High Serum Erythropoietin and Ferritin Levels in Conjunction with Anemia Response in Malignant Lymphoma
Sofia Omari1,2 Alhossain Khalafallah,1,3 Mahmoud Ayesh,4 Ismail Matalka2 and Al- Raji Hadithi4
3Department of Haematology, Launceston General Hospital, Tasmania, Australia.
4Department of Internal Medicine, Jordan University of Science and Technology, Irbid, Jordan.
Published: May 16, 2011
Received: January 18, 2011
Accepted: April 10, 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011018, DOI 10.4084/MJHID.2011.018
This article is available from: http://www.mjhid.org/article/view/7867
Abstract
Our findings suggest that ferritin estimation in lymphoma patients may predict the level of erythropoiesis and possibly the degree of anemia. Further studies to confirm these findings are warranted.
Anemia is common in patients with cancer, especially Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphomas (NHL).[1,2] About 30-40 % of lymphoma patients present with anemia prior to commencement of chemotherapy. The anemia is mainly attributed to bone marrow replacement by lymphoma cells or inadequate erythropoietin (EPO) production which leads to suppressed erythropoiesis.[3] The frequency of anemia increases in all malignancies, with the largest increase in patients with NHL (from 35.1% at baseline to 73.7%) and HL (from 21.9% to 54.5%) under the age of 60.[4-6] EPO stimulates the proliferation and maturation of erythroid precursors in the bone marrow. The earliest progenitor form that can be identified is the erythroid burst-forming unit (BFU-E), which subsequently evolve into erythroid colony-forming units (CFU-E). EPO acts primarily on these cells by stimulating cell division and maturation as well as inhibiting apoptosis.[7] Tissue hypoxia triggers endogenous EPO production when hemoglobin (Hb) falls below 12 g/dL. [8,9] When hypoxia resolves, EPO production decreases.[9]
Initial studies in patients with cancer found that most of the patients had disproportionately low levels of endogenous EPO for the degree of their anemia, especially after chemotherapy.[10-12] Some studies showed that cancer-related anemia patients had serum EPO levels above the normal range which shows their strong ability into self-body regulation in response to anemia. [10-12] Although some studies suggested that EPO levels decrease in malignant lymphoma,[11,13] however, others reported that EPO levels increase in anemic lymphoma patients, indicating that anemia did not depend on defective EPO secretion.[14] Ferritin is synthesised and released from malignant lymphocytes in a faster manner than normal lymphocytes causing ferritin concentration to increase in those patients.[15-17] However, there is very few studies that address EPO response in association with ferritin levels in conjunction with anemia response in malignant lymphoma. The aims of our study were to determine (a) the EPO and ferritin levels in de novo and treated lymphoma patients, and (b) whether there is a relationship between EPO level and ferritin response to anemia.
Patients and Methods
Fifty-five serum samples were obtained from patients who were diagnosed with malignant lymphomas (29 HL and 26 NHL) during the period between November 2006 to March 2008 at the King Abdullah University Teaching Hospital. Written consent was obtained from all patients. This study was ethically approved by the Conjoint Ethics Committee of Jordan University of Science and Technology. Thirty samples were obtained from newly-diagnosed patients and twenty-five from patients who were receiving chemotherapy after at least 3 cycles of chemotherapy. Both groups included anemic and non-anemic patients. The specific diagnosis and the stage of the disease for each lymphoma patient were obtained from patients clinical notes. We included in the study newly diagnosed patients with malignant lymphoma and patients who had received at least three cycles of chemotherapy for malignant lymphoma and had not received blood transfusions for at least 12 weeks.
There were an equal number of samples collected at the time of diagnosis and after 3 cycles of chemotherapy. Bone marrow studies were performed for all patients and showed that 13 cases had bone marrow involvement (stage IV lymphoma disease) without a significant compromise of the hemopoeitic reserve.
We excluded patients with hepatitis, infections, liver disease, renal failure, iron, B12 and folate deficiencies and patients with hemolytic anemia as these conditions may influence the levels of ferritin and EPO. Infections markers were negative for the patients included in the study. Furthermore, no patients received erythropoietin therapy in this study.
Fifty-five healthy, sex and age-matched volunteers were randomly selected as a control group. All control group participants provided a written consent.
Non-fasting 5-10 ml serum samples were obtained from all participants during the morning at room temperature. Samples were centrifuged within 30 minutes of collection at 2000 rpm for 10 minutes at room temperature and sera were isolated from each sample. For each patient, the following tests were performed: serum EPO, Hb, creatinine, lactate dehydrogenase (LDH), B12, folate and ferritin. Serum vitamin B12 was obtained to exclude megaloblastic anemia. EPO was measured by ELISA (IBL-Hamburg kit, Germany). Ferritin levels were measured by electrochemiluminescence immunoassay (ECLIA) using an Elecsys 1020-immunoassay analyzer (Roche Diagnostics, Indianapolis, Indiana, USA).
Definitions: Stage A lymphoma disease is defined by absence of constitutional symptoms, while stage B means presence of B-constitutional symptoms such as weight loss, night sweats or fever.
Stage I-II lymphoma represent a localized nodal disease, while stage III lymphoma disease represents wide-speared nodal disease but with absence of non-reticuloendothelial organ involvement, and stage IV lymphoma disease reflects organ involvement such as bone marrow, liver or lung involvement. Extra-nodal disease is defined as involvement of other organs that are not related to the reticulo-endothelial system. Severe anemia is defined by a level of Hb <8 g/dl, while moderate anemia; Hb=8-10 g/dl and mild anemia in males; Hb=10-13 g/dl and in females; Hb=10-12 g/dl.
Statistical analysis: Means, standard deviations (SD) and difference of means were estimated for illustrative purposes using general linear modelling. Assumptions of linear regression were violated in most of these analyses due to heteroskedasticity and skewness of residuals; therefore, non-parametric analyses (ordinal logistic regression) were performed to test differences of distributions. P-values were corrected for multiple comparisons where appropriate by the Holm method. All statistical analyses were performed using Stata/SE 11.0 (StataCorp, College Station, Tx USA).
Results
We estimated serum EPO levels in 55 normal healthy controls where the EPO mean was 7 mU/L (SD± 5.3). We observed that lymphoma patients had higher EPO levels compared with the healthy control group. Hb was significantly lower in lymphoma patients compared with the control group (P<0.001). Lymphoma patients had significantly higher ferritin levels than the control group (P<0.001; Table 1). Also, Hb was lower in newly-diagnosed lymphoma patients when compared to the lymphoma patients in the chemotherapy group (P=0.003). EPO, LDH and ferritin concentrations were similar for newly-diagnosed lymphoma patients and patients who underwent chemotherapy. Stage B lymphoma (symptomatic) patients comprised most of the lymphoma study group (73.9 %) compared with 19.6 % for stage A lymphoma (asymptomatic) and 6.5% for extranodal involvement patients. Stage A lymphoma patients had significantly higher Hb concentrations compared with those with stage B (P=0.028). However, EPO levels were not significantly higher in stage B lymphoma patients compared with stage A patients (P=0.4), and there were no significant differences observed in LDH and ferritin levels between stage A and stage B lymphoma patients. There was no statistical difference seen between HL and NHL patients in regard to EPO, Hb, ferritin, and LDH levels.
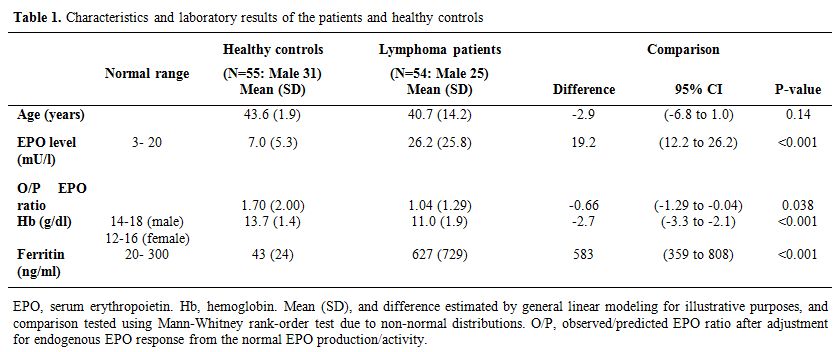
Table 1. Characteristics and laboratory results of the patients and healthy controls
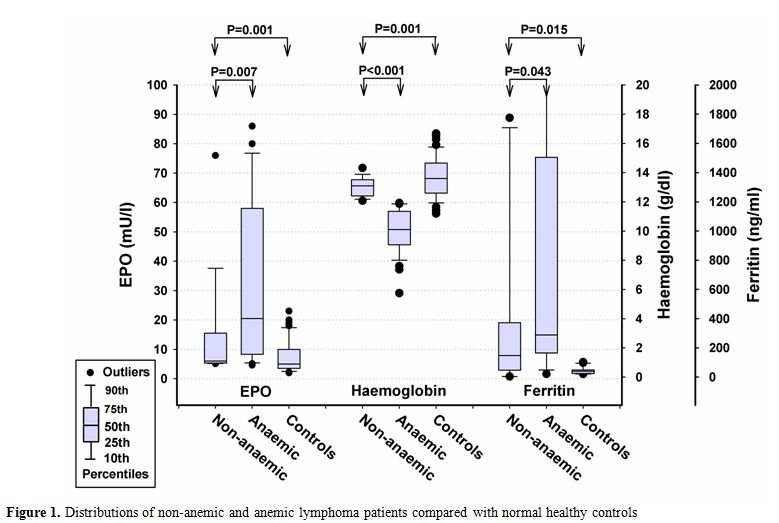
Figure 1. Distributions of non-anemic and anemic lymphoma patients compared with normal healthy controls
Discussion
Our analysis showed that serum EPO levels were higher in lymphoma patients compared with the healthy control group. This is most likely due to EPO response to anemia in lymphoma patients. Furthermore, anemic lymphoma patients (74.4%) had higher EPO levels compared to non-anemic lymphoma patients. Similar findings have been reported by Birgegård et al;[5] Li et al [12] and others. [18] Serum EPO concentrations in lymphoma patients exhibited wide variation, ranging from normal levels to high levels in association with anemia. Some studies showed a decreased level of EPO in treated lymphoma patients,[3,11,13] whereas the present study demonstrats no difference in EPO levels between newly-diagnosed lymphoma patients and patients on chemotherapy. Our results are consistent with a previous study reported by Ozguroglu et al.[18] However, Lee [11] reported low levels of EPO in patients receiving chemotherapy compared with newly-diagnosed patients. An inverse relationship between Hb level and EPO was seen in our series in accordance with other studies.[3,18] Furthermore, the current study showed an improvement in Hb level in lymphoma patients treated with chemotherapy for at least three cycles as compared with patients who were newly-diagnosed (P=0.003). This is largely due to the fact that most of the treated patients for lymphoma disease were in advanced clinical stage III-IV (65%) that has responded with improvement of Hb secondary to the commenced treatment. Furthermore, stage A lymphoma patients had significantly higher initial Hb levels compared with stage B patients (P=0.028). Higher EPO levels were found in stage B patients in comparison with stage A but was not statistically significant. We observed higher ferritin levels in lymphoma patients with high EPO levels compared to healthy controls (P<0.001), that has been explained by erythropoietic response to anemia. Therefore, the ferritin level may indicate the increase of EPO activity among lymphoma patients.
Conclusions
In summary, an adequate EPO and
ferritin response to anemia in patients with lymphoma was demonstrated
in our
study. This may indicate that recombinant human EPO (rhuEPO) treatment
is not
required in anemic lymphoma patients who have acceptable EPO and
ferritin
response. Results obtained from this study would help in understanding
different factors that contribute in the degree of anemia among
lymphoma
patients. Furthermore, most of the patients with high ferritin levels
had
significantly high EPO levels. Perhaps more studies can address
utilization of
ferritin as a possible marker for EPO activity in addition to measuring
the
response to anemia in this cohort of patients. Of note, the cost of
ferritin
measurement is much lower than that of EPO. Moreover, ferritin testing
is more
accessible in most laboratories compared to EPO. However, one of the
important
limitations is that ferritin level can be affected as an acute phase
reactant
by the degree of infection or inflammation that may coincide in
lymphoma patients.
It is worth noting that we excluded all patients with infections from
the
current study. Nevertheless, further studies to confirm our findings
are
warranted.
Acknowledgements
This work was supported by a grant from Jordan University
of Science
and
Technology (J.U.S.T), Jordan. All thanks to the King Abdullah
University
Teaching Hospital which allowed access to diagnostic materials and
pathology
reports
References
- Smith Jr R, Tchekmedyian S. Practitioners'
practical model for managing cancer-related anemia. Oncology (Williston
Park, NY) 2002;16(S10):55-63.

- Ludwig H, Strasser K. Symptomatology of
anemia. Semin Oncol 2001;28(S8):7-14. doi:10.1016/S0093-7754(01)90206-4

- Kostova G, Siljanovski N. Erythropoietin
production in patients with malignant lymphoma. Prilozi
2005;26:157-68.PMid:16400237

- Coiffier B. The impact and management of anaemia in haematological malignancies. Medical oncology 2000;17:S2-10
- Birgegård G, Gascón P, Ludwig H. Evaluation
of anaemia in patients with multiple myeloma and lymphoma: findings of
the European CANCER ANAEMIA SURVEY. European journal of haematology
2006;77:378-86. doi:10.1111/j.1600-0609.2006.00739.xPMid:17044835
PMCid:1618958

- Steurer M, Wagner H, Gastl G. Prevalence
and management of anaemia in haematologic cancer patients receiving
cyclic nonplatinum chemotherapy: Results of a prospective national
chart survey. Wiener Klinische Wochenschrift 2004;116:367-72.doi:10.1007/BF03040915
PMid:15291288

- Sathyanarayana P, Dev A, Fang J, Houde E,
Bogacheva O, Bogachev O, et al. EPO receptor circuits for primary
erythroblast survival. Blood 2008; 11:5390-9.doi:10.1182/blood-2007-10-119743
PMid:18349318 PMCid:2396729

- Gabrilove J. Overview: erythropoiesis,
anemia, and the impact of erythropoietin. Semin Hematol 2000;37(S6):1-3.doi:10.1016/S0037-1963(00)90060-X

- Haase VH. Hypoxic regulation of
erythropoiesis and iron metabolism. American Journal of Physiology -
Renal Physiology 2010; 299:1-13. doi:10.1152/ajprenal.00174.2010
PMid:20444740

- Schwartz R. Anemia in patients with
cancer: Incidence, causes, impact, management, and use of treatment
guidelines and protocols. American Journal of Health-System Pharmacy
2007;64:5-13. doi:10.2146/ajhp060601
PMid:17244886

- Lee S, Kwon J, Jung C. Erythropoietin
response is inadequate in cancer patients receiving chemotherapy.
International journal of hematology 2001;74:416-20. doi:10.1007/BF02982085 PMid:11794697

- Li LB, Luo R. Clinical significance of
serum erythropoietin detection in patients with cancer-related anemia.
Di Yi Jun Yi Da Xue Xue Bao 2003;23:954-55. PMid:13129732

- Han B, Shi Y, Zhu J, He X, Lin N, Li S, et
al. Study on serum erythropoietin levels in patients with hematologic
malignancies. Zhonghua xue ye xue za zhi 2006;27:543-45.PMid:17172129

- Rokicka-Piotrowicz M, Paszkowska M,
Kuratowska Z. Anemia and erythropoietin level in some hematology
malignancies. Polskie archiwum medycyny wewn 1994;92:409-16

- Sarcione E, Smalley J, Lema M, Stutzman L.
Increased ferritin synthesis and release by hodgkin's disease
peripheral blood lymphocytes1. International Journal of Cancer
2006;20(3):339-46

- Yildirim R, Gundogdu M, Erdem F, Kiki I, Bilici M. The Levels of Serum C-Reactive Protein, Beta 2 Microglobulin, Ferritin, Lactate Dehydrogenase and Some Specific Proteins in Patients with Non-Hodgkin’s Lymphoma Before and After Treatment. The Eurasian Journal of Medicine 2009;41:165-68
- Knutson M, Wessling-Resnick M. Iron
metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol
2003;38:61-88. doi:10.1080/713609210
PMid:12641343

- Ozguroglu M, Arun B, Demir G, Demirelli F,
Mandel N, Buyukunal E, et al. Serum erythropoietin level in anemic
cancer patients. Medical Oncology 2000;17:29-34. doi:10.1007/BF02826213
PMid:10713657
