs to a new class of tridentate iron chelators. It was selected among more than 700 molecules because it is orally administrable and it gave the best therapeutic results [34,35]. Two molecules of Deferasirox are able to generate a stable complex with one molecule of iron. Its half life (t1/2) is between 8 and 16 hours, allowing a once-daily administration. After the oral administration (dissolved in water or orange or apple juice) it is rapidly absorbed and its plasma level is in the therapeutic range for 18 to 24 hours [36]. Thus, after a dose, the chelating effect lasts all day. The lowest plasma level of the drug, during the day, corresponds to 25% of the peak of drug plasma concentration.
Deferasirox protects cells from the toxicity of Non Transferrin-Bound serum Iron (NTBI) and of Labile Plasma Iron (LPI). The last generates Reactive Oxigen Species (ROS) able to damage cells [37]. High LPI values, if they frequently occur, can affect the most important organ function and the patient survival [38]. Also the other chelators (DFO and Deferiprone) have this protective action but, for their shorter half-life, this is not continuous. On the contrary, Deferasirox assures a more constant chelation during the day. Daar et al demonstrated that Deferasirox, after two hours of the oral intake in the morning, reduces LPI levels. During the treatment, if the drug intake is regular, the LPI, measured before the daily dose, tends to decrease and subsequently LPI values reach the normal range 30,40.
If Deferasirox is taken with food, its bioavaibility changes overall according to the fat content of the meal. For this reason the drug must be taken at least 30 minute before eating, preferably at the same time every day [41]. The patient age is another parameter influencing the bioavailability which is lower for the adolescents (12-17 years old) and children (2-12 years old).
Metabolism and elimination of deferasirox and the iron chelate (Fe-[deferasirox]2) is primarily by glucuronidation followed by hepatobiliary excretion into the feces (83%). A enterohepatic circulation, after a decojugation of glucuronidated drug in small bowel, is supposed.
Many clinical trials, performed in patient groups with transfusion-dependent anemias, demonstrated Deferasirox efficacy [36,43,46]. For this purpose serum ferritin and Liver Iron Concentration (LIC) were monitored. These study showed that a relation between the drug dose administered and the reduction of body iron index is present and this is a function of iron intake though transfusions [47,48]. In fact, treating, multitransfused β-thalassemia patients with 20 and 30mg/kg/die, a mean LIC reduction ranging from -0.4 and -8.9 mg Fe/g of dry weight and a mean ferritin reduction ranging from -36 and -926 μg/l were observed. These result obtained after an year were confirmed after a follow-up of 5 years- Moreover, during this time, the number of patients who reached a LIC<7 mg Fe/g dw and serum ferritin < 1000 mg/l progressively raised (35% versus 45% for LIC and 12% versus 33% for ferritin)[49].
Analysis of registration study results, indicated that the Deferasirox dose must be set in function of transfusion regimen and in function of the therapeutic target: reduction of iron overload or maintenance of iron balance. The daily dose of 20 mg /kg/ was able to obtain a neutre/negative iron balance in 47% of patients with high blood supply, in 55% of patients with intermediate blood supply and in 75% of patients with low blood supply. The daily dose of 30 mg/kg was sufficient to reduce iron overload in 82% of patients with high blood supply, in 83% of patients with intermediate blood supply and in 96% of patients with low blood supply. Thereafter 30 mg/kg/die is the starting dose to reduced iron overload in regularly highly multitransfused patients [50] .
Recently EPIC study results were published [51]. This study was performed examining 1744 multitransfused overloaded patients: 1115 β thalassemic (63.9%); 341 with MDS (19.6%); 116 with Aplastic Anemia (6.7%); 80 SCD (4.6%); 43 with rare anemias (2.5%) and other anemias including cancer anemias (2.9%). For a mean Deferasirox dose of 22,2 mg/kg/die and an iron intake of 0.41 mg Fe/kg/die, the mean serum ferritin reduction was 264 ng/ml. In a cohort of patients treated with 30 mg/kg/die or more the highest reduction of ferritin was registered (-882 ng/ml with an iron intake of 0.37 mg Fe/kg/die).
The ESCALATOR study [52] included 237 patients with high overload and not responders to other chelators. They were treated with Deferasirox dose > 30mg/kg/die. The mean basal LIC was 20.1 mg Fe/g dw; at the end of the study ( after 2,7 years) the mean LIC was significantly lower: 11.8 mg Fe/g dw (P<0.0001)[53].The cardiac T2* was evaluated in 19 patients of the ESCALATOR study: this parameter showed a significant improvement, independently to the mean basal values [54].
Moreover, in multitransfused patients Deferasirox is efficacious in reducing cardiac overload and in preventing it [55-57]. In fact, in 100 β thalassemic, in a period of 2 years, an improvement of T2* of 40,8% was observed (mean T2* 11.2 msec versus 12,9 msec p<0.0001). In 78 β thalassemic with normal basal T2* after a year no change was observed. Finally in those patients showing a basal T2* ranging between 10 and 20 msec, a mean dose of 34.5±4.8 mg/kg/die for two years normalized the T2*: Δ% = +48.1%; basal T2* 14.6 msec versus final T2* 20,4 msec; p<0.001.
Moreover Deferasirox is a safe and well tolerated drug in adults and children both in short and long treatment periods. In registration studies after 5 years, no new side effects were reported and no change in the incidence of those already reported. The frequency of the most common side effects has been progressively decreasing [36, 43-46,51-57].
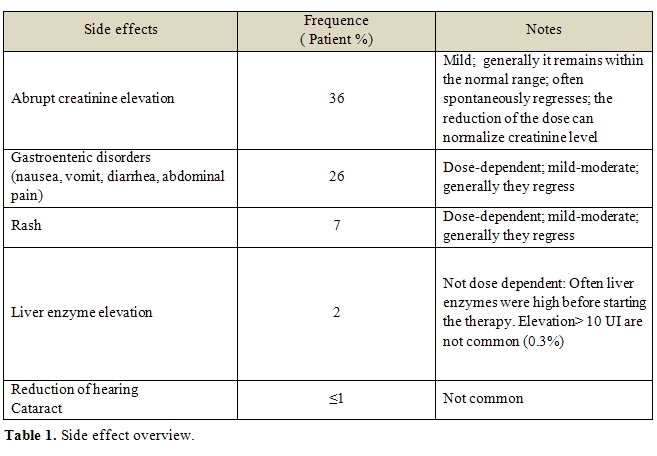
Table 1 summarized the most important reported side effects. At present, this drug is largely employed. Among the side effects not reported in registration studies, cytopenias occurred, although mainly in patients with predisposing clinical conditions. The exact role of Deferasirox in inducing cytopenias is not defined. Nevertheless, regular controls of complete and differential blood counts are recommended. Renal and liver failures and bleeding from the gastroenteric tract were reported. Similarly to cytopenias, in the clinical history of these patients, predisposing conditions able to induce these complications are often recognizable. Before starting Deferasirox therapy, the careful patient physical examination, the accurate medical history registration and the detection of the most common laboratory tests are crucial for a safe use of the drug [58].

The results of many clinical trials and studies are consistent with the evidence that Deferasirox is efficacious in removing and in preventing the liver and cardiac iron overload, in chelating free iron for 24 hours and it is safe and well tolerated also if high doses (up to 40 mg/kg/die) are used.
Table 2 show the most important feature of iron chelators commercially available
Conclusions
The recent expansion of erythrocyte transfusional therapy to pediatric patients with sickle cell disease and an elevated risk of stroke [59] and in some type of thalassemia intermedia associated with bone deformations and growth defects, has increased the population of pediatric patients requiring chelation therapy. The knowledge of the properties of the available iron chelating agents and of individual patient requirements, coupled with effective methods to accurately monitor iron levels, has enabled iron chelation therapy to be highly personalized. The recently available oral chelators offer a convenient administration regimen, overcoming the burden of a subcutaneous infusions of DFO and potentially improving compliance. Treating patients with tailored therapy will eventually lead to improvement in morbidity and mortality induced by iron toxicity.
Iron overload occurs when the intake of iron is increased over a prolonged period of time and is commonly seen in patients with hereditary or refractory anemias (e.g. b-thalassaemia major, sickle cell anemia and myelodysplastic syndromes) who receive frequent blood transfusions. The iron excess is initially stored in the reticuloendotelial system, which has a capacity of about 10-15 g, and then in all parechymas [1], resulting in life-threatening complications, namely cardiopathy, liver and endocrine dysfunction and reduced patient’s survival [2,3,4]. Iron excess also increases cell concentration of iron-binding proteins such as ferritin and haemosiderin complexes in lysosomes [5]. Non-transferrin-bound-iron (NTBI), iron in low molecular weight forms, may initiate free radicals reactions6. Patients with b-thalassaemia require regular blood transfusion in order to have a normal life. Correct management inhibits bone marrow hyperactivity and delays the appearance of hypersplenism. Their mean annual intake is 165 or 140 mg of pure red cells/Kg (for non splenectomized and splenectomized patients, respectively), which corresponds to 0,49-0,44 mg/Kg/day [7]. Prior to the introduction of chelating therapy, most patients did not reach the second decade of life, mainly owing to heart disease [8].
Desferrioxamine:
The hexadentate chelator desferrioxamine B (DFO) was identified as the first effective biologically active Fe chelator. Released in the 1960s as the first clinically approved chelator for the treatment of iron overload, DFO has significantly improved the life expectancy and the quality of life of patients with iron overload [9] who previously would not have survived after their teen years. Moreover, it had been the gold standard in iron chelation therapy. The ability of a chelating agent to penetrate cells depends on its molecular size and affinity for lipids. For its large molecular size (molecular weight 656 Daltons) and low affinity for lipid, DFO is poorly absorbed from the gastrointestinal tract [10]. It is not orally active, undergoes rapid renal elimination. Thus, it must be administrated only parenterally. Its plasma half-life is short ( ~20 minutes). It binds iron very strongly with a ratio of 1:1. The usual dose of DFO is 20 to 60 mg/Kg/die. It is subcutaneously administered via a battery-operated portable pump over a period of 8-12 hours overnight, for 5 to 7 nights per week. This has resulted in a large proportion of patients (~ 33%) failing to comply with this regimen [11].
Deferiprone:
Deferiprone is a synthetic, bidentate iron chelator. Three molecules of deferiprone are required to bind one atom of iron. Deferiprone also binds other metals including zinc; zinc deficiency has been reported in a small number of patients with iron overload receiving long-term deferiprone [12]. It is orally administered at 75 mg/Kg/day in three divided doses. Compliance rates with deferiprone are generally higher than those associated with subcutaneous infusions of desferrioxamine [13]. The molecule undergoes extensive liver metabolism and > 85% of the administered dose is recovered in the urine as a non-chelating O-glicuronide conjugate. It is excreted predominantly via the renal system as the parent compound, its conjugate and as an iron-bound complex. The urinary iron excretion obtained with a dose of 75 mg/Kg is comparable with that obtained with 40-50 mg/Kg DFO infusion, while iron excretion in the stool is negligible. Deferiprone was introduced in Europe in 2000 as second-line therapy for b-thalassemia patients with DFO–related adverse events or contraindications to DFO. In general, the above indicated oral daily dose is required for the treatment of iron overload condition, although some investigators have used up to 100 mg/Kg/day. The most frequent deferiprone-related adverse reactions are gastrointestinal adverse reactions (nausea, abdominal pain, vomiting) and arthralgia, while the most serious adverse event is agranulocytosis [12]. In a multi-centre study designed to evaluate the incidence of agranulocytosis (neutrophil count of < 0.5 x 109/L ) in patients treated with deferiprone, 1 patient out of 187 (0,5%) was affected by this condition and 9 patients (4,5%) had moderate neutropenia (two consecutive neutrophil counts of 1.5 x 10 9 /L ) within the first year of treatment [14]. Over the following three years, there were no cases of agranulocytosis observed and seven new cases of moderate neutropenia were reported [15]. In an Italian study conducted in 532 patients with b-thalassemia treated for a total of 1154 patients-years, the rates of agranulocytosis and neutropenia were 0.43 and 2.08 respectively, per 100 patients-years [16]. An idiosyncrasic pathogenesis can be hypothesized [17]. Generally it is more frequent during the first year of deferiprone treatment. Agranulocitosys is sometimes reversible when the drug is stopped but sometimes can be necessary to quickly begin therapy with GCS-F. For elevated incidence of relapse, it is not suitable to submit the patient to the same therapy. An interesting advantage of deferiprone over DFO seem to be its ability to reduce cardiac iron levels in patients with b-thalassemia, wich is probably due to the small size of the molecule (molecular weight 139 versus 656 Dalton for DFO) and to its lipophilic properties[14,15,18]. Anderson et al. demonstrated that patients with b-thalassemia treated with deferiprone (n=15) presented a significantly (p=0.02) elevated myocardial T2* value, an RMI variable with inverse correlation to tissue iron levels, compared with that of mached control patients ( n=30) treated with DFO, and that more than half (67%) of the patients treated with DFO were not protected against cardiac siderosis. In contrast, almost three quartes (73%) of deferiprone recipients were protected against cardiac siderosis [19]. Furthermore in such patients who were randomized to open-label deferiprone at mean dose of 92 mg/Kg (n=29) or who continued to receive subcutaneous DFO at standard dose (n=32) , improvements from baseline in myocardial T2* values at 12 months (primary endpoint) were significant for both groups (p< 0.001 for both groups), but the between-group difference was significantly in favor to deferiprone (27% versus 13% improvement; p< 0.0023). In the same study, in patients treated with deferiprone, left ventricular ejection fraction improved by 3.1% ( absolute units), compared with 0.32% improvement in DFO-treated patients (p=0.003 versus DFO)20. Recently, some authors in a multicenter, prospective, long-term (7 years and 4 months) randomized trial on 265 enrolled thalassemic patients, have shown an improving of survival in patients with deferiprone treatment (alone or in sequential or in association with DFO) versus DFO21. Recently El-Beshlawy et al. in a multi-centre 24 week study period on 100 young thalassemic patients £ 10 years old showed that the a new liquid formulation had a lower incidence of gastrointestinal adverse reactions that previously reported with the tablet formulation (13% vs 42%), no important changes about neutropenia or agranulocytosis were observed [22]. Hoffbrand’s metanalysis18 demonstrated that treatment with deferiprone leads to a negative iron balance in some but not in all patients. Factors that may contribute to inter-individual response variation are the degree of iron overload, therapy duration, dosage, and compliance. One explanation for individual variation in response to the drug is that deferiprone is inactivated by glucuronidation, which may be very rapid in some individual [12].
Combined therapy:
Deferiprone can be associated to DFO when the chelation response is unsatisfactory or under conditions of serious cardiac siderosis with or without heart failure, in conditions with high risk of morbility or mortality. The efficacy of the combination of a low molecular weight chelating agent that is able to penetrate cells efficiently, with a high molecular weight chelating agent that is able to form a stable association with iron and thus achieve a satisfactory urinary iron excretion, has been shown in several clinical studies [23-25].Combination therapy leads a reduction of plasma ferritin levels in patients in which monotherapy with deferiprone failed to produce a satisfactory outcome [26] and shows an additive effect on the urinary excretion of iron [27]. Tanner et al demonstrated that, compared with DFO monotherapy, combination therapy significantly improved myocardial T2* values (primary endpoint), plasma ferritin levels, LVEF, endothelial function [23]. This approach has also proven effective in the acute phase treatment of heart failure caused by iron overload [29,30]. Recently, the beneficial effect of combination therapy on the pancreatic endocrine damage secondary to hemosiderosis has been described [30]. The mortality due to cardiac damage is strongly reduced by means of T2* cardiovascular magnetic resonance and combined therapy with deferiprone and desferrioxamine [31].
Deferasirox:
Deferasirox (ICL670) is a new iron chelator orally bioavailable. Well as Thalassemia Major and Intermedia, it has been used in many others chronic anemias transfusions dependent such as Sickle Cell Disease (SCD), Diamond Blackfan anemia, Myelodysplastic Syndromes (MDS) and other rare anemias. In 2005, Food and Drug Administation (FDA)[32], and afterward the European Medicine Agency (EMEA)[33] approved its use in Thalassemia Major patients (aged ≥ 6 years), transfused with ≥ 7ml/kg/month of packed red blood cells. Deferasirox therapy is allowed also in the following groups of multitransfused iron overloaded patients when treatment with DFO is contraindicated or inadeguate: Thalassemia Major patients transfused with <7ml/kg/month of packed red blood cells, Thalassemia Major patients aged 2-5 years, patients affected by other anemias. Actually, Deferasirox is available in 90 nations [34].
Deferasirox, a N-substituted bis-hydroxyphenyl-triazoles compound, belongs to a new class of tridentate iron chelators. It was selected among more than 700 molecules because it is orally administrable and it gave the best therapeutic results [34,35]. Two molecules of Deferasirox are able to generate a stable complex with one molecule of iron. Its half life (t1/2) is between 8 and 16 hours, allowing a once-daily administration. After the oral administration (dissolved in water or orange or apple juice) it is rapidly absorbed and its plasma level is in the therapeutic range for 18 to 24 hours [36]. Thus, after a dose, the chelating effect lasts all day. The lowest plasma level of the drug, during the day, corresponds to 25% of the peak of drug plasma concentration.
Deferasirox protects cells from the toxicity of Non Transferrin-Bound serum Iron (NTBI) and of Labile Plasma Iron (LPI). The last generates Reactive Oxigen Species (ROS) able to damage cells [37]. High LPI values, if they frequently occur, can affect the most important organ function and the patient survival [38]. Also the other chelators (DFO and Deferiprone) have this protective action but, for their shorter half-life, this is not continuous. On the contrary, Deferasirox assures a more constant chelation during the day. Daar et al demonstrated that Deferasirox, after two hours of the oral intake in the morning, reduces LPI levels. During the treatment, if the drug intake is regular, the LPI, measured before the daily dose, tends to decrease and subsequently LPI values reach the normal range 30,40.
If Deferasirox is taken with food, its bioavaibility changes overall according to the fat content of the meal. For this reason the drug must be taken at least 30 minute before eating, preferably at the same time every day [41]. The patient age is another parameter influencing the bioavailability which is lower for the adolescents (12-17 years old) and children (2-12 years old).
Metabolism and elimination of deferasirox and the iron chelate (Fe-[deferasirox]2) is primarily by glucuronidation followed by hepatobiliary excretion into the feces (83%). A enterohepatic circulation, after a decojugation of glucuronidated drug in small bowel, is supposed.
Many clinical trials, performed in patient groups with transfusion-dependent anemias, demonstrated Deferasirox efficacy [36,43,46]. For this purpose serum ferritin and Liver Iron Concentration (LIC) were monitored. These study showed that a relation between the drug dose administered and the reduction of body iron index is present and this is a function of iron intake though transfusions [47,48]. In fact, treating, multitransfused β-thalassemia patients with 20 and 30mg/kg/die, a mean LIC reduction ranging from -0.4 and -8.9 mg Fe/g of dry weight and a mean ferritin reduction ranging from -36 and -926 μg/l were observed. These result obtained after an year were confirmed after a follow-up of 5 years- Moreover, during this time, the number of patients who reached a LIC<7 mg Fe/g dw and serum ferritin < 1000 mg/l progressively raised (35% versus 45% for LIC and 12% versus 33% for ferritin)[49].
Analysis of registration study results, indicated that the Deferasirox dose must be set in function of transfusion regimen and in function of the therapeutic target: reduction of iron overload or maintenance of iron balance. The daily dose of 20 mg /kg/ was able to obtain a neutre/negative iron balance in 47% of patients with high blood supply, in 55% of patients with intermediate blood supply and in 75% of patients with low blood supply. The daily dose of 30 mg/kg was sufficient to reduce iron overload in 82% of patients with high blood supply, in 83% of patients with intermediate blood supply and in 96% of patients with low blood supply. Thereafter 30 mg/kg/die is the starting dose to reduced iron overload in regularly highly multitransfused patients [50] .
Recently EPIC study results were published [51]. This study was performed examining 1744 multitransfused overloaded patients: 1115 β thalassemic (63.9%); 341 with MDS (19.6%); 116 with Aplastic Anemia (6.7%); 80 SCD (4.6%); 43 with rare anemias (2.5%) and other anemias including cancer anemias (2.9%). For a mean Deferasirox dose of 22,2 mg/kg/die and an iron intake of 0.41 mg Fe/kg/die, the mean serum ferritin reduction was 264 ng/ml. In a cohort of patients treated with 30 mg/kg/die or more the highest reduction of ferritin was registered (-882 ng/ml with an iron intake of 0.37 mg Fe/kg/die).
The ESCALATOR study [52] included 237 patients with high overload and not responders to other chelators. They were treated with Deferasirox dose > 30mg/kg/die. The mean basal LIC was 20.1 mg Fe/g dw; at the end of the study ( after 2,7 years) the mean LIC was significantly lower: 11.8 mg Fe/g dw (P<0.0001)[53].The cardiac T2* was evaluated in 19 patients of the ESCALATOR study: this parameter showed a significant improvement, independently to the mean basal values [54].
Moreover, in multitransfused patients Deferasirox is efficacious in reducing cardiac overload and in preventing it [55-57]. In fact, in 100 β thalassemic, in a period of 2 years, an improvement of T2* of 40,8% was observed (mean T2* 11.2 msec versus 12,9 msec p<0.0001). In 78 β thalassemic with normal basal T2* after a year no change was observed. Finally in those patients showing a basal T2* ranging between 10 and 20 msec, a mean dose of 34.5±4.8 mg/kg/die for two years normalized the T2*: Δ% = +48.1%; basal T2* 14.6 msec versus final T2* 20,4 msec; p<0.001.
Moreover Deferasirox is a safe and well tolerated drug in adults and children both in short and long treatment periods. In registration studies after 5 years, no new side effects were reported and no change in the incidence of those already reported. The frequency of the most common side effects has been progressively decreasing [36, 43-46,51-57].
Table 1 summarized the most important reported side effects. At present, this drug is largely employed. Among the side effects not reported in registration studies, cytopenias occurred, although mainly in patients with predisposing clinical conditions. The exact role of Deferasirox in inducing cytopenias is not defined. Nevertheless, regular controls of complete and differential blood counts are recommended. Renal and liver failures and bleeding from the gastroenteric tract were reported. Similarly to cytopenias, in the clinical history of these patients, predisposing conditions able to induce these complications are often recognizable. Before starting Deferasirox therapy, the careful patient physical examination, the accurate medical history registration and the detection of the most common laboratory tests are crucial for a safe use of the drug [58].


The results of many clinical trials and studies are consistent with the evidence that Deferasirox is efficacious in removing and in preventing the liver and cardiac iron overload, in chelating free iron for 24 hours and it is safe and well tolerated also if high doses (up to 40 mg/kg/die) are used.
Table 2 show the most important feature of iron chelators commercially available
Conclusions
The recent expansion of erythrocyte transfusional therapy to pediatric patients with sickle cell disease and an elevated risk of stroke [59] and in some type of thalassemia intermedia associated with bone deformations and growth defects, has increased the population of pediatric patients requiring chelation therapy. The knowledge of the properties of the available iron chelating agents and of individual patient requirements, coupled with effective methods to accurately monitor iron levels, has enabled iron chelation therapy to be highly personalized. The recently available oral chelators offer a convenient administration regimen, overcoming the burden of a subcutaneous infusions of DFO and potentially improving compliance. Treating patients with tailored therapy will eventually lead to improvement in morbidity and mortality induced by iron toxicity.
References
- Porter JB. Practical management of iron
overload. Br J Haematol 2001;115:239-252.

- Modell B: Total management of thalassemia.
Arch Dis Child 1977;52:489-500

- Modell B, Berdoukas V. The Clinical Approach to Thalassemia.London, Grune & Stratton,1984
- Buja LM, Roberts WC. Iron in the
heart: etiology and clinical significance. Am J Med 1971;51:209-221

- Unger A, Hershko C: Hepatocellular uptake
of ferritin in the rat. Br J Haematol 1974;28:169-179.

- Hershko C, Graham G, Bates GW, Rachmilewitz
EA. Non-specific serum
iron in thalasemia: An abnormal serum iron fraction of potential
toxicity. Br J Haematol 1978;40:255-263.

- Rebulla P, Modell B. Transfusion
requirements and effects in patients
with thalassemia major.Cooleycare programme. Lancet 1991;337:277-280

- Zurlo MG, De Stefano P. Borgna-Pignatti C
Piga A, Melevendi C, Di
Gregorio F, Burattini MG, Teroli S. Survival and causes of death in
thalassemia. Lancet 1989;i:27-30

- Borgna-Pignatti C, Rugolotto S, D Stefano
P, Piga A, Di Gregorio F,
Gamberini MR, Sabato V, Melevendi C, Cappellini MD, Verlato G. Survival
and disease complications in thalassemia major. Ann NY Acad Sci 1998;
850:227-231

- Hider RC. Design of therapeutic chelating
agents. Biochem.Soc. Trans.2002;30:751-754

- Kontoghiorghes
G.J, Eracleus E., Economides C. and Kolnagou A. Advances in iron
overload therapies. prospects for effective use of deferiprone (L1),
deferoxamine, the new experimental chelators ICL670, GT56-252, L1NA11
and their combinations. Curr Med Chem 2005;12:2663-2681

- Barman Balfour, Foster RH. Deferiprone: a
review of its clinical
potential in iron overload in beta thalassemia major and other
transfusion-dependent diseases. Drugs 1999;58:553-578

- Guidelines for the clinical management of thalassemia.2007(cited 2008 June 8); 2nd Edition: (Availablefrom: http://www.thalassemia.org)
- Cohen AR,Galanello R, Piga A, DiPalma A,
Vullo C, Tricta F. Safety
profile of the oral iron chelator deferiprone: a multicentre study. Br
J
Haematol 2000;08:305-312

- Cohen AR, Galanello R, Piga A, De Sancis
V, Tricta F, Safety and
effectiveness of long-term therapy with the oral iron chelator
deferiprone. Blood 2003;1;102:1583-1587

- Ceci A, Baiardi P, Felisi M, Cappellini
MD, Carnelli V, De Sanctis V,
Galanello R, Maggio A, Masera G, Piga A, Schettini F, Stefano I, Tricta
F. The safety and effectiveness of deferiprone in a large-scale, 3
–year study in Italian patients. Br J Haematol.2002;118:330-336

- al-Refaie FN, Veys PA, Wilkes S, Wonke B,
Hoffbrand AV. Agranulocytosis
in a patient with thalassaemia major during treatmente with the oral
iron chelator, 1,2-dimethyl-3-hydroxypyrid-4-one. Acta Haematol.
1993;89(2):86-90

- Hoffbrand AV, Cohen A, Hershko C, Role of
deferiprone in chelation
therapy for transfusional iron overload. Blood 2003;1;102:17-24

- Anderson LJ,Wonke B, Prescott E; Holden S,
Walker JM, Pennell DJ.
Comparison of effects of oral deferiprone and subcutaneous
desferrioxamine on myocardial iron concentrations and ventricular
function in beta-thalassemia.Lancet 2002;360:516-520

- Pennell DJ, Berdoukas V, Karagiorga M,
Ladis V, Piga A, Aessopos A,
Gotsis ED, Tanner MA, Smith GC, Westwood MA, Wonke B, Galanello R.
Randomized controlled trial of deferiprone or deferoxamine in
beta-thalassemia major patients with asymptomatic myocardial
siderosis.Blood 2006;1:107:3738-374

- Maggio A; Vitrano A, Capra M, Cuccia L,
Gagliardotto F, Filosa A,
Magnano C et al. Improving survival with deferiprone treatment in
patients with thalassemia major: a prospective multicenter randomized
clinical trial under the auspices of Italian society for thalassemia
and hemoglobinopathies. Blood Cells, Molecules and diseases 2009;
42:247-251.

- El-Beshlawy A, Sari TT, Chan LL, Tricta F, El-Alfy Mohsen: 'The safety and efficacy of a new formulation of deferiprone (Ferriprox®) in children with transfusional iron overload'. Poster in 11th International Conference on Thalassaemia & Haemoglobinopathies / 13th International TIF Conference for Thalassaemia Patients & Parents. Singapore; October 8-11, 2008. (available from: http://www.ferriprox.com/News/20081015_1.asp)
- Tanner MA, Galanello R, Dessì C, Smith GC,
Westwood MA, Agus A,
Roughton M, Assomull R, Nair SV, Walker Jm, Pennel DJ. A randomized,
placebo-controlled, double-blind trial of the effect of combined
therapy with deferoxamine and deferiprone on myocardial iron in
thalassemia major using cardiovascular magnetic resonance. Circulation
2007;115:1876-1884

- Wonke B,Wright C, Hoffbrand AV,Combined
Therapy with deferiprone and desferrioxamine. Br J Haematol
1998;103:361-364

- Kattamis A, Kassou C, Berdousi H, Ladis V,
Papassotiriou I, Kattamis C.
Combined therapy with desferrioxamine and deferiprone in thalassemic
patients: effect on urinary iron excretion. Haematologca
2003;88:1423-1425

- Wonke B,Wright C, Hoffbrand AV,Combined
Therapy with deferiprone and desferrioxamine. Br J Haematol
1998;103:361-364

- Kattamis A, Kassou C, Berdousi H, Ladis V,
Papassotiriou I, Kattamis C.
Combined therapy with desferrioxamine and deferiprone in thalassemic
patients: effect on urinary iron excretion. Haematologca
2003;88:1423-1425

- Tsironi M, Deftereos S, Andriopoulos P,
Farmakis D, Meletis J, Aessopos
A. Reversal of heart failure in thalassemia major by combined chelation
therapy: a case report. Eur J Haematol 2005;74:84-85.

- Porcu M, Landis N, Salis S, Corda M, Orru
P, Serra E, Usai B, Matta G,
Galanello R. Effects of combined deferiprone and desferrioxamine iron
chelatoing therapy in beta-thalassemia major end-stage heart failure: a
case report. Eur J Heart Fail 2007;9:320-322

- Farmaki K, Angelopoulos N, Anagnostopoulos
G, Gotsis E.Rombopoulos G,
Tolis G.Effect of enhanced iron chelation therapy on glucose metabolism
in patients with beyta-thalassemia major.Br J Haematol 2006;134:438-444.

- Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassemia major in the UK and relation to T2* cardiovascular magnetic resonance. J of Card Magn. Res. 2008;10(1):42
- US Food and Drug administration. FDA approves first oral drug for chronic iron overload (online). Available from URL: http://www.fda.gov/bbs/topics/news/2005/NEW01258.html (Accessed 2007 April 1)
- European Medicine Agency.Committee for Medicinal Products for Uman Use summary of positive opinion for Exjade (online). Available from URL: http://www.emea.europa.eu/pdfs/human/opinion/22855406en.pdf ( Accessed 2007 Aug 6).
- Nick H, Acklin P, Lattmann R, Lattmann R,
Buehlmayer P, Hauffe S,
Schupp J and Alberti D. Development of tridentate iron chelators: from
desferriothiocin to ICL670.Curr Med Chem 2003;10:1065-1076

- Steinhauser S, Heinz U, Bartoloma M, et al
.Complex formation of ICL670
and related ligands with FeIII and FeII. Eur J Inorg Chem 2004;
21:4177-92

- Piga A, Galanello R, , Forni GL, Bertrand
Y, Foschini MLfile:///E:/Cianciulli.html,
Bordone E,
Leoni G, Lavagetto A, Zappu A, Longo F, Maseruka H, Hewson N, Sechaud
R, Belleli R, Alberti D. Randomized phase II trial of deferasirox (
Exjade, ICL670), a once-daily, orally-administered iron chelator, in
comparison to deferoxamine in thalassemia patients with transfusional
iron overload. Haematologica 2006; 91: 873-880

- Esposito BP, Breuer W, Sirankapracha P,
Pootrakul P, Hershko C,
Cabantchik ZI. Labile plasma iron in iron overload: redox activity and
susceptibility to chelation. Blood 2003;102:2670-2677

- Cabantchik ZI, Breuer W, Zanninelli G,
Cianciulli P. LPI- labile plasma
iron in iron overload. Best Pract Res Clin Haematol. 2005
Jun;18(2):277-87.

- Daar S, Pathare A, Nick H, Kriemler-Krahn
U, Hmissi A, Habr D, Taher A.
Reduction in labile plasma iron during treatment with deferasirox, a
once-daily oral iron chelator, in heavily iron-overloaded patients with
b-thalassaemia Eur J Haematol 2009; 82:454-457

- Porter JB Cappellini MD, El-Beshlavy A, Kattamis A, Seymur JF, Wook Lee J, Nick H, Habr D, Domokos G, Hmissi A,and Taher A. Effect of Deferasirox (Exjade®) on Labile Plasma Iron Levels in Heavily Iron-Overloaded Patients with Transfusion-Dependent Anemias Enrolled in the Large-Scale, Prospective 1-Year EPIC Trial. Blood 2008; 112 (11): abstr 3881
- Galanello R ,Piga A, Cappellini MD, Forni
GL, Zappu A, Origa R, Dutreix
C, Belleli R, Ford JM, Rivière GJ, Balez S, Alberti D, Séchaud R.
Effect of Food, Type of Food, and Time of Food Intake on Deferasirox
Bioavailability: Recommendations for an Optimal Deferasirox
Administration Regimen. Journal of Clinical Pharmacology,
2008;18:428-435

- Galanello R, Piga A, Forni GL, Bertrand Y,
Foschini ML, Bordone E,
Leoni G, Lavagetto A, Zappu A, Longo F, Maseruka H, Hewson N, Sechaud
R, Belleli R, Alberti D. Phase II clinical evaluation of deferasirox, a
once-daily oral chelating agent, in pediatric patients with β-
thalassemia major Haematologica. Haematologica. 2006 Oct;91(10):1343-51.

- Cappellini MD, Cohen A, Piga A, Bejaoui M,
Perrotta S, Agaoglu L,
Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R,
Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M,
Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen
C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti
D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron
chelator, in patients with β-thalassemia. Blood. 2006;107(9):3455–3462.

- Galanello R, Piga A, Forni GL, Bertrand Y,
Foschini ML, Bordone E,
Leoni G, Lavagetto A, Zappu A, Longo F, Maseruka H, Hewson N, Sechaud
R, Belleli R, Alberti D. Phase II clinical evaluation of deferasirox, a
once-daily oral chelating agent, in pediatric patients with
β-thalassemia major. Haematologica. 2006;91(10):1343–1351.

- Vichinsky E, Onyekwere O, Porter J,. A
rSwerdlow P, Eckman J, Lane P,
Files B, Hassell K, Kelly P, Wilson F, Bernaudin F, Forni GL, Okpala I,
Ressayre-Djaffer C, Alberti D, Holland J, Marks P, Fung E, Fischer R,
Mueller BU, Coates T. Randomized comparison of deferasirox versus
deferoxamine for the treatment of transfusional iron overload in sickle
cell disease. Br J Haematol. 2007;136(3):501–508.

- Porter J, Galanello R, Saglio G,. Neufeld
EJ, Vichinsky E, Cappellini
MD, Olivieri N, Piga A, Cunningham MJ, Soulières D, Gattermann N,
Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M,
Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M,
Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose
C. Relative response of patients with myelodysplastic syndromes and
other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr
prospective study. Eur J Haematol.2008;80(2):168–176.

- Taher A, Cappellini MD. Update on the use
of deferasirox in the
management of iron overload. Ther Clin Risk Manag. 2009:5 857–868

- Cappellini MD, Taher A. Deferasirox
(Exjade ®) for the Treatment of Iron Overload. Acta Haematol
2009;122:165–173

- Cappellini MD, Perrotta S, Agaoglu L, Aydinok Y, Capra M, Canatan D, Drelichman G, Kilinc Y, Magnano C, Porter JB, Piga A, Griffel L, Lagrone D, Clark J,and Kattamis A. Efficacy and Safety of Deferasirox (Exjade®) in Patients with β-Thalassemia Major Treated for up to 5 Years. Blood 2009; 114 (22) : abstr 4063
- Cohen AR , Glimm E, Porter JB.
Effect of transfusional iron intake on
response to chelation therapy in beta-thalassemia major Blood.
2008;111:583-587

- Cappellini MD, Porter J, El-Beshlawy A, Li
CK, Seymour JF, Elalfy M,
Gattermann N, Giraudier S, Lee JW, Chan LL, Lin KH, Rose C, Taher A,
Thein SL, Viprakasit V, Habr D, Domokos G, Roubert B, Kattamis A; on
behalf of the EPIC study investigators. Tailoring iron chelation by
iron intake and serum ferritin: Prospective EPIC study of deferasirox
in 1744 patients with transfusion-dependent anemias.
Haematologica
2009; doi:10.3324/haematol.2009.014696

- Taher A, El-Beshlawy A, Elalfy MS, Al Zir
K, Daar S, Habr D,
Kriemler-Krahn U, Hmissi A, Al Jefri A. Efficacy and safety of
deferasirox, an oral iron chelator, in heavily iron-overloaded patients
with β-thalassaemia: the ESCALATOR study. Eur J Haematol.
2009;82(6):458–465.

- Taher A, El-Beshlawy A, Elalfy M, et al. Deferasirox significantly reduces iron burden in heavily iron-overloaded patients with beta-thalassaemia: 2.7 year results from the ESCALATOR study. Haematologica. 2009; 94(Suppl 2):abstr 209.
- Pathare A, Taher A, Daar S. Deferasirox
(Exjade®) significantly
improves cardiac T2* in heavily iron-overloaded patients with
β-thalassemia major. Ann Hematol DOI 10.1007/s00277-009-0838-z

- Pennell DJ, Porter JB, Cappellini MD,
El-Beshlawy A, Chan LL, Aydinok
Y, Elalfy MS, Sutcharitchan P, Li CK, Ibrahim H, Viprakasit V, Kattamis
A, Smith G, Habr D, Domokos G, Roubert B, Taher A. Efficacy of
deferasirox in reducing and preventing cardiac iron overload in
β-thalassemia Prepublished online Dec 8, 2009;
doi:10.1182/blood-2009-04-217455

- Pennel DJ, Porter JB, Cappellini MD, Chan LL, El-Beshlawy A,Aydinok Y, Ibrahim H, Kong-Li C, Viprakasit V, Elalfy MS, Kattamis A, Smith G, Habr D, Domokos G, Roubert B and Taher A. Efficacy and Safety of Deferasirox (Exjade®) in β-Thalassemia Patients with Myocardial Siderosis: 2-Year Results From the EPIC Cardiac Sub-Study. Blood 2009; 114 (22): abstr 4062.
- Pennell D, Sutcharitchan P, El-Beshlawy A, Aydinok Y, Taher A, Smith G, Hbr D, Kriemler-Krahan U, Hmissi A,and Porter JB. Efficacy and safety of deferasirox (Exjade®) in preventing cardiac iron overload in β-thalassemia patients with normal baseline cardiac iron: results from the cardiac substudy of the EPIC trial. Blood. 2008;112(11): abstr 3874.
- Vichinsky E Clinical application of
deferasirox: practical patient management. Am J Hematol.
2008;83(5):398-402.

- Barton JC. Optimal management strategies
for chronic iron overload. Drugs 2007; 67: 239-252.
