Role of Comorbidities in Optimizing Decision-Making for Allogeneic Hematopoietic Cell Transplantation
Mohamed L. Sorror1,2 and Rainer F. Storb1,2
1Fred Hutchinson Cancer Research Center
2University of Washington School of Medicine, Seattle, WA.
Correspondence to: Mohamed
Sorror, M.D., M.Sc., Fred Hutchinson Cancer Research Center, Seattle,
WA 98109-1024, e-mail: msorror@fhcrc.org
Published: June 9, 2010
Received: June 1, 2010
Accepted: June 7, 2010
Medit J Hemat Infect Dis 2010, 2(2): e2010015, DOI 10.4084/MJHID.2010.015
This article is available from: http://www.mjhid.org/article/view/6042
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Allogeneic
conventional hematopoietic cell transplantation (HCT) following
high-dose, myeloablative conditioning regimens has been used since the
1970’s as potentially curative treatment for patients with malignant,
hematological disorders. The toxicities of conditioning regimens have
limited conventional HCT to relatively young patients in otherwise good
medical condition. With the development of less toxic nonmyeloablative
regimens and improvements in supportive care, increasing numbers of
older and medically infirm patients have been treated by allogeneic
HCT. Until recently, there has been almost no effort to evaluate the
prevalence of comorbidities among HCT recipients and their impact on
outcomes. We first evaluated the Charlson Comorbidity Index (CCI)
developed for patients with solid malignancies, for this purpose. While
useful, it lacked sensitivity and specificity for the HCT setting. We
next introduced the HCT-specific comorbidity index (HCT-CI) which was
based on objective laboratory data to better define comorbidities.
Here, we describe this development and illustrate the usefulness of the
HCT-CI in predicting HCT outcomes in patients with myeloid and lymphoid
malignancies undergoing allogeneic transplantation.
Introduction
Conclusions:
The HCT-CI provided simple and reliable scoring of pre-transplant comorbidities that predicted NRM and survival. The index still needs validation among larger patient samples in multi-center settings. Comorbidity data used in the index will likely become as important as defining cancer diagnosis, disease stage and other, more familiar prognostic variables.[31]
Introduction: Allogeneic
conventional HCT is considered potentially curative for patients with
malignant or non-malignant hematological diseases. Conditioning
regimens for conventional HCT have been intensified to the limits of
organ tolerance in order to optimize disease eradication. Consequently,
serious toxicities to organs, such as gut, lung, kidney, heart, and
liver have been observed which, additionally, have limited the ability
to deliver adequate doses of postgrafting immunosuppression needed for
control of GVHD. Until recently, these regimen-related toxicities
associated with myeloablative conditioning have limited allogeneic HCT
to patients without significant co-morbidities who were less than 55 to
60 years old. This age restriction has been unfortunate since the
median ages of patients with most candidate diseases for HCT, e.g.,
acute and chronic leukemias, myelodysplasia (MDS), multiple myeloma,
and lymphomas, have ranged from 65 to 70 years.
In an effort to expand treatment options for patients with hematological malignancies and based on results from a series of canine studies,[1-4] a truly nonmyeloablative regimen of 2 Gy TBI with or without fludarabine, 90 mg/m2, has been introduced to older and medically infirm patients before allogeneic HCT from related or unrelated donors.[5,6] The conditioning regimen’s major role has been host immunosuppression. Effective postgrafting immunosuppression with MMF and CSP has been crucial in this approach with the aim of both enhancing hematopoietic engraftment and controlling GVHD. There has been little direct antitumor effect from the conditioning regimen. Instead, the approach has relied predominantly on the generation of donor T cell (and/or NK cell)-mediated graft-versus-tumor effects for eradication of cancer. The use of this nonmyeloablative regimen has expanded the use of HCT to include elderly and medically infirm patients with various hematological disorders.[7-9]
Age has been frequently cited as an important prognostic variable in HCT. Historical age cutoffs have been 55 and 60 years, respectively, largely influenced by the type of HCT donor (related versus unrelated). The reason for the age cutoffs has been prohibitive regimen-related toxicity and mortality in older patients. It has also been suggested that older patients were at higher risk of GVHD resulting in worse survivals. Most reports on age and HCT outcomes, however, have ignored comorbidities, which might have been confounding factors. Several investigators have studied single organ comorbidities in the context of predicting same organ toxicity after HCT. Comprehensive assessment of the interaction between multiple comorbidities and their impacts on HCT outcomes has become increasingly important given both increasing age of the Western population along with increasing prevalence of cancer and comorbidities[10] and the increasing enrollment of patients aged >60 years in HCT clinical trials.[11]
Comorbidities using the Charlson Comorbidity Index (CCI):
In the field of cancer, investigators have found variable interactions between a given primary disease and different comorbidities based on type and severity of organ involvements. As a result, several indices have been created to rate the impacts of different comorbidities on the primary disease. The Charlson Comorbidity Index (CCI)[12] included 19 comorbidities which have been selected and weighted based on their strength of associations with mortality. The CCI has been the most widely used comorbidity index to predict mortality risks in various solid malignancies[13-21].
We used the CCI in a retrospective study to compare pretransplant comorbidity differences among recipients of nonmyeloablative (n=60) and myeloablative HCT (n=72) from unrelated donors.[22] At the time of HCT, nonmyeloablative patients had more often high-risk diseases (P=0.02); were older (median age, 54 versus 41 years, P<0.0001); had more preceding chemotherapy regimens (3 versus 1, P=0.01); had more frequently failed myeloablative HCT (P<0.0001); and received more often peripheral blood stem cell grafts (P<0.0001) than myeloablative patients. In addition, nonmyeloablative patients had higher CCI scores compared to myeloablative patients (scores of 1-2 and 3, 35% and 18% compared to 12% and 0%, respectively, P<0.0001) at the time of HCT.
After HCT, nonmyeloablative patients experienced less gastrointestinal (P<0.0001), hepatic (P=0.02), hemorrhagic (P=0.005), infectious (P=0.09), and metabolic (P=0.03) grades III-IV toxicities. Further, there were trends for less neurological, renal, and pulmonary grades III-IV toxicities (P=0.1 for each). In particular, nonmyeloablative patients had less (32% versus 69%, P<0.0001) overall grade IV (life-threatening) toxicities than myeloablative patients. No single cases of veno-occlusive disease or mucositis was detected among nonmyeloablative compared to 18% and 72% among myeloablative patients, respectively. Also, nonmyeloablative patients experienced less grades III-IV acute GVHD (P=0.03). The lessened cumulative incidences of day 100 (12% versus 18%, P=1.4) and 1-year (20% versus 32%, P=1.4) NRM among nonmyeloablative patients did not reach statistical significance. After adjustment for pretransplant differences, including comorbidity scores, statistically suggestive or significant lower hazard ratios (HR) for day 100 (0.2, P=0.07) and 1-year (0.3, P=0.04) NRM were found for nonmyeloablative patients, confirming the importance of a single scoring system for comorbidities. In multivariate analyses of risk factors for outcomes, comorbidities as scored by the CCI, proved to be the only independent factor for predicting overall grade IV toxicity (HR were 2.9 and 5.5 for scores 1-2 and ≥ 3, respectively, p=0.06) and NRM (HR were 2.4 and 10.5, respectively, p=0.04). Cumulative incidence and Kaplan Maier curves showed linear increases in overall grade IV toxicities, NRM, and worsening survival with increasing CCI scores, whereby better outcomes were observed among nonmyeloablative compared to myeloablative patients with similar CCI scores. In a concurrent study, the CCI was important in predicting NRM among recipients of HLA-matched related HCT.[23]
An HCT-specific comorbidity index (HCT-CI):
The CCI lacked sensitivity in detecting several comorbidities among HCT recipients, given that scores >0 were detected among only 35% of all HCT patients (12% among myeloablative patients).[22] This was thought to be due to not well-defined definitions of some comorbidities, such as hepatic and pulmonary. In addition, relatively frequent comorbidities among HCT patients, such as infections, were not included in the CCI.
In order to improve sensitivity, a study was designed which included 1055 consecutive recipients of allogeneic HCT between 1998 and 2004 who had various hematological diseases, and of whom 249 received nonmyeloablative and 761 myeloablative conditioning. Patients were randomly assigned to training (n=708) and validation (n=347) sets.[24] Novel definitions were modeled for hepatic and renal comorbidities by using actual laboratory data and for pulmonary and cardiac comorbidities by using test results of organ function. Also, new integer weights of comorbidities were calculated based on HRs from Cox proportional hazard models of 2-year NRM, which were adjusted for disease risk, age, and conditioning regimen intensity. The new HCT-CI consisted of 17 comorbidities including three comorbidities that were not represented in the CCI, obesity, peritransplant infections, and psychiatric disturbances. HCT-CI scores of 0, 1, 2, 3, and ≥ 4 predicted 2-year NRM of 9%, 14%, 27%, 41%, and 43%, respectively, among patients of the training set.
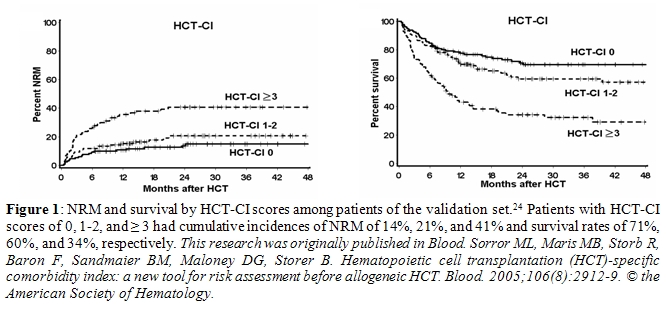
When applied to data from the validation set, HCT-CI scores of 1-2 and ≥ 3 were found in 34% and 28% of patients compared to CCI scores of 1 and 2 in only 10% and 3% of patients, respectively. Most importantly, HCT-CI scores of 0, 1-2, and ≥ 3 showed linear predictions of NRM (14%, 21%, and 41%) and survival (71%, 60%, and 34%), respectively (Figure 1). In addition, HCT-CI scores had higher discriminative power than CCI scores both for NRM (c statistic of 0.692 versus 0.546, P < 0.001) and survival (c statistic of 0.661 versus 0.561, P < 0.001).
HCT-CI and outcomes after conditioning regimens of different intensities:
Patients with acute myeloid leukemia (AML) or myelodysplasia (MDS). We compared outcomes among patients with AML (n=391) or MDS (n=186) given either nonmyeloablative (n=125) or myeloablative HCT (n=452).[25] The median age of nonmyeloablative patients was 60 years compared to 46 years among myeloablative patients. In an initial analysis of outcomes among all patients, high HCT-CI scores and high disease risk independently predicted non-relapse mortality (NRM, p<0.0001 and p=0.004), overall survival (OS, p<0.0001 and p<0.0001), and relapse-free survival (RFS, p<0.0001 and p<0.0001), respectively. This allowed us to divide patients into four risk groups based both on comorbidities and disease risks (Table 1).
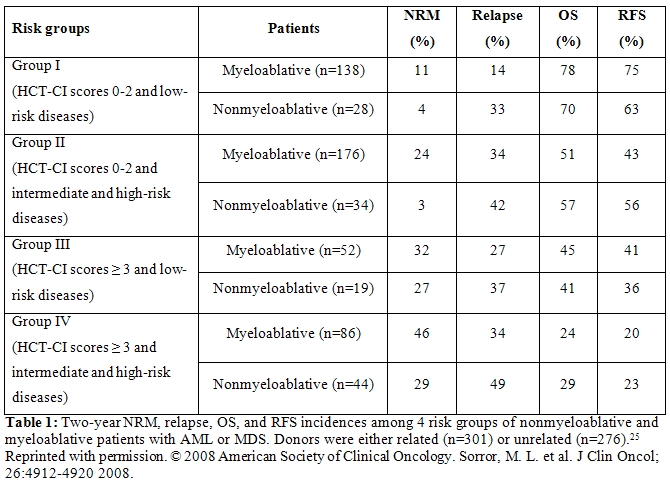
Cumulative incidences of NRM tended to be lower and relapse rates higher among nonmyeloablative compared to myeloablative patients resulting in comparable rates of OS and RFS across all risk groups, even though nonmyeloablative patients were older than those given myeloablative conditioning. Novel anti-tumor agents combined with nonmyeloablative HCT should be explored among patients with high comorbidity scores and advanced disease.[25]
Patients with lymphoma or chronic lymphocytic leukemia (CLL). Myeloablative allogeneic HCT has been associated with high regimen-related mortality (up to 60%) among patients with lymphoma or CLL.[26-29] In order to get around this problem, nonmyeloablative conditioning regimens have been explored. A recent analysis compared outcomes among 152 older (median age, 60 years) patients given nonmyeloablative conditioning to those among 68 younger (median age, 46 years) patients given myeloablative conditioning, stratifying for the HCT-CI.[30]
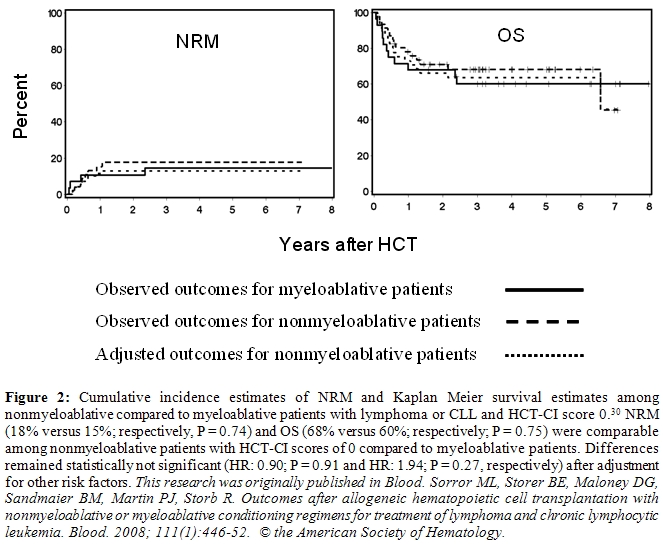
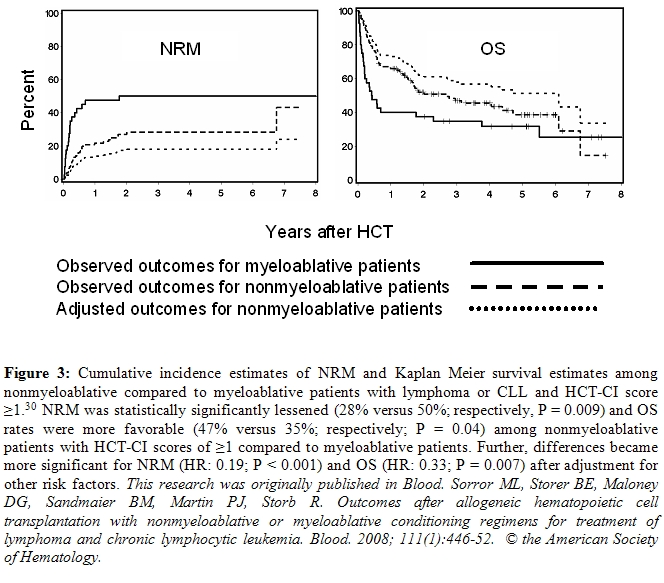
We found that patients without comorbidities both in the nonmyeloablative and myeloablative cohorts had comparable NRM, OS, and progression-free survivals (Figure 2). However, nonmyeloablative patients with comorbidities had lower NRM (p = 0.009) and better OS (p = 0.04) than myeloablative patients (Figure 3). These differences became more significant after adjusting for other variables; also adjusted progression-free survival was better (p = 0.01). This suggests that younger patients with comorbidities would benefit from reducing conditioning intensity.
In an effort to expand treatment options for patients with hematological malignancies and based on results from a series of canine studies,[1-4] a truly nonmyeloablative regimen of 2 Gy TBI with or without fludarabine, 90 mg/m2, has been introduced to older and medically infirm patients before allogeneic HCT from related or unrelated donors.[5,6] The conditioning regimen’s major role has been host immunosuppression. Effective postgrafting immunosuppression with MMF and CSP has been crucial in this approach with the aim of both enhancing hematopoietic engraftment and controlling GVHD. There has been little direct antitumor effect from the conditioning regimen. Instead, the approach has relied predominantly on the generation of donor T cell (and/or NK cell)-mediated graft-versus-tumor effects for eradication of cancer. The use of this nonmyeloablative regimen has expanded the use of HCT to include elderly and medically infirm patients with various hematological disorders.[7-9]
Age has been frequently cited as an important prognostic variable in HCT. Historical age cutoffs have been 55 and 60 years, respectively, largely influenced by the type of HCT donor (related versus unrelated). The reason for the age cutoffs has been prohibitive regimen-related toxicity and mortality in older patients. It has also been suggested that older patients were at higher risk of GVHD resulting in worse survivals. Most reports on age and HCT outcomes, however, have ignored comorbidities, which might have been confounding factors. Several investigators have studied single organ comorbidities in the context of predicting same organ toxicity after HCT. Comprehensive assessment of the interaction between multiple comorbidities and their impacts on HCT outcomes has become increasingly important given both increasing age of the Western population along with increasing prevalence of cancer and comorbidities[10] and the increasing enrollment of patients aged >60 years in HCT clinical trials.[11]
Comorbidities using the Charlson Comorbidity Index (CCI):
In the field of cancer, investigators have found variable interactions between a given primary disease and different comorbidities based on type and severity of organ involvements. As a result, several indices have been created to rate the impacts of different comorbidities on the primary disease. The Charlson Comorbidity Index (CCI)[12] included 19 comorbidities which have been selected and weighted based on their strength of associations with mortality. The CCI has been the most widely used comorbidity index to predict mortality risks in various solid malignancies[13-21].
We used the CCI in a retrospective study to compare pretransplant comorbidity differences among recipients of nonmyeloablative (n=60) and myeloablative HCT (n=72) from unrelated donors.[22] At the time of HCT, nonmyeloablative patients had more often high-risk diseases (P=0.02); were older (median age, 54 versus 41 years, P<0.0001); had more preceding chemotherapy regimens (3 versus 1, P=0.01); had more frequently failed myeloablative HCT (P<0.0001); and received more often peripheral blood stem cell grafts (P<0.0001) than myeloablative patients. In addition, nonmyeloablative patients had higher CCI scores compared to myeloablative patients (scores of 1-2 and 3, 35% and 18% compared to 12% and 0%, respectively, P<0.0001) at the time of HCT.
After HCT, nonmyeloablative patients experienced less gastrointestinal (P<0.0001), hepatic (P=0.02), hemorrhagic (P=0.005), infectious (P=0.09), and metabolic (P=0.03) grades III-IV toxicities. Further, there were trends for less neurological, renal, and pulmonary grades III-IV toxicities (P=0.1 for each). In particular, nonmyeloablative patients had less (32% versus 69%, P<0.0001) overall grade IV (life-threatening) toxicities than myeloablative patients. No single cases of veno-occlusive disease or mucositis was detected among nonmyeloablative compared to 18% and 72% among myeloablative patients, respectively. Also, nonmyeloablative patients experienced less grades III-IV acute GVHD (P=0.03). The lessened cumulative incidences of day 100 (12% versus 18%, P=1.4) and 1-year (20% versus 32%, P=1.4) NRM among nonmyeloablative patients did not reach statistical significance. After adjustment for pretransplant differences, including comorbidity scores, statistically suggestive or significant lower hazard ratios (HR) for day 100 (0.2, P=0.07) and 1-year (0.3, P=0.04) NRM were found for nonmyeloablative patients, confirming the importance of a single scoring system for comorbidities. In multivariate analyses of risk factors for outcomes, comorbidities as scored by the CCI, proved to be the only independent factor for predicting overall grade IV toxicity (HR were 2.9 and 5.5 for scores 1-2 and ≥ 3, respectively, p=0.06) and NRM (HR were 2.4 and 10.5, respectively, p=0.04). Cumulative incidence and Kaplan Maier curves showed linear increases in overall grade IV toxicities, NRM, and worsening survival with increasing CCI scores, whereby better outcomes were observed among nonmyeloablative compared to myeloablative patients with similar CCI scores. In a concurrent study, the CCI was important in predicting NRM among recipients of HLA-matched related HCT.[23]
An HCT-specific comorbidity index (HCT-CI):
The CCI lacked sensitivity in detecting several comorbidities among HCT recipients, given that scores >0 were detected among only 35% of all HCT patients (12% among myeloablative patients).[22] This was thought to be due to not well-defined definitions of some comorbidities, such as hepatic and pulmonary. In addition, relatively frequent comorbidities among HCT patients, such as infections, were not included in the CCI.
In order to improve sensitivity, a study was designed which included 1055 consecutive recipients of allogeneic HCT between 1998 and 2004 who had various hematological diseases, and of whom 249 received nonmyeloablative and 761 myeloablative conditioning. Patients were randomly assigned to training (n=708) and validation (n=347) sets.[24] Novel definitions were modeled for hepatic and renal comorbidities by using actual laboratory data and for pulmonary and cardiac comorbidities by using test results of organ function. Also, new integer weights of comorbidities were calculated based on HRs from Cox proportional hazard models of 2-year NRM, which were adjusted for disease risk, age, and conditioning regimen intensity. The new HCT-CI consisted of 17 comorbidities including three comorbidities that were not represented in the CCI, obesity, peritransplant infections, and psychiatric disturbances. HCT-CI scores of 0, 1, 2, 3, and ≥ 4 predicted 2-year NRM of 9%, 14%, 27%, 41%, and 43%, respectively, among patients of the training set.
When applied to data from the validation set, HCT-CI scores of 1-2 and ≥ 3 were found in 34% and 28% of patients compared to CCI scores of 1 and 2 in only 10% and 3% of patients, respectively. Most importantly, HCT-CI scores of 0, 1-2, and ≥ 3 showed linear predictions of NRM (14%, 21%, and 41%) and survival (71%, 60%, and 34%), respectively (Figure 1). In addition, HCT-CI scores had higher discriminative power than CCI scores both for NRM (c statistic of 0.692 versus 0.546, P < 0.001) and survival (c statistic of 0.661 versus 0.561, P < 0.001).

HCT-CI and outcomes after conditioning regimens of different intensities:
Patients with acute myeloid leukemia (AML) or myelodysplasia (MDS). We compared outcomes among patients with AML (n=391) or MDS (n=186) given either nonmyeloablative (n=125) or myeloablative HCT (n=452).[25] The median age of nonmyeloablative patients was 60 years compared to 46 years among myeloablative patients. In an initial analysis of outcomes among all patients, high HCT-CI scores and high disease risk independently predicted non-relapse mortality (NRM, p<0.0001 and p=0.004), overall survival (OS, p<0.0001 and p<0.0001), and relapse-free survival (RFS, p<0.0001 and p<0.0001), respectively. This allowed us to divide patients into four risk groups based both on comorbidities and disease risks (Table 1).

Cumulative incidences of NRM tended to be lower and relapse rates higher among nonmyeloablative compared to myeloablative patients resulting in comparable rates of OS and RFS across all risk groups, even though nonmyeloablative patients were older than those given myeloablative conditioning. Novel anti-tumor agents combined with nonmyeloablative HCT should be explored among patients with high comorbidity scores and advanced disease.[25]
Patients with lymphoma or chronic lymphocytic leukemia (CLL). Myeloablative allogeneic HCT has been associated with high regimen-related mortality (up to 60%) among patients with lymphoma or CLL.[26-29] In order to get around this problem, nonmyeloablative conditioning regimens have been explored. A recent analysis compared outcomes among 152 older (median age, 60 years) patients given nonmyeloablative conditioning to those among 68 younger (median age, 46 years) patients given myeloablative conditioning, stratifying for the HCT-CI.[30]
We found that patients without comorbidities both in the nonmyeloablative and myeloablative cohorts had comparable NRM, OS, and progression-free survivals (Figure 2). However, nonmyeloablative patients with comorbidities had lower NRM (p = 0.009) and better OS (p = 0.04) than myeloablative patients (Figure 3). These differences became more significant after adjusting for other variables; also adjusted progression-free survival was better (p = 0.01). This suggests that younger patients with comorbidities would benefit from reducing conditioning intensity.


Conclusions:
The HCT-CI provided simple and reliable scoring of pre-transplant comorbidities that predicted NRM and survival. The index still needs validation among larger patient samples in multi-center settings. Comorbidity data used in the index will likely become as important as defining cancer diagnosis, disease stage and other, more familiar prognostic variables.[31]
References
- Storb R, Raff RF, Appelbaum FR, Schuening
FW, Sandmaier BM, Graham TC, Thomas ED. What radiation dose for
DLA-identical canine marrow grafts? Blood 1988;72:1300-1304.

- Storb R, Raff RF, Appelbaum FR, Deeg HJ,
Graham TC, Schuening FG, Shulman H, Yu C, Bryant E, Burnett R, Seidel
K. DLA-identical bone marrow grafts after low-dose total body
irradiation: the effect of canine recombinant hematopoietic growth
factors. Blood 1994;84:3558-3566.

- Yu C, Storb R, Mathey B, Deeg HJ, Schuening
FG, Graham TC, Seidel K, Burnett R, Wagner JL, Shulman H, Sandmaier
BM. DLA-identical bone marrow grafts after low-dose total body
irradiation: Effects of high-dose corticosteroids and cyclosporine on
engraftment. Blood 1995;86:4376-4381.

- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA,
Kiem H-P, Leisenring W, Shulman H. Stable mixed hematopoietic
chimerism in DLA-identical littermate dogs given sublethal total body
irradiation before and pharmacological immunosuppression after marrow
transplantation. Blood 1997;89(8):3048-3054.

- McSweeney PA, Niederwieser D, Shizuru JA,
Sandmaier BM, Molina AJ, Maloney DG, Chauncey TR, Gooley TA, Hegenbart
U, Nash RA, Radich J, Wagner JL, Minor S, Appelbaum FR, Bensinger WI,
Bryant E, Flowers MED, Georges GE, Grumet FC, Kiem H-P, Torok-Storb B,
Yu C, Blume KG, Storb RF. Hematopoietic cell transplantation in
older patients with hematologic malignancies: replacing high-dose
cytotoxic therapy with graft-versus-tumor effects. Blood
2001;97(11):3390-3400.

- Maris MB, Niederwieser D, Sandmaier BM,
Storer B, Stuart M, Maloney D, Petersdorf E, McSweeney P, Pulsipher M,
Woolfrey A, Chauncey T, Agura E, Heimfeld S, Slattery J, Hegenbart U,
Anasetti C, Blume K, Storb R. HLA-matched unrelated donor
hematopoietic cell transplantation after nonmyeloablative conditioning
for patients with hematologic malignancies. Blood 2003;102(6):2021-2030.

- Maris MB, Sandmaier BM, Storer BE, Chauncey
T, Stuart MJ, Maziarz RT, Agura E, Langston AA, Pulsipher M, Storb R,
Maloney DG. Allogeneic hematopoietic cell transplantation after
fludarabine and 2 Gy total body irradiation for relapsed and refractory
mantle cell lymphoma. Blood 2004;104(12):3535-3542.

- Sorror ML, Maris MB, Sandmaier BM, Storer
BE, Stuart MJ, Hegenbart U, Agura E, Chauncey TR, Leis J, Pulsipher M,
McSweeney P, Radich JP, Bredeson C, Bruno B, Langston A, Loken MR,
Al-Ali H, Blume KG, Storb R, Maloney DG. Hematopoietic cell
transplantation after nonmyeloablative conditioning for advanced
chronic lymphocytic leukemia. J Clin Oncol 2005;23(16):3819-3829.

- Maloney DG, Molina AJ, Sahebi F,
Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, Storer B, Hegenbart
U, Somlo G, Chauncey T, Bruno B, Appelbaum FR, Blume KG, Forman SJ,
McSweeney P, Storb R. Allografting with nonmyeloablative
conditioning following cytoreductive autografts for the treatment of
patients with multiple myeloma. Blood 2003;102(9):3447-3454.

- Piccirillo JF, Vlahiotis A, Barrett LB,
Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence
of comorbidity across the age spectrum. Critical Reviews in
Oncology-Hematology 2008;67(2):124-132.

- Sorror ML, Storer B, Sandmaier BM, Maloney DG, Franke G, Shizuru J, Chauncey T, Agura E, Maziarz RT, Sahebi F, Langston AA, Wade JC, Maris M, Bruno B, Yeager AM, Pulsipher M, Petersen F, Bethge WA, McSweeney PA, Niederwieser D, Blume KG, Storb RF. Allogeneic hematopoietic cell transplantation (HCT) after nonmyeloablative conditioning for patients (pts) aged > or = to 60 years [abstract]. Blood. 2008;112(11):753-754, #2162.
- Charlson ME, Pompei P, Ales KL, MacKenzie
CR. A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic Dis
1987;40(5):373-383.

- Di Iorio B, Cillo N, Cirillo M, De Santo
NG. Charlson Comorbidity Index is a predictor of outcomes in
incident hemodialysis patients and correlates with phase angle and
hospitalization. Int J Artif Organs 2004;27(4):330-336.

- Goldstein LB, Samsa GP, Matchar DB, Horner
RD. Charlson Index comorbidity adjustment for ischemic stroke
outcome studies. Stroke 2004;35(8):1941-1945.

- Hemmelgarn BR, Manns BJ, Quan H, Ghali
WA. Adapting the Charlson Comorbidity Index for use in patients
with ESRD. American Journal of Kidney Diseases 2003;42(1):125-132.

- Sachdev M, Sun JL, Tsiatis AA, Nelson CL,
Mark DB, Jollis JG. The prognostic importance of comorbidity for
mortality in patients with stable coronary artery disease. J Am Coll
Cardiol 2004;43(4):576-582.

- Lubke T, Monig SP, Schneider PM, Holscher AH, Bollschweiler E. Does Charlson-comorbidity index correlate with short-term outcome in patients with gastric cancer? [German]. Zentralblatt fur Chirurgie 2003;128(11):970-976.
- Firat S, Byhardt RW, Gore E. Comorbidity
and Karnofksy performance score are independent prognostic factors in
stage III non-small-cell lung cancer: an institutional analysis of
patients treated on four RTOG studies. Radiation Therapy Oncology
Group. Int J Radiat Oncol Biol Phys 2002;54(2):357-364.

- Sabin SL, Rosenfeld RM, Sundaram K, Har-el
G, Lucente FE. The impact of comorbidity and age on survival with
laryngeal cancer. Ear, Nose, & Throat Journal 1999;78(8):578-4.

- Singh B, Bhaya M, Stern J, Roland JT,
Zimbler M, Rosenfeld RM, Har-el G, Lucente FE. Validation of the
Charlson comorbidity index in patients with head and neck cancer: a
multi-institutional study. Laryngoscope 1997;107(11 Pt 1):1469-1475.

- Extermann M. Measuring comorbidity in
older cancer patients (Review). Eur J Cancer 2000;36(4):453-471.

- Sorror ML, Maris MB, Storer B, Sandmaier
BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing
morbidity and mortality of HLA-matched unrelated donor hematopoietic
cell transplantation after nonmyeloablative and myeloablative
conditioning: influence of pretransplant comorbidities. Blood
2004;104(4):961-968.

- Diaconescu R, Flowers CR, Storer B, Sorror
ML, Maris MB, Maloney DG, Sandmaier BM, Storb R. Morbidity and
mortality with nonmyeloablative compared to myeloablative conditioning
before hematopoietic cell transplantation from HLA matched related
donors. Blood 2004;104(5):1550-1558.

- Sorror ML, Maris MB, Storb R, Baron F,
Sandmaier BM, Maloney DG, Storer B. Hematopoietic cell
transplantation (HCT)-specific comorbidity index: a new tool for risk
assessment before allogeneic HCT. Blood 2005;106(8):2912-2919.

- Sorror ML, Sandmaier BM, Storer BE, Maris
MB, Baron F, Maloney DG, Scott BL, Deeg HJ, Appelbaum FR, Storb
R. Comorbidity and disease status-based risk stratification of
outcomes among patients with acute myeloid leukemia or myelodysplasia
receiving allogeneic hematopoietic cell transplantation. J Clin Oncol
2007;25(27):4246-4254.

- Michallet M, Archimbaud E, Bandini G,
Rowlings PA, Deeg HJ, Gahrton G, Montserrat E, Rozman C, Gratwohl A,
Gale RP. HLA-identical sibling bone marrow transplantation in
younger patients with chronic lymphocytic leukemia. European Group for
Blood and Marrow Transplantation and the International Bone Marrow
Transplant Registry. Ann Intern Med 1996;124(3):311-315.

- Gribben JG, Zahrieh D, Stephans K,
Bartlett-Pandite L, Alyea EP, Fisher DC, Freedman AS, Mauch P,
Schlossman R, Sequist LV, Soiffer RJ, Marshall B, Neuberg D, Ritz J,
Nadler LM. Autologous and allogeneic stem cell transplantations
for poor-risk chronic lymphocytic leukemia. Blood
2005;106(13):4389-4396.

- Akpek G, Ambinder RF, Piantadosi S, Abrams
RA, Brodsky RA, Vogelsang GB, Zahurak ML, Fuller D, Miller CB, Noga SJ,
Fuchs E, Flinn IW, O'Donnell P, Seifter EJ, Mann RB, Jones RJ.
Long-term results of blood and marrow transplantation for Hodgkin's
lymphoma. J Clin Oncol 2001;19(23):4314-4321.

- de Lima M, van Besien KW, Giralt SA,
Khouri IF, Mehra R, Andersson BS, Przepiorka D, Gajewski JL, Korbling
M, Champlin RE. Bone marrow transplantation after failure of
autologous transplant for non-Hodgkin's lymphoma. Bone Marrow
Transplant 1997;19:121-127.

- Sorror ML, Storer BE, Maloney DG,
Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic
hematopoietic cell transplantation with nonmyeloablative or
myeloablative regimens for treatment of lymphoma and chronic
lymphocytic leukemia. Blood 2008;111(1):446-452.

- Jones RB. HCT outcomes: a new tool?
Blood 2005;106(8):2602-2603.
