Hematopietic Stem Cell Transplantation in Thalassemia and Related Disorders
Emanuele Angelucci1, Pilo Federica1, Targhetta Clara1, Pettinau Martina1, Depau Cristina1, Cogoni Claudia1, Usai Sara1, Pani Mario2 , Desś Laura1 and Donatella Baronciani1
1Struttura Complessa Disciplina di Ematologia e Centro Trapianto Cellule Staminali Emopoietiche “Wilma Deplano”. Ospedale Oncologico di Riferimento Regionale “Armando Businco” Cagliari.
2Servizio Immmunoematologia e Trasfusionale, azienda Ospedaliera “Brotzu”, Cagliari.
Correspondence
to:
Emanuele Angelucci, MD. Unità Operativa di Ematologia, Ospedale
Oncologico “Armando Businco”, via Edward Jenner, 09121 Cagliari, Italy.
E-mail: emnang@tin.it
Published: December 03, 2009
Received: November 18, 2009
Accepted: November 28, 2009
Medit J Hemat Infect Dis 2009, 1(1): e2009015 DOI 10.4084/MJHID.2009.015
This article is available from: http://www.mjhid.org/article/view/5155
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
The
basis of allogeneic hemopoietic stem cell (HSC) transplantation in
thalassemia consists in substituting the ineffective thalassemic
erythropoiesis with and allogeneic effective one. This cellular
replacement therapy is an efficient way to obtain a long lasting,
probably permanent, clinical effective correction of the anaemia
avoiding transfusion requirement and subsequent complications like iron
overload. The first HSC transplant for thalassemia was
performed in Seattle on Dec 2, 1981. In the early eighties
transplantation procedure was limited to very few centres worldwide.
Between 17 December 1981 and 31 January 2003, over 1000
consecutive patients, aged from 1 to 35 years, underwent
transplantation in Pesaro. After the pioneering work by the
Seattle and Pesaro groups, this therapeutic approach is now
widely applied worldwide. Medical therapy of thalassemia is one of the
most spectacular successes of the medical practice in the last decades.
In recent years advances in knowledge of iron overload
patho-physiopathology, improvement and diffusion of diagnostic
capability together with the development of new effective and safe oral
chelators promise to further increase success of medical therapy.
Nevertheless situation is dramatically different in non-industrialized
countries were the very large majority of patients live today.
Transplantation technologies have improved substantially during the
last years and transplantation outcome is likely to be much better
today than in the ‘80s. Recent data indicated a probability of overall
survival and thalassemia free survival of 97% and 89% for patients with
no advanced disease and of 87% and 80% for patients with advanced
disease. Thus the central role of HSC in thalassemia has now been
fully established. HSC remains the only definitive curative therapy for
thalassemia and other hemoblobinopathies. The development of oral
chelators has not changed this position. However this has not settled
the controversy on how this curative but potentially lethal treatment
stands in front of medical therapy for adults and advanced disease
patients. In sickle cell disease HSC transplantation
currently is reserved almost exclusively for patients with clinical
features that indicate a poor outcome or significant sickle-related
morbidity.
Introduction
Conclusion
In
easy words the rationale basis of allogeneic hemopoietic stem cell
(HSC) transplantation in thalassemia consists in sub-stituting the
ineffective thalassemic erythropoiesis with and allogeneic effective
one1. This approach is an efficient way to obtain a long lasting,
probably permanent, clinical effective correction of the haemolytic
anaemia [1]. Thus avoiding transfusion requirement
and subsequent
complications like iron overload. Allogeneic hemopoietic stem cell
transplantation in genetic disease has been a cornerstone of the
development of HSC transplantation [2] and a topic of
passionate debate
during the past decades [3,4]. Although
transplantation role in
thalassemia is today well established this debate would be more
important today in the oral chelator era and in the era of a worldwide
diffuse medicine with increasing attention to the problem of the costs.
HSC
transplantation in a not malignant disease
The issue of HSC transplant in hemo-globinopathies is conceptually more similar to solid organ transplantation than to HSC transplant in haematological malignancies. In this setting there is not a malignant clone to eradicate and a Graft versus Leukemia is not required. On the other hand there is not a malignant progressive disease to be included in the risk/benefit ratio analyses. Patients have not received previous chemotherapy and immunosuppression but have iron-related tissue damage. This cellular replacement has been improperly defined “gene therapy today”.
All these findings must be considered in patients’ selection and transplant execution.
The issue of HSC transplant in hemo-globinopathies is conceptually more similar to solid organ transplantation than to HSC transplant in haematological malignancies. In this setting there is not a malignant clone to eradicate and a Graft versus Leukemia is not required. On the other hand there is not a malignant progressive disease to be included in the risk/benefit ratio analyses. Patients have not received previous chemotherapy and immunosuppression but have iron-related tissue damage. This cellular replacement has been improperly defined “gene therapy today”.
All these findings must be considered in patients’ selection and transplant execution.
Results
of transplantation
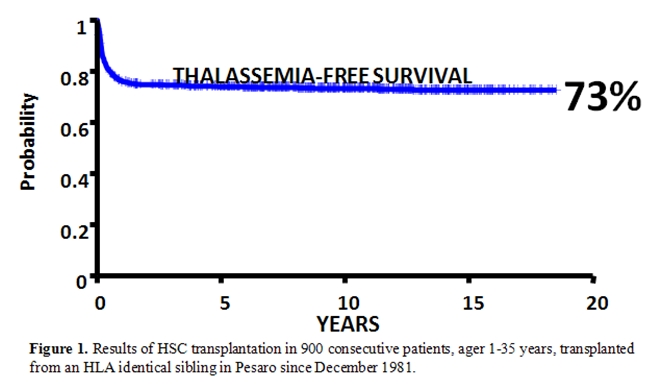
The first HSC transplant for thalassemia was performed in Seattle on Dec 2, 1981 [5]. Subsequently between 17 December 1981 and 31 January 2003, over 1000 consecutive patients, aged from 1 to 35 years, underwent transplantation in Pesaro. In fig 1 is reported the thalassemia free survival obtained in 900 consecutive patients transplanted from an HLA identical sibling. Overall, the greater than 20-year Kaplan–Meier probability of thalassemia-free survival was 73% (Figure 1) [6].
The first HSC transplant for thalassemia was performed in Seattle on Dec 2, 1981 [5]. Subsequently between 17 December 1981 and 31 January 2003, over 1000 consecutive patients, aged from 1 to 35 years, underwent transplantation in Pesaro. In fig 1 is reported the thalassemia free survival obtained in 900 consecutive patients transplanted from an HLA identical sibling. Overall, the greater than 20-year Kaplan–Meier probability of thalassemia-free survival was 73% (Figure 1) [6].

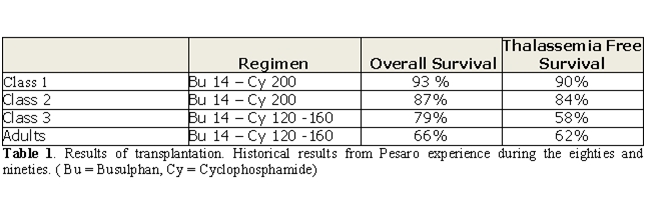
With regard to predictors of
transplant outcome, in the historical
experience, patients younger than 17 years were stratified on the basis
of the risk factors of hepatomegaly, a history of irregular chelation,
and hepatic fibrosis [7]. With the combination of
oral Busulfan 14 mg/kg
and Cyclophosphamide 200 mg/kg as preparative regimen results were
satisfactory for patients with no advanced disease (Class 1 and Class
2) while were unacceptable for patients with advanced disease (Class 3)
[7]. For the first two category of patients this
conditioning regimen
has remained unmodified since 1985 [8]. For the
discouraging
results with this conditioning regimen in patients with advanced
disease, after 1990 class 3 patients underwent transplantation based on
protocols that included lower dosages of cyclophosphamide (120–160
mgkg) [9]. Transplantation in class 3 children, with
this regimen was
characterized by a 30% risk of thalassemia recurrence [9].
In contrast,
in adults with the same dose of cyclophosphamide, thalassemia
recurrence was only 4%, but there was a 35% transplant-related
mortality [10]. These historical Pesaro results are
reported in Table 1.

Because class 3 children had
the problem of thalassemia recurrence
whereas adults had the problem of transplant-related mortality a new
preparative regimen was introduced for class 3 patients with the aim of
increasing the rate of sustained engraftment while decreasing
transplant-related mortality. This regimen was characterized by a
30-day preconditioning period designed to produce erythroid
cytoreduction and immune-suppression before a conditioning regimen
using a reduced dose of cyclophosphamide. In a pilot study of 33
consecutive class 3 thalassemia children, the 5-year Kaplan–Meier
probability of survival and disease free survival was 93% and 85%,
respectively [11].
Application
of transplantation world-wide
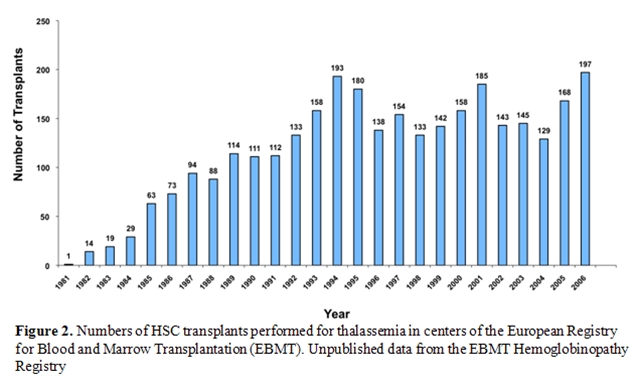
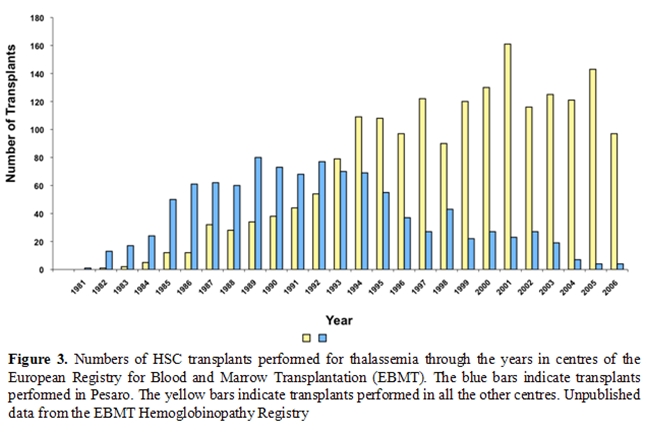
After have been pioneered by the Seattle group [5], the Pesaro group [4, 7, 8, 12-15] and the Pescara group [16]this therapeutic approach is now widely applied worldwide [6]. In the early eighties transplantation procedure was limited to very few centres worldwide. The European Group for Blood and Marrow Transplantation has recently established the hemoglobinopathy registry and it is possible today to a have detailed epidemiology data on over 3,000 patients registered. After the early ninety a constant numbers of transplants raging between 133 and 197 per year have been registered with a constant behaviour during the years (Figure 2) [6]. The pioneering role of the Pesaro group is today clear (Figure 3) and the wide dissemination of this practice after 1993 is the best demonstration of its success.
After have been pioneered by the Seattle group [5], the Pesaro group [4, 7, 8, 12-15] and the Pescara group [16]this therapeutic approach is now widely applied worldwide [6]. In the early eighties transplantation procedure was limited to very few centres worldwide. The European Group for Blood and Marrow Transplantation has recently established the hemoglobinopathy registry and it is possible today to a have detailed epidemiology data on over 3,000 patients registered. After the early ninety a constant numbers of transplants raging between 133 and 197 per year have been registered with a constant behaviour during the years (Figure 2) [6]. The pioneering role of the Pesaro group is today clear (Figure 3) and the wide dissemination of this practice after 1993 is the best demonstration of its success.


Transplantation
versus medical therapy
Medical therapy of thalassemia is one of the most spectacular successes of the medical practice in the last decades. Thalassemia has been transformed from an infant lethal disease in a chronic disease permitting prolonged survival. An Italian retro-spective study [17] clearly demonstrated which has been the medical progress in this setting comparing survival of patients born in different decades. Nevertheless situation is dramatically different in non-industrialized countries were the very large majority of patients live today.
In recent years advances in knowledge of iron overload patho-physiopathology, improvement and diffusion of diagnostic capability together with the development of new effective and safe oral chelators [18,19] promise to further increase success of medical therapy [20]. Particularly today well-studied oral chelators offer a concrete alternative to deferoxamine infusion therapy.
The problem of the choice between transplantation versus medical therapy is a kind of question, which cannot be resolved, for several, obvious, reasons by a prospective randomized clinical trial. In absence of such data the choice should be done on analyses of large prospective (transplant) and retrospective (medical therapy) phase II trials and individual preference. In this kind of clinical question several aspects must be kept in consideration making the decisional process different in individual cases: age, clinical status, capability and compliance to adhere a correct transfusional-chelation regimen and resources. In case of children the dilemma dramatically relies on parents shoulders.
Medical therapy of thalassemia is one of the most spectacular successes of the medical practice in the last decades. Thalassemia has been transformed from an infant lethal disease in a chronic disease permitting prolonged survival. An Italian retro-spective study [17] clearly demonstrated which has been the medical progress in this setting comparing survival of patients born in different decades. Nevertheless situation is dramatically different in non-industrialized countries were the very large majority of patients live today.
In recent years advances in knowledge of iron overload patho-physiopathology, improvement and diffusion of diagnostic capability together with the development of new effective and safe oral chelators [18,19] promise to further increase success of medical therapy [20]. Particularly today well-studied oral chelators offer a concrete alternative to deferoxamine infusion therapy.
The problem of the choice between transplantation versus medical therapy is a kind of question, which cannot be resolved, for several, obvious, reasons by a prospective randomized clinical trial. In absence of such data the choice should be done on analyses of large prospective (transplant) and retrospective (medical therapy) phase II trials and individual preference. In this kind of clinical question several aspects must be kept in consideration making the decisional process different in individual cases: age, clinical status, capability and compliance to adhere a correct transfusional-chelation regimen and resources. In case of children the dilemma dramatically relies on parents shoulders.
Resources
availability
Even if not properly medical issue resources availability is a fundamental issue. A modern complete transfusion – chelation therapy is a high technology therapy requiring important expertise. Even not considering complication, medical therapy is very expensive clearly not widely available worldwide [21]. Even transplantation is a high technology therapy but the diffusion of transplant centres is greatly increased today [22]. It has been calculated that in term of resources availability and utilization HSC transplantation corresponds to approximately 3 years of transfusion- chelation therapy with deferoxamine not including complications costs.
In large part of the world, were today lives the large part of thalassemia patients, the choice between transplant and medical therapy cannot consider just reported literature but resources availability also.
Even if not properly medical issue resources availability is a fundamental issue. A modern complete transfusion – chelation therapy is a high technology therapy requiring important expertise. Even not considering complication, medical therapy is very expensive clearly not widely available worldwide [21]. Even transplantation is a high technology therapy but the diffusion of transplant centres is greatly increased today [22]. It has been calculated that in term of resources availability and utilization HSC transplantation corresponds to approximately 3 years of transfusion- chelation therapy with deferoxamine not including complications costs.
In large part of the world, were today lives the large part of thalassemia patients, the choice between transplant and medical therapy cannot consider just reported literature but resources availability also.
Alternative
source of stem cells
The large majority of transplant in thalassemia has been performed using bone marrow derived stem cells. In the EBMT survey more than 80% of the transplants have been performed using bone marrow derided cells. Even in the most recent years, although less pronounced, bone marrow derived stem cells continue to be the preferred source of stem cells for transplantation in thalassemia.
In 1998 Franco Locatelli first proposed the use of identical sibling derived hemopoietic stem cells for transplantation in hemoglobinopathies [27]. Since that time use of cord blood derived hemopoietic stem cell has much expanded [28] with outstanding results in the field of hemo-globinopathies particularly regards a very low mortality rate.
The large majority of transplant in thalassemia has been performed using bone marrow derived stem cells. In the EBMT survey more than 80% of the transplants have been performed using bone marrow derided cells. Even in the most recent years, although less pronounced, bone marrow derived stem cells continue to be the preferred source of stem cells for transplantation in thalassemia.
In 1998 Franco Locatelli first proposed the use of identical sibling derived hemopoietic stem cells for transplantation in hemoglobinopathies [27]. Since that time use of cord blood derived hemopoietic stem cell has much expanded [28] with outstanding results in the field of hemo-globinopathies particularly regards a very low mortality rate.
Alternative
donor
As very well known in transplantation only approximately 1/3 of patients has an HLA identical sibling inside the family [23]. Use of alternative donors includes matched unrelated donor, mismatched donors. The first approach has been applied by a multicentre Italian study and demonstrated satisfactory results [24]. However the limited number of cases reported (< 100 in ore than 10 years) and the limitation in donor selection makes this procedure be reserved to specialized centres and controlled trials.
Unrelated cord blood transplantation is standard practice today for HSC therapy in malignancies. Tolerance of 1-2 HLA antigens mismatch, fast availability and low incidence of graft versus host disease makes this option very attractive for not malignant diseases including thalassemia. Preliminary experience is promising but limited [36] reported cases with a 77% overall survival and 65% thalassemia free survival) [25] and, therefore, should be considered a experimental approach. Mismatched donor transplantation has been issue of a very limited number of trials [26]. Because of the experimental role of this procedure and the alternative of medical therapy available today this procedure should be not recommended unless in very selected clinical cases in which medical therapy in has been formally documented to be not feasible.
As very well known in transplantation only approximately 1/3 of patients has an HLA identical sibling inside the family [23]. Use of alternative donors includes matched unrelated donor, mismatched donors. The first approach has been applied by a multicentre Italian study and demonstrated satisfactory results [24]. However the limited number of cases reported (< 100 in ore than 10 years) and the limitation in donor selection makes this procedure be reserved to specialized centres and controlled trials.
Unrelated cord blood transplantation is standard practice today for HSC therapy in malignancies. Tolerance of 1-2 HLA antigens mismatch, fast availability and low incidence of graft versus host disease makes this option very attractive for not malignant diseases including thalassemia. Preliminary experience is promising but limited [36] reported cases with a 77% overall survival and 65% thalassemia free survival) [25] and, therefore, should be considered a experimental approach. Mismatched donor transplantation has been issue of a very limited number of trials [26]. Because of the experimental role of this procedure and the alternative of medical therapy available today this procedure should be not recommended unless in very selected clinical cases in which medical therapy in has been formally documented to be not feasible.
Alternative
conditioning regimens
Be-cause of the lack of a malignant clone to eradicate and the need to reduce toxicity non-ablative transplantation would have theoretically a role in thalassemia and hemoglobinopathies. However attempts performed in this direction failed. This was recently reported in two limited experiences [29,30]. Overall eleven patients with Thalassemia or Sickle cell Disease received a non-myeloablative regimen before HSC transplant. As expected transplant related mortality was very limited but only one patient had a sustained engraftment. This was probably due to expanded erythroid clone and the immunological situation demonstrating that a stable allogeneic engraftment is difficult to obtain in hemoglobinopathies and additional immunological tools would be necessary. However in the setting of a not-malignant disease the addiction of post transplant donor lymphocyte infusion does not appears to a rational approach in terms of risk/benefit ratio.
Be-cause of the lack of a malignant clone to eradicate and the need to reduce toxicity non-ablative transplantation would have theoretically a role in thalassemia and hemoglobinopathies. However attempts performed in this direction failed. This was recently reported in two limited experiences [29,30]. Overall eleven patients with Thalassemia or Sickle cell Disease received a non-myeloablative regimen before HSC transplant. As expected transplant related mortality was very limited but only one patient had a sustained engraftment. This was probably due to expanded erythroid clone and the immunological situation demonstrating that a stable allogeneic engraftment is difficult to obtain in hemoglobinopathies and additional immunological tools would be necessary. However in the setting of a not-malignant disease the addiction of post transplant donor lymphocyte infusion does not appears to a rational approach in terms of risk/benefit ratio.
Mixed
chimerism
In the setting of HSC transplantation mixed chimerism is defined as the concurrent presence of donor and host hemopoietic cells. Mixed chimerism is commonly reported after HSC transplant for thalassemia after a myelo-ablative-conditioning regimen. In a prospective study by Andreani in over 300 consecutive thalassemia patients [31] early mixed chimerism was demonstrated in 28% of the patients. Of them only approximately one fourth (11% of the surviving patients) confirmed mixed chimerism in a prolonged follow up extending over the second year after transplant (persistent chimerism). In a recent study from Pavia this behaviour has not been confirmed in the setting of cord blood transplantation [28].
Very interesting and surprising in those patients who presented persistent mixed chimerism a partial engraftment, up to the 20% of marrow cells, was still able to maintain a normal hemoglobin level avoiding blood transfusions. In this population, no sign of increasing iron overload or other clinical complication of thalassemia or thalassemia intermedia were detectable, thus obtaining a complete clinical control of the disease.
Regardless of mechanism correction of anemia by relatively low amount of mixed chimerism provide the rationale for development of minimally–ablative conditioning regimens to obtain a partial engraftment and at the same time abolish or drastically decrease transplant related mortality. The evidence that a partial engraftment of a normal erythropoiesis provide clinical control of the disease could also be the basis for a gene therapy program (when safely available) since eradication of the thalassemic marrow is not necessary to clinical control of the disease.
In the setting of HSC transplantation mixed chimerism is defined as the concurrent presence of donor and host hemopoietic cells. Mixed chimerism is commonly reported after HSC transplant for thalassemia after a myelo-ablative-conditioning regimen. In a prospective study by Andreani in over 300 consecutive thalassemia patients [31] early mixed chimerism was demonstrated in 28% of the patients. Of them only approximately one fourth (11% of the surviving patients) confirmed mixed chimerism in a prolonged follow up extending over the second year after transplant (persistent chimerism). In a recent study from Pavia this behaviour has not been confirmed in the setting of cord blood transplantation [28].
Very interesting and surprising in those patients who presented persistent mixed chimerism a partial engraftment, up to the 20% of marrow cells, was still able to maintain a normal hemoglobin level avoiding blood transfusions. In this population, no sign of increasing iron overload or other clinical complication of thalassemia or thalassemia intermedia were detectable, thus obtaining a complete clinical control of the disease.
Regardless of mechanism correction of anemia by relatively low amount of mixed chimerism provide the rationale for development of minimally–ablative conditioning regimens to obtain a partial engraftment and at the same time abolish or drastically decrease transplant related mortality. The evidence that a partial engraftment of a normal erythropoiesis provide clinical control of the disease could also be the basis for a gene therapy program (when safely available) since eradication of the thalassemic marrow is not necessary to clinical control of the disease.
Transplantation
for thalassemia in the era of oral chelators
The central role of HSC in thalassemia has now been fully established. Transplantation remains the only definitive curative therapy for thalassemia and other hemoglobino-pathies. The development of oral chelators has not changed this position. However this has not settled the controversy on how this curative but potentially lethal treatment stands in front of medical therapy for adults and advanced disease patients. Trans-plantation technologies have improved substantially during the last years and transplantation outcome is likely to be much better today than in the ‘80s. Recent data indicated a probability of overall survival and thalassemia free survival of 97% and 89% for patients with no advanced disease and of 87% and 80% for patients with advanced disease [32]. Similar data are coming from the EBMT survey on over 1000 patients transplanted in the EBMT centres during the last 10 years with a over 90% overall survival at 5 years.
The central role of HSC in thalassemia has now been fully established. Transplantation remains the only definitive curative therapy for thalassemia and other hemoglobino-pathies. The development of oral chelators has not changed this position. However this has not settled the controversy on how this curative but potentially lethal treatment stands in front of medical therapy for adults and advanced disease patients. Trans-plantation technologies have improved substantially during the last years and transplantation outcome is likely to be much better today than in the ‘80s. Recent data indicated a probability of overall survival and thalassemia free survival of 97% and 89% for patients with no advanced disease and of 87% and 80% for patients with advanced disease [32]. Similar data are coming from the EBMT survey on over 1000 patients transplanted in the EBMT centres during the last 10 years with a over 90% overall survival at 5 years.
Transplantation
for Sickle Cell Disease
Similar to β thalassemia major, the objective of HSC transplantation for sickle cell disease is to substitute recipient erythropoiesis, or to reduce its clinical impact, by the expression of donor β-globin chains. The clinical benefit of this cellular replacement is the elimination, or noteworthy amelioration, of the clinical complications caused by polymerized sickle hemoglobin. Since the initial clinical trials of HSC transplantation for sickle cell disease it was clear that the replacement of donor for sickle erythropoiesis might abolish not only the hematological manifestations of the underlying disorder, but also stabilize and even decrease the organ damage caused by previous recurrent vessel occlusion and haemolysis [33-36]. As in thalassemia there is usefulness in assigning a risk-based approach to applying transplantation for sickle cell disease. Unfortunately, defining risk characteristics is difficult in the absence of prospective trials. Nonetheless, the appropriate broader application of HSC transplantation hinges on two important objectives.
Similar to β thalassemia major, the objective of HSC transplantation for sickle cell disease is to substitute recipient erythropoiesis, or to reduce its clinical impact, by the expression of donor β-globin chains. The clinical benefit of this cellular replacement is the elimination, or noteworthy amelioration, of the clinical complications caused by polymerized sickle hemoglobin. Since the initial clinical trials of HSC transplantation for sickle cell disease it was clear that the replacement of donor for sickle erythropoiesis might abolish not only the hematological manifestations of the underlying disorder, but also stabilize and even decrease the organ damage caused by previous recurrent vessel occlusion and haemolysis [33-36]. As in thalassemia there is usefulness in assigning a risk-based approach to applying transplantation for sickle cell disease. Unfortunately, defining risk characteristics is difficult in the absence of prospective trials. Nonetheless, the appropriate broader application of HSC transplantation hinges on two important objectives.
- To identify those patients who have the greatest risk of developing sickle-related complications and who are most likely to benefit from hematopoietic cell transplantation.
- To reduce transplant-related complications by minimizing the short- and long-term toxicities of HSC transplantation.
Unlike β-thalassemia major,
where the genotype directs a reasonably
reliable phenotype in the vast majority of cases, the clinical
expression of sickle cell anemia is quite variable and difficult to
predict based upon the hemoglobin genotype alone. Thus, in standard
practice, HSC transplantation for sickle cell disease currently is
reserved almost exclusively for patients with clinical features that
indicate a poor outcome or significant sickle-related morbidity, in
part due to the toxicity of this intensive therapy [37].
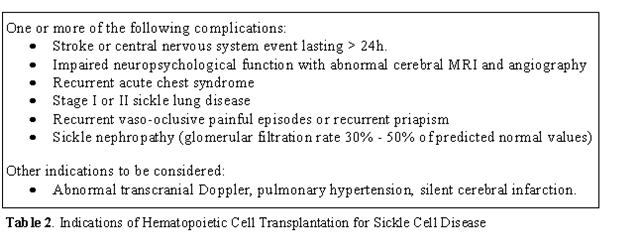
These
clinical indications are listed in Table
2.

These criteria have been
developed and applied almost exclusively on
children, for whom the risk–benefit ratio is most advantageous in terms
of years-of-life gained. . Less certain is how to apply inclusion
criteria to adults with sickle cell anemia, in whom the experience of
transplantation is limited, but for whom the risk of significant
transplantation-related toxicity remains substantial. For all patients,
clinicians must carefully weigh therapeutic alternatives to
hematopoietic cell trans-plantation, with particular attention to
safety, efficacy, availability, and the cost of intervention [38-40].
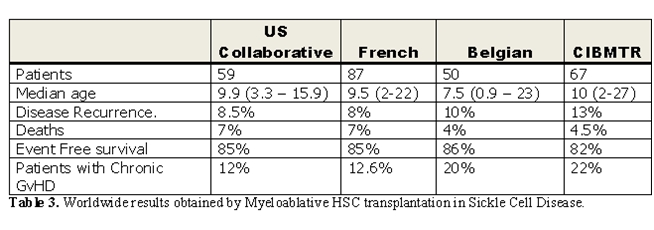
The worldwide experience of conventional myeloablative hematopoietic cell transplantation for sickle cell disease [34-36, 41-44] is summarized in Table 3. In the collective experiences of these studies, HSC transplantation moved from an experimental intervention reserved for severely affected patients, to one in which younger children with early signs of sickle-related morbidity are targeted. The results of transplantation were best when performed in children with sickle cell disease who had HLA-identical sibling donors. Even though many children who received allografts had significant sickle-related complications such as stroke and recurrent episodes of acute chest syndrome, the disease-free survival rate was 80% to 85% quite similar in the different experiences; however, 5%–10% of patients died of complications related to transplantation, with GVHD and its treatment the leading cause of death
The worldwide experience of conventional myeloablative hematopoietic cell transplantation for sickle cell disease [34-36, 41-44] is summarized in Table 3. In the collective experiences of these studies, HSC transplantation moved from an experimental intervention reserved for severely affected patients, to one in which younger children with early signs of sickle-related morbidity are targeted. The results of transplantation were best when performed in children with sickle cell disease who had HLA-identical sibling donors. Even though many children who received allografts had significant sickle-related complications such as stroke and recurrent episodes of acute chest syndrome, the disease-free survival rate was 80% to 85% quite similar in the different experiences; however, 5%–10% of patients died of complications related to transplantation, with GVHD and its treatment the leading cause of death

As in thalassemia major, the
observation of donor–host hematopoietic
chimerism after conventional myeloablative hematopoietic cell
transplantation has lent substantial support to the notion that
persistence of even a fraction of normal erythropoiesis might elicit a
curative clinical effect [31, 44]
leading to the same potentiality for
a gene therapy approach as described for thalassemia.
Conclusion
HSC
transplantation in hemoglobinopathies has today a well-recognized place
as therapeutic option for these inherited diseases. As elsewhere
described in this journal, these diseases have a tremendous impact with
hundred of thousands of patients (mostly children) affected worldwide
of whom few have the transplant option. As above described the
transplant decision is much different in the two diseases requiring
deep knowledge of the diseases and at the same time of the transplant
possibility and limitation. Any effort should be done to further
decrease transplant related mortality and to extend transplantation
option to patients so far lacking an HLA identical donor and to those
without modern healthcare resources.
References
- Schrier SL, Angelucci E. New strategies in
the treatment of the thalassemias. Annu Rev Med 2005;56:157-71.

- Appelbaum FR. Hematopoietic-cell
transplantation at 50. N Engl J Med 2007;357:1472-5.

- Lucarelli G, Clift R, Angelucci E.
Deferoxamine in thalassemia major. N Engl J Med 1995;332:271; author
reply 2-3.

- Lucarelli G, Weatherall DJ. For debate:
bone marrow transplantation for severe thalassaemia (1). The view from
Pesaro (2). To be or not to be. Br J Haematol 1991;78:300-3.

- Thomas ED, Buckner CD, Sanders JE, et al.
Marrow transplantation for thalassaemia. Lancet 1982;2:227-9.

- Angelucci E, Baronciani D. Allogeneic stem
cell transplantation for thalassemia major. Haematologica
2008;93:1780-4.

- Lucarelli G, Galimberti M, Polchi P, et al.
Bone marrow transplantation in patients with thalassemia. N Engl J Med
1990;322:417-21.

- Lucarelli G, Galimberti M, Polchi P, et al.
Marrow transplantation in patients with thalassemia responsive to iron
chelation therapy. N Engl J Med 1993;329:840-4.

- Lucarelli G, Clift RA, Galimberti M, et al.
Marrow transplantation for patients with thalassemia: results in class
3 patients. Blood 1996;87:2082-8.

- Lucarelli G, Clift RA, Galimberti M, et
al. Bone marrow transplantation in adult thalassemic patients. Blood
1999;93:1164-7.

- Sodani P, Gaziev D, Polchi P, et al. New
approach for bone marrow transplantation in patients with class 3
thalassemia aged younger than 17 years. Blood 2004;104:1201-3.

- Lucarelli G, Polchi P, Izzi T, et al.
Allogeneic marrow transplantation for thalassemia. Exp Hematol
1984;12:676-81.

- Lucarelli G, Polchi P, Galimberti M, et
al. Marrow transplantation for thalassaemia following busulphan and
cyclophosphamide. Lancet 1985;1:1355-7.

- Lucarelli G, Galimberti M, Polchi P, et
al. Marrow transplantation in patients with advanced thalassemia. N
Engl J Med 1987;316:1050-5.

- Lucarelli G, Galimberti M, Polchi P, et
al. Bone marrow transplantation in adult thalassemia. Blood
1992;80:1603-7.

- Di Bartolomeo P, Santarone S, Di
Bartolomeo E, et al. Long-term results of survival in patients with
thalassemia major treated with bone marrow transplantation. Am J
Hematol 2008;83:528-30.

- Borgna-Pignatti C, Rugolotto S, De Stefano
P, et al. Survival and complications in patients with thalassemia major
treated with transfusion and deferoxamine. Haematologica
2004;89:1187-93.

- Hoffbrand AV, Cohen A, Hershko C. Role of
deferiprone in chelation therapy for transfusional iron overload. Blood
2003;102:17-24.

- Cappellini MD, Cohen A, Piga A, et al. A
phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator,
in patients with beta-thalassemia. Blood 2006;107:3455-62.

- Angelucci E, Barosi G, Camaschella C, et
al. Italian Society of Hematology practice guidelines for the
management of iron overload in thalassemia major and related disorders.
Haematologica 2008;93:741-52.

- Scalone L, Mantovani LG, Krol M, et al.
Costs, quality of life, treatment satisfaction and compliance in
patients with beta-thalassemia major undergoing iron chelation therapy:
the ITHACA study. Curr Med Res Opin 2008;24:1905-17.

- Miano M, Labopin M, Hartmann O, et al.
Haematopoietic stem cell transplantation trends in children over the
last three decades: a survey by the paediatric diseases working party
of the European Group for Blood and Marrow Transplantation. Bone Marrow
Transplant 2007;39:89-99.

- Delfini C, Donati M, Marchionni D, et al.
HLA compatibility for patients with thalassemia: implications for bone
marrow transplantation. Int J Cell Cloning 1986;4:274-8.

- La Nasa G, Argiolu F, Giardini C, et al.
Unrelated bone marrow transplantation for beta-thalassemia patients:
The experience of the Italian Bone Marrow Transplant Group. Ann N Y
Acad Sci 2005;1054:186-95.

- Jaing T-H, Tan P, Rosenthal J, et al. Unrelated cord blood transplantation (CBT) for thalassemia. Blood (ASH Annual meeting abstract) 2006;108:554.
- Gaziev D, Galimberti M, Lucarelli G, et
al. Bone marrow transplantation from alternative donors for
thalassemia: HLA-phenotypically identical relative and HLA-nonidentical
sibling or parent transplants. Bone Marrow Transplant 2000;25:815-21.

- Locatelli F, Rocha V, Reed W, et al.
Related umbilical cord blood transplantation in patients with
thalassemia and sickle cell disease. Blood 2003;101:2137-43.

- Lisini D, Zecca M, Giorgiani G, et al.
Donor/recipient mixed chimerism does not predict graft failure in
children with beta-thalassemia given an allogeneic cord blood
transplant from an HLA-identical sibling. Haematologica 2008;93:1859-67.

- Jacobsohn DA, Duerst R, Tse W, Kletzel M.
Reduced intensity haemopoietic stem-cell transplantation for treatment
of non-malignant diseases in children. Lancet 2004;364:156-62.

- Iannone R, Casella JF, Fuchs EJ, et al.
Results of minimally toxic nonmyeloablative transplantation in patients
with sickle cell anemia and beta-thalassemia. Biol Blood Marrow
Transplant 2003;9:519-28.

- Andreani M, Nesci S, Lucarelli G, et al.
Long-term survival of ex-thalassemic patients with persistent mixed
chimerism after bone marrow transplantation. Bone Marrow Transplant
2000;25:401-4.

- Lucarelli G, Gaziev J. Advances in the
allogeneic transplantation for thalassemia. Blood Rev 2008;22:53-63.

- Bernaudin F, Souillet G, Vannier JP, et
al. Bone marrow transplantation (BMT) in 14 children with severe sickle
cell disease (SCD): the French experience. GEGMO. Bone Marrow
Transplant 1993;12 Suppl 1:118-21.

- Vermylen C, Cornu G, Ferster A, et al.
Haematopoietic stem cell transplantation for sickle cell anaemia: the
first 50 patients transplanted in Belgium. Bone Marrow Transplant
1998;22:1-6.

- Walters MC, Patience M, Leisenring W, et
al. Bone marrow transplantation for sickle cell disease. N Engl J Med
1996;335:369-76.

- Walters MC, Storb R, Patience M, et al.
Impact of bone marrow transplantation for symptomatic sickle cell
disease: an interim report. Multicenter investigation of bone marrow
transplantation for sickle cell disease. Blood 2000;95:1918-24.

- Hoppe CC, Walters MC. Bone marrow
transplantation in sickle cell anemia. Curr Opin Oncol 2001;13:85-90.

- Nietert PJ, Abboud MR, Silverstein MD,
Jackson SM. Bone marrow transplantation versus periodic prophylactic
blood transfusion in sickle cell patients at high risk of ischemic
stroke: a decision analysis. Blood 2000;95:3057-64.

- Nietert PJ, Silverstein MD, Abboud MR.
Sickle cell anaemia: epidemiology and cost of illness.
Pharmacoeconomics 2002;20:357-66.

- Wayne AS, Schoenike SE, Pegelow CH.
Financial analysis of chronic transfusion for stroke prevention in
sickle cell disease. Blood 2000;96:2369-72.

- Panepinto JA, Walters MC, Carreras J, et
al. Matched-related donor transplantation for sickle cell disease:
report from the Center for International Blood and Transplant Research.
Br J Haematol 2007;137:479-85.

- Bernaudin F, Socie G, Kuentz M, et al.
Long-term results of related myeloablative stem-cell transplantation to
cure sickle cell disease. Blood 2007;110:2749-56.

- Horan JT, Liesveld JL, Fenton P, Blumberg
N, Walters MC. Hematopoietic stem cell transplantation for multiply
transfused patients with sickle cell disease and thalassemia after
low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte
globulin. Bone Marrow Transplant 2005;35:171-7.

- Walters MC, Patience M, Leisenring W, et
al. Stable mixed hematopoietic chimerism after bone marrow
transplantation for sickle cell anemia. Biol Blood Marrow Transplant
2001;7:665-73.
