Allogeneic Transplantation for Chronic Lymphocytic Leukemia
Luca Laurenti, Michela Tarnani, Patrizia Chiusolo, Federica Sorà and Simona Sica.
Istituto di Ematologia, Policlinico "A. Gemelli", Universita' Cattolica del Sacro Cuore, Rome, Italy.
Correspondence to: Luca Laurenti, MD. Department of Haematology, Catholic University Hospital "A. Gemelli", Rome, Italy. Largo A. Gemelli 8. 00168, Rome-Italiy. Phone: 39-06-35503953, Fax: 39-06-3017319. E-mail: l.laurenti@rm.unicatt.it
Published: September 7, 2010
Received: July 13, 2010
Accepted: August 8, 2010
Medit J Hemat Infect Dis 2010, 2(2): e2010026, DOI 10.4084/MJHID.2010.026
This article is available from: http://www.mjhid.org/article/view/6184
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Even
if Chronic lymphocytic leukemia (CLL) often has an indolent behavior
with good responsiveness to cytoreductive treatment, about 20% of the
patients, so called “poor-risk” patients, show an aggressive course and
die within a few years despite early intensive therapies. Criteria for
poor-risk disease according to the European Bone Marrow Transplantation
(EBMT) CLL Transplant Consensus are: purine analogue refractoriness,
early relapse after purine analogue combination therapy, CLL with p53
lesion requiring treatment.
Allogeneic transplant has potential curative role in CLL, however burden with very high transplant related mortality (TRM) rates of 38–50%. A major advance in reducing the short-term morbidity and mortality of allogeneic stem cell transplantation (SCT) has been the introduction of non-myeloablative or reduced intensity conditioning (RIC) regimens to allow engraftment of allogeneic stem cells. There is no doubt that the crucial therapeutic principle of allo-SCT in CLL is graft versus leukemia (GVL) activity.
The major complications of allogeneic SCT in CLL are: chronic graft-versus-host-disease (GVHD) affecting quality of life, high graft rejection and infection rates correlated with preexisting immunosuppression. Disease relapse remains the major cause of failure after RIC allo-HCT in CLL patients.
Sensitive minimal residual disease (MRD) quantification has strong prognostic impact after transplant.
Allogeneic transplant has potential curative role in CLL, however burden with very high transplant related mortality (TRM) rates of 38–50%. A major advance in reducing the short-term morbidity and mortality of allogeneic stem cell transplantation (SCT) has been the introduction of non-myeloablative or reduced intensity conditioning (RIC) regimens to allow engraftment of allogeneic stem cells. There is no doubt that the crucial therapeutic principle of allo-SCT in CLL is graft versus leukemia (GVL) activity.
The major complications of allogeneic SCT in CLL are: chronic graft-versus-host-disease (GVHD) affecting quality of life, high graft rejection and infection rates correlated with preexisting immunosuppression. Disease relapse remains the major cause of failure after RIC allo-HCT in CLL patients.
Sensitive minimal residual disease (MRD) quantification has strong prognostic impact after transplant.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukaemia in the Western World[1] and has long been considered an indolent disease affecting elderly patients, with a median age at diagnosis of approximately 72 years.
In parallel with improvements in treatment outcome, recent advances in the understanding of CLL biology have allowed us to identify selected subgroups of patients with adverse characteristics such as chromosomal abnormalities,[2] immunoglobulin heavy chain (IgVh) gene mutational status,[3] zeta associated protein 70 (ZAP70)[4] and CD38 expression,[5] some of whom have projected survival as short as 3 years.
Even if CLL often has an indolent behavior with good responsiveness to cytoreductive treatment, about 20% of the patients who need treatment show an aggressive course and die within a few years of diagnosis despite early institution of intensive therapies. These so called “poor-risk” patients are characterized by preexisting or rapidly developing resistance to conventional chemotherapy, including modern purine analogue-antibody combination regimens[6,7] with a dismal survival of less than 12 months.[8,9]
Although alemtuzumab, lenalidomide, and flavopiridol, show promising activity in some patients with poor-risk CLL, none of these compounds seem to be able to change the natural course of poor-risk CLL.[10-14]
Transplant in CLL
Myeloablative allogeneic SCT has several theoretical advantages over autologous SCT. Firstly, any potential tumor contamination of the stem cell product is eliminated even if new data are emerging about the potential possibility of CLL transmission by unrecognized CLL/MBL (Monoclonal B Lymphocytosis) donor.[15] Secondly, there is the potential for the graft versus leukaemia (GVL) effect to eliminate chemotherapy-resistant leukaemia cells by immune mechanisms. Moreover, in contradistinction with the results of autologous SCT plateaus in the survival curves emerge 1–2 years following allogeneic transplantation.
The enthusiasm for myeloablative allogeneic SCT in CLL, using either related or unrelated donors, is however tempered by registry reports showing very high TRM rates of 38–50%: these published data showed that approximately two-thirds of allotransplanted CLL patients will succumb either to TRM or to recurrent disease, and approximately one-third will be cured of their disease.[16,17] Moreover patients with chemosensitive disease have significantly better outcomes than patients with refractory disease, suggesting that an earlier application of allogeneic SCT may further improve transplantation outcomes.
A major advance in reducing the short-term morbidity and mortality of allogeneic SCT has been the introduction of non-myeloablative or reduced intensity conditioning (RIC) regimens to allow engraftment of allogeneic stem cells. So the RIC regimens were developed in the 1990s to allow allo-SCT in older patients or younger patients with comorbidities and the total number of allogeneic transplantations for CLL patients in Europe, has almost treble in the last 7 years. In the last decade, many reports, which enrolled primarily patients with chemorefractory end-stage disease, have stressed the potential curative role of RIC allo-SCT in CLL.
There is no doubt that the crucial therapeutic principle of allo-SCT in CLL is GVL activity. This evidence derives from some interesting observations such as: decreasing relapse incidence over time even in RIC Allo-SCT, in contrast to autologous SCT or other intensive therapies,[16-22] achievement of durable clinical and molecular responses due to antitumor activity,[23] reduced relapse rates in patients with chronic graft-versus-host disease (GVHD),[20,24] increased relapse rates associated with T cell–depleted grafts[25,26] and efficacy of donor lymphocyte infusions (DLIs) in the post-transplant relapse.[25,19,27]
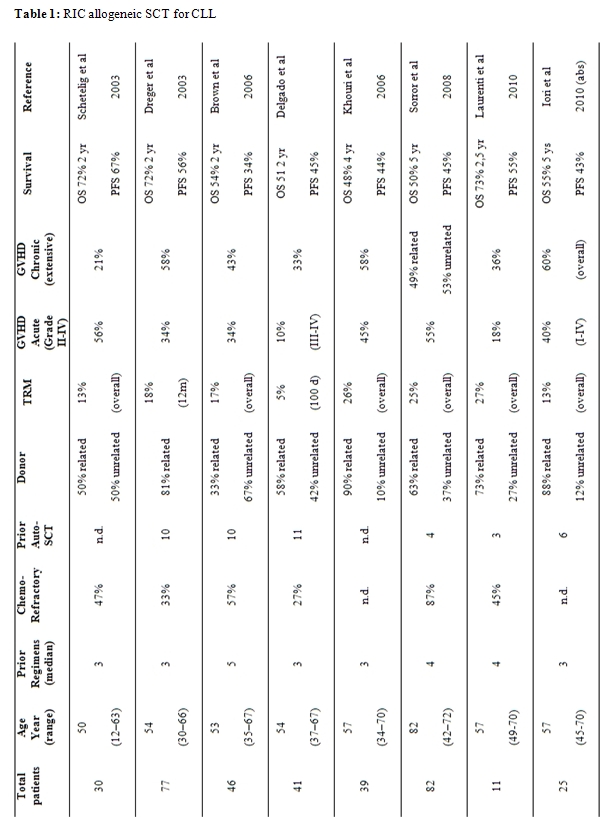
Multiple single-center phase 2 studies have evaluated several different RIC regimens with different myeloablative and immunoablative potential (Table 1).
Ritgen et al demonstrated that in the majority of cases achievement of minimal residual disease (MRD) negativity was clearly linked to immune intervention, such as tapering of immunosuppression or donor lymphocyte infusions that lead to develop chronic GVHD, also he suggests that a “GVL escape” mechanism might be acquired during the post-transplantation course probably caused by survival of clonogenic cells at “GVL sanctuary” sites.[23] Farina et al confirmed GVL activity in patients in clinical complete response (CR) after RIC Allo-SCT showing delayed clearance or decrease of MRD levels upon chronic GVHD in clinical CR.[24] Both studies also detected that MRD negativity 1 year post transplant was durable over the entire follow-up period in more than 90% of patients and predictive for the virtual absence of clinical relapse. In conclusion, MRD kinetics studies consistently indicate that permanent MRD negativity after Allo-SCT for CLL can be reached in the context of chronic GVHD and/or immunomodulating intervention. Both the durability of MRD remission and its sensitivity to immunomodulation strongly suggest that GVL is effective in CLL.
Ex-vivo and in-vivo T-cell depletion are effective means of preventing acute and chronic GVHD after Allo-SCT. In CLL, however, T-cell depletion has been associated with increased relapse and rejection rates.[26-28] Now-a-day, considering the scanty data, the role of this type of transplantation in CLL is controversial.
“Poor risk” patients: indications and Allo-SCT timing in CLL
European Bone Marrow Transplant (EBMT) guidelines have now been established outlining indications for SCT in CLL [29] (Table 1). In the absence of prospective randomized trials, the Consensus recommendations were based on evidence of grade II or less.
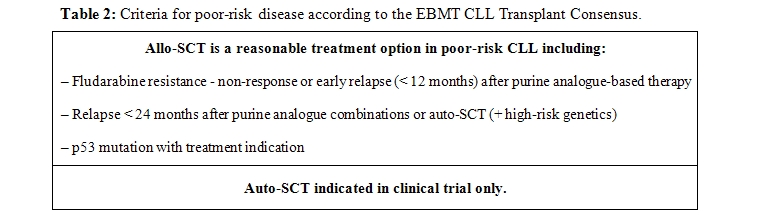
Criteria for poor-risk disease according to the EBMT CLL Transplant Consensus are: purine analogue refractoriness, early relapse after purine analogue combination therapy, and CLL with p53 lesion requiring treatment.
However considering the recent improvements in CLL treatment outcome, now-a-day one should test other innovative and very promising therapeutically approach before giving the indication to Allo-SCT, such as: antibody-purine analogue combination regimens as first-line or salvage treatment,[30,31] combined alemtuzumab therapies useful in fludarabine refractory and 17p-/p53 patients as showed by the German CLL Study Group (GCLLSG) CLL2H study,[12] and novel agent flavopiridol and lenalidomide capable to achieve 30% to 40% response rate when used as salvage therapy in fludarabine-refractory or del 17p- CLL.[13,14] Moreover these approaches may be helpful to achieve remission, a basic prerequisite for successful Allo-SCT.[32]
The EBMT guidelines conclude that there is evidence-based efficacy of allogeneic SCT in CLL and that this procedure is indicated in high-risk CLL patients. It should be stressed that none of the EBMT categories requires assessment of biologic risk factors except for cytogenetics for detection of p53 deletions, who deserves allogeneic SCT in first response. Ongoing prospective clinical studies will determine the impact of biomarkers including IgVh mutational status and other cytogenetic abnormalities in identification of patients at sufficiently high risk to deserve use of allogeneic SCT in first CR.
Another crucial point in the allogeneic transplant is a better selection of patient, as recently suggested by Sorror.[21] The FHCRC analysis revealed that comorbidities were more important than CLL-related variables for predicting progression free survival (PFS) and overall survival (OS).[21] Another risk factor for non-relapse-mortality (NRM), usually correlated with the presence of comorbidities, is refractory disease at transplantation.[22] Thus, it would appear prudent to limit allo-SCT to fit patients without evidence of refractory disease.
In conclusion, allo-SCT is the only therapy with curative potential in CLL and, in contrast to conventional treatment, with potential of providing long-term disease control even in patients with a very unfavorable biological and clinical risk profile (Table 2). However in addition to the disease risk, patient-related risk factors, such as age and comorbidity, have to be considered when the decision about allo-SCT is made.[21,33]
The larger RIC prospective trials uniformly show that in CLL the results of Allo-SCT are considerably impaired if the disease is not in remission at the time of transplantation, due to nodal bulks and/or chemotherapy resistance. Thus, Allo-SCT should be performed before CLL has advanced to a status of complete refractoriness or large resistant tumor burden in order not to miss the “window of opportunity” for a successful outcome. In eligible patients, Allo-SCT should be considered as soon as the first and/or the third EBMT criterion is met; on the contrary, the optimum timing for Allo-SCT in patients with early relapse (within 2 years) after purine analogue combination therapy (second EBMT criterion) needs to be defined by systematic studies.
On the other hand, RIC allo-HCT is able to mitigate the adverse prognosis conferred by purine analog resistance and unfavorable genetics. The EBMT guidelines identify a group of patients in whom available therapies are unlikely to achieve a prolonged disease-free survival.
The choice of Conditioning regimen and stem cell source
Several prospective RIC studies with a combined number of more than 300 patients included on the basis of modern CLL-specific risk stratification give relatively concordant results. In contrast, the experience with myeloablative conditioning in CLL largely relies on registry analyses and a few small single-center studies.[15-18,34,28] Therefore, RIC rather than myeloablative conditioning represents the standard procedure for Allo-SCT in CLL.
The choice of RIC Allo-SCT as the standard procedure rather than myeloablative conditioning emerged by the fact that the crucial therapeutic principle of allotransplantation in CLL is GVL activity. That evidence for superior direct cytotoxic activity of myeloablative conditioning over RIC is lacking, and also one should consider that CLL is an elderly disease.[35] At the same time an increased incidence of relapse after RIC in comparison with myeloablative conditioning was observed in a registry analysis, thus impaired disease control by RIC cannot be clearly ruled out.[20] Thus, according to the individual situation, the optimum choice of conditioning regimens may vary: in the presence of comorbidity and sensitive disease reduced-intensity regimens appear to be more appropriate, high-intensity regimens might be preferable in younger patients with good performance status but poorly controlled disease.[36,29]
A matched sibling donor should be preferred for Allo-SCT in CLL whenever possible, but a matched unrelated donor (MUD) is a reasonable alternative if an HLA-identical sibling is not available. In fact from published RIC studies in CLL, unfavorable effect of MUD respect to sibling donor did not significantly emerge.[19,21-22,27,37-38]
Due to the median age of patients, experience with haploidentical transplant and cord blood transplant is very scanty in CLL, and it is unlikely that disease-specific evidence for benefit of these procedures can ever be obtained.
RIC allo-SCT was associated with a NRM of 11%-34%, a PFS of 34%-67%, and OS of 48%-72% depending on the conditioning regimen and follow-up. Long-term disease control was obtained in a substantial proportion of patients, particularly in those with chemosensitive and non-bulky disease at the time of transplantation.
In the CLL3X trial of the German CLL Study Group (GCLLSG) and also in Schetelig data, poor risk CLL as defined by purine-analogue refractoriness or presence of deletion 17p- had a similar outcome to patients without poor-risk characteristics in terms of PFS, with a median follow up of 2 years.[19]
The outcome of 82 patients treated at the FHCRC consortium using a non-ablative conditioning regimen (fludarabine and low-dose total body irradiation) was recently updated [20]. Eighty-seven percent of patients had fludarabine refractory disease and 9% had del(17p).
At 5 years after RIC allo transplant, the NRM and relapse rate were 23% and 38% respectively, which translated into a OS of 50% and PFS of 39%. Patients with bulky lymphadenopathy (>5 cm) at the time of transplantation had a particularly poor outcome, with a 5-year relapse rate and PFS of 71% and 8% respectively. Extensive chronic GVHD was diagnosed in 50% of patients, but it resolved in most of them.[21]
A retrospective analysis of the EBMT on Allo-SCT in 44 patients with del 17p- CLL showed a 3-year PFS of 37% with no event occurring later than 3.5 years after transplant.[26]
We recently reported in a single centre experience the efficacy and feasibility of RIC allo-SCT in heavily pre-treated CLL patients, even if in a small number of patients and with a relatively short follow-up. In our series, 45% of patients were considered “high-risk” for fludarabine-refractory disease or 17p deletion. At median follow-up of 20 months (range 12-44) 80% of them are alive, confirming literature data.[39]
In conclusion, RIC Allo-SCT seems to be effective in poor-risk CLL, thereby overcoming the adverse prognostic impact of purine analogue refractoriness and del 17p-. However, active, unresponsive and bulky disease at the time of Allo-SCT still remains a predictor of an unfavorable outcome.[20-22,37]
Complication of allogeneic SCT in CLL and post-transplant follow-up
The major determinant of long-term morbidity affecting quality of life after Allo-SCT is chronic GVHD. Active chronic GVHD documented a significantly reduced long-term health status in patients allografted for various hematological malignancies.[40] Sorror et al. reported a 5-year cumulative incidence of extensive chronic GVHD of 49% for related and 53% for unrelated recipients. However, in the majority of affected patients clinical symptoms of chronic GVHD resolved over time, allowing discontinuation of systemic therapeutic immunosuppression after a median of 25 months [21]. Transplant-related long-term morbidity after Allo-SCT for CLL can be significant but is mainly restricted to those patients who have ongoing active chronic GVHD.
The high graft rejection rates remain a complication either of myeloablative or non-myeloablative transplant in CLL: it ranges between 18%- 20% in conventional conditioning,[16,41] while in case of RIC regimen between 12-25% in T cell–depleted grafts and 5-6% in unmanipulated grafts.[21,38,42] The possible explanations for this phenomenon could be the significant marrow infiltration in CLL patients at the time of transplantation, inversely correlated with outcome[37,42] and the role played by host dendritic cells, which are seriously defective in CLL patients.[43]
Another complication is represented by the high infection rates correlated with preexisting immunosuppression. Infections are the cause of about 50% of all CLL-related deaths,[44-45] primarily in fludarabine and/or alemtuzumab-refractory patients.[7,8] The base of susceptibility to development infections is the immunosuppression before SCT both disease and therapy-related, rarely encountered in patients with other lymphoproliferative disorders, such as follicular lymphoma, whose main cause of death is disease progression.[46] The multifactorial genesis should be secondary to hypogammaglobulinemia, defective T and natural killer cell function, neutropenia, and deficient complement activity.[47] Moreover in recent reports the risk of infections has been clearly correlated with presence of GVHD[21,38,43,48] and refractory disease.[8,49]
Disease relapse remains the major cause of failure after RIC allo-HCT in CLL patients. Early relapses are correlated with chemorefractory disease at the time of transplantation, perhaps due to the ineffectiveness of RIC regimens in controlling the disease before the graft vesus CLL (GVCLL) effect can take place.
Late relapse an be explaned with different mechanism including: CLL clonal evolution, the development of tolerance[25] and the survival of tumor cells in “GVCLL sanctuary sites” based on the negative impact of bulky lymphadenopathy at the time of transplant on PFS,[21] inadequate GVL effect to allow a complete disease eradication. It should be noted that a significant percentage of these late relapses occurred in lymph nodes in the absence of bone marrow or peripheral blood involvement, or even in patients with MRD negative status.[9,28,34,50-52]
Sensitive MRD quantification can be studied in CLL by polymerase chain reaction (PCR) or flow cytometry-based assays and has strong prognostic impact after transplant. The generally delayed decline of the MRD level and its close correlation with immune-relevant events strongly supports the assumption that GVL activity is the crucial contribution to tumor control in the allo-SCT. GVL-induced MRD negativity after allogeneic transplant is highly predictive of freedom from relapse.[24]
Moreover in CLL quantitative MRD monitoring seems to be a valid instrument for sensitive guidance of immune interventions aimed at disease eradication after allo-SCT. In contrast to donor’s lymphocyte infusion (DLI) upon clinical relapse which often shows only limited benefit in CLL,[19,33] MRD-triggered pre-emptive DLI can be highly effective.[53]
The best approach to post transplant immunotherapy, including monoclonal antibody[38] in CLL needs further study. Some of them, although a still short follow-up, show very promising results and the use of monoclonal antibody in the conditioning or just after transplant, could improve the results of allogeneic stem cell transplantation.[54] Rituximab given concomitantly with RIC allo-SCT or DLI may facilitate disease control[43] probably due , not only to direct cytotoxicity, but also to modulation of the GVCLL effect. In addition, maintenance therapy with monoclonal antibodies or immunomodulatory drugs with proven activity in poor-risk CLL, such as lenalidomide, could be explored in future trials to minimize the risk of relapse.
Chronic lymphocytic leukemia (CLL) is the most common adult leukaemia in the Western World[1] and has long been considered an indolent disease affecting elderly patients, with a median age at diagnosis of approximately 72 years.
In parallel with improvements in treatment outcome, recent advances in the understanding of CLL biology have allowed us to identify selected subgroups of patients with adverse characteristics such as chromosomal abnormalities,[2] immunoglobulin heavy chain (IgVh) gene mutational status,[3] zeta associated protein 70 (ZAP70)[4] and CD38 expression,[5] some of whom have projected survival as short as 3 years.
Even if CLL often has an indolent behavior with good responsiveness to cytoreductive treatment, about 20% of the patients who need treatment show an aggressive course and die within a few years of diagnosis despite early institution of intensive therapies. These so called “poor-risk” patients are characterized by preexisting or rapidly developing resistance to conventional chemotherapy, including modern purine analogue-antibody combination regimens[6,7] with a dismal survival of less than 12 months.[8,9]
Although alemtuzumab, lenalidomide, and flavopiridol, show promising activity in some patients with poor-risk CLL, none of these compounds seem to be able to change the natural course of poor-risk CLL.[10-14]
Transplant in CLL
Myeloablative allogeneic SCT has several theoretical advantages over autologous SCT. Firstly, any potential tumor contamination of the stem cell product is eliminated even if new data are emerging about the potential possibility of CLL transmission by unrecognized CLL/MBL (Monoclonal B Lymphocytosis) donor.[15] Secondly, there is the potential for the graft versus leukaemia (GVL) effect to eliminate chemotherapy-resistant leukaemia cells by immune mechanisms. Moreover, in contradistinction with the results of autologous SCT plateaus in the survival curves emerge 1–2 years following allogeneic transplantation.
The enthusiasm for myeloablative allogeneic SCT in CLL, using either related or unrelated donors, is however tempered by registry reports showing very high TRM rates of 38–50%: these published data showed that approximately two-thirds of allotransplanted CLL patients will succumb either to TRM or to recurrent disease, and approximately one-third will be cured of their disease.[16,17] Moreover patients with chemosensitive disease have significantly better outcomes than patients with refractory disease, suggesting that an earlier application of allogeneic SCT may further improve transplantation outcomes.
A major advance in reducing the short-term morbidity and mortality of allogeneic SCT has been the introduction of non-myeloablative or reduced intensity conditioning (RIC) regimens to allow engraftment of allogeneic stem cells. So the RIC regimens were developed in the 1990s to allow allo-SCT in older patients or younger patients with comorbidities and the total number of allogeneic transplantations for CLL patients in Europe, has almost treble in the last 7 years. In the last decade, many reports, which enrolled primarily patients with chemorefractory end-stage disease, have stressed the potential curative role of RIC allo-SCT in CLL.
There is no doubt that the crucial therapeutic principle of allo-SCT in CLL is GVL activity. This evidence derives from some interesting observations such as: decreasing relapse incidence over time even in RIC Allo-SCT, in contrast to autologous SCT or other intensive therapies,[16-22] achievement of durable clinical and molecular responses due to antitumor activity,[23] reduced relapse rates in patients with chronic graft-versus-host disease (GVHD),[20,24] increased relapse rates associated with T cell–depleted grafts[25,26] and efficacy of donor lymphocyte infusions (DLIs) in the post-transplant relapse.[25,19,27]
Multiple single-center phase 2 studies have evaluated several different RIC regimens with different myeloablative and immunoablative potential (Table 1).

Ritgen et al demonstrated that in the majority of cases achievement of minimal residual disease (MRD) negativity was clearly linked to immune intervention, such as tapering of immunosuppression or donor lymphocyte infusions that lead to develop chronic GVHD, also he suggests that a “GVL escape” mechanism might be acquired during the post-transplantation course probably caused by survival of clonogenic cells at “GVL sanctuary” sites.[23] Farina et al confirmed GVL activity in patients in clinical complete response (CR) after RIC Allo-SCT showing delayed clearance or decrease of MRD levels upon chronic GVHD in clinical CR.[24] Both studies also detected that MRD negativity 1 year post transplant was durable over the entire follow-up period in more than 90% of patients and predictive for the virtual absence of clinical relapse. In conclusion, MRD kinetics studies consistently indicate that permanent MRD negativity after Allo-SCT for CLL can be reached in the context of chronic GVHD and/or immunomodulating intervention. Both the durability of MRD remission and its sensitivity to immunomodulation strongly suggest that GVL is effective in CLL.
Ex-vivo and in-vivo T-cell depletion are effective means of preventing acute and chronic GVHD after Allo-SCT. In CLL, however, T-cell depletion has been associated with increased relapse and rejection rates.[26-28] Now-a-day, considering the scanty data, the role of this type of transplantation in CLL is controversial.
“Poor risk” patients: indications and Allo-SCT timing in CLL
European Bone Marrow Transplant (EBMT) guidelines have now been established outlining indications for SCT in CLL [29] (Table 1). In the absence of prospective randomized trials, the Consensus recommendations were based on evidence of grade II or less.
Criteria for poor-risk disease according to the EBMT CLL Transplant Consensus are: purine analogue refractoriness, early relapse after purine analogue combination therapy, and CLL with p53 lesion requiring treatment.
However considering the recent improvements in CLL treatment outcome, now-a-day one should test other innovative and very promising therapeutically approach before giving the indication to Allo-SCT, such as: antibody-purine analogue combination regimens as first-line or salvage treatment,[30,31] combined alemtuzumab therapies useful in fludarabine refractory and 17p-/p53 patients as showed by the German CLL Study Group (GCLLSG) CLL2H study,[12] and novel agent flavopiridol and lenalidomide capable to achieve 30% to 40% response rate when used as salvage therapy in fludarabine-refractory or del 17p- CLL.[13,14] Moreover these approaches may be helpful to achieve remission, a basic prerequisite for successful Allo-SCT.[32]
The EBMT guidelines conclude that there is evidence-based efficacy of allogeneic SCT in CLL and that this procedure is indicated in high-risk CLL patients. It should be stressed that none of the EBMT categories requires assessment of biologic risk factors except for cytogenetics for detection of p53 deletions, who deserves allogeneic SCT in first response. Ongoing prospective clinical studies will determine the impact of biomarkers including IgVh mutational status and other cytogenetic abnormalities in identification of patients at sufficiently high risk to deserve use of allogeneic SCT in first CR.
Another crucial point in the allogeneic transplant is a better selection of patient, as recently suggested by Sorror.[21] The FHCRC analysis revealed that comorbidities were more important than CLL-related variables for predicting progression free survival (PFS) and overall survival (OS).[21] Another risk factor for non-relapse-mortality (NRM), usually correlated with the presence of comorbidities, is refractory disease at transplantation.[22] Thus, it would appear prudent to limit allo-SCT to fit patients without evidence of refractory disease.
In conclusion, allo-SCT is the only therapy with curative potential in CLL and, in contrast to conventional treatment, with potential of providing long-term disease control even in patients with a very unfavorable biological and clinical risk profile (Table 2). However in addition to the disease risk, patient-related risk factors, such as age and comorbidity, have to be considered when the decision about allo-SCT is made.[21,33]

The larger RIC prospective trials uniformly show that in CLL the results of Allo-SCT are considerably impaired if the disease is not in remission at the time of transplantation, due to nodal bulks and/or chemotherapy resistance. Thus, Allo-SCT should be performed before CLL has advanced to a status of complete refractoriness or large resistant tumor burden in order not to miss the “window of opportunity” for a successful outcome. In eligible patients, Allo-SCT should be considered as soon as the first and/or the third EBMT criterion is met; on the contrary, the optimum timing for Allo-SCT in patients with early relapse (within 2 years) after purine analogue combination therapy (second EBMT criterion) needs to be defined by systematic studies.
On the other hand, RIC allo-HCT is able to mitigate the adverse prognosis conferred by purine analog resistance and unfavorable genetics. The EBMT guidelines identify a group of patients in whom available therapies are unlikely to achieve a prolonged disease-free survival.
The choice of Conditioning regimen and stem cell source
Several prospective RIC studies with a combined number of more than 300 patients included on the basis of modern CLL-specific risk stratification give relatively concordant results. In contrast, the experience with myeloablative conditioning in CLL largely relies on registry analyses and a few small single-center studies.[15-18,34,28] Therefore, RIC rather than myeloablative conditioning represents the standard procedure for Allo-SCT in CLL.
The choice of RIC Allo-SCT as the standard procedure rather than myeloablative conditioning emerged by the fact that the crucial therapeutic principle of allotransplantation in CLL is GVL activity. That evidence for superior direct cytotoxic activity of myeloablative conditioning over RIC is lacking, and also one should consider that CLL is an elderly disease.[35] At the same time an increased incidence of relapse after RIC in comparison with myeloablative conditioning was observed in a registry analysis, thus impaired disease control by RIC cannot be clearly ruled out.[20] Thus, according to the individual situation, the optimum choice of conditioning regimens may vary: in the presence of comorbidity and sensitive disease reduced-intensity regimens appear to be more appropriate, high-intensity regimens might be preferable in younger patients with good performance status but poorly controlled disease.[36,29]
A matched sibling donor should be preferred for Allo-SCT in CLL whenever possible, but a matched unrelated donor (MUD) is a reasonable alternative if an HLA-identical sibling is not available. In fact from published RIC studies in CLL, unfavorable effect of MUD respect to sibling donor did not significantly emerge.[19,21-22,27,37-38]
Due to the median age of patients, experience with haploidentical transplant and cord blood transplant is very scanty in CLL, and it is unlikely that disease-specific evidence for benefit of these procedures can ever be obtained.
RIC allo-SCT was associated with a NRM of 11%-34%, a PFS of 34%-67%, and OS of 48%-72% depending on the conditioning regimen and follow-up. Long-term disease control was obtained in a substantial proportion of patients, particularly in those with chemosensitive and non-bulky disease at the time of transplantation.
In the CLL3X trial of the German CLL Study Group (GCLLSG) and also in Schetelig data, poor risk CLL as defined by purine-analogue refractoriness or presence of deletion 17p- had a similar outcome to patients without poor-risk characteristics in terms of PFS, with a median follow up of 2 years.[19]
The outcome of 82 patients treated at the FHCRC consortium using a non-ablative conditioning regimen (fludarabine and low-dose total body irradiation) was recently updated [20]. Eighty-seven percent of patients had fludarabine refractory disease and 9% had del(17p).
At 5 years after RIC allo transplant, the NRM and relapse rate were 23% and 38% respectively, which translated into a OS of 50% and PFS of 39%. Patients with bulky lymphadenopathy (>5 cm) at the time of transplantation had a particularly poor outcome, with a 5-year relapse rate and PFS of 71% and 8% respectively. Extensive chronic GVHD was diagnosed in 50% of patients, but it resolved in most of them.[21]
A retrospective analysis of the EBMT on Allo-SCT in 44 patients with del 17p- CLL showed a 3-year PFS of 37% with no event occurring later than 3.5 years after transplant.[26]
We recently reported in a single centre experience the efficacy and feasibility of RIC allo-SCT in heavily pre-treated CLL patients, even if in a small number of patients and with a relatively short follow-up. In our series, 45% of patients were considered “high-risk” for fludarabine-refractory disease or 17p deletion. At median follow-up of 20 months (range 12-44) 80% of them are alive, confirming literature data.[39]
In conclusion, RIC Allo-SCT seems to be effective in poor-risk CLL, thereby overcoming the adverse prognostic impact of purine analogue refractoriness and del 17p-. However, active, unresponsive and bulky disease at the time of Allo-SCT still remains a predictor of an unfavorable outcome.[20-22,37]
Complication of allogeneic SCT in CLL and post-transplant follow-up
The major determinant of long-term morbidity affecting quality of life after Allo-SCT is chronic GVHD. Active chronic GVHD documented a significantly reduced long-term health status in patients allografted for various hematological malignancies.[40] Sorror et al. reported a 5-year cumulative incidence of extensive chronic GVHD of 49% for related and 53% for unrelated recipients. However, in the majority of affected patients clinical symptoms of chronic GVHD resolved over time, allowing discontinuation of systemic therapeutic immunosuppression after a median of 25 months [21]. Transplant-related long-term morbidity after Allo-SCT for CLL can be significant but is mainly restricted to those patients who have ongoing active chronic GVHD.
The high graft rejection rates remain a complication either of myeloablative or non-myeloablative transplant in CLL: it ranges between 18%- 20% in conventional conditioning,[16,41] while in case of RIC regimen between 12-25% in T cell–depleted grafts and 5-6% in unmanipulated grafts.[21,38,42] The possible explanations for this phenomenon could be the significant marrow infiltration in CLL patients at the time of transplantation, inversely correlated with outcome[37,42] and the role played by host dendritic cells, which are seriously defective in CLL patients.[43]
Another complication is represented by the high infection rates correlated with preexisting immunosuppression. Infections are the cause of about 50% of all CLL-related deaths,[44-45] primarily in fludarabine and/or alemtuzumab-refractory patients.[7,8] The base of susceptibility to development infections is the immunosuppression before SCT both disease and therapy-related, rarely encountered in patients with other lymphoproliferative disorders, such as follicular lymphoma, whose main cause of death is disease progression.[46] The multifactorial genesis should be secondary to hypogammaglobulinemia, defective T and natural killer cell function, neutropenia, and deficient complement activity.[47] Moreover in recent reports the risk of infections has been clearly correlated with presence of GVHD[21,38,43,48] and refractory disease.[8,49]
Disease relapse remains the major cause of failure after RIC allo-HCT in CLL patients. Early relapses are correlated with chemorefractory disease at the time of transplantation, perhaps due to the ineffectiveness of RIC regimens in controlling the disease before the graft vesus CLL (GVCLL) effect can take place.
Late relapse an be explaned with different mechanism including: CLL clonal evolution, the development of tolerance[25] and the survival of tumor cells in “GVCLL sanctuary sites” based on the negative impact of bulky lymphadenopathy at the time of transplant on PFS,[21] inadequate GVL effect to allow a complete disease eradication. It should be noted that a significant percentage of these late relapses occurred in lymph nodes in the absence of bone marrow or peripheral blood involvement, or even in patients with MRD negative status.[9,28,34,50-52]
Sensitive MRD quantification can be studied in CLL by polymerase chain reaction (PCR) or flow cytometry-based assays and has strong prognostic impact after transplant. The generally delayed decline of the MRD level and its close correlation with immune-relevant events strongly supports the assumption that GVL activity is the crucial contribution to tumor control in the allo-SCT. GVL-induced MRD negativity after allogeneic transplant is highly predictive of freedom from relapse.[24]
Moreover in CLL quantitative MRD monitoring seems to be a valid instrument for sensitive guidance of immune interventions aimed at disease eradication after allo-SCT. In contrast to donor’s lymphocyte infusion (DLI) upon clinical relapse which often shows only limited benefit in CLL,[19,33] MRD-triggered pre-emptive DLI can be highly effective.[53]
The best approach to post transplant immunotherapy, including monoclonal antibody[38] in CLL needs further study. Some of them, although a still short follow-up, show very promising results and the use of monoclonal antibody in the conditioning or just after transplant, could improve the results of allogeneic stem cell transplantation.[54] Rituximab given concomitantly with RIC allo-SCT or DLI may facilitate disease control[43] probably due , not only to direct cytotoxicity, but also to modulation of the GVCLL effect. In addition, maintenance therapy with monoclonal antibodies or immunomodulatory drugs with proven activity in poor-risk CLL, such as lenalidomide, could be explored in future trials to minimize the risk of relapse.
References
- Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2001.
- Dohner H, Stilgenbauer S, Benner A, Leupolt
E, Kröber A,
Bullinger L, Döhner K, Bentz M, Lichter P. Genomic aberrations and
survival in chronic lymphocytic leukemia. N Engl J Med. 2000; 343:
1910-1916.

- Damle RN, Wasil T, Fais F, Ghiotto F,
Valetto A, Allen SL,
Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P,
Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation
status and CD38 expression as novel prognostic indicators in chronic
lymphocytic leukemia. Blood. 1999; 94: 1840-1847.

- Rassenti LZ, Huynh L, Toy TL, Chen L,
Keating MJ, Gribben
JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A,
Kipps TJ. ZAP-70 compared with immunoglobulin heavy-chain gene mutation
status as a predictor of disease progression in chronic lymphocytic
leukemia. N Engl J Med. 2004; 351: 893-901.

- Rassenti LZ, Jain S, Keating MJ, Wierda WG,
Grever MR, Byrd
JC, Kay NE, Brown JR, Gribben JG, Neuberg DS, He F, Greaves AW, Rai KR,
Kipps TJ. Relative value of ZAP-70, CD38, and immunoglobulin mutation
status in predicting aggressive disease in chronic lymphocytic
leukemia. Blood. 2008; 112: 1923-1930.

- Montserrat E, Moreno C, Esteve J,
Urbano-Ispizua A, Giné E, Bosch F. How I treat refractory CLL. Blood.
2006; 107: 1276-1283.

- Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F,
Dighiero G, Döhner H, Hillmen P, Keating MJ, Montserrat E, Rai KR,
Kipps TJ. Guidelines for the diagnosis and treatment of chronic
lymphocytic leukemia: a report from the International Workshop on
Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer
Institute Working Group (NCIWG) 1996 guidelines. Blood. 2008; 111:
5446-5456.

- Keating MJ, O’Brien S, Kontoyiannis D,
Plunkett W, Koller
C, Beran M, Lerner S, Kantarjian H. Results of first salvage therapy
for patients refractory to a fludarabine regimen in chronic lymphocytic
leukemia. Leuk Lymphoma. 2002; 43: 1755-1762.

- Tam CS, O’Brien S, Lerner S, Khouri
I,
Ferrajoli A, Faderl S, Browning M, Tsimberidou AM,
Kantarjian H, Wierda WG. The natural history of
fludarabine-refractory chronic lymphocytic leukemia patients who fail
alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma. 2007; 48:
1931-1939.

- Lozanski G, Heerema NA, Flinn IW, Smith L,
Harbison J, Webb
J, Moran M, Lucas M, Lin T, Hackbarth ML, Proffitt JH, Lucas D, Grever
MR, Byrd JC. Alemtuzumab is an effective therapy for chronic
lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;
103: 3278-3281.

- Moreton P, Kennedy B, Lucas G, Leach M,
Rassam SMB, Haynes
A, Tighe J, Oscier D, Fegan C, Rawstron A, Hillmen P.
Eradication of minimal residual disease in B-cell chronic lymphocytic
leukemia after alemtuzumab therapy is associated with prolonged
survival. J Clin Oncol. 2005; 23: 2971-2979.

- Stilgenbauer S, Zenz T, Winkler D, Bühler
A, Schlenk RF,
Groner S, Busch R, Hensel M, Dührsen U, Finke J, Dreger P, Jäger U,
Lengfelder E, Hohloch K, Söling U, Schlag R, Kneba M, Hallek M, Döhner
H. Subcutaneous alemtuzumab in fludarabine-refractory chronic
lymphocytic leukemia: clinical results and prognostic marker analyses
from the CLL2H Trial of the GCLLSG. J Clin Oncol. 2009; 27: 3994-4001.

- Ferrajoli A, Lee BN, Schlette EJ, O'Brien
SM, Gao H, Wen S,
Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ.
Lenalidomide induces complete and partial remissions in patients with
relapsed and refractory chronic lymphocytic leukemia. Blood. 2008; 111:
5291-5297.

- Phelps MA, Lin TS, Johnson AJ, Hurh E,
Rozewski DM, Farley
KL, Wu D, Blum KA, Fischer B, Mitchell SM, Moran ME, Brooker-McEldowney
M, Heerema NA, Jarjoura D, Schaaf LJ, Byrd JC, Grever MR, Dalton JT.
Clinical response and pharmacokinetics from a phase 1 study of an
active dosing schedule of flavopiridol in relapsed chronic lymphocytic
leukemia. Blood. 2009; 113: 2637-2645.

- Rachel JM, Zucker ML, Fox CM, Plapp FV,
Menitove JE, Abbasi
F, Marti GE. Monoclonal B-cell lymphocytosis in blood donors. Br J
Haematol. 2007; 139: 832-836.

- Michallet M, Archimbaud E, Bandini G,
Rowlings PA, Deeg HJ,
Gahrton G, Montserrat E, Rozman C, Gratwohl A, Gale RP.
HLA-identical sibling bone marrow transplantation in younger patients
with chronic lymphocytic leukemia. European Group for Blood and Marrow
Transplantation and the International Bone Marrow Transplant Registry.
Ann Intern Med. 1996; 124: 311-315.

- Pavletic SZ, Khouri IF, Haagenson M, King
RJ, Bierman PJ,
Bishop MR, Carston M, Giralt S, Molina A, Copelan EA, Ringdén O, Roy V,
Ballen K, Adkins DR, McCarthy P, Weisdorf D, Montserrat E, Anasetti C.
Unrelated donor marrow transplantation for B-cell chronic lymphocytic
leukemia after using myeloablative conditioning: results from the
Center for International Blood and Marrow Transplant research. J Clin
Oncol. 2005; 23: 5788-5794.

- Moreno C, Villamor N, Colomer D, Esteve J,
Martino R,
Nomdedéu J, Bosch F, López-Guillermo A, Campo E, Sierra J, Montserrat
E. Allogeneic stem-cell transplantation may overcome the adverse
prognosis of Unmutated VH gene in patients with chronic lymphocytic
leukemia. J Clin Oncol. 2005; 23: 3433-3438.

- Schetelig J, Thiede C, Bornhäuser M,
Schwerdtfeger R, Kiehl
M, Beyer J, Sayer HG, Kröger N, Hensel M, Scheffold C, Held TK, Höffken
K, Ho AD, Kienast J, Neubauer A, Zander AR, Fauser AA, Ehninger
G, Siegert W. Evidence of a graft-versus-leukemia effect in chronic
lymphocytic leukemia after reduced-intensity conditioning and
allogeneic stem-cell transplantation: the Cooperative German Transplant
Study Group. J Clin Oncol. 2003; 21: 2747-2753.

- Dreger P, Brand R, Milligan D, Corradini
P, Finke J,
Lambertenghi Deliliers G, Martino R, Russell N, van Biezen A, Michallet
M, Niederwieser D. Reduced-intensity conditioning lowers
treatment-related mortality of allogeneic stem cell transplantation for
chronic lymphocytic leukemia: a population-matched analysis. Leukemia.
2005; 19: 1029-1033.

- Sorror ML, Storer BE, Sandmaier BM, Maris
M, Shizuru J,
Maziarz R, Agura E, Chauncey TR, Pulsipher MA, McSweeney PA, Wade JC,
Bruno B, Langston A, Radich J, Niederwieser D, Blume KG, Storb R,
Maloney DG. Five-year follow-up of patients with advanced chronic
lymphocytic leukemia treated with allogeneic hematopoietic cell
transplantation after nonmyeloablative conditioning. J Clin Oncol.
2008; 26: 4912-4920.

- Dreger P, Stilgenbauer S, Böttcher S, Ritgen M, Dietrich S, Bunjes D, Wilhelm Beelen D, Cohen S, Hegenbart U, Schubert J, Stadler M, Glass B, Burchert A, Uharek L, Hallek M, Kneba M, Schmitz N, Döhner H. Prognostic factors for outcome of nonmyeloablative allogeneic stem cell transplantation (NST) in poor-risk chronic lymphocytic leukemia (CLL): final results from a prospective multicenter trial (GCLLSG CLL3X study) [abstract]. Blood. 2008; 112: 212.
- Ritgen M, Böttcher S, Stilgenbauer S,
Bunjes D, Schubert J,
Cohen S, Humpe A, Hallek M, Kneba M, Schmitz N, Döhner H, Dreger P.
Quantitative MRD monitoring identifies distinct GVL response patterns
after allogeneic stem cell transplantation for chronic lymphocytic
leukemia: results from the GCLLSG CLL3X trial. Leukemia. 2008; 22(7):
1377-1386.

- Farina L, Carniti C, Dodero A, Vendramin
A, Raganato A,
Spina F, Patriarca F, Narni F, Benedetti F, Olivieri A, Corradini
P. Qualitative and quantitative polymerase chain reaction monitoringof
minimal residual disease in relapsed chronic lymphocytic leukemia:
early assessment can predict long-term outcome after reduced intensity
allogeneic transplantation. Haematologica. 2009; 94(5): 654-662.

- Hoogendoorn M, Jedema I, Barge RMY,
van
Luxemburg-Heijs SAP, Beaumont F, Marijt EWA, Willemze R, Falkenburg
JHF. Characterization of graft-versus-leukemia responses in patients
treated for advanced chronic lymphocytic leukemia with donor lymphocyte
infusions after in vitro T-cell depleted allogeneic stem cell
transplantation following reduced-intensity conditioning. Leukemia.
2007; 21(12): 2569-2574.

- Schetelig J, van Biezen A, Brand R,
Caballero D, Martino R,
Itala M, García-Marco JA, Volin L, Schmitz N, Schwerdtfeger R, Ganser
A, Onida F, Mohr B, Stilgenbauer S, Bornhäuser M, de Witte T, Dreger P.
Allogeneic hematopoietic stem-cell transplantation for chronic
lymphocytic leukemia with 17p deletion: a retrospective European Group
for Blood and Marrow Transplantation analysis. J Clin Oncol.
2008; 26(31): 5094-5100.

- Delgado J, Thomson K, Russell N, Ewing J,
Stewart W, Cook
G, Devereux S, Lovell R, Chopra R, Marks DI, Mackinnon S, Milligan DW.
Results of alemtuzumab-based reduced-intensity allogeneic
transplantation for chronic lymphocytic leukemia:a British Society of
Blood and Marrow Transplantation Study. Blood. 2006; 107(4): 1724-1730.

- Gribben JG, Zahrieh D, Stephans K,
Bartlett-Pandite L,
Alyea EP, Fisher DC, Freedman AS, Mauch P, Schlossman R, Sequist LV,
Soiffer RJ, Marshall B, Neuberg D, Ritz J, Nadler LM. Autologous and
allogeneic stem cell transplantation for poor risk chronic lymphocytic
leukemia. Blood. 2005; 106: 4389-4396.

- Dreger P, Corradini P, Kimby E, Michallet
M, Milligan
D, Schetelig J, Wiktor-Jedrzejczak W, Niederwieser D, Hallek M,
Montserrat F. Indications for allogeneic stem cell transplantation in
chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia.
2007; 21: 12-17.

- Robak T, Moiseev SI, Dmoszynska A, Solal-Céligny P, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Geisler C, Montillo M, Ganly P, Dartigeas C, Rosta A, Janssens A, Mendila M, Maurer J, Wenger MK. Rituximab, Fludarabine, and Cyclophosphamide (R-FC) Prolongs Progression Free Survival in Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) Compared with FC Alone: Final Results from the International Randomized Phase III REACH Trial. Blood (abstract). Nov 2008; 112: lba-1.
- Hallek M, Fingerle-Rowson G, Fink A, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Gruenhagen U, Bergmann MA, Catalano J, Zinzani PL, Caligaris Cappio F, Seymour JF, Berrebi A, Jaeger U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Boettcher S, Ritgen M, Stilgenbauer S, Mendila M, Kneba M, Döhner H, Fischer K. Immunochemotherapy with fludarabine, cyclophosphamide, and Rituximab (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia [abstract]. Blood. 2008; 112:125.
- Tsimberidou AM, Wierda WG, Plunkett W,
Kurzrock R, O’Brien
S, Wen S, Ferrajoli A, Ravandi-Kashani F, Garcia-Manero G, Estrov Z,
Kipps TJ, Brown JR, Fiorentino A, Lerner S, Kantarjian HM, Keating MJ.
Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab
combination therapy in patients with Richter’s syndrome or
fludarabine-refractory chronic lymphocytic leukemia. J Clin
Oncol. 2008; 26: 196-203.

- Sorror ML, Maris MB, Storb R, Baron F,
Sandmaier BM,
Maloney DG, Storer B. Hematopoietic cell transplantation-specific
comorbidity index (HCTCI): a new tool for risk assessment before
allogeneic HCT. Blood. 2005; 106: 2912-2919.

- Toze CL, Galal A, Barnett MJ, Shepherd JD,
Conneally EA,
Hogge DE, Nantel SH, Nevill TJ, Sutherland HJ, Connors JM, Voss NJ,
Kiss TL, Messner HA, Lavoie JC, Forrest DL, Song KW, Smith CA, Lipton
J. Myeloablative allografting for chronic lymphocytic leukemia:
evidence for a potent graft-versus leukemia effect associated with
graft-versus-host disease. Bone Marrow Transplant. 2005; 36: 825-830.

- Dreger P. Chronic lymphocytic leukemia: the role of stem cell transplantation. Hematology Education: the education programme for the annual congress of the EHA. 2008; 2: 314-319.
- Sorror ML, Storer BE, Maloney DG,
Sandmaier BM , Martin PJ,
Storb R. Outcomes after allogeneic hematopoietic cell transplantation
with nonmyeloablative or myeloablative conditioning regimens for
treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;
111: 446-452.

- Brown JR, Kim HT, Li S, Stephans K, Fisher
DC, Cutler C, Ho
V, Lee SJ, Milford EL, Ritz J, Antin JH, Soiffer RJ, Gribben JG, Alyea
EP. Predictors of improved progression-free survival after
nonmyeloablative allogeneic stem cell transplantation for advanced
chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2006; 12:
1056-1064.

- Khouri IF, Saliba RM, Admirand J,
O'Brien S, Lee MS ,
Korbling M, Samuels BI, Giralt S, Lima deM , Keating MJ, Champlin RE,
Bueso-Ramos C. Graft-versusleukaemia effect after non-myeloablative
haematopoietic transplantation can overcome the unfavourable expression
of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol.
2007; 137: 355-363.

- Laurenti L, Chiusolo P, Tarnani M, Balducci M, Piccirillo N, Sorà F, Sica S, Leone G. Reduced-Intensity conditioning allogeneic transplant in heavily pre-treated chronic lymphocytic leukaemia patients: a single centre experience. Hemat Oncol. 2010 (in press).
- Fraser CJ, Bhatia S, Ness K, Carter A,
Francisco L, Arora
M, Parker P, Forman S, Weisdorf D, Gurney JG, Baker KS. Impact of
chronic graft-versus-host disease on the health status of hematopoietic
cell transplantation survivors: a report from the Bone Marrow
Transplant Survivor Study. Blood. 2006;108:2867-2873.

- Khouri IF, Przepiorka D, van Besien K,
O'Brien S, Palmer
JL, Lerner S, Mehra RC, Vriesendorp HM, Andersson BS, Giralt S,
Körbling M, Keating MJ, Champlin RE. Allogeneic blood or marrow
transplantation for chronic lymphocytic leukaemia: timing of
transplantation and potential effect of fludarabine on acute
graft-versus-host disease. Br J Haematol. 1997; 97(2): 466-473.

- Sorror ML, Maris MB, Sandmaier BM, Storer
BE, Stuart MJ,
Hegenbart U, Agura E, Chauncey TR, Leis J, Pulsipher M, McSweeney P,
Radich JP, Bredeson C, Bruno B, Langston A, Loken MR, Al-Ali H, Blume
KG, Storb R, Maloney DG. Hematopoietic cell transplantation after
nonmyeloablative conditioning for advanced chronic lymphocytic
leukemia. J Clin Oncol. 2005; 23(16): 3819-3829.

- Delgado J, Pillai S, Benjamin R, Caballero
D, Martino R,
Nathwani A, Lovell R, Thomson K, Perez-Simon JA, Sureda A, Kottaridis
P, Vazquez L, Peggs K, Sierra J, Milligan D, Mackinnon S. The effect of
in vivo T cell depletion with alemtuzumab on reduced-intensity
allogeneic hematopoietic cell transplantation for chronic lymphocytic
leukemia. Biol Blood Marrow Transplant. 2008; 14(11): 1288-1297.

- Oscier D, Fegan C, Hillmen P, Illidge T,
Johnson S, Maguire
P, Matutes E, Milligan D. Guidelines on the diagnosis and management of
chronic lymphocytic leukaemia. Br J Haematol. 2004; 125(3): 294-317.

- Francis S, Karanth M, Pratt G, Starczynski
J, Hooper L,
Fegan C, Pepper C, Valcarcel D, Milligan DW, Delgado J. The effect of
immunoglobulin VH gene mutation status and other prognostic factors on
the incidence of major infections in patients with chronic lymphocytic
leukemia. Cancer. 2006; 107(5): 1023-1033.

- Johnson PW, Rohatiner AZ, Whelan JS, Price
CG, Love S, Lim
J, Matthews J, Norton AJ, Amess JA, Lister TA. Patterns of survival in
patients with recurrent follicular lymphoma: a 20-year study from a
single center. J Clin Oncol. 1995; 13(1): 140-147.

- Hensel M, Kornacker M, Yammeni S,
Egerer G, Ho AD.
Disease activity and pretreatment, rather than hypogammaglobulinaemia,
are major risk factors for infectious complications in patients with
chronic lymphocytic leukaemia. Br J Haematol. 2003; 122(4): 600-606.

- Caballero D, Garcia-Marco JA, Martino R,
Mateos V, Ribera
JM, Sarrá J, León A, Sanz G, de la Serna J, Cabrera R, González M,
Sierra J, San Miguel J. Allogeneic transplant with reduced intensity
conditioning regimens may overcome the poor prognosis of B-cell chronic
lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain
gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res.
2005; 11(21): 7757-7763.

- Perkins JG, Flynn JM, Howard RS, Byrd JC.
Frequency and
type of serious infections in fludarabine-refractory B-cell chronic
lymphocytic leukemia and small lymphocytic lymphoma: implications for
clinical trials in this patient population. Cancer. 2002; 94(7):
2033-2039.

- Khouri IF, Keating MJ, Saliba RM, Champlin
RE. Long-term
follow-up of patients with CLL treated with allogeneic hematopoietic
transplantation. Cytotherapy. 2002; 4(3): 217-221.

- Moreno C, Villamor N, Colomer D, Esteve J,
Giné E,
Muntañola A, Campo E, Bosch F, Montserrat E.. Clinical significance of
minimal residual disease, as assessed by different techniques, after
stem cell transplantation for chronic lymphocytic leukemia. Blood.
2006; 107(11): 4563-4569.

- Malhotra P, Hogan WJ, Litzow MR, Elliott
MA, Gastineau DA,
Ansell SM, Dispenzieri A, Gertz MA, Hayman SR, Inwards DJ, Lacy MQ,
Micallef IN, Porrata LF, Tefferi A. Longterm outcome of allogeneic stem
cell transplantation in chronic lymphocytic leukemia: analysis after a
minimum follow-up of 5 years. Leuk Lymphoma. 2008; 49(9): 1724-1730.

- Ritgen M, Stilgenbauer S, von Neuhoff N,
Humpe A,
Brüggemann M, Pott C, Raff T, Kröber A, Bunjes D, Schlenk R, Schmitz N,
Döhner H, Kneba M, Dreger P. Graft-versus-leukemia activity may
overcome therapeutic resistance of chronic lymphocytic leukemia with
unmutated immunoglobulin variable heavy-chain gene status: implications
of minimal residual disease measurement with quantitative PCR. Blood.
2004 Oct 15; 104(8): 2600-2.

- Khouri IF, Lee MS, Saliba RM, Andersson B,
Anderlini P,
Couriel D, Hosing C, Giralt S, Korbling M, McMannis J, Keating MJ,
Champlin RE. Nonablative allogeneic stem cell transplantation for
chronic lymphocytic leukemia: impact of rituximab on immunomodulation
and survival. Exp Hematol. 2004;32:28-35.
