Clinical Analysis and Optimization of Postremission Therapy for Acute Myeloid Leukemia Patients with Minimal Residual Disease as Determined by Flow Cytometry.
Daichi Inoue1, Hayato Maruoka2 and Takayuki Takahashi1
Department of Hematology and Clinical Immunology1, Clinical Laboratory2, Kobe City Medical Center General Hospital, Kobe, Japan.
Correspondence to: Takayuki Takahashi, Department of Hematology and Clinical Immunology, Kobe City Medical Center General Hospital, 4-6 Minatojima-Nakamachi, Chuo-ku, Kobe 650-0046, Japan. Tel: 81-78-302-4321, Fax: 81-78-302-7537. e-mail: sonata53@kcgh.gr.jp
Published: August 5, 2010
Received: June 6, 2010
Accepted: August 2, 2010
Medit J Hemat Infect Dis 2010, 2(2): e2010020, DOI 10.4084/MJHID.2010.020
This article is available from: http://www.mjhid.org/article/view/6036
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Background: Although
several
prognostic indicators of de novo acute myeloid leukemia (AML) patients
have
been identified, the clinical significance of minimal residual disease
(MRD)
needs to be evaluated further in Japanese adult patients.
Methods: Using three color flow cytometry, we identified leukemia-associated phenotypes (LAP) in bone marrow specimens at diagnosis and assessed the relationship between clinical outcomes and the presence of marrow MRD in 33 patients who achieved a morphologic complete remission (CR) and were followed after CR.
Results: Of 33 consecutive patients, we detected MRD in 20 patients after achieving CR (Group A) and did not in 13 patients (Group B), with 2-year overall survival (OS) rates of 49.0% and 84.6%, respectively (P =.0317), and relapse-free survival (RFS) rates of 13.7% and 91.7%, respectively (P=.0010). By multivariate analysis, MRD-positivity at post-induction was found to be associated with a shorter duration of RFS (P=.0042). Notably, we achieved MRD negativity in only 2 patients (10%) of Group A in spite of subsequent intensive consolidation therapies and found that the fluctuation of the MRD level during consolidation therapies was not a significant prognostic factor. Four patients in Group A underwent allogeneic hematopoietic stem-cell transplantation (HSCT) when in the CR state and did not experience relapse at a median follow-up period of 20.5 months after HSCT.
Conclusions: MRD is critical for predicting de novo AML outcomes. Most MRD-positive patients cannot achieve MRD negativity with conventional chemotherapy. Thus, HSCT may be the primary therapeutic option for these patients.
Methods: Using three color flow cytometry, we identified leukemia-associated phenotypes (LAP) in bone marrow specimens at diagnosis and assessed the relationship between clinical outcomes and the presence of marrow MRD in 33 patients who achieved a morphologic complete remission (CR) and were followed after CR.
Results: Of 33 consecutive patients, we detected MRD in 20 patients after achieving CR (Group A) and did not in 13 patients (Group B), with 2-year overall survival (OS) rates of 49.0% and 84.6%, respectively (P =.0317), and relapse-free survival (RFS) rates of 13.7% and 91.7%, respectively (P=.0010). By multivariate analysis, MRD-positivity at post-induction was found to be associated with a shorter duration of RFS (P=.0042). Notably, we achieved MRD negativity in only 2 patients (10%) of Group A in spite of subsequent intensive consolidation therapies and found that the fluctuation of the MRD level during consolidation therapies was not a significant prognostic factor. Four patients in Group A underwent allogeneic hematopoietic stem-cell transplantation (HSCT) when in the CR state and did not experience relapse at a median follow-up period of 20.5 months after HSCT.
Conclusions: MRD is critical for predicting de novo AML outcomes. Most MRD-positive patients cannot achieve MRD negativity with conventional chemotherapy. Thus, HSCT may be the primary therapeutic option for these patients.
Introduction
Despite the high remission rate (approaching 80% in younger adults) seen after intensive chemotherapy in patients with de novo acute myeloid leukemia (AML), only 30% to 40% of these patients survive for 5 years after diagnosis.[1] Unfortunately, many patients experience relapse, probably due to the presence of minimal residual disease (MRD). Regarding the prognostic factors for post-remission relapse, a great effort has been made to detect and characterize MRD using various prognostic and therapeutic strategies. For example, specific gene rearrangements such as PML/RARα, AML1/ETO, and CBFB/MYH11 have been used to represent MRD using nested reverse transcriptase-polymerase chain reaction (RT-PCR).[2-4] However, this technique applicable in only 20% to 30% of AML patients.[5] Furthermore, AML1/ETO or CBFB/MYH11 transcripts are often detectable in patients who were considered to be cured of leukemia.[4,6] Therefore, PCR for the detection of these transcripts after complete remission (CR) may be an equivocal tool because the significance of MRD positivity is undetermined, although MRD positivity on PCR closely correlates with a subsequent relapse in patients with the PML/RARα fusion gene.[2,3] On the other hand, multiparameter flow cytometry (MFC) may be a useful method for MRD detection as it defines leukemia-associated phenotypes (LAP) in AML cells at diagnosis in almost all AML patients.[7] Several reports have been published showing that MRD detection using MFC at post-induction/-consolidation is a valuable tool for predicting relapse.[8-15] However, to our best knowledge, there is little information regarding the use of MRD detection by MFC in Japanese AML patients for the optimization of post-remission therapy. Therefore, we attempted to identify characteristic LAP from bone marrow specimens taken at diagnosis in individual patients using three color flow cytometry and assessed the relationship between LAP and clinical outcomes.
Patients and Method
Eligibility: A total of 33 consecutive adult de novo AML patients with LAP who were diagnosed between April 2006 and March 2009 and treated to accomplish a morphologic CR at the Department of Hematology and Clinical Immunology, Kobe City Medical Center General Hospital (Kobe, Japan) were analyzed. We experienced a total of 49 AML patients in the period; 40 with LAP and 9 without LAP. The achievement of a morphologic CR after inductinon chemotherapy was a criterion for inclusion in this study, while the exclusion criteria were AML without LAP; myelodysplastic syndrome (MDS)-derived overt AML; therapy-related AML; and PML/RARα-associated AML (AML-M3), in which RT-PCR has been reported to be a most valuable tool as described above [2,3]. Approval for this study was obtained from the institutional review board.
Treatment Protocols
As the induction chemotherapy, combined cytarabine (100 mg/m2/day, days 1-7) and daunorubicin (45 mg/m2/day, days 1-3) were administered. In the case of induction failure, re-induction chemotherapy composed of enocitabine (200 mg/m2/day, days 1-7), mitoxantrone (8 mg/m2/day, days 1-3), and etoposide (100 mg/m2/day, days 1-5) was performed. For the refractory cases, which could not achieve a hematologic CR after 2 courses of induction therapy, salvage regimens depended on the physicians’ choice. As consolidation chemotherapy, the patients received high-dose cytarabine (6 g/m2/day, days 1-3) for 4 cycles. When the patients were more than 70 years old, had impaired kidney, heart, lung, or liver function, the dosage of chemotherapeutic agents was reduced 30% to 50% when considered necessary by the physicians. We performed allogeneic hematopoietic stem cell transplantation (allo-HSCT) for suitable cases, which were chosen according to the Japan Society for Hematopoietic Cell Transplantation (JSHCT) guidelines (JSHCT monograph Vol. 6: http://www.jshct.com/guidline/ , The Japan Society for Hematopoietic Cell Transplantation Web).
Detection of LAP and MRD
At presentation, immunophenotypic studies using 3-color flow cytometry were performed to detect LAP in bone marrow specimens. AML cells were identified using a CD45/side scatter gating strategy, and LAP were detected by staining leukemia cells with a triple fluorescence assay, which consisted of staining for CD45 and that for other two antigens, including myeloid/monocytic markers (CD11b, 13, 14, 33, 36, 86, 117), lymphoid markers (CD2, 4, 7, 19, 56), and miscellaneous markers (CD34, HLA-DR),[7] as shown in table 1.
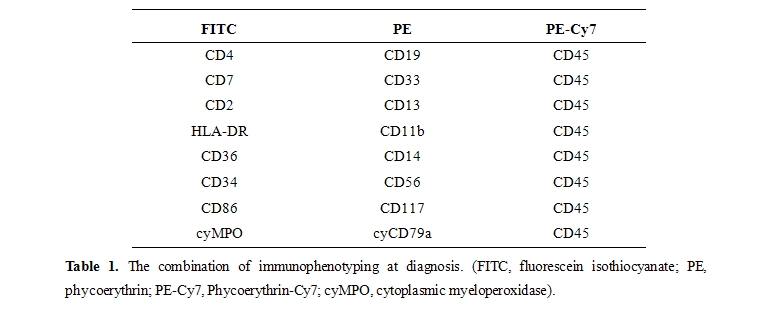
A given combination of markers was regarded as relevant if it was expressed in at least 50% of blasts. Two or more antibody combinations, when possible, were employed to determine the LAP for each patient. The CellQuest software (Becton Dickinson, Mountain View, CA) was used for MFC data acquisition.16 MRD studies during CR were performed on erythrocyte-lysed whole bone marrow cells using the same antibody combination as used for each LAP at diagnosis. To abstract myeloblast group efficiently, instead of erythroblasts, monocytes, or promyelocytes, we adopted allophycocyanin (APC)-conjugated anti-CD34 and phycoerythrin-Cy7 (PE-Cy7)-conjugated anti-CD45 monoclonal antibodies gating strategy after CD45/side scatter gating. We determined a cell group as MRD only when the group was definitely different from its normal counterpart in the expression of antigens described as above and comprised more than 0.10% of total nucleated cells.
Statistical analysis
Overall survival (OS) and relapse free survival (RFS) were calculated from the date on which we first accomplished morphologic CR. CR, relapse, and RFS were defined by standardized criteria.[17] The Kaplan-Meier method was used to estimate OS and RFS. For comparisons of OS and RFS between two groups, the log-rank test was applied. To evaluate the independent effects of different variables on the duration of OS and RFS, multivariate analysis was performed, using the Cox proportional hazards model with predictive variables that were significant in the univariate analysis. The other statistical tests included t tests, χ2 tests, and Fisher’s exact tests. All calculations were made using the program JMP 8.0 (SAS Institute, Cary, NC, US). All P values of <0.05 were considered to be significant.
Results
Clinical Characteristics of AML Patient: The clinical characteristics of 33 AML patients are shown in table 2.
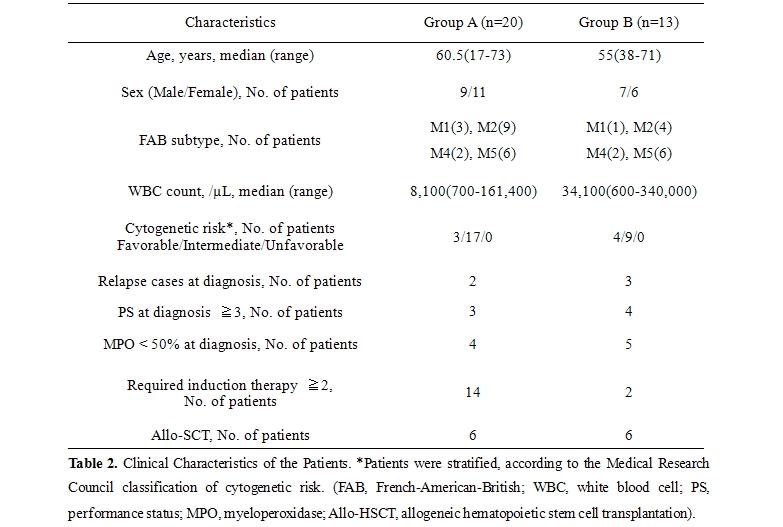
We classified the patients into Group A (20 patients, MRD positive) and B (13 patients, MRD negative), according to the detection of MRD by MFC at the first hematologic CR. The median follow up period was 16 months (4-42 months). Between Group A and B, there was no significant difference regarding age, sex predominance, French-American-British (FAB) subtype, white blood cell (WBC) count, cytogenetic risk, performance status before treatment, or the proportion of patients in which less than 50% of AML cells were positive for myeloperoxidase (MPO), although the number of patients who required 2 or more remission induction therapies to achieve hematologic CR was 14 in Group A and 2 in Group B (P=.0067). All of these factors other than sex were previously suggested as unfavorable prognostic factors in Japanese patients.[18] We were obliged to reduce the treatment intensity for 9 and 5 patients in Group A and B, respectively. Allogeneic stem-cell transplantation (allo-SCT) was performed in 6 patients of Group A and 6 of Group B, without statistical significance. Seven of 9 AML patients without LAP achieved morphological CR within 2 courses of chemotherapy, with 2-year OS rate of 55.6%. On the other hand, 33 of 40 patients with LAP accomplished a morphologic CR, with 2-year OS rate of 63.0%.
Impact of MRD After CR Induction: As shown in figure 1, Group A exhibited a significantly lower survival rate than Group B (P=.0317) with 2-year OS rates of 49.0% and 84.6%, respectively. Notably, in Group A, the patients who underwent single induction therapy were found to be more likely to show better survival than those who required 2 or more rounds of induction therapy (P=.1475) with 2-year OS rates of 75.0% and 39.4% respectively. Moreover, Group A showed much worse RFS than Group B (P=.0010) with 2-year RFS rates of 13.7% and 91.7%, respectively.
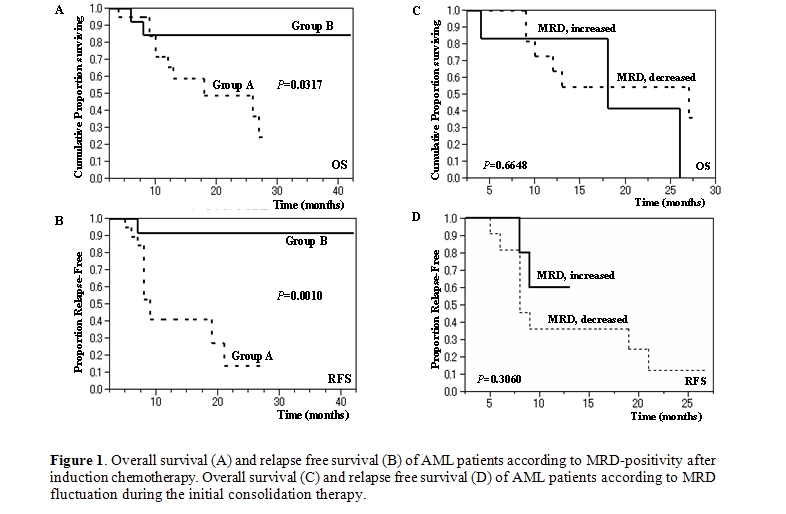
Impact of MRD Fluctuation After CR Induction: We divided Group A into 2 subgroups: ‘MRD increased (11 patients)’ and ‘MRD decreased (6 patients)’ according to the effects of the first consolidation chemotherapy administered when the patient was in a morphologic CR state. The remaining patients consisted of one patient who relapsed after the first post-induction therapy and two who had a nearly stable MRD level. The level of MRD at postinduction and postconsolidation (3 courses) was 0.10 to 1.28% with a median value of 0.60% and 0.25 to 3.44% with a median value of 1.04% among ‘MRD increased’ group, respectively; 0.18 to 1.75% with a median value of 0.32% and 0.00 to 0.47% with a median value of 0.14% among ‘MRD decreased’ group, respectively. As shown in figure 1 (C,D), there was no difference between the 2 subgroups with regard to OS or RFS (P=.6648 and P=.3060, respectively). Of note, intensive consolidation chemotherapy eradicated MRD in only 2 of 20 cases in Group A.
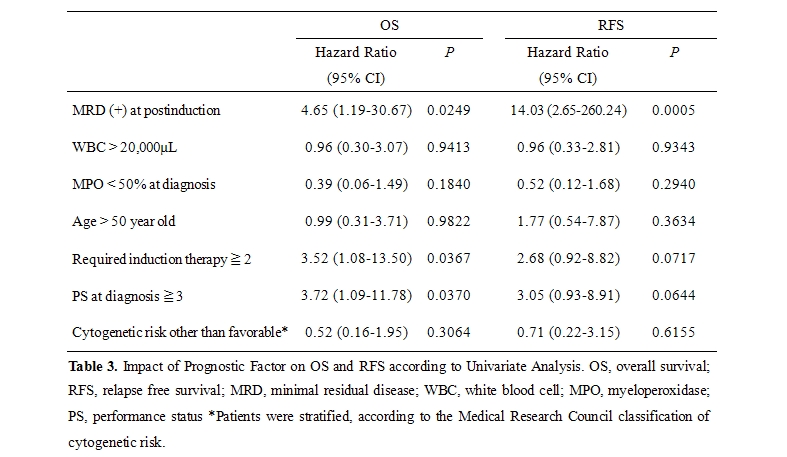
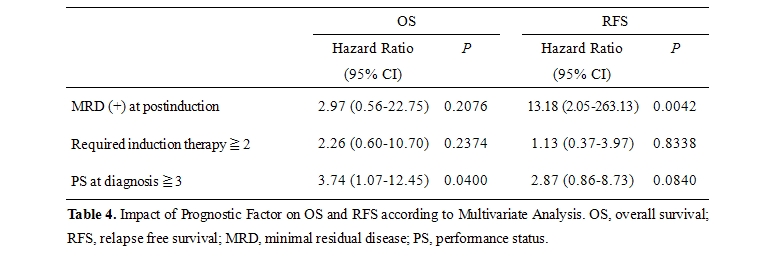
Impact of Prognostic Factors on OS and RFS: As shown in table 3, several significant factors were highlighted by univariate analysis, which led to the conclusion that OS was correlated with MRD positivity at post-induction, 2 or more courses of required induction therapy, and 3 or more PS at diagnosis (P=.0249, P=.0367, and P=.0370, respectively) and that RFS was related to MRD positivity (P=.0005). Although we found relatively higher cytogenetic risk and lower WBC count in GroupA compared to those of Group B in table 2, these variables did not exhibit any significance in univariate analysis. All of these relevant prognostic variables were subjected to a multivariate model. In this analysis (table 4), MRD-positivity and a high level of PS (3 or more) were found to be independent variables significantly associated with a shorter duration of RFS and OS, respectively (P=.0042 and P=.0400, respectively).
Discussion
The MRD studies reported in the literature indicate that 60% to 94% of AML patients have an aberrant phenotype of AML cells, as evaluated by MFC at diagnosis,[7,19] which are much higher frequencies than those demonstrated by PCR detection of specific gene rearrangements such as PML/RARα, AML1/ETO, and CBFB/MYH11. LAP have traditionally been classified into three groups: cross-lineage expression of lymphoid antigens on AML cells; asynchronous expression of antigens, for example, when blasts express both immature and mature antigens; and under-expression or over-expression of antigens.[5] In our analysis, a total of 75 LAP were identified; 25 cross-lineage expressions, 10 asynchronous expressions, and 40 under-expressions. Unlike PCR, in which sensitivity is affected by variations in the expression levels of the target genes in AML cells, MFC directly measures cell number and can be combined with cell sorting of selected cell populations for further characterization.5 To increase the sensitivity of LAP detection, the exploitation of 6-8 color antibody panels and uncommon markers such as CD15 and CD65 may have a positive effect.[7]
Regarding the impact of MRD positivity on clinical outcomes, several reports have been published providing evidence that MRD studies using MFC are valuable tools for predicting relapse.[8-15] In our study, it was shown that MRD detection by 3-color flow cytometry after successful remission induction therapy had a great impact on the OS and RFS of patients with AML. Therefore, information regarding MRD positivity is very useful for physicians for evaluating clinical outcomes in relation to the necessity of allo-SCT at a relatively early stage of the clinical course.[15] Notably, only a few patients in Group A obtained MRD negativity in the present study, even after several courses of intensive consolidation chemotherapy strongly suggesting that Group A type patients require allo-SCT because of their high risk of early relapse. This concept is supported by the fact that 4 patients of Group A who underwent allo-HSCT when in the CR state were still alive without relapse at a median follow-up period of 20.[5] months after HSCT, while 2 patients who necessarily received allo-SCT during the chemotherapy-refractory state died 2 or 3 months after the transplantation. In our cohort, however, we need to take into account that we analyzed the data from relatively limited number of patients and that the result might be confused by the potential effect of allo-HSCT. Therefore, in the future, it may be reasonable that we determine the indication of allo-HSCT based on LAP-determined MRD in a larger cohort of patients. Similarly, although we found the fact that MRD fluctuation during consolidation therapy has no significant impact on the prognosis as shown in Figure 1 (C,D), the accumulation of cases should be needed. Although Al-Mawali et al. and San Miguel et al. demonstrated a correlation between the relapse rate and the levels of MRD after induction,[20,21] Venditti et al. found that the level of MRD after consolidation was the most important prognostic factor.[13] The differences in the therapeutic induction regimens between these studies may explain the contradictory results; the formers adopted less intensive therapy for induction, as employed in the present study. We also observed a reduction in the importance of MRD positivity after 3 courses of consolidation therapy regarding OS and RFS (P=.1864 and P=.0025, respectively) compared with MRD positivity after induction. In previous reports, the threshold discriminating MRD-negative from MRD-positive cases was set at 0.15% to 1% at postinduction9,20,21 and 0.035% to 0.2% at postconsolidation.9,15,19,21 Given that 3-color flow cytometry has the ability to achieve a sensitivity of 0.1% to 0.01% (one leukemic cell in 1,000 to 10,000 nucleated cells),19,22 we considered whether the detection of MRD by even 3-color flow cytometry is a reliable marker for predicting the outcome of AML patients who have achieved hematologic CR. In fact, the level of MRD at postinduction in the present study was 0.10 to 1.75% with a median value of 0.28%. In patients with the AML1/ETO or CBFB/MYH11fusion gene, we examined bone marrow samples using RT-PCR in addition to MFC. RT-PCR exhibited higher sensitivity; all 13 samples (3 patients) without LAP-associated MRD turned out to be positive for AML1/ETO or CBFB/MYH11 fusion transcript. However, 2 of 3 patients (one with AML1/ETO and another with CBFB/MYH11) survived without relapse in spite of prolonged PCR-positivity at a follow-up period of 26 and 13 months, respectively, suggesting that LAP-associated MRD is a more valuable tool for predicting prognosis, although the third case subsequently suffered a relapse.
Nevertheless, we have some problems with the specificity and sensitivity of MFC as a diagnostic tool for MRD detection. For example, LAP may undergo phenotypic shifts,[7] which result in false negativity. Also, considering the specificity of LAP, we cannot deny the possibility that the normal counterpart of AML cells may have identical of similar antigen profiles. To overcome these defects, some reports have suggested the feasibility of the real-time quantitative PCR (RQ-PCR) assay for detecting nucleophosmin (NPM1) mutations and Wilms’ Tumor gene (WT1) overexpression as well as established genetic markers including PML/RARα.[23-27] Although WT1 is overexpressed in more than 70% of AML cases,[28] the levels of WT1 expression in normal bone marrow cells are relatively high.[27] Thus, low levels of WT1 may not be useful for distinguishing MRD from the background levels of WT1 expression derived from normal hematopoietic cells. On the other hand, NPM1 mutations are observed exclusively in leukemic cells, being identified in approximately 35% of de novo AML and in 50% of AML cases with a normal karyotype.[29] We have recently established nested RQ-PCR assays for detecting MRD by the use of NPM1 mutations using patient-specific primers, which yield assay sensitivities of 0.0001%. Thus, RQ-PCR is considered a more valuable method than MFC especially in cases with NPM1 mutation. These techniques may be useful for detecting MRD as a means of predicting clinical outcomes.
Conclusions
MRD detected by 3-color flow cytometry at a relatively early stage of treatment may provide a lot of valuable information to physicians concerning the risk of AML recurrence and indications for allo-HSCT. The accumulation of studies employing 6-or-more color MFC and PCR assays for novel gene targets is necessary to establish more precise optimization of postremission therapy for AML patients.
Despite the high remission rate (approaching 80% in younger adults) seen after intensive chemotherapy in patients with de novo acute myeloid leukemia (AML), only 30% to 40% of these patients survive for 5 years after diagnosis.[1] Unfortunately, many patients experience relapse, probably due to the presence of minimal residual disease (MRD). Regarding the prognostic factors for post-remission relapse, a great effort has been made to detect and characterize MRD using various prognostic and therapeutic strategies. For example, specific gene rearrangements such as PML/RARα, AML1/ETO, and CBFB/MYH11 have been used to represent MRD using nested reverse transcriptase-polymerase chain reaction (RT-PCR).[2-4] However, this technique applicable in only 20% to 30% of AML patients.[5] Furthermore, AML1/ETO or CBFB/MYH11 transcripts are often detectable in patients who were considered to be cured of leukemia.[4,6] Therefore, PCR for the detection of these transcripts after complete remission (CR) may be an equivocal tool because the significance of MRD positivity is undetermined, although MRD positivity on PCR closely correlates with a subsequent relapse in patients with the PML/RARα fusion gene.[2,3] On the other hand, multiparameter flow cytometry (MFC) may be a useful method for MRD detection as it defines leukemia-associated phenotypes (LAP) in AML cells at diagnosis in almost all AML patients.[7] Several reports have been published showing that MRD detection using MFC at post-induction/-consolidation is a valuable tool for predicting relapse.[8-15] However, to our best knowledge, there is little information regarding the use of MRD detection by MFC in Japanese AML patients for the optimization of post-remission therapy. Therefore, we attempted to identify characteristic LAP from bone marrow specimens taken at diagnosis in individual patients using three color flow cytometry and assessed the relationship between LAP and clinical outcomes.
Patients and Method
Eligibility: A total of 33 consecutive adult de novo AML patients with LAP who were diagnosed between April 2006 and March 2009 and treated to accomplish a morphologic CR at the Department of Hematology and Clinical Immunology, Kobe City Medical Center General Hospital (Kobe, Japan) were analyzed. We experienced a total of 49 AML patients in the period; 40 with LAP and 9 without LAP. The achievement of a morphologic CR after inductinon chemotherapy was a criterion for inclusion in this study, while the exclusion criteria were AML without LAP; myelodysplastic syndrome (MDS)-derived overt AML; therapy-related AML; and PML/RARα-associated AML (AML-M3), in which RT-PCR has been reported to be a most valuable tool as described above [2,3]. Approval for this study was obtained from the institutional review board.
Treatment Protocols
As the induction chemotherapy, combined cytarabine (100 mg/m2/day, days 1-7) and daunorubicin (45 mg/m2/day, days 1-3) were administered. In the case of induction failure, re-induction chemotherapy composed of enocitabine (200 mg/m2/day, days 1-7), mitoxantrone (8 mg/m2/day, days 1-3), and etoposide (100 mg/m2/day, days 1-5) was performed. For the refractory cases, which could not achieve a hematologic CR after 2 courses of induction therapy, salvage regimens depended on the physicians’ choice. As consolidation chemotherapy, the patients received high-dose cytarabine (6 g/m2/day, days 1-3) for 4 cycles. When the patients were more than 70 years old, had impaired kidney, heart, lung, or liver function, the dosage of chemotherapeutic agents was reduced 30% to 50% when considered necessary by the physicians. We performed allogeneic hematopoietic stem cell transplantation (allo-HSCT) for suitable cases, which were chosen according to the Japan Society for Hematopoietic Cell Transplantation (JSHCT) guidelines (JSHCT monograph Vol. 6: http://www.jshct.com/guidline/ , The Japan Society for Hematopoietic Cell Transplantation Web).
Detection of LAP and MRD
At presentation, immunophenotypic studies using 3-color flow cytometry were performed to detect LAP in bone marrow specimens. AML cells were identified using a CD45/side scatter gating strategy, and LAP were detected by staining leukemia cells with a triple fluorescence assay, which consisted of staining for CD45 and that for other two antigens, including myeloid/monocytic markers (CD11b, 13, 14, 33, 36, 86, 117), lymphoid markers (CD2, 4, 7, 19, 56), and miscellaneous markers (CD34, HLA-DR),[7] as shown in table 1.

A given combination of markers was regarded as relevant if it was expressed in at least 50% of blasts. Two or more antibody combinations, when possible, were employed to determine the LAP for each patient. The CellQuest software (Becton Dickinson, Mountain View, CA) was used for MFC data acquisition.16 MRD studies during CR were performed on erythrocyte-lysed whole bone marrow cells using the same antibody combination as used for each LAP at diagnosis. To abstract myeloblast group efficiently, instead of erythroblasts, monocytes, or promyelocytes, we adopted allophycocyanin (APC)-conjugated anti-CD34 and phycoerythrin-Cy7 (PE-Cy7)-conjugated anti-CD45 monoclonal antibodies gating strategy after CD45/side scatter gating. We determined a cell group as MRD only when the group was definitely different from its normal counterpart in the expression of antigens described as above and comprised more than 0.10% of total nucleated cells.
Statistical analysis
Overall survival (OS) and relapse free survival (RFS) were calculated from the date on which we first accomplished morphologic CR. CR, relapse, and RFS were defined by standardized criteria.[17] The Kaplan-Meier method was used to estimate OS and RFS. For comparisons of OS and RFS between two groups, the log-rank test was applied. To evaluate the independent effects of different variables on the duration of OS and RFS, multivariate analysis was performed, using the Cox proportional hazards model with predictive variables that were significant in the univariate analysis. The other statistical tests included t tests, χ2 tests, and Fisher’s exact tests. All calculations were made using the program JMP 8.0 (SAS Institute, Cary, NC, US). All P values of <0.05 were considered to be significant.
Results
Clinical Characteristics of AML Patient: The clinical characteristics of 33 AML patients are shown in table 2.

We classified the patients into Group A (20 patients, MRD positive) and B (13 patients, MRD negative), according to the detection of MRD by MFC at the first hematologic CR. The median follow up period was 16 months (4-42 months). Between Group A and B, there was no significant difference regarding age, sex predominance, French-American-British (FAB) subtype, white blood cell (WBC) count, cytogenetic risk, performance status before treatment, or the proportion of patients in which less than 50% of AML cells were positive for myeloperoxidase (MPO), although the number of patients who required 2 or more remission induction therapies to achieve hematologic CR was 14 in Group A and 2 in Group B (P=.0067). All of these factors other than sex were previously suggested as unfavorable prognostic factors in Japanese patients.[18] We were obliged to reduce the treatment intensity for 9 and 5 patients in Group A and B, respectively. Allogeneic stem-cell transplantation (allo-SCT) was performed in 6 patients of Group A and 6 of Group B, without statistical significance. Seven of 9 AML patients without LAP achieved morphological CR within 2 courses of chemotherapy, with 2-year OS rate of 55.6%. On the other hand, 33 of 40 patients with LAP accomplished a morphologic CR, with 2-year OS rate of 63.0%.
Impact of MRD After CR Induction: As shown in figure 1, Group A exhibited a significantly lower survival rate than Group B (P=.0317) with 2-year OS rates of 49.0% and 84.6%, respectively. Notably, in Group A, the patients who underwent single induction therapy were found to be more likely to show better survival than those who required 2 or more rounds of induction therapy (P=.1475) with 2-year OS rates of 75.0% and 39.4% respectively. Moreover, Group A showed much worse RFS than Group B (P=.0010) with 2-year RFS rates of 13.7% and 91.7%, respectively.

Impact of MRD Fluctuation After CR Induction: We divided Group A into 2 subgroups: ‘MRD increased (11 patients)’ and ‘MRD decreased (6 patients)’ according to the effects of the first consolidation chemotherapy administered when the patient was in a morphologic CR state. The remaining patients consisted of one patient who relapsed after the first post-induction therapy and two who had a nearly stable MRD level. The level of MRD at postinduction and postconsolidation (3 courses) was 0.10 to 1.28% with a median value of 0.60% and 0.25 to 3.44% with a median value of 1.04% among ‘MRD increased’ group, respectively; 0.18 to 1.75% with a median value of 0.32% and 0.00 to 0.47% with a median value of 0.14% among ‘MRD decreased’ group, respectively. As shown in figure 1 (C,D), there was no difference between the 2 subgroups with regard to OS or RFS (P=.6648 and P=.3060, respectively). Of note, intensive consolidation chemotherapy eradicated MRD in only 2 of 20 cases in Group A.
Impact of Prognostic Factors on OS and RFS: As shown in table 3, several significant factors were highlighted by univariate analysis, which led to the conclusion that OS was correlated with MRD positivity at post-induction, 2 or more courses of required induction therapy, and 3 or more PS at diagnosis (P=.0249, P=.0367, and P=.0370, respectively) and that RFS was related to MRD positivity (P=.0005). Although we found relatively higher cytogenetic risk and lower WBC count in GroupA compared to those of Group B in table 2, these variables did not exhibit any significance in univariate analysis. All of these relevant prognostic variables were subjected to a multivariate model. In this analysis (table 4), MRD-positivity and a high level of PS (3 or more) were found to be independent variables significantly associated with a shorter duration of RFS and OS, respectively (P=.0042 and P=.0400, respectively).


Discussion
The MRD studies reported in the literature indicate that 60% to 94% of AML patients have an aberrant phenotype of AML cells, as evaluated by MFC at diagnosis,[7,19] which are much higher frequencies than those demonstrated by PCR detection of specific gene rearrangements such as PML/RARα, AML1/ETO, and CBFB/MYH11. LAP have traditionally been classified into three groups: cross-lineage expression of lymphoid antigens on AML cells; asynchronous expression of antigens, for example, when blasts express both immature and mature antigens; and under-expression or over-expression of antigens.[5] In our analysis, a total of 75 LAP were identified; 25 cross-lineage expressions, 10 asynchronous expressions, and 40 under-expressions. Unlike PCR, in which sensitivity is affected by variations in the expression levels of the target genes in AML cells, MFC directly measures cell number and can be combined with cell sorting of selected cell populations for further characterization.5 To increase the sensitivity of LAP detection, the exploitation of 6-8 color antibody panels and uncommon markers such as CD15 and CD65 may have a positive effect.[7]
Regarding the impact of MRD positivity on clinical outcomes, several reports have been published providing evidence that MRD studies using MFC are valuable tools for predicting relapse.[8-15] In our study, it was shown that MRD detection by 3-color flow cytometry after successful remission induction therapy had a great impact on the OS and RFS of patients with AML. Therefore, information regarding MRD positivity is very useful for physicians for evaluating clinical outcomes in relation to the necessity of allo-SCT at a relatively early stage of the clinical course.[15] Notably, only a few patients in Group A obtained MRD negativity in the present study, even after several courses of intensive consolidation chemotherapy strongly suggesting that Group A type patients require allo-SCT because of their high risk of early relapse. This concept is supported by the fact that 4 patients of Group A who underwent allo-HSCT when in the CR state were still alive without relapse at a median follow-up period of 20.[5] months after HSCT, while 2 patients who necessarily received allo-SCT during the chemotherapy-refractory state died 2 or 3 months after the transplantation. In our cohort, however, we need to take into account that we analyzed the data from relatively limited number of patients and that the result might be confused by the potential effect of allo-HSCT. Therefore, in the future, it may be reasonable that we determine the indication of allo-HSCT based on LAP-determined MRD in a larger cohort of patients. Similarly, although we found the fact that MRD fluctuation during consolidation therapy has no significant impact on the prognosis as shown in Figure 1 (C,D), the accumulation of cases should be needed. Although Al-Mawali et al. and San Miguel et al. demonstrated a correlation between the relapse rate and the levels of MRD after induction,[20,21] Venditti et al. found that the level of MRD after consolidation was the most important prognostic factor.[13] The differences in the therapeutic induction regimens between these studies may explain the contradictory results; the formers adopted less intensive therapy for induction, as employed in the present study. We also observed a reduction in the importance of MRD positivity after 3 courses of consolidation therapy regarding OS and RFS (P=.1864 and P=.0025, respectively) compared with MRD positivity after induction. In previous reports, the threshold discriminating MRD-negative from MRD-positive cases was set at 0.15% to 1% at postinduction9,20,21 and 0.035% to 0.2% at postconsolidation.9,15,19,21 Given that 3-color flow cytometry has the ability to achieve a sensitivity of 0.1% to 0.01% (one leukemic cell in 1,000 to 10,000 nucleated cells),19,22 we considered whether the detection of MRD by even 3-color flow cytometry is a reliable marker for predicting the outcome of AML patients who have achieved hematologic CR. In fact, the level of MRD at postinduction in the present study was 0.10 to 1.75% with a median value of 0.28%. In patients with the AML1/ETO or CBFB/MYH11fusion gene, we examined bone marrow samples using RT-PCR in addition to MFC. RT-PCR exhibited higher sensitivity; all 13 samples (3 patients) without LAP-associated MRD turned out to be positive for AML1/ETO or CBFB/MYH11 fusion transcript. However, 2 of 3 patients (one with AML1/ETO and another with CBFB/MYH11) survived without relapse in spite of prolonged PCR-positivity at a follow-up period of 26 and 13 months, respectively, suggesting that LAP-associated MRD is a more valuable tool for predicting prognosis, although the third case subsequently suffered a relapse.
Nevertheless, we have some problems with the specificity and sensitivity of MFC as a diagnostic tool for MRD detection. For example, LAP may undergo phenotypic shifts,[7] which result in false negativity. Also, considering the specificity of LAP, we cannot deny the possibility that the normal counterpart of AML cells may have identical of similar antigen profiles. To overcome these defects, some reports have suggested the feasibility of the real-time quantitative PCR (RQ-PCR) assay for detecting nucleophosmin (NPM1) mutations and Wilms’ Tumor gene (WT1) overexpression as well as established genetic markers including PML/RARα.[23-27] Although WT1 is overexpressed in more than 70% of AML cases,[28] the levels of WT1 expression in normal bone marrow cells are relatively high.[27] Thus, low levels of WT1 may not be useful for distinguishing MRD from the background levels of WT1 expression derived from normal hematopoietic cells. On the other hand, NPM1 mutations are observed exclusively in leukemic cells, being identified in approximately 35% of de novo AML and in 50% of AML cases with a normal karyotype.[29] We have recently established nested RQ-PCR assays for detecting MRD by the use of NPM1 mutations using patient-specific primers, which yield assay sensitivities of 0.0001%. Thus, RQ-PCR is considered a more valuable method than MFC especially in cases with NPM1 mutation. These techniques may be useful for detecting MRD as a means of predicting clinical outcomes.
Conclusions
MRD detected by 3-color flow cytometry at a relatively early stage of treatment may provide a lot of valuable information to physicians concerning the risk of AML recurrence and indications for allo-HSCT. The accumulation of studies employing 6-or-more color MFC and PCR assays for novel gene targets is necessary to establish more precise optimization of postremission therapy for AML patients.
References
- Lowenberg, B., Griffin, J.D., &
Tallman, M.S. Acute myeloid leukemia and acute promyelocytic leukemia.
Hematology Am Soc Hematol Educ Program 2003;82-101.

- Lo-Coco, F. & Ammatuna, E. The biology
of acute promyelocytic leukemia and its impact on diagnosis and
treatment. Hematology Am Soc Hematol Educ Program 2006;156-161, 514.

- Reiter, A., Lengfelder, E., & Grimwade,
D. Pathogenesis, diagnosis and monitoring of residual disease in acute
promyelocytic leukaemia. Acta Haematol 2004;112:55-67.

- Yin, J.A. & Grimwade, D. Minimal
residual disease evaluation in acute myeloid leukaemia. Lancet
2002;360:160-162.

- Freeman, S.D., Jovanovic, J.V., &
Grimwade, D. Development of minimal residual disease-directed therapy
in acute myeloid leukemia. Semin Oncol 2008;35:388-400.

- Miyamoto, T., Weissman, I.L., & Akashi,
K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous
leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A
2000;97:7521-7526.

- Al-Mawali, A., Gillis, D., & Lewis, I.
The role of multiparameter flow cytometry for detection of minimal
residual disease in acute myeloid leukemia. Am J Clin Pathol
2009;131:16-26.

- Coustan-Smith, E., Ribeiro, R.C., Rubnitz,
J.E., Razzouk, B.I., Pui, C.H., Pounds, S., Andreansky, M., Behm, F.G.,
Raimondi, S.C., Shurtleff, S.A., Downing, J.R., & Campana, D.
Clinical significance of residual disease during treatment in childhood
acute myeloid leukaemia. Br J Haematol 2003;123:243-252.

- Feller, N., van der Pol, M.A., van Stijn,
A., Weijers, G.W., Westra, A.H., Evertse, B.W., Ossenkoppele, G.J.,
& Schuurhuis, G.J. MRD parameters using immunophenotypic detection
methods are highly reliable in predicting survival in acute myeloid
leukaemia. Leukemia 2004;18:1380-1390.

- Kern, W., Voskova, D., Schoch, C.,
Hiddemann, W., Schnittger, S., & Haferlach, T. Determination of
relapse risk based on assessment of minimal residual disease during
complete remission by multiparameter flow cytometry in unselected
patients with acute myeloid leukemia. Blood 2004;104:3078-3085.

- San Miguel, J.F., Vidriales, M.B.,
Lopez-Berges, C., Diaz-Mediavilla, J., Gutierrez, N., Canizo, C.,
Ramos, F., Calmuntia, M.J., Perez, J.J., Gonzalez, M., & Orfao, A.
Early immunophenotypical evaluation of minimal residual disease in
acute myeloid leukemia identifies different patient risk groups and may
contribute to postinduction treatment stratification. Blood
2001;98:1746-1751.

- Sievers, E.L., Lange, B.J., Alonzo, T.A.,
Gerbing, R.B., Bernstein, I.D., Smith, F.O., Arceci, R.J., Woods, W.G.,
& Loken, M.R. Immunophenotypic evidence of leukemia after induction
therapy predicts relapse: results from a prospective Children's Cancer
Group study of 252 patients with acute myeloid leukemia. Blood
2003;101:3398-3406.

- Venditti, A., Buccisano, F., Del Poeta,
G., Maurillo, L., Tamburini, A., Cox, C., Battaglia, A., Catalano, G.,
Del Moro, B., Cudillo, L., Postorino, M., Masi, M., & Amadori, S.
Level of minimal residual disease after consolidation therapy predicts
outcome in acute myeloid leukemia. Blood 2000;96:3948-3952.

- Buccisano, F., Maurillo, L., Gattei, V.,
Del Poeta, G., Del Principe, M.I., Cox, M.C., Panetta, P., Consalvo,
M.I., Mazzone, C., Neri, B., Ottaviani, L., Fraboni, D., Tamburini, A.,
Lo-Coco, F., Amadori, S., & Venditti, A. The kinetics of reduction
of minimal residual disease impacts on duration of response and
survival of patients with acute myeloid leukemia. Leukemia
2006;20:1783-1789.

- Maurillo, L., Buccisano, F., Del Principe,
M.I., Del Poeta, G., Spagnoli, A., Panetta, P., Ammatuna, E., Neri, B.,
Ottaviani, L., Sarlo, C., Venditti, D., Quaresima, M., Cerretti, R.,
Rizzo, M., de Fabritiis, P., Lo Coco, F., Arcese, W., Amadori, S.,
& Venditti, A. Toward optimization of postremission therapy for
residual disease-positive patients with acute myeloid leukemia. J Clin
Oncol 2008;26:4944-4951.

- Venditti, A., Tamburini, A., Buccisano,
F., Del Poeta, G., Maurillo, L., Panetta, P., Scornajenghi, K.A., Cox,
C., & Amadori, S. Clinical relevance of minimal residual disease
detection in adult acute myeloid leukemia. J Hematother Stem Cell Res
2002;11:349-357.

- Cheson, B.D., Bennett, J.M., Kopecky,
K.J., Buchner, T., Willman, C.L., Estey, E.H., Schiffer, C.A., Doehner,
H., Tallman, M.S., Lister, T.A., Lo-Coco, F., Willemze, R., Biondi, A.,
Hiddemann, W., Larson, R.A., Lowenberg, B., Sanz, M.A., Head, D.R.,
Ohno, R., & Bloomfield, C.D. Revised recommendations of the
International Working Group for Diagnosis, Standardization of Response
Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic
Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21:4642-4649.

- Ackerstein, A., Kedar, E., & Slavin,
S. Use of recombinant human interleukin-2 in conjunction with syngeneic
bone marrow transplantation in mice as a model for control of minimal
residual disease in malignant hematologic disorders. Blood
1991;78:1212-1215.

- Al-Mawali, A., Gillis, D., Hissaria, P.,
& Lewis, I. Incidence, sensitivity, and specificity of
leukemia-associated phenotypes in acute myeloid leukemia using specific
five-color multiparameter flow cytometry. Am J Clin Pathol
2008;129:934-945.

- Al-Mawali, A., Gillis, D., & Lewis, I.
The use of receiver operating characteristic analysis for detection of
minimal residual disease using five-color multiparameter flow cytometry
in acute myeloid leukemia identifies patients with high risk of
relapse. Cytometry B Clin Cytom 2009;76:91-101.

- San Miguel, J.F., Martinez, A., Macedo,
A., Vidriales, M.B., Lopez-Berges, C., Gonzalez, M., Caballero, D.,
Garcia-Marcos, M.A., Ramos, F., Fernandez-Calvo, J., Calmuntia, M.J.,
Diaz-Mediavilla, J., & Orfao, A. Immunophenotyping investigation of
minimal residual disease is a useful approach for predicting relapse in
acute myeloid leukemia patients. Blood 1997;90:2465-2470.

- Campana, D. & Coustan-Smith, E.
Detection of minimal residual disease in acute leukemia by flow
cytometry. Cytometry 1999;38:139-152.

- Gorello, P., Cazzaniga, G., Alberti, F.,
Dell'Oro, M.G., Gottardi, E., Specchia, G., Roti, G., Rosati, R.,
Martelli, M.F., Diverio, D., Lo Coco, F., Biondi, A., Saglio, G.,
Mecucci, C., & Falini, B. Quantitative assessment of minimal
residual disease in acute myeloid leukemia carrying nucleophosmin
(NPM1) gene mutations. Leukemia 2006;20:1103-1108.

- Chou, W.C., Tang, J.L., Wu, S.J., Tsay,
W., Yao, M., Huang, S.Y., Huang, K.C., Chen, C.Y., Huang, C.F., &
Tien, H.F. Clinical implications of minimal residual disease monitoring
by quantitative polymerase chain reaction in acute myeloid leukemia
patients bearing nucleophosmin (NPM1) mutations. Leukemia
2007;21:998-1004.

- Papadaki, C., Dufour, A., Seibl, M.,
Schneider, S., Bohlander, S.K., Zellmeier, E., Mellert, G., Hiddemann,
W., & Spiekermann, K. Monitoring minimal residual disease in acute
myeloid leukaemia with NPM1 mutations by quantitative PCR: clonal
evolution is a limiting factor. Br J Haematol 2009;144:517-523.

- Barragan, E., Pajuelo, J.C., Ballester,
S., Fuster, O., Cervera, J., Moscardo, F., Senent, L., Such, E., Sanz,
M.A., & Bolufer, P. Minimal residual disease detection in acute
myeloid leukemia by mutant nucleophosmin (NPM1): comparison with WT1
gene expression. Clin Chim Acta 2008;395:120-123.

- Lapillonne, H., Renneville, A., Auvrignon,
A., Flamant, C., Blaise, A., Perot, C., Lai, J.L., Ballerini, P.,
Mazingue, F., Fasola, S., Dehee, A., Bellman, F., Adam, M., Labopin,
M., Douay, L., Leverger, G., Preudhomme, C., & Landman-Parker, J.
High WT1 expression after induction therapy predicts high risk of
relapse and death in pediatric acute myeloid leukemia. J Clin Oncol
2006;24:1507-1515.

- Steinbach, D., Schramm, A., Eggert, A.,
Onda, M., Dawczynski, K., Rump, A., Pastan, I., Wittig, S.,
Pfaffendorf, N., Voigt, A., Zintl, F., & Gruhn, B. Identification
of a set of seven genes for the monitoring of minimal residual disease
in pediatric acute myeloid leukemia. Clin Cancer Res 2006;12:2434-2441.

- Falini, B., Mecucci, C., Tiacci, E.,
Alcalay, M., Rosati, R., Pasqualucci, L., La Starza, R., Diverio, D.,
Colombo, E., Santucci, A., Bigerna, B., Pacini, R., Pucciarini, A.,
Liso, A., Vignetti, M., Fazi, P., Meani, N., Pettirossi, V., Saglio,
G., Mandelli, F., Lo-Coco, F., Pelicci, P.G., & Martelli, M.F.
Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal
karyotype. N Engl J Med 2005;352:254-266.
