Case Report
Coagulopathy in
Beta-Thalassemia: Current Understanding and Future PerspectivesAlfredo Marzano MD1, Andrea Marengo MD1, Michela di Fonzo MD2, Paola Begini MD2, Antonella Ferrari MD2, Bruno Monarca MD3, Gianfranco delle Fave MD3 and Massimo Marignani MD32
1Gastroenterology
and Hepatology , San Giovanni Battista Hospital, Turin , Italy. Corso
Bramante 88, 10125 Torino, Italy. 2Digestive and Liver
Disease Dpt., Second Medical Faculty, University of Rome “La Sapienza”
at S. Andrea Hospital. Via di Grottarossa 1035-1039, 00189 Rome, Italy.
3Hematology Dpt., Second Medical Faculty University of
Rome “La Sapienza” at S. Andrea Hospital. Via di Grottarossa 1035-1039,
00189 Rome, Italy
Correspondence
to: Massimo Marignani MD. Digestive and Liver Disease Dpt.,
Second Medical Faculty University of Rome “Sapienza”. S. Andrea
Hospital, Via di Grottarossa 1035-1039, 00189 Rome, Italy. Tel.
+390633775691. e-mail mmarignani@hotmail.com
Published: December 13, 2010
Received: October 06, 2010
Accepted: November 25, 2010
Medit J Hemat Infect Dis 2010, 2(1): e2010035, DOI 10.4084/MJHID.2010.035
This article is available from: http://www.mjhid.org/article/view/6547
This is an Open Access article
distributed under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited
Abstract
Patients with inactive or
occult hepatitis B virus infection and onco-hematological malignancies
are at risk of hepatitis flare, hepatic failure and death due to
chemotherapy-mediated reactivation. Nucleot(s)ide analogues can reduce
reactivation risks and/or hepatitis. However, immuno-mediated phenomena
combine to determine liver damage and clinical outcome. We describe in
this report two patients with onco-hematological malignancies and
hepatitis B reactivation after chemotherapy in whom glucocorticoids
were added to nucleot(s)ide. Antiviral therapy was effective on
replication, while glucocorticoids managed hyperergic response. One
patient without underlying liver disease survived, while the second
died and the autopsy demonstrated cirrhosis undetected before death.
This clinical trial suggests that in patients with onco-hematological
malignancies and altered liver function tests in spite of effective
antiviral response, glucocorticoids could control the effects of immune
response. However prognosis and survival are related to the underlying
liver status.
Introduction
In overt or occult HBV carriers with onco-hematological malignancies, viral reactivation can be a consequence of chemotherapy, influencing the risk of developing hepatitis flare, hepatic failure and death.1 Nucleot(s)ide analogues (NUC) are used to reduce the risk of reactivation in overt carriers controlling viral replication. Prophylaxis in HBsAg-negative/anti-HBc-positive remains controversial.[1]
Nevertheless, immuno-mediated phenomena, occurring as the result of the host’s immune system reconstitution after chemotherapy, also concur to determine the extent of liver damage and clinical outcome.[2]No clear indications exist for the management of patients with HBV reactivation in whom biochemical disorders remain and the clinical course deteriorates, in spite of an effective control over viral replication obtained with NUC, possibly as the result of this immuno-mediated hyperergic response.[2] Glucocorticoids have been sporadically used to control the immuno-mediated effects of HBV reactivation,[3,4] but their role in the management strategies of patients with onco-hematological malignancies has not been defined. Underlying liver disease status could also influence clinical events and survival, with more advanced liver disease determining the worst outcomes .[1]Our report consists of two patients with onco-hematological malignancies and HBV reactivation in whom glucocorticoids were added to NUC to manage reactivation.
Case reports
Case 1: In July 2006 a 73 year-old man was diagnosed with myelo-monoblastic acute leukemia (AML-M4). Before chemotherapy, ultrasound (US) study of the liver, serum alanine aminotransferase (ALT), total bilirubin, International Normalized Ratio (INR) were normal; anti-HCV, HBsAg and HBV-DNA were negative, anti-HBc, anti-HBs and anti-HBe positive. HBV monitoring was instituted [1]. FLANG (fludarabine, aracytin, novantrone) was administered (August 2006-January 2007), which resulted in the patient going into remission. The patient did not attend the scheduled follow up visit for personal reasons, and in July 2007, sero-reversion to HBsAg and increased ALT levels were detected and he was admitted to the Liver Unit. Table 1 summarizes disease course. ALT were >15 times higher than the upper normal value, HBV-DNA positive (266,700 IU/mL), US study of the liver was normal. High dose lamivudine (LAM, 300 mg/day) was started immediately due to significant viremia and the diagnosis of leukemia. Twenty days later bilirubin, ALT and INR increased in spite of HBV-DNA decrease to 31,000 IU/mL; firstly Adefovir (ADV) and then prednisolone (PDN, 50 mg/day) were added to LAM, speculating that a hyperergic immune response was the cause of liver damage and jaundice. ADV (10 mg/day) was introduced to control mutants eventually escaping LAM. ALT, bilirubin, and INR began to improve after three days with the introduction of PDN. HBV-DNA became negative one month later, and biochemical profile normalized after two months of treatment. During this period LAM and PDN were reduced to 100 and 30 mg/day respectively, and ADV continued. Thereafter, PDN was further reduced and discontinued in November, maintaining LAM and ADV. In May 2008, the patient reverted to HBsAg-negative and anti-HBs positive (9,5 UI/mL). NUC were discontinued on September 2009. One year later the patient is in hematological remission, while both HBsAg and HBV-DNA remain negative.
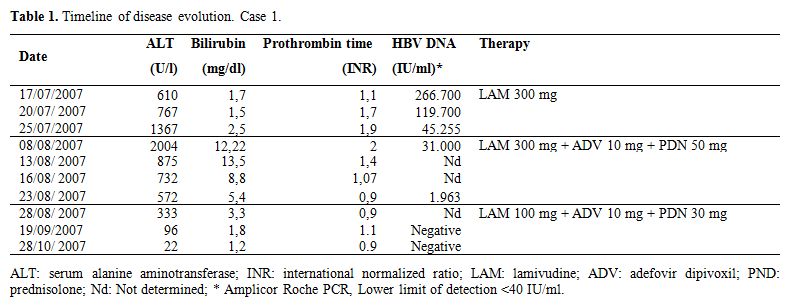 Table 1. Timeline of disease
evolution. Case 1.
Table 1. Timeline of disease
evolution. Case 1.
Case 2: In September 2006 a 73 year-old man was diagnosed with follicular lymphoma (grade-3a, stage III). Past medical history was unremarkable and baseline US liver study was normal, ALT were normal, HBsAg and anti-HBe positive, and HBV-DNA persistently below 2,000 IU/mL. He was categorized as inactive carrier and started on LAM before chemotherapy (6R-MINE: rituximab, mesna, ifosfamide, mitroxantrone, etoposide), taken until March 2007. During LAM therapy hematological remission was obtained, ALT were normal, while bilirubin and HBV-DNA remained slightly elevated. In July 2007, the patient continued the LAM treatment, and was admitted to the Liver Unit due to the development of jaundice. Lymphoma relapse was ruled out, but signs of viral and biochemical breakthroughs were present. Table 2 summarizes the disease course.
Hepatic failure, mild portal encephalopathy, and ascites developed. LAM resistance (L80V, L180M, M204I) was demonstrated by genotypic analysis (INNO-LiPA DR2), and ADV (10 mg/day) was added. Two weeks later viremia significantly decreased but bilirubin, INR and ALT increased. The addition of PDN (50 mg/day) determined a slight ALT decrease, however bilirubin and INR progressively worsened and the patient died because of irreversible liver failure. The autopsy revealed liver cirrhosis.
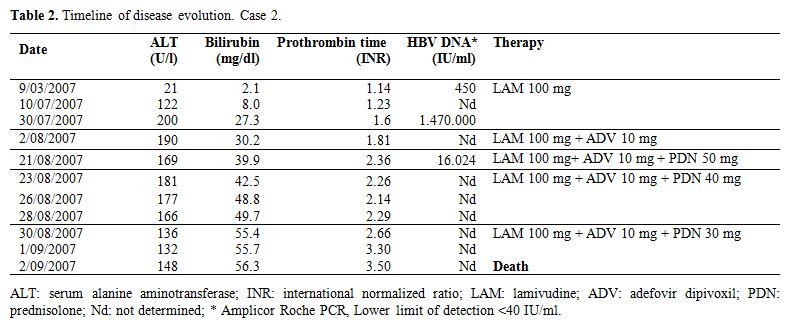 Table 2. Timeline of disease
evolution. Case 2.
Table 2. Timeline of disease
evolution. Case 2.
Discussion: During chemotherapy administration for onco-hematological malignancies, HBV replication can reactivate in overt or occult HBV carriers. Active viral replication reappears during immunosuppression, and hepatocytes infection occurs.[5] After the restoration of immune response, immune-mediated destruction of infected hepatocytes can cause hepatitis, hepatic failure and even death.[2] Pre-emptive LAM treatment can reduce reactivation in HBsAg-positive, while universal prophylaxis in HBsAg-negative/anti-HBc-positive remains controversial.1 The available data regarding HBV reactivation rates in these patients range from 12% to 25%,6-8 and have been obtained mainly from studies conducted on B-cell lymphoma patients, many of whom had been treated with the anti CD20 monoclonal antibody rituximab. In this setting LAM usually controls HBV replication and disease evolution.[1,6,7] However, a severe course despite adequate inhibition of viral replication can occur.[7,9-10] In fact, in the study by Yeo, the reported mortality in lymphoma patients sero-reverting to HBsAg was 20%, despite LAM treatment.[7] In this study HBV reactivation was diagnosed when an increase in ALT (3-fold or greater or more than 100 IU/L) was associated to HBsAg seroreversion and HBV-DNA increase.[7] Surveillance protocols based on HBV-DNA and anti-HBs level monitoring have been proposed to detect reactivation of viral replication at an earlier stage.[8] In the recent Niitsu paper, this approach allowed to detect virological reactivation before ALT increase, even prior to HBsAg reappearance. Patient in whom HBV reactivation was detected by this surveillance protocol were treated with entecavir, a more potent antiviral, and none developed acute hepatitis or died of liver-related causes.[8] Nevertheless, the cost-effectiveness of this approach, based on high cost technologies and medications, remains to be determined. At any rate, delayed detection of HBV reactivation, and the consequent unnoticed progression to acute hepatitis should be considered a risk factor for a worse clinical outcome, due to the late commencement of treatment,[7] as it happened in patient 1 due to his inability to attend the follow up appointments. Similarly, the late identification of HBV mutants by genotypic analysis in suboptimal responders to NUC plays a pivotal role, delaying appropriate rescue therapy and supporting HBV reactivation, as it happened in patient [2]. Therefore, these situations should be avoided, appropriate surveillance protocols should be developed, enforcing patients to adhere to follow up. Glucocorticoids addition could be useful in this clinical setting3-5 as these drugs blunt T-cell response targeting HBV-infected hepatocytes.[11]
Glucocorticoids use in the management of HBV reactivation remains anecdotal. Early glucocorticoids administration to manage HBV reactivation has been described in a heterogeneous group of patients, some with onco-hematological malignancies.[3,4] In these trials pre-treatment baseline HBV-DNA and liver disease status were unknown, no case of isolated anti-HBc positive sero-reversion was included, management protocols varied widely, different NUC were used with a variable sequence of glucocorticoids and NUC introduction.[3,4] Nevertheless, these reports seem to suggest that a key strategy is the timely consideration of glucocorticoids addition in those cases in which liver functional status worsens in spite of good virological response to NUC. In this case, adding glucocorticoids might combat the harmful effects of immune reconstitution against HBV infected hepatocytes in onco-hematological malignancies, since NUC alone might not be able to control the progression to liver failure resulting from hyperergic reactions.[9,10] As HBV contains a glucocorticoid-responsive element that may enhance viral replication favoring diffusion and the possible emergence of resistant strains,[12] these drugs should be introduced when one or more NUC have been started to control replication.
What emerges from our experience, is that the presence of underlying liver disease strongly influences survival, even when a compound treatment (virologic and immunologic) is adopted. Thus, a careful evaluation of HBV markers, clinical history and direct/indirect signs of silent liver disease is mandatory in all onco-hematological malignancies patients eligible for chemotherapy.[1]
Conclusion: Our experience supports the policy of baseline screening of onco-hematological malignancies patients for HBV and liver disease status.[1]Prophylaxis is indicated in inactive carriers, and could be considered in anti-HBc positive subjects with onco-hematological malignancies and/or bone marrow transplantation. When biochemical changes do not improve in spite of effective virologic response to NUC(s), glucocorticoids addition should be considered early in order to control hyperergic immune response. However, prognosis is strictly related to the underlying liver disease status, and to the timely addition of glucocorticoids.
Aknowledgements: We thank Jennifer Cox for kindly providing free assistance in revising the English language.
In overt or occult HBV carriers with onco-hematological malignancies, viral reactivation can be a consequence of chemotherapy, influencing the risk of developing hepatitis flare, hepatic failure and death.1 Nucleot(s)ide analogues (NUC) are used to reduce the risk of reactivation in overt carriers controlling viral replication. Prophylaxis in HBsAg-negative/anti-HBc-positive remains controversial.[1]
Nevertheless, immuno-mediated phenomena, occurring as the result of the host’s immune system reconstitution after chemotherapy, also concur to determine the extent of liver damage and clinical outcome.[2]No clear indications exist for the management of patients with HBV reactivation in whom biochemical disorders remain and the clinical course deteriorates, in spite of an effective control over viral replication obtained with NUC, possibly as the result of this immuno-mediated hyperergic response.[2] Glucocorticoids have been sporadically used to control the immuno-mediated effects of HBV reactivation,[3,4] but their role in the management strategies of patients with onco-hematological malignancies has not been defined. Underlying liver disease status could also influence clinical events and survival, with more advanced liver disease determining the worst outcomes .[1]Our report consists of two patients with onco-hematological malignancies and HBV reactivation in whom glucocorticoids were added to NUC to manage reactivation.
Case reports
Case 1: In July 2006 a 73 year-old man was diagnosed with myelo-monoblastic acute leukemia (AML-M4). Before chemotherapy, ultrasound (US) study of the liver, serum alanine aminotransferase (ALT), total bilirubin, International Normalized Ratio (INR) were normal; anti-HCV, HBsAg and HBV-DNA were negative, anti-HBc, anti-HBs and anti-HBe positive. HBV monitoring was instituted [1]. FLANG (fludarabine, aracytin, novantrone) was administered (August 2006-January 2007), which resulted in the patient going into remission. The patient did not attend the scheduled follow up visit for personal reasons, and in July 2007, sero-reversion to HBsAg and increased ALT levels were detected and he was admitted to the Liver Unit. Table 1 summarizes disease course. ALT were >15 times higher than the upper normal value, HBV-DNA positive (266,700 IU/mL), US study of the liver was normal. High dose lamivudine (LAM, 300 mg/day) was started immediately due to significant viremia and the diagnosis of leukemia. Twenty days later bilirubin, ALT and INR increased in spite of HBV-DNA decrease to 31,000 IU/mL; firstly Adefovir (ADV) and then prednisolone (PDN, 50 mg/day) were added to LAM, speculating that a hyperergic immune response was the cause of liver damage and jaundice. ADV (10 mg/day) was introduced to control mutants eventually escaping LAM. ALT, bilirubin, and INR began to improve after three days with the introduction of PDN. HBV-DNA became negative one month later, and biochemical profile normalized after two months of treatment. During this period LAM and PDN were reduced to 100 and 30 mg/day respectively, and ADV continued. Thereafter, PDN was further reduced and discontinued in November, maintaining LAM and ADV. In May 2008, the patient reverted to HBsAg-negative and anti-HBs positive (9,5 UI/mL). NUC were discontinued on September 2009. One year later the patient is in hematological remission, while both HBsAg and HBV-DNA remain negative.
 Table 1. Timeline of disease
evolution. Case 1.
Table 1. Timeline of disease
evolution. Case 1. Case 2: In September 2006 a 73 year-old man was diagnosed with follicular lymphoma (grade-3a, stage III). Past medical history was unremarkable and baseline US liver study was normal, ALT were normal, HBsAg and anti-HBe positive, and HBV-DNA persistently below 2,000 IU/mL. He was categorized as inactive carrier and started on LAM before chemotherapy (6R-MINE: rituximab, mesna, ifosfamide, mitroxantrone, etoposide), taken until March 2007. During LAM therapy hematological remission was obtained, ALT were normal, while bilirubin and HBV-DNA remained slightly elevated. In July 2007, the patient continued the LAM treatment, and was admitted to the Liver Unit due to the development of jaundice. Lymphoma relapse was ruled out, but signs of viral and biochemical breakthroughs were present. Table 2 summarizes the disease course.
Hepatic failure, mild portal encephalopathy, and ascites developed. LAM resistance (L80V, L180M, M204I) was demonstrated by genotypic analysis (INNO-LiPA DR2), and ADV (10 mg/day) was added. Two weeks later viremia significantly decreased but bilirubin, INR and ALT increased. The addition of PDN (50 mg/day) determined a slight ALT decrease, however bilirubin and INR progressively worsened and the patient died because of irreversible liver failure. The autopsy revealed liver cirrhosis.
 Table 2. Timeline of disease
evolution. Case 2.
Table 2. Timeline of disease
evolution. Case 2. Discussion: During chemotherapy administration for onco-hematological malignancies, HBV replication can reactivate in overt or occult HBV carriers. Active viral replication reappears during immunosuppression, and hepatocytes infection occurs.[5] After the restoration of immune response, immune-mediated destruction of infected hepatocytes can cause hepatitis, hepatic failure and even death.[2] Pre-emptive LAM treatment can reduce reactivation in HBsAg-positive, while universal prophylaxis in HBsAg-negative/anti-HBc-positive remains controversial.1 The available data regarding HBV reactivation rates in these patients range from 12% to 25%,6-8 and have been obtained mainly from studies conducted on B-cell lymphoma patients, many of whom had been treated with the anti CD20 monoclonal antibody rituximab. In this setting LAM usually controls HBV replication and disease evolution.[1,6,7] However, a severe course despite adequate inhibition of viral replication can occur.[7,9-10] In fact, in the study by Yeo, the reported mortality in lymphoma patients sero-reverting to HBsAg was 20%, despite LAM treatment.[7] In this study HBV reactivation was diagnosed when an increase in ALT (3-fold or greater or more than 100 IU/L) was associated to HBsAg seroreversion and HBV-DNA increase.[7] Surveillance protocols based on HBV-DNA and anti-HBs level monitoring have been proposed to detect reactivation of viral replication at an earlier stage.[8] In the recent Niitsu paper, this approach allowed to detect virological reactivation before ALT increase, even prior to HBsAg reappearance. Patient in whom HBV reactivation was detected by this surveillance protocol were treated with entecavir, a more potent antiviral, and none developed acute hepatitis or died of liver-related causes.[8] Nevertheless, the cost-effectiveness of this approach, based on high cost technologies and medications, remains to be determined. At any rate, delayed detection of HBV reactivation, and the consequent unnoticed progression to acute hepatitis should be considered a risk factor for a worse clinical outcome, due to the late commencement of treatment,[7] as it happened in patient 1 due to his inability to attend the follow up appointments. Similarly, the late identification of HBV mutants by genotypic analysis in suboptimal responders to NUC plays a pivotal role, delaying appropriate rescue therapy and supporting HBV reactivation, as it happened in patient [2]. Therefore, these situations should be avoided, appropriate surveillance protocols should be developed, enforcing patients to adhere to follow up. Glucocorticoids addition could be useful in this clinical setting3-5 as these drugs blunt T-cell response targeting HBV-infected hepatocytes.[11]
Glucocorticoids use in the management of HBV reactivation remains anecdotal. Early glucocorticoids administration to manage HBV reactivation has been described in a heterogeneous group of patients, some with onco-hematological malignancies.[3,4] In these trials pre-treatment baseline HBV-DNA and liver disease status were unknown, no case of isolated anti-HBc positive sero-reversion was included, management protocols varied widely, different NUC were used with a variable sequence of glucocorticoids and NUC introduction.[3,4] Nevertheless, these reports seem to suggest that a key strategy is the timely consideration of glucocorticoids addition in those cases in which liver functional status worsens in spite of good virological response to NUC. In this case, adding glucocorticoids might combat the harmful effects of immune reconstitution against HBV infected hepatocytes in onco-hematological malignancies, since NUC alone might not be able to control the progression to liver failure resulting from hyperergic reactions.[9,10] As HBV contains a glucocorticoid-responsive element that may enhance viral replication favoring diffusion and the possible emergence of resistant strains,[12] these drugs should be introduced when one or more NUC have been started to control replication.
What emerges from our experience, is that the presence of underlying liver disease strongly influences survival, even when a compound treatment (virologic and immunologic) is adopted. Thus, a careful evaluation of HBV markers, clinical history and direct/indirect signs of silent liver disease is mandatory in all onco-hematological malignancies patients eligible for chemotherapy.[1]
Conclusion: Our experience supports the policy of baseline screening of onco-hematological malignancies patients for HBV and liver disease status.[1]Prophylaxis is indicated in inactive carriers, and could be considered in anti-HBc positive subjects with onco-hematological malignancies and/or bone marrow transplantation. When biochemical changes do not improve in spite of effective virologic response to NUC(s), glucocorticoids addition should be considered early in order to control hyperergic immune response. However, prognosis is strictly related to the underlying liver disease status, and to the timely addition of glucocorticoids.
Aknowledgements: We thank Jennifer Cox for kindly providing free assistance in revising the English language.
References
- Marzano A, Angelucci E, Andreone P,
Brunetto M, Bruno R, Burra P, Caraceni P, Daniele B, Di Marco V,
Fabrizi F, Fagiuoli S, Grossi P, Lampertico P, Meliconi R, Mangia A,
Puoti M, Raimondo G, Smedile A. Prophylaxis and treatment of hepatitis
B in immunocompromised patients. Dig Liver Dis 2007; 39: 397-408.
doi:10.1016/j.dld.2006.12.017.
- Perrillo RP. Acute flares in chronic
hepatitis B: the natural and unnatural history of an
immunologically-mediated liverdisease.Gastroenterology 2001; 120:
1009–22. doi:10.1053/gast.2001.22461.
- Fujiwara K, Yokosuka O, Kojima H, Kanda T,
Saisho H, Hirasawa H, Suzuki H. Importance of adequate
immunosuppressive therapy for the recovery of patients with
“life-threatening” severe exacerbation of chronic hepatitis B. World J
Gastroenterol 2005; 11: 1109–14.
- Fujiwara K, Yasui S, Yonemitsu Y, Fukai K,
Arai M, Imazeki F, Suzuki A, Sadahiro T, Oda S, Yokosuka O. Efficacy of
combination therapy of antiviral and immunosuppressive drugs for the
treatment of severe acute exacerbation of chronic hepatitis B. J
Gastroenterol 2008;43:711-19. doi:10.1007/s00535-008-2222-5.
- Tillmann HL, Wedemeyer H, Manns MP.
Treatment of hepatitis B in special patient groups: hemodialysis, heart
and renal transplant, fulminant hepatitis, hepatitis B virus
reactivation. J Hepatol 2003; 39 (Suppl 1): S206-11.
doi:10.1016/S0168-8278(03)00364-7.
- Yeo W, Johnson PJ. Diagnosis, prevention
and management of hepatitis B virus reactivation during anticancer
therapy. Hepatology 2006; 43: 209–220. doi:10.1002/hep.21051.
- Yeo W, Chan TC, Leung NW, Lam WY, Mo FK,
Chu MT, Chan HL, Hui EP, Lei KI, Mok TS, Chan PK. Hepatitis B virus
reactivation in lymphoma patients with prior resolved hepatitis B
undergoing anticancer therapy with or without rituximab. J Clin Oncol.
2009; 27:605-11. doi:10.1200/JCO.2008.18.0182.
- Nozomi N, Hagiwara Y, Tanae K, Kohri M,
Takahashi N. Prospective analysis of hepatitis B virus reactivation in
patients with diffuse large B-cell lymphoma after rituximab combination
chemotheraphy. J Clin Oncol 2010 Sept 13. [Epub ahead of print].
- Tsubota A, Arase Y, Suzuki Y, Suzuki F,
Sezaki H, Hosaka T, Akuta N, Someya T, Kobayashi M, Saitoh S, Ikeda K,
Kumada H. Lamivudine monotherapy for spontaneous severe acute
exacerbation of chronic hepatitis B. J Gastroenterol Hepatol 2005; 20:
426-32. doi:10.1111/j.1440-1746.2004.03534.x.
- Chan HL, Tsang SW, Hui Y, Leung NW, Chan
FK, Sung JJ. The role of lamivudine and predictors of mortality in
severe flare-up of chronic hepatitis B with jaundice. J Viral Hepat
2002; 9: 424-8. doi:10.1046/j.1365-2893.2002.00385.x.
- Tornatore KM, Venuto RC, Logue G, Davis
PJ. CD4+ and CD8+ lymphocyte and cortisol response patterns in
elderly and young males after methylprednisolone exposure. J Med 1998;
29: 159-83.
- Tur-Kaspa R, Burk RD, Shaul Y, Shafritz
DA. Hepatitis B virus DNA contains glucocorticoid-reponsive
element. Proc Natl Acad Sci USA 1986; 83: 1627-31.
doi:10.1073/pnas.83.6.1627