Therapy-Related Myeloid Neoplasms in Chronic Lymphocytic Leukemia and Waldenstrom’s Macroglobulinemia
Ricci Francesca, Tedeschi Alessandra, Montillo Marco and Morra Enrica.
1Division of
Hematology, Niguarda Ca’ Granda Hospital, Milano, Italy
Correspondence
to: Francesca
Ricci, M.D. Division of Hematology, Niguarda Ca’ Granda Hospital.
Piazza
Ospedale Maggiore 3, 20162 Milano, Italy. Tel. +39-0264442668, Fax:
+39-0264442033. e-mail: francesca.ricci@ospedaleniguarda.it
Published: July 9, 2011
Received: March 24, 2011
Accepted: June 13, 2011
Mediterr J Hematol Infect Dis 2011, 3: e2011031, DOI 10.4084/MJHID.2011.031
This article is available from: http://www.mjhid.org/article/view/8251
This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Secondary
myelodysplasia (MDS) and acute myeloid leukemia (AML) are frequent long
term complications in Chronic Lymphocytic Leukemia (CLL) and
Waldenström Macroglobulinemia (WM) patients. Although disease-related
immune-suppression plays a crucial role in leukemogenesis there is
great concern that therapy may further increase the risk of developing
these devastating complications.
Nucleoside analogs (NA) and alkylating agents are considered appropriate agents in the treatment of both CLL and WM patients. Prolonged immunosuppression related to NA therapy and the incorporation of these agents or their metabolites into DNA, with potentially mutagenic action, leads to speculation that their therapeutic use might be responsible for an increased incidence of second cancer especially when combined with other DNA damaging agents like alkylating agents.
In this review the published studies considering the occurrence of secondary MDS and AML in CLL and WM patients are reported and the potential role of chemotherapeutic agents in leukemogenesis is discussed.
Nucleoside analogs (NA) and alkylating agents are considered appropriate agents in the treatment of both CLL and WM patients. Prolonged immunosuppression related to NA therapy and the incorporation of these agents or their metabolites into DNA, with potentially mutagenic action, leads to speculation that their therapeutic use might be responsible for an increased incidence of second cancer especially when combined with other DNA damaging agents like alkylating agents.
In this review the published studies considering the occurrence of secondary MDS and AML in CLL and WM patients are reported and the potential role of chemotherapeutic agents in leukemogenesis is discussed.
Introduction
Advances in the treatment of Chronic Lymphocytic Leukemia (CLL) and Waldenström Macroglobulinemia (WM) have resulted in higher response rates and more durable remissions. Chemoimmunotherapy has become the standard of care and the addition of rituximab to combination chemotherapy is associated with improved outcomes.[1-5] As a result, the number of cancer survivors is rapidly growing and the late toxicities of treatment particularly therapy-related myeloid neoplasms (t-MN) have become a more important concern.
Some authors have reported an increasing incidence of these late complications over the years6 probably due to the better diagnostic criteria employed, increased age of population and widespread and successful use of drug combination in cancer patients.
A comprehensive review of reported myelodysplasia and acute myeloid leukemia (MDS/AML) rates concluded that up to 10% of patients treated for indolent non-Hodgkin’s lymphoma (NHL) using either conventional dose alkylating agents-based regimens or high-dose therapy develop MDS or AML within a decade of primary therapy.[7] Based on these data the risk of therapy-related MDS/AML is an important consideration in the initial choice of therapy in these conditions.
In this review we have summarized the possible mechanisms of leukemogenesis, analyzed studies conducted to delineate the incidence of secondary MDS/AML in patients with CLL and WM and discussed the potential relationship between these events and treatment with NA and alkylating agents.
Drugs Inducing Secondary Myeloid Neoplasms and Mechanism of Leukemogenesis
Important discoveries have been made over recent years that have contributed to a better understanding of the pathogenesis of neoplasm and in particular of secondary AML/MDS. There are several lines of evidence showing that different mechanisms cooperate in leukemogenesis defining a multistep process.
Disease related immunodeficiency, resulting from both cell mediated and humoral-mediated immunity defects, has been postulated as a possible mechanism predisposing to second cancers in non Hodgkin lymphoma (NHL) and in particular in CLL and WM patients. B-cell impairment, low gammaglobulin levels and quantitative and functional defects of various subsets of T cells have all been documented in lymphoprolipherative diseases and are implicated in defective immune surveillance favouring second cancers. In line with this hypothesis is the documented incremented occurrence of malignant tumours in patients having a background of humoral immunodeficiency or abnormalities in T-cell function such as common variable immunodeficiency, ataxia-telangectasia, HIV disease.[8,9] Furthermore, some authors underline the crucial role of the immune system in the oncogenesis reporting an increased risk of second cancers also in untreated CLL and WM patients.[10,11]
However, several studies have raised concerns about the role of therapy in the development of second malignancies and t-MN are a well recognized distinct entity in the 2008 WHO classification of tumors of hematopoietic and lymphoid tissues.[12]
Cytotoxic agents associated with this complication include alkylating agents, topoisomerase II inhibitors, ionizing radiation, antimetabolites and antitubulin agents.[12,13] The WHO classification considers therapy-related MDS/AML as a unique clinical syndrome even though some cases may satisfy the morphological or cytogenetic criteria for other entities. Indeed, particular cytotoxic agents are associated with therapy-related MDS/AML with characteristic biological and clinical features.
Alkylating agents damage DNA either by methylation or DNA inter-strand crosslink formation. The latency between treatment and t-MN is generally long, between 5 and 7 years and may be preceded by a myelodysplastic phase. Most cases present deletion or loss of the long arm or total loss of chromosome 5 (5q-/-5) and/or deletion or loss of the long arm or total loss of chromosome 7 (7q-/-7).[14-16]
Topoisomerase II is an essential enzyme in eukaryotic cells and is thought to play an important role in DNA replication, transcription and cell division. This enzyme catalyzes the breaking and rejoining of both DNA strands. Topoisomerase II inhibitors block the enzymatic reaction through relegation and enzyme release, leaving the DNA with a permanent strand break. Multiple DNA-strand breaks lead to cell death. Both the antineoplastic effect and the leukemogenic effect are due to chromosomal break-age and this breakage, resolved by chromosomal translocation, is the cause of the leukaemic transformation. A shorter latency of approximately 2 years characterized t-MN secondary to topoisomerase II inhibitors. Balanced chromosome translocations frequently reported after exposure to this chemotherapeutic agents involve 11q23 (MLL) or 21q22 (RUNX1). However, others may also occur, for example t(8;21) or t(15;17) in the case of therapy-related acute promyelocytic leukemia (t-APL).[12,17,18]
Antimetabolites share structural similarities with nucleotides, and can be incorporated into DNA or RNA, causing inhibition of cell proliferation. Among antimetabolite agents fludarabine is the nucleoside analogs (NA) more frequently used in the treatment of indolent lymphoproliferative disorders.[19] The combination of fludarabine with cyclophosphamide or other DNA damaging agents may increase the risk of MDS/AML due to synergistic effects in the ability to produce DNA damage. Fludarabine inhibits DNA repair and augments the cytotoxic effect of DNA damaging agents such as cyclophosphamide.[20] This enhancement of DNA damage may also affect marrow progenitor cells. Such a mechanism could explain the observed impairment of peripheral blood progenitor cell collection after prior fludarabine treatment and could predispose to an increased risk of therapy-related MDS/AML.[21] High occurrence of abnormalities of chromosome 5 and 7 has also been described in MDS/AML secondary to NA therapy.[22-24]
In addition to typical genetic abnormalities, t-MN are characterized by molecular changes which may cooperate in the pathogenesis of the disease. FLT3 and p53 mutations are the most frequent described. Chimeric rearrangement of transcription factors, including AML1, CBFB, MLL or RARA and NPM1 mutations have also been reported.[25]
An important role in the multistep secondary
leukemogenesis has been recently attributed to the epigenetic changes. Epigenetic mechanisms such as DNA methylation, post-translational modifications of histone proteins and remodelling of nucleosomes affect chromatin structure and contribute to define heritable changes in gene expression. The abnormalities in DNA methylation profile have been described more commonly in therapy-related rather than de novo MDS/AML.[26]
Since only a small fraction of patients exposed to cytotoxic therapy developed t-MN, one of the important questions in the field is what predisposes individuals to develop the disease. A large number of studies have offered evidence demonstrating the importance of genetic variation in key genes, which include those involved in drug metabolism, protection of cellular entities from damage and repair of DNA damage. Mutations in these vital genes are rare while much more common are genetic polymorphisms in detoxification genes and in genes belonging to the major DNA repair pathways.[18]
Although the multistep leukemogenesis mechanism has not been completely clarified, the important knowledge achieved in the last years in this matter has laid the groundwork to a risk adapted therapeutic approach. An adequate screening of individual susceptibility to develop t-MN could influence initial treatment strategies with the goal of decreasing the incidence of these serious complications.
Waldenstrom Macroglobulinemia
WM patients have a well documented increased risk of second cancers and in particular of secondary MDS/AML. Although disease-related immune-suppression has been suggested as one of the main causes of this increasing risk,10 the role of therapy in this important matter is under investigation.
Several studies have been conducted in Europe and in the United States to retrospectively examine the incidence of secondary MDS/AML in treated WM patients.
Alkylating agents and NA such as fludarabine and 2-chlorodeoxyadenosine (2-CdA) have been considered appropriate first line agents for the treatment of WM.[27-28]
Among patients receiving alkylating agents the incidence of such events ranged from 0 to 6% (Table 1) in different retrospective studies.[29-33] Among them the most consistent data have been published by Ghobrial et al33 on 337 patients with newly diagnosed symptomatic WM. After a median duration of follow up of 11 years death occurred in 237 (70%) patients. The cause of death was due to WM or complications of therapy in 125 cases (37%) cases. Of these, death due to MDS/AML occurred in 16 patients (4.7%) indicating that this is a serious and fatal complication of alkylating agents therapy.
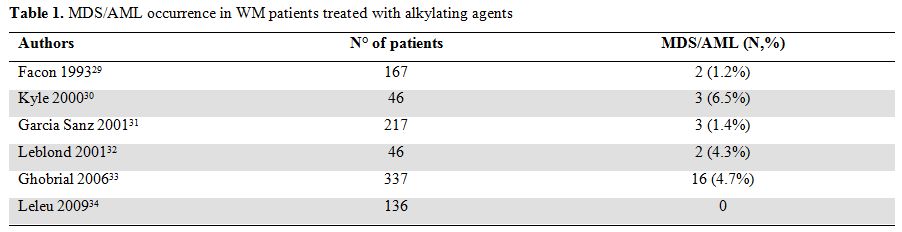
Table 1. MDS/AML occurrence in WM patients treated with alkylating agents
More recently attention has been focused on long term toxicity of NA-based therapy [34,35] and retrospective comparative data has been reported by the French Cooperative Group on CLL/WM36 and by the Dana-Farber Cancer Institute.[37]
Leblond et al36 reviewed the incidence of secondary MDS/AML in 165 WM patients treated with NA agents in 3 trials performed from 1993 to 2003 by the French Cooperative Group on CLL/WM.
In the first trial [38] 71 relapsed/refractory WM patients initially treated with alkylating agents received fludarabine. In the second trial [32] 92 patients in first relapse after alkylating agents were randomized to receive fludarabine and CAP regimen (doxorubicine, cyclophosphamide, prednisone). In the third trial [39] 49 patients with untreated or pretreated disease received fludarabine combined with cyclophosphamide.
The crude incidence of secondary MDS/AML varied from 1.4 to 8.9% in patients treated with fludarabine alone and was 6% in patients treated with fludarabine combined with cyclophosphamide. Incidence of such events were not different in the two groups of patients. More recently Leleu et al [37] carried out a retrospective study to delineate the incidence of secondary MDS/AML in a large population of patients with WM and to determine the potential relationship between these events and treatment with NA. Among the 439 consecutive patients considered in this study 329 were treated with (193) or without (136) NA and 110 patients remained untreated. The median follow-up for all patients was 5 years. Overall 3 patients (1.6%) developed secondary MDS/AML among NA treated patients while no events were reported in both patients receiving other chemotherapeutic drugs and in untreated patients The 15-year probabilities of developing secondary MDS/AML were 8%. These data demonstrate an increased incidence of developing secondary MDS/AML among patients with WM treated with NA. The incidence of MDS/AML was not statistically different in patients treated with NA as first line treatment versus patients treated later in their disease history, in patients treated with either one or with multiple courses of NA, and in patients with history versus no history of treatment with an oral alkylating agent. Among the three patients who developed MDS/AML in this series, only one patient had previously received oral alkylating agents therapy. Furthermore, none of the clinical and laboratory features studied (gender, age, family history of haematological malignancies, markers of tumor burden, prognosis markers including WM-ISS) significantly predicted the occurrence of secondary MDS/AML events. However, no definite conclusion can be drawn because of the low number of events and therefore a lack of power in multivariate analysis might also explain that no risk factors except treatment with NA were observed.
Data reported in all these retrospective studies (Table 2) lead to conclude that although a role of NA in leukemogenesis has been wide recognized other data and prospective studies are needed to clarify the impact of fludarabine and alkylating agent combination compared to fludarabine alone in the increased risk of t-MN in WM patients.
In the last decade the use of rituximab has also been extensively evaluated in patients with WM and high activity of fludarabine and rituximab combination with or without cyclophosphamide has been demonstrated.
Treon et al [40] reported the long term outcome of a multicenter prospective study examining fludarabine and rituximab association in 43 WM patients with less than two prior therapies. With a median follow-up of 40.3 months, 3 patients (7%) developed myeloid malignancies: MDS in one case and AML in two cases. Among them one had previously received chlorambucil and another had previously undergone high-dose chemotherapy with an autologous transplantation. The median time from protocol therapy to secondary MDS/AML was 39.4 months.
More recently toxicities data of fludarabine, cyclophosphamide and rituximab combination (FCR) have been reported by Leblond et al [41] in 55 WM patients. Among them 40 had been previously treated with a median of 2 lines of therapy (range 1-4), including 24 patients with relapsed disease and 16 patients with refractory disease. After a median follow-up of 28 months secondary MDS/AML were observed in two heavily treated patients (3.6%). Similar results have been observed by Tedeschi et al in a group of 43 untreated and pretreated patients receiving FCR schedule: MDS occurred in three patients (6.9%), all of them previously heavily pretreated with alkylating agents, after 5, 24 and 60 months from FCR, respectively.[42] Based on these data, addition of rituximab to fludarabine containing regimens does not seem to increase the incidence of t-MN (Table 2).
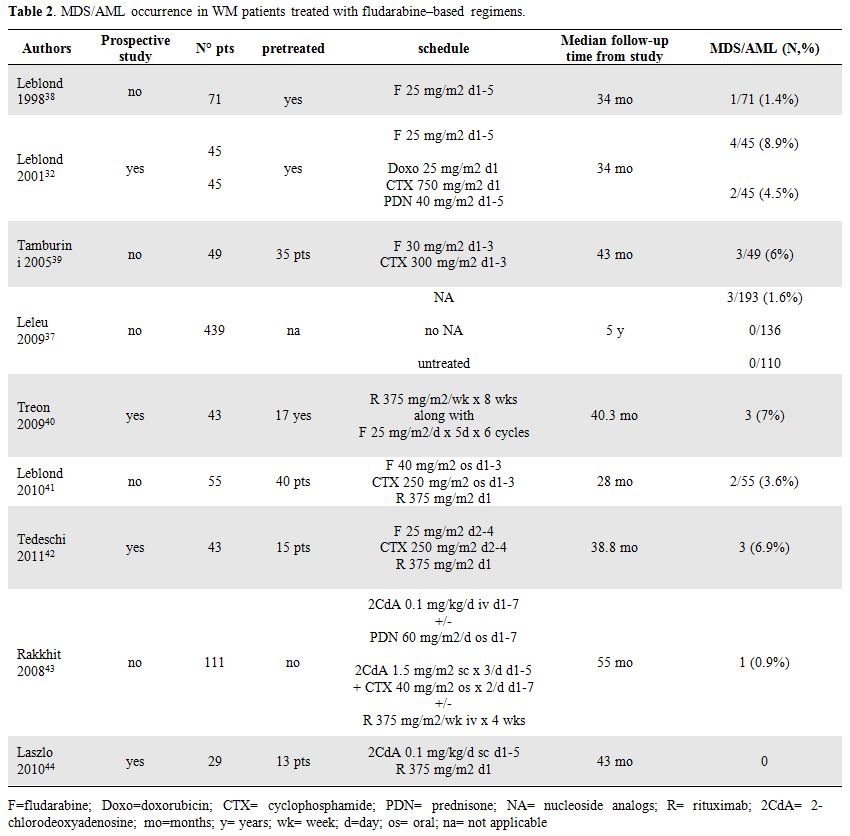
Table 2. MDS/AML occurrence in WM patients treated with fludarabine–based regimens.
Although data on fludarabine treatment are more consistent, recent analysis of long term toxicity after 2-CdA therapy has also been published.
The MD Anderson Cancer Center performed a retrospective analysis of 111 consecutive, previously untreated patients with symptomatic WM who received 2 consecutive 4–6-week courses of either 2-CdA alone or in combination with other agents including prednisone, cyclophosphamide, and rituximab. Thirteen patients (12%) developed second malignancies, among them one patient had AML (0.9%). The median time to development of a second malignancy was 85.5 months.[43]
Laszlo et al [44] recently reported the results with 2CdA and rituximab combination in 29 WM patients in first line therapy or previously treated without NA. With a median follow-up of 43 months no secondary MDS/AML was observed.
Chronic Lymphocytic Leukemia
Second malignancies are frequent complications in patients with CLL. The most frequent event is CLL transformation to large B-cell non-Hodgkin’s lymphoma also known as Richter’s syndrome.[45-47] However, the secondary development of myeloproliferative disorders as well as solid tumors has also been documented in CLL patients.[48,49]
An increased incidence of these late complications has occasionally been reported in patients receiving alkylating agents. However, occurrence of therapy-related MDS/AML after exposure to alkylating agents seems to be lower when they are used intermittently as opposed to prolonged, continuous exposure.[50]
The study of French Cooperative Group on CLL reported data of two randomized trials in 1535 patients with previously untreated stage A CLL.[50] In the first trial, 609 patients were randomly assigned to receive either daily chlorambucil or no treatment; in the second trial, 926 patients were randomly assigned to receive either intermittent chlorambucil plus prednisone or no treatment. Median follow-up for the first and second trials exceeded 11 and 6 years, respectively. CLL patients receiving either intermittent chlorambucil and prednisone or no treatment showed no increase of second tumors; in particular AML was observed in only one case (0.2%). However, a higher frequency of second cancers was reported in the group of patients treated with daily chlorambucil and AML occurred in 4 cases (1.3%).
No evidence of an increased risk of secondary MDS/AML was reported at M.D Anderson Cancer Center in a retrospective analysis of 1374 consecutive CLL patients, even though nearly three quarters of these patients had received prior treatment with alkylating agents.[51] Authors suggest that the suppressive properties of disease–related cytokines acting on myeloid clones may be responsible for the lack of an increased rate of secondary AML/MDS in CLL despite prolonged treatment with alkylating agents and disease related immunosuppression.
In contrast to the published experience with alkylating agents, secondary MDS/AML are rarely reported following NA as monotherapy in CLL patients.[52-56] However, the combination of NA with alkylating agents or other DNA damaging agents may increase the t-MN risk due to their synergistic effects (Table 3).
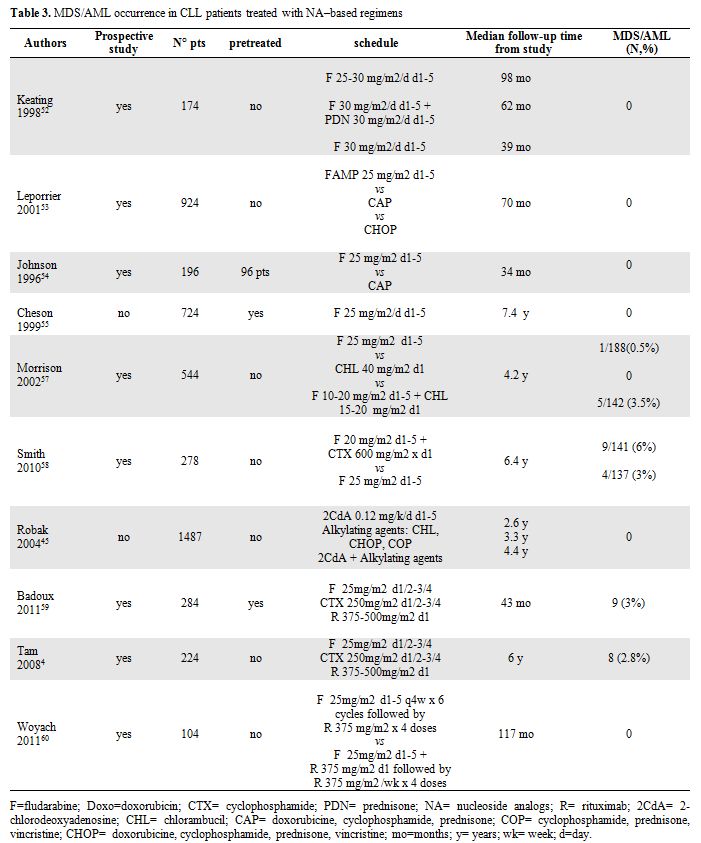
Table 3. MDS/AML occurrence in CLL patients treated with NA–based regimens.
Morrison et al. presented data from intergroup CLL treatment trial comparing treatment with chlorambucil, fludarabine or chlorambucil plus fludarabine, in which a possible increase in the number of cases of therapy-
These data have been recently confirmed by the prospective randomized phase 3 trial, E2997, comparing FC with F alone as initial therapy in 278 CLL patients. With a median follow-up of 6.4 years, there have been 13 cases of t-MN, 9 cases after FC and 4 after fludarabine alone. The increased incidence of t-MN after FC, usually in the absence of additional treatment, suggests that FC is more leukemogenic than fludarabine.[58]
In contrast Robak et al.[45] did not observed any MDS or AML in a retrospective series of 1487 CLL patients treated with 2-CdA, alkylating agents or both.
In the last five years chemoimmunotherapy regimens including rituximab showed high activity in CLL patients improving quality and duration of responses. The chemoimmunotherapy FCR has become a standard treatment for CLL based on the German CLL Study Group (GCLLSG) frontline CLL8 trial and the International REACH trial for patients in first relapse.[1,2]
Data of long term follow-up of FCR-therapy has been published by Badoux et al59 in a group of 284 previously treated patients followed in M. D. Anderson Cancer Center. Among them nine patients (3%) developed secondary MDS or AML (n=1) with a median of 20 months from starting FCR. Eight of these nine patients died after a median OS time of 23 months from FCR therapy. Comparable data has been reported by the same group4 with first-line FCR therapy: after a median follow-up of 6 years MDS occurred during first remission in 8 of 224 patients (2.8%) receiving immunochemotherapy.
More recently results published by Cancer and Leukemia Group Study60 suggest that incidence of t-MN does not increase with Fludarabine-rituximab combination (FR) without cyclophosphamide. After a median follow-up of 117 months none of untreated patients receiving fludarabine-rituximab concurrent or sequential combination developed t-MN during remission. Only one patient experienced therapy-related MDS, however, this occurred 45 months after FR treatment and 9 months after salvage treatment with FCR combination.
Studies examining consolidative autologous stem cell transplantation in CLL have also reported an increased risk of t-MN. Gribben et al identified in the Dana Farber series, an actuarial incidence of MDS/AML of 12% at 8 years.[61 ]The 137 patients considered in this series received conditioning regimen with cyclophosphamide and total body irradiation. Previous debulking therapy consisted of alkylating agents and/or fludarabine and/or rituximab.
A lower incidence of secondary MDS/AML was reported by the German CLL-3 trial with three cases in 150 patients who underwent autograft.[62]
As well as the Dana Farber group a high occurrence of MDS/AML has also been described by Milligan et al in patients enrolled in the Medical Research Council Chronic Lymphocytic Leukaemia-5 trial.[63] Of 115 newly diagnosed patients treated with fludarabine, 65 patients proceeded to autologous transplant. Conditioning was cyclophosphamide and total body irradiation in 49 (75%) patients and chemotherapy in 12 (18%). Ten patients developed MDS/AML; eight had undergone an autograft. Risk of MDS/AML was not associated with type of prior therapy. Five-year actuarial risk of developing MDS/AML postautograft was 12.4%. No analysed potential risk factor was predictive for MDS/AML development. Authors hypothesise that potential causative factors are fludarabine, low cell dose and transplant conditioning. The UK and German data differ in that there is an increased exposure to fludarabine and a lower infused cell dose in the UK. It has been suggested that the relatively low cell dose infused permitted a preferential expansion of damaged residual host stem cells, and that the myeloablative conditioning treatment will not destroy all hematopoietic cells with proliferative potential.
Recently results published of a phase 3 randomized trial of autografting in CLL versus observation for responding patients after first or second line treatment reported MDS in 4 patients: 3 in the autografiting arm (2.7%) and 1 in the observation arm (0.9%). The relatively low occurrence of MDS in the autotransplantation arm may be related to the limited follow-up of 5 years after randomization.[64]
Conclusions
The increased risk of secondary malignancies occurring in WM and CLL patients is well documented.
Overall published data reported frequently higher occurrence of t-MN in WM rather than in CLL patients, while in CLL patients transformation to Richter’s syndrome or the evolution to prolymphocytic leukemia is the more frequent long term complication.
The complex immunodeficiency resulting from B-cell impairment, hypogammaglobulinemia, T-cell dysfunction and decreased natural killer-cell activity, associated with these diseases may play a crucial role in leukemogenesis. However, there is great concern that therapy, especially with alkylating agents and purine nucleoside analogs, may further contribute to increase the risk of this devastating complication.
Although in the last few years several retrospective studies have been published on this topic, the real impact on risk of secondary MDS/AML complication of the single therapeutic agents has not been definitively clarified.
Indeed assessment of the specific risk is often confounded in patients with indolent lymphoprolipherative diseases by frequent exposure to multiple lines of cytotoxic therapy. Rates of secondary myeloid malignancies also vary with the length of follow-up and estimation may be falsely high if early reversible dysplastic features and cytopenia associated with cytotoxic treatment are misinterpreted.
Furthermore, the role played by additional immunosuppression due to the introduction of monoclonal antibodies in clinical practice needs to be better understood.
These considerations lead to conclude that the impact of secondary MDS/AML and the role of old and new drugs in the development of these late complications needs to be better evaluated in larger prospective studies, especially in young patients. In view of these data, we suggest that risk versus benefit should be considered when treating younger WM and CLL patients.
Advances in the treatment of Chronic Lymphocytic Leukemia (CLL) and Waldenström Macroglobulinemia (WM) have resulted in higher response rates and more durable remissions. Chemoimmunotherapy has become the standard of care and the addition of rituximab to combination chemotherapy is associated with improved outcomes.[1-5] As a result, the number of cancer survivors is rapidly growing and the late toxicities of treatment particularly therapy-related myeloid neoplasms (t-MN) have become a more important concern.
Some authors have reported an increasing incidence of these late complications over the years6 probably due to the better diagnostic criteria employed, increased age of population and widespread and successful use of drug combination in cancer patients.
A comprehensive review of reported myelodysplasia and acute myeloid leukemia (MDS/AML) rates concluded that up to 10% of patients treated for indolent non-Hodgkin’s lymphoma (NHL) using either conventional dose alkylating agents-based regimens or high-dose therapy develop MDS or AML within a decade of primary therapy.[7] Based on these data the risk of therapy-related MDS/AML is an important consideration in the initial choice of therapy in these conditions.
In this review we have summarized the possible mechanisms of leukemogenesis, analyzed studies conducted to delineate the incidence of secondary MDS/AML in patients with CLL and WM and discussed the potential relationship between these events and treatment with NA and alkylating agents.
Drugs Inducing Secondary Myeloid Neoplasms and Mechanism of Leukemogenesis
Important discoveries have been made over recent years that have contributed to a better understanding of the pathogenesis of neoplasm and in particular of secondary AML/MDS. There are several lines of evidence showing that different mechanisms cooperate in leukemogenesis defining a multistep process.
Disease related immunodeficiency, resulting from both cell mediated and humoral-mediated immunity defects, has been postulated as a possible mechanism predisposing to second cancers in non Hodgkin lymphoma (NHL) and in particular in CLL and WM patients. B-cell impairment, low gammaglobulin levels and quantitative and functional defects of various subsets of T cells have all been documented in lymphoprolipherative diseases and are implicated in defective immune surveillance favouring second cancers. In line with this hypothesis is the documented incremented occurrence of malignant tumours in patients having a background of humoral immunodeficiency or abnormalities in T-cell function such as common variable immunodeficiency, ataxia-telangectasia, HIV disease.[8,9] Furthermore, some authors underline the crucial role of the immune system in the oncogenesis reporting an increased risk of second cancers also in untreated CLL and WM patients.[10,11]
However, several studies have raised concerns about the role of therapy in the development of second malignancies and t-MN are a well recognized distinct entity in the 2008 WHO classification of tumors of hematopoietic and lymphoid tissues.[12]
Cytotoxic agents associated with this complication include alkylating agents, topoisomerase II inhibitors, ionizing radiation, antimetabolites and antitubulin agents.[12,13] The WHO classification considers therapy-related MDS/AML as a unique clinical syndrome even though some cases may satisfy the morphological or cytogenetic criteria for other entities. Indeed, particular cytotoxic agents are associated with therapy-related MDS/AML with characteristic biological and clinical features.
Alkylating agents damage DNA either by methylation or DNA inter-strand crosslink formation. The latency between treatment and t-MN is generally long, between 5 and 7 years and may be preceded by a myelodysplastic phase. Most cases present deletion or loss of the long arm or total loss of chromosome 5 (5q-/-5) and/or deletion or loss of the long arm or total loss of chromosome 7 (7q-/-7).[14-16]
Topoisomerase II is an essential enzyme in eukaryotic cells and is thought to play an important role in DNA replication, transcription and cell division. This enzyme catalyzes the breaking and rejoining of both DNA strands. Topoisomerase II inhibitors block the enzymatic reaction through relegation and enzyme release, leaving the DNA with a permanent strand break. Multiple DNA-strand breaks lead to cell death. Both the antineoplastic effect and the leukemogenic effect are due to chromosomal break-age and this breakage, resolved by chromosomal translocation, is the cause of the leukaemic transformation. A shorter latency of approximately 2 years characterized t-MN secondary to topoisomerase II inhibitors. Balanced chromosome translocations frequently reported after exposure to this chemotherapeutic agents involve 11q23 (MLL) or 21q22 (RUNX1). However, others may also occur, for example t(8;21) or t(15;17) in the case of therapy-related acute promyelocytic leukemia (t-APL).[12,17,18]
Antimetabolites share structural similarities with nucleotides, and can be incorporated into DNA or RNA, causing inhibition of cell proliferation. Among antimetabolite agents fludarabine is the nucleoside analogs (NA) more frequently used in the treatment of indolent lymphoproliferative disorders.[19] The combination of fludarabine with cyclophosphamide or other DNA damaging agents may increase the risk of MDS/AML due to synergistic effects in the ability to produce DNA damage. Fludarabine inhibits DNA repair and augments the cytotoxic effect of DNA damaging agents such as cyclophosphamide.[20] This enhancement of DNA damage may also affect marrow progenitor cells. Such a mechanism could explain the observed impairment of peripheral blood progenitor cell collection after prior fludarabine treatment and could predispose to an increased risk of therapy-related MDS/AML.[21] High occurrence of abnormalities of chromosome 5 and 7 has also been described in MDS/AML secondary to NA therapy.[22-24]
In addition to typical genetic abnormalities, t-MN are characterized by molecular changes which may cooperate in the pathogenesis of the disease. FLT3 and p53 mutations are the most frequent described. Chimeric rearrangement of transcription factors, including AML1, CBFB, MLL or RARA and NPM1 mutations have also been reported.[25]
An important role in the multistep secondary
leukemogenesis has been recently attributed to the epigenetic changes. Epigenetic mechanisms such as DNA methylation, post-translational modifications of histone proteins and remodelling of nucleosomes affect chromatin structure and contribute to define heritable changes in gene expression. The abnormalities in DNA methylation profile have been described more commonly in therapy-related rather than de novo MDS/AML.[26]
Since only a small fraction of patients exposed to cytotoxic therapy developed t-MN, one of the important questions in the field is what predisposes individuals to develop the disease. A large number of studies have offered evidence demonstrating the importance of genetic variation in key genes, which include those involved in drug metabolism, protection of cellular entities from damage and repair of DNA damage. Mutations in these vital genes are rare while much more common are genetic polymorphisms in detoxification genes and in genes belonging to the major DNA repair pathways.[18]
Although the multistep leukemogenesis mechanism has not been completely clarified, the important knowledge achieved in the last years in this matter has laid the groundwork to a risk adapted therapeutic approach. An adequate screening of individual susceptibility to develop t-MN could influence initial treatment strategies with the goal of decreasing the incidence of these serious complications.
Waldenstrom Macroglobulinemia
WM patients have a well documented increased risk of second cancers and in particular of secondary MDS/AML. Although disease-related immune-suppression has been suggested as one of the main causes of this increasing risk,10 the role of therapy in this important matter is under investigation.
Several studies have been conducted in Europe and in the United States to retrospectively examine the incidence of secondary MDS/AML in treated WM patients.
Alkylating agents and NA such as fludarabine and 2-chlorodeoxyadenosine (2-CdA) have been considered appropriate first line agents for the treatment of WM.[27-28]
Among patients receiving alkylating agents the incidence of such events ranged from 0 to 6% (Table 1) in different retrospective studies.[29-33] Among them the most consistent data have been published by Ghobrial et al33 on 337 patients with newly diagnosed symptomatic WM. After a median duration of follow up of 11 years death occurred in 237 (70%) patients. The cause of death was due to WM or complications of therapy in 125 cases (37%) cases. Of these, death due to MDS/AML occurred in 16 patients (4.7%) indicating that this is a serious and fatal complication of alkylating agents therapy.

Table 1. MDS/AML occurrence in WM patients treated with alkylating agents
More recently attention has been focused on long term toxicity of NA-based therapy [34,35] and retrospective comparative data has been reported by the French Cooperative Group on CLL/WM36 and by the Dana-Farber Cancer Institute.[37]
Leblond et al36 reviewed the incidence of secondary MDS/AML in 165 WM patients treated with NA agents in 3 trials performed from 1993 to 2003 by the French Cooperative Group on CLL/WM.
In the first trial [38] 71 relapsed/refractory WM patients initially treated with alkylating agents received fludarabine. In the second trial [32] 92 patients in first relapse after alkylating agents were randomized to receive fludarabine and CAP regimen (doxorubicine, cyclophosphamide, prednisone). In the third trial [39] 49 patients with untreated or pretreated disease received fludarabine combined with cyclophosphamide.
The crude incidence of secondary MDS/AML varied from 1.4 to 8.9% in patients treated with fludarabine alone and was 6% in patients treated with fludarabine combined with cyclophosphamide. Incidence of such events were not different in the two groups of patients. More recently Leleu et al [37] carried out a retrospective study to delineate the incidence of secondary MDS/AML in a large population of patients with WM and to determine the potential relationship between these events and treatment with NA. Among the 439 consecutive patients considered in this study 329 were treated with (193) or without (136) NA and 110 patients remained untreated. The median follow-up for all patients was 5 years. Overall 3 patients (1.6%) developed secondary MDS/AML among NA treated patients while no events were reported in both patients receiving other chemotherapeutic drugs and in untreated patients The 15-year probabilities of developing secondary MDS/AML were 8%. These data demonstrate an increased incidence of developing secondary MDS/AML among patients with WM treated with NA. The incidence of MDS/AML was not statistically different in patients treated with NA as first line treatment versus patients treated later in their disease history, in patients treated with either one or with multiple courses of NA, and in patients with history versus no history of treatment with an oral alkylating agent. Among the three patients who developed MDS/AML in this series, only one patient had previously received oral alkylating agents therapy. Furthermore, none of the clinical and laboratory features studied (gender, age, family history of haematological malignancies, markers of tumor burden, prognosis markers including WM-ISS) significantly predicted the occurrence of secondary MDS/AML events. However, no definite conclusion can be drawn because of the low number of events and therefore a lack of power in multivariate analysis might also explain that no risk factors except treatment with NA were observed.
Data reported in all these retrospective studies (Table 2) lead to conclude that although a role of NA in leukemogenesis has been wide recognized other data and prospective studies are needed to clarify the impact of fludarabine and alkylating agent combination compared to fludarabine alone in the increased risk of t-MN in WM patients.
In the last decade the use of rituximab has also been extensively evaluated in patients with WM and high activity of fludarabine and rituximab combination with or without cyclophosphamide has been demonstrated.
Treon et al [40] reported the long term outcome of a multicenter prospective study examining fludarabine and rituximab association in 43 WM patients with less than two prior therapies. With a median follow-up of 40.3 months, 3 patients (7%) developed myeloid malignancies: MDS in one case and AML in two cases. Among them one had previously received chlorambucil and another had previously undergone high-dose chemotherapy with an autologous transplantation. The median time from protocol therapy to secondary MDS/AML was 39.4 months.
More recently toxicities data of fludarabine, cyclophosphamide and rituximab combination (FCR) have been reported by Leblond et al [41] in 55 WM patients. Among them 40 had been previously treated with a median of 2 lines of therapy (range 1-4), including 24 patients with relapsed disease and 16 patients with refractory disease. After a median follow-up of 28 months secondary MDS/AML were observed in two heavily treated patients (3.6%). Similar results have been observed by Tedeschi et al in a group of 43 untreated and pretreated patients receiving FCR schedule: MDS occurred in three patients (6.9%), all of them previously heavily pretreated with alkylating agents, after 5, 24 and 60 months from FCR, respectively.[42] Based on these data, addition of rituximab to fludarabine containing regimens does not seem to increase the incidence of t-MN (Table 2).

Table 2. MDS/AML occurrence in WM patients treated with fludarabine–based regimens.
Although data on fludarabine treatment are more consistent, recent analysis of long term toxicity after 2-CdA therapy has also been published.
The MD Anderson Cancer Center performed a retrospective analysis of 111 consecutive, previously untreated patients with symptomatic WM who received 2 consecutive 4–6-week courses of either 2-CdA alone or in combination with other agents including prednisone, cyclophosphamide, and rituximab. Thirteen patients (12%) developed second malignancies, among them one patient had AML (0.9%). The median time to development of a second malignancy was 85.5 months.[43]
Laszlo et al [44] recently reported the results with 2CdA and rituximab combination in 29 WM patients in first line therapy or previously treated without NA. With a median follow-up of 43 months no secondary MDS/AML was observed.
Chronic Lymphocytic Leukemia
Second malignancies are frequent complications in patients with CLL. The most frequent event is CLL transformation to large B-cell non-Hodgkin’s lymphoma also known as Richter’s syndrome.[45-47] However, the secondary development of myeloproliferative disorders as well as solid tumors has also been documented in CLL patients.[48,49]
An increased incidence of these late complications has occasionally been reported in patients receiving alkylating agents. However, occurrence of therapy-related MDS/AML after exposure to alkylating agents seems to be lower when they are used intermittently as opposed to prolonged, continuous exposure.[50]
The study of French Cooperative Group on CLL reported data of two randomized trials in 1535 patients with previously untreated stage A CLL.[50] In the first trial, 609 patients were randomly assigned to receive either daily chlorambucil or no treatment; in the second trial, 926 patients were randomly assigned to receive either intermittent chlorambucil plus prednisone or no treatment. Median follow-up for the first and second trials exceeded 11 and 6 years, respectively. CLL patients receiving either intermittent chlorambucil and prednisone or no treatment showed no increase of second tumors; in particular AML was observed in only one case (0.2%). However, a higher frequency of second cancers was reported in the group of patients treated with daily chlorambucil and AML occurred in 4 cases (1.3%).
No evidence of an increased risk of secondary MDS/AML was reported at M.D Anderson Cancer Center in a retrospective analysis of 1374 consecutive CLL patients, even though nearly three quarters of these patients had received prior treatment with alkylating agents.[51] Authors suggest that the suppressive properties of disease–related cytokines acting on myeloid clones may be responsible for the lack of an increased rate of secondary AML/MDS in CLL despite prolonged treatment with alkylating agents and disease related immunosuppression.
In contrast to the published experience with alkylating agents, secondary MDS/AML are rarely reported following NA as monotherapy in CLL patients.[52-56] However, the combination of NA with alkylating agents or other DNA damaging agents may increase the t-MN risk due to their synergistic effects (Table 3).

Table 3. MDS/AML occurrence in CLL patients treated with NA–based regimens.
Morrison et al. presented data from intergroup CLL treatment trial comparing treatment with chlorambucil, fludarabine or chlorambucil plus fludarabine, in which a possible increase in the number of cases of therapy-
These data have been recently confirmed by the prospective randomized phase 3 trial, E2997, comparing FC with F alone as initial therapy in 278 CLL patients. With a median follow-up of 6.4 years, there have been 13 cases of t-MN, 9 cases after FC and 4 after fludarabine alone. The increased incidence of t-MN after FC, usually in the absence of additional treatment, suggests that FC is more leukemogenic than fludarabine.[58]
In contrast Robak et al.[45] did not observed any MDS or AML in a retrospective series of 1487 CLL patients treated with 2-CdA, alkylating agents or both.
In the last five years chemoimmunotherapy regimens including rituximab showed high activity in CLL patients improving quality and duration of responses. The chemoimmunotherapy FCR has become a standard treatment for CLL based on the German CLL Study Group (GCLLSG) frontline CLL8 trial and the International REACH trial for patients in first relapse.[1,2]
Data of long term follow-up of FCR-therapy has been published by Badoux et al59 in a group of 284 previously treated patients followed in M. D. Anderson Cancer Center. Among them nine patients (3%) developed secondary MDS or AML (n=1) with a median of 20 months from starting FCR. Eight of these nine patients died after a median OS time of 23 months from FCR therapy. Comparable data has been reported by the same group4 with first-line FCR therapy: after a median follow-up of 6 years MDS occurred during first remission in 8 of 224 patients (2.8%) receiving immunochemotherapy.
More recently results published by Cancer and Leukemia Group Study60 suggest that incidence of t-MN does not increase with Fludarabine-rituximab combination (FR) without cyclophosphamide. After a median follow-up of 117 months none of untreated patients receiving fludarabine-rituximab concurrent or sequential combination developed t-MN during remission. Only one patient experienced therapy-related MDS, however, this occurred 45 months after FR treatment and 9 months after salvage treatment with FCR combination.
Studies examining consolidative autologous stem cell transplantation in CLL have also reported an increased risk of t-MN. Gribben et al identified in the Dana Farber series, an actuarial incidence of MDS/AML of 12% at 8 years.[61 ]The 137 patients considered in this series received conditioning regimen with cyclophosphamide and total body irradiation. Previous debulking therapy consisted of alkylating agents and/or fludarabine and/or rituximab.
A lower incidence of secondary MDS/AML was reported by the German CLL-3 trial with three cases in 150 patients who underwent autograft.[62]
As well as the Dana Farber group a high occurrence of MDS/AML has also been described by Milligan et al in patients enrolled in the Medical Research Council Chronic Lymphocytic Leukaemia-5 trial.[63] Of 115 newly diagnosed patients treated with fludarabine, 65 patients proceeded to autologous transplant. Conditioning was cyclophosphamide and total body irradiation in 49 (75%) patients and chemotherapy in 12 (18%). Ten patients developed MDS/AML; eight had undergone an autograft. Risk of MDS/AML was not associated with type of prior therapy. Five-year actuarial risk of developing MDS/AML postautograft was 12.4%. No analysed potential risk factor was predictive for MDS/AML development. Authors hypothesise that potential causative factors are fludarabine, low cell dose and transplant conditioning. The UK and German data differ in that there is an increased exposure to fludarabine and a lower infused cell dose in the UK. It has been suggested that the relatively low cell dose infused permitted a preferential expansion of damaged residual host stem cells, and that the myeloablative conditioning treatment will not destroy all hematopoietic cells with proliferative potential.
Recently results published of a phase 3 randomized trial of autografting in CLL versus observation for responding patients after first or second line treatment reported MDS in 4 patients: 3 in the autografiting arm (2.7%) and 1 in the observation arm (0.9%). The relatively low occurrence of MDS in the autotransplantation arm may be related to the limited follow-up of 5 years after randomization.[64]
Conclusions
The increased risk of secondary malignancies occurring in WM and CLL patients is well documented.
Overall published data reported frequently higher occurrence of t-MN in WM rather than in CLL patients, while in CLL patients transformation to Richter’s syndrome or the evolution to prolymphocytic leukemia is the more frequent long term complication.
The complex immunodeficiency resulting from B-cell impairment, hypogammaglobulinemia, T-cell dysfunction and decreased natural killer-cell activity, associated with these diseases may play a crucial role in leukemogenesis. However, there is great concern that therapy, especially with alkylating agents and purine nucleoside analogs, may further contribute to increase the risk of this devastating complication.
Although in the last few years several retrospective studies have been published on this topic, the real impact on risk of secondary MDS/AML complication of the single therapeutic agents has not been definitively clarified.
Indeed assessment of the specific risk is often confounded in patients with indolent lymphoprolipherative diseases by frequent exposure to multiple lines of cytotoxic therapy. Rates of secondary myeloid malignancies also vary with the length of follow-up and estimation may be falsely high if early reversible dysplastic features and cytopenia associated with cytotoxic treatment are misinterpreted.
Furthermore, the role played by additional immunosuppression due to the introduction of monoclonal antibodies in clinical practice needs to be better understood.
These considerations lead to conclude that the impact of secondary MDS/AML and the role of old and new drugs in the development of these late complications needs to be better evaluated in larger prospective studies, especially in young patients. In view of these data, we suggest that risk versus benefit should be considered when treating younger WM and CLL patients.
References
- Robak T, Moiseev S, Dmoszynska A, Solal-CélignyP, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Geisler C, Montillo M, Ganly P, Dartigeas C, Rosta A, Janssens A, Mendila M, Maurer J, Wenger MK. Rituximab, Fludarabine, and Cyclophosphamide (R-FC) Prolongs Progression Free Survival in Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) Compared with FC Alone: Final Results from the International Randomized Phase III REACH Trial. Blood (ASH Annual Meeting Abstracts) 2008; 112: lba-1
- Hallek M, Fischer K, Fingerle-Rowson G,
Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grünhagen
U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF,
Berrebi A, Jäger U, Cazin B, Trneny M, Westermann A, Wendtner CM,
Eichhorst BF, Staib P, Bühler A, Winkler D, Zenz T, Böttcher S, Ritgen
M, Mendila M, Kneba M, Döhner H, Stilgenbauer S. International Group of
Investigators; German Chronic Lymphocytic Leukaemia Study
Group.Addition of rituximab to fludarabine and cyclophosphamide in
patients with chronic lymphocytic leukaemia: a randomised, open-label,
phase 3 trial. Lancet 2010;
376:1164-74.doi:10.1016/S0140-6736(10)61381-5

- Dimopoulos MA, Anagnostopoulos A, Kyrtsonis
MC, Zervas K, Tsatalas C, Kokkinis G, Repoussis P, Symeonidis A,
Delimpasi S, Katodritou E, Vervessou E, Michali E, Pouli A, Gika D,
Vassou A, Terpos E, Anagnostopoulos N, Economopoulos T, Pangalis G.
Primary treatment of Waldenström macroglobulinemia with dexamethasone,
rituximab, and cyclophosphamide. J Clin Oncol. 2007; 25:
3344-9.doi:10.1200/JCO.2007.10.9926
PMid:17577016

- Tam CS, O'Brien S, Wierda W, Kantarjian H,
Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term
results of the fludarabine, cyclophosphamide, and rituximab regimen as
initial therapy of chronic lymphocytic leukemia. Blood 2008;112:
975-80. doi:10.1182/blood-2008-02-140582
PMid:18411418

- Wierda W, O'Brien S, Wen S, Faderl S,
Garcia-Manero G, Thomas D, Do KA, Cortes J, Koller C, Beran M,
Ferrajoli A, Giles F, Lerner S, Albitar M, Kantarjian H, Keating M.
Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab
for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol.
2005; 23: 4070-8. doi:10.1200/JCO.2005.12.516
PMid:15767647

- Leone G, Mele L, Pulsoni A, Equitani F,
Pagano L.The incidence of secondary leukemias. Haematologica. 1999;
84:937-45. PMid:10509043

- Armitage JO, Carbone PP, Connors JM, Levine
A, Bennett JM, Kroll S. Treatment-related myelodysplasia and acute
leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol. 2003;
21:897-906. doi:10.1200/JCO.2003.07.113
PMid:12610191

- Dasanu CA, Alexandrescu DT. Risk for second
nonlymphoid neoplasms in chronic lymphocytic leukemia. MedGenMed. 2007;
9: 35.

- Greene, M.H., Hoover, R.N. and Fraumeni,
J.F. Subsequent cancer in patients with chronic lymphocytic leucemia a
possible immunologic mechanism. J Natl Cancer Inst. 1978; 61:
337-340.PMid:277720

- Varettoni M, Tedeschi A, Arcaini L,
Pascutto C, Vismara E, Orlandi E, Ricci F, Corso A, Greco A,
Mangiacavalli S, Lazzarino M, Morra E. Risk of second cancers in
Waldenstrom macroglobulinemia. Ann Oncol. 2011; Apr 27;--(--):--Robak
T. Second malignancies and Richter's syndrome in patients with chronic
lymphocytic leukemia. Hematology. 2004;
9:387-400 doi:10.1080/10245330400018599
PMid:15763979

- Robak T. Second malignancies and Richter's
syndrome in patients with chronic lymphocytic leukemia. Hematology.
2004; 9:387-400. doi:10.1080/10245330400018599
PMid:15763979

- Vardiman JW, Arber DA, Brunning RD, Larson RA, Matutes E, Baumann I, Thiele J. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, ed. Therapy-related myeloid neoplasms - WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth Ed. Lyon, France: IARC Press. 2008; 127-9.
- Seymour JF, Juneja SK, Campbell LJ, Ellims
PH, Estey EH, Prince HM. Secondary acute myeloid leukemia with inv(16):
report of two cases following paclitaxel-containing chemotherapy and
review of the role of intensified ara-C therapy. Leukemia. 1999; 13:
1735-40. doi:10.1038/sj.leu.2401552
PMid:10557046

- Pedersen-Bjergaard J, Pedersen M, Roulston
D, Philip P. Different genetic pathways in leukemogenesis for patients
presenting with therapy-related myelodysplasia and therapy-related
acute myeloid leukemia. Blood. 1995; 86: 3542–52.PMid:7579462

- Mauritzson N, Albin M, Rylander L,
Billstrom R, Ahlgren T, Mikoczy Z, Björk J, Strömberg U, Nilsson PG,
Mitelman F, Hagmar L, Johansson B. Pooled analysis of clinical and
cytogenetic features in treatment-related and de novo adult myeloid
leukaemia and myelodysplastic syndromes based on a consecutive series
of 761 patients analyzed 1976-1993 and on 5098 unselected cases
reported in the literature 1974-2001. Leukemia. 2002; 12: 2366–78. doi:10.1038/sj.leu.2402713
PMid:12454741

- Smith SM, Le Beau MM, Huo D, Karrison T,
Sobecks RM, Anastasi J, Vardiman JW, Rowley JD, Larson RA.
Clinical-cytogenetic associations in 306 patients with therapy-related
myelodysplasia and myeloid leukemia: the University of Chicago series.
Blood. 2003; 102: 43–52. doi:10.1182/blood-2002-11-3343
PMid:12623843

- Rowley JD, Olney HJ. International
workshop on the relationship of prior therapy to balanced chromosome
aberrations in therapy-related myelodysplastic syndromes and acute
leukemia: overview report. Genes Chromosomes Cancer 2002; 33:331-45. doi:10.1002/gcc.10040
PMid:11921269

- Leone G, Fianchi L, Pagano L, Voso MT.
Incidence and susceptibility to therapy-related myeloid neoplasms. Chem
Biol Interact. 2010; 184:39-45. doi:10.1016/j.cbi.2009.12.013PMid:20026017

- Keating MJ, O'Brien S, Lerner S, Koller C,
Beran M, Robertson LE, Freireich EJ, Estey E, Kantarjian H. Long term
follow-up of patients with chronic lymphocytic leukemia (CLL) receiving
fludarabine regimens as initial therapy. Blood 1998; 92:
1165-71.PMid:9694704

- Yamauchi T, Nowak BJ, Keating MJ, Plunkett
W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by
4-hydroperoxycyclophosphamide is inhibited by fludarabine and
clofarabine. Clin Cancer Res. 2001; 7: 3580-9. PMid:11705880

- Morgan SJ, Seymour JF, Grigg A, Matthews
JP, Prince HM, Wolf MM, Januszewicz EH. Predictive factors for
successful stem cell mobilization in patients with indolent
lymphoproliferative disorders previously treated with fludarabine.
Leukemia. 2004;18: 1034-8.doi:10.1038/sj.leu.2403326
PMid:14990978

- Morrison VA, Rai KR, Peterson BL, Kolitz
JE, Elias L, Appelbaum FR, Hines JD, Shepherd L, Larson RA, Schiffer
CA. Therapy-related myeloid leukemias are observed in patients with
chronic lymphocytic leukemia after treatment with fludarabine and
chlorambucil: results of an intergroup study, cancer and leukemia group
B 9011. J Clin Oncol. 2002; 20: 3878-84.doi:10.1200/JCO.2002.08.128
PMid:12228208

- McLaughlin P, Estey E, Glassman A,
Romaguera J, Samaniego F, Ayala A, Hayes K, Maddox AM, Preti HA,
Hagemeister FB. Myelodysplasia and acute myeloid leukemia following
therapy for indolent lymphoma with fludarabine, mitoxantrone, and
dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;
105:4573-5. doi:10.1182/blood-2004-08-3035
PMid:15741224 PMCid:1895007

- Carney DA, Westerman DA, Tam CS, Milner A,
Prince HM, Kenealy M, Wolf M, Januszewicz EH, Ritchie D, Came N,
Seymour JF. Therapy-related myelodysplastic syndrome and acute myeloid
leukemia following fludarabine combination chemotherapy. Leukemia.
2010; 24: 2056-62. doi:10.1038/leu.2010.218
PMid:20962860

- Pedersen-Bjergaard J, Christiansen DH,
Desta F, Andersen MK. Alternative genetic pathways and cooperating
genetic abnormalities in the pathogenesis of therapy-related
myelodysplasia and acute myeloid leukemia. Leukemia. 2006; 20:1943-9. doi:10.1038/sj.leu.2404381
PMid:16990778

- Voso MT, D'Alò F, Greco M, Fabiani E,
Criscuolo M, Migliara G, Pagano L, Fianchi L, Guidi F, Hohaus S, Leone
G. Epigenetic changes in therapy-related MDS/AML. Chem Biol Interact,
2010; 184:46-9. doi:10.1016/j.cbi.2009.10.013
PMid:19874806

- Treon SP, Gertz MA, Dimopoulos M,
Anagnostopoulos A, Blade J, Branagan AR, Garcia-Sanz R, Johnson S,
Kimby E, Leblond V, Fermand JP, Maloney DG, Merlini G, Morel P, Morra
E, Nichols G, Ocio EM, Owen R, Stone MJ. Update on treatment
recommendations from the Third International Workshop on Waldenstrom's
macroglobulinemia. Blood 2006; 107: 3442-6. doi:10.1182/blood-2005-02-0833
PMid:16410453

- Vijay A. Gertz MA, Waldenstrom
Macroglobulinemia. Blood 2007; 109: 5096-103.

- Facon T, Brouillard M, Duhamel A, Morel P,
Simon M, Jouet JP, Bauters F, Fenaux P. Prognostic factors in
Waldenström's macroglobulinemia: a report of 167 cases. J Clin Oncol
1993; 11: 1553-8. PMid:8336194

- Kyle RA, Greipp PR, Gertz MA, Witzig TE,
Lust JA, Lacy MQ, Therneau TM Waldenström's macroglobulinaemia: a
prospective study comparing daily with intermittent oral chlorambucil.
Br J Haematol. 2000; 108: 737-42. doi:10.1046/j.1365-2141.2000.01918.x
PMid:10792277

- García-Sanz R, Montoto S, Torrequebrada A,
de Coca AG, Petit J, Sureda A, Rodríguez-García JA, Massó P,
Pérez-Aliaga A, Monteagudo MD, Navarro I, Moreno G, Toledo C, Alonso A,
Besses C, Besalduch J, Jarque I, Salama P, Rivas JA, Navarro B, Bladé
J, Miguel JF. Waldenström macroglobulinaemia: presenting features and
outcome in a series with 217 cases. Br J Haematol 2001, 115: 575-82. doi:10.1046/j.1365-2141.2001.03144.x
PMid:11736938

- Leblond V, Lévy V, Maloisel F, Cazin B,
Fermand JP, Harousseau JL, Remenieras L, Porcher R, Gardembas M, Marit
G, Deconinck E, Desablens B, Guilhot F, Philippe G, Stamatoullas A,
Guibon O. Multicenter, randomized comparative trial of fludarabine and
the combination of cyclophosphamide-doxorubicin-prednisone in 92
patients with Waldenström macroglobulinemia in first relapse or with
primary refractory disease. Blood 2001; 98: 2640-4. doi:10.1182/blood.V98.9.2640
PMid:11675332

- Ghobrial IM, Fonseca R, Gertz MA, Plevak
MF, Larson DR, Therneau TM, Wolf RC, Hoffmann RJ, Lust JA, Witzig TE,
Lacy MQ, Dispenzieri A, Vincent Rajkumar S, Zeldenrust SR, Greipp PR,
Kyle RA. Prognostic model for disease-specific and overall mortality in
newly diagnosed symptomatic patients with Waldenstrom
macroglobulinaemia. Br J Haematol. 2006; 132: 158-64. doi:10.1111/j.1365-2141.2006.06003.x
PMid:16611306

- Leleu X, Soumerai J, Roccaro A,
Hatjiharissi E, Hunter ZR, Manning R, Ciccarelli BT, Sacco A,
Ioakimidis L, Adamia S, Moreau AS, Patterson CJ, Ghobrial IM, Treon SP.
Increased incidence of transformation and myelodysplasia/acute leukemia
in patients with Waldenström macroglobulinemia treated with nucleoside
analogs. J Clin Oncol. 2009; 27: 250-5.doi:10.1200/JCO.2007.15.1530
PMid:19064987

- Tedeschi A, Alamos SM, Ricci F, Greco A,
Morra E Fludarabine-based combination therapies for Waldenström's
macroglobulinemia. Clin Lymphoma Myeloma. 2009; 9:
67-70.doi:10.3816/CLM.2009.n.017

- Leblond V, Tamburini J, Levy V, Choquet S, Taksin AL, Maloisel F and Morel P. Incidence of Disease Transformation and Development of MDS/AML in 165 Patients with Waldenström’s Macroglobulinemia (WM) Treated with Fludarabine (F)-Based Regimen in Three Studies (French Cooperative Group on CLL/WM). Blood (ASH Annual Meeting Abstracts) 2007; 110: 1291. PMid:17485551
- Leleu X, Soumerai J, Roccaro A,
Hatjiharissi E, Hunter ZR, Manning R, Ciccarelli BT, Sacco A,
Ioakimidis L, Adamia S, Moreau AS, Patterson CJ, Ghobrial IM, Treon SP.
Increased incidence of transformation and myelodysplasia/acute leukemia
in patients with Waldenström macroglobulinemia treated with nucleoside
analogs. J Clin Oncol. 2009; 27: 250-5. doi:10.1200/JCO.2007.15.1530
PMid:19064987

- Leblond V, Ben-Othman T, Deconinck E,
Taksin AL, Harousseau JL, Delgado MA, Delmer A, Maloisel F, Mariette X,
Morel P, Clauvel JP, Duboisset P, Entezam S, Hermine O, Merlet M,
Yakoub-Agha I, Guibon O, Caspard H, Fort N. Activity of fludarabine in
previously treated Waldenström's macroglobulinemia: a report of 71
cases. Groupe Coopératif Macroglobulinémie. J Clin Oncol. 1998; 16:
2060-4.PMid:9626204

- Tamburini J, Lévy V, Chaleteix C, Fermand
JP, Delmer A, Stalniewicz L, Morel P, Dreyfus F, Grange MJ, Christian
B, Choquet S, Leblond V. Fludarabine plus cyclophosphamide in
Waldenström's macroglobulinemia: results in 49 patients. Leukemia 2005;
19:1831-4. doi:10.1038/sj.leu.2403885
PMid:16121217

- Treon SP, Branagan AR, Ioakimidis L,
Soumerai JD, Patterson CJ, Turnbull B, Wasi P, Emmanouilides C, Frankel
SR, Lister A, Morel P, Matous J, Gregory SA, Kimby E. Long-term
outcomes to fludarabine and rituximab in Waldenström macroglobulinemia.
Blood 2009; 113: 3673-8.doi:10.1182/blood-2008-09-177329 PMid:19015393
PMCid:2670786

- Leblond V, Compain L, Levy V, Tamburini J, Delmer A, Vargaftig J, Pégourié-Bandelier B, Mahe B, Le Guill S, Tournilhac O, Brottier-Mancini E, Delarue R, Buzyn A, Maigre M, Gardin C Choquet S. Fludarabine Plus Cyclophosphamide and Rituximab In Waldenström's Macroglobulinemia: Results In 55 Patients. Blood (ASH Annual Meeting Abstracts) 2010; 116: 1757.
- Tedeschi A, Benevolo G, Varettoni M, Battista M, Zinzani P, Visco C, Meneghini V, Pioltelli P, Sacchi S, Ricci F, Zaja F, Nichelatti M, Lazzarino M, Vitolo U, Morra E. Fludarabine, plus cyclophosphamide and rituximab in Waldenstrom’s Macroglobulinemia: an effective but myelosuppressive regimen to be offered to advanced disease patients. Cancer in press. 2011.
- Rakkhit R, Delasalle KB, Gavino MB, Thomas SK, Dimopoulos MA, Hagemeister FB, Rodriguez MA, McLaughlin P, Alexanian R, Weber DM. Incidence of Transformation to Large Cell Lymphoma and to Second Malignancies in Symptomatic Patients with Waldenstrom’s Macroglobulinemia (WM) Treated with Cladribine (2-CdA) Combination Induction Therapy. Blood (ASH Annual Meeting Abstracts) 2008; 112: 3065.PMid:18650451
- Laszlo D, Andreola G, Rigacci L, Fabbri A,
Rabascio C, Mancuso P, Pruneri G, Radice D, Pinto A, Frigeri F,
Calabrese L, Billio A, Bertolini F, Martinelli G. Rituximab and
subcutaneous 2-chloro-2'-deoxyadenosine combination treatment for
patients with Waldenstrom macroglobulinemia: clinical and biologic
results of a phase II multicenter study. J Clin Oncol. 2010; 28:
2233-8. doi:10.1200/JCO.2009.23.6315
PMid:20368573

- Robak T, Blonski JZ, Gora-Tybor J,
Kasznicki M, Konopka L, Ceglarek B, Komarnicki M, Lewandowski K,
Hellmann A, Lewandowski K, Moskwa A, Dmoszynska A, Sokolowska B,
Dwilewicz-Trojaczek A, Tomaszewska A, Sulek K, Calbecka M. Second
malignancies and Richter's syndrome in patients with chronic
lymphocytic leukaemia treated with cladribine. Eur J Cancer 2004; 40:
383-9. doi:10.1016/j.ejca.2003.09.031PMid:14746857

- Seymour J. and Campbell J. In: Cheson B.D, ed., Richter’s Syndrome in Chronic Lymphocytic Leukemia (Marcel Dekker, Nek York, Basel). 2002; 459-83.
- Giles FJ, O'Brien SM, Keating MJ. Chronic
lymphocytic leukemia in (Richter's) transformation. Semin Oncol. 1998;
25: 117-25. PMid:9482533

- Wiernik PH. Second neoplasms in patients
with chronic lymphocytic leukemia. Curr Treat Options Oncol. 2004; 5:
215-23. doi:10.1007/s11864-004-0013-7
PMid:15115650

- Travis LB, Curtis RE, Hankey BF, Fraumeni
JF. Second cancers in patients with chronic lymphocytic leukemia. J
Natl Cancer Inst. 1992; 84: 1422-7. doi:10.1093/jnci/84.18.1422

- Dighiero G, Maloum K, Desablens B, Cazin
B, Navarro M, Leblay R, Leporrier M, Jaubert J, Lepeu G, Dreyfus B,
Binet JL, Travade P. Chlorambucil in indolent chronic lymphocytic
leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N
Engl J Med. 1998; 338: 1506-14.doi:10.1056/NEJM199805213382104
PMid:9593789

- Robertson LE, Estey E, Kantarjian H,
Koller C, O'Brien S, Brown B, Keating MJ. Therapy-related leukemia and
myelodysplastic syndrome in chronic lymphocytic leukemia. Leukemia
1994; 8: 2047-51. PMid:7807993

- Keating MJ, O'Brien S, Lerner S, Koller C,
Beran M, Robertson LE, Freireich EJ, Estey E, Kantarjian H. Long term
follow-up of patients with chronic lymphocytic leukemia (CLL) receiving
fludarabine regimens as initial therapy. Blood 1998; 92: 1165-71.
PMid:9694704

- Leporrier M, Chevret S, Cazin B, Boudjerra
N, Feugier P, Desablens B, Rapp MJ, Jaubert J, Autrand C, Divine M,
Dreyfus B, Maloum K, Travade P, Dighiero G, Binet JL, Chastang C.
Randomized comparison of fludarabine, CAP, and ChOP in 938 previously
untreated stage B and C chronic lymphocytic leukemia patients. Blood
2001; 98:2319-25.doi:10.1182/blood.V98.8.2319
PMid:11588025

- Johnson S, Smith AG, Löffler H, Osby E,
Juliusson G, Emmerich B, Wyld PJ, Hiddemann W. Multicentre prospective
randomised trial of fludarabine versus cyclophosphamide, doxorubicin,
and prednisone (CAP) for treatment of advanced-stage chronic
lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet
1996; 347:1432-8. PMid:8676625

- Cheson BD, Vena DA, Barrett J, Freidlin B.
Second malignancies as a consequence of nucleoside analog therapy for
chronic lymphoid leukemias. J Clin Oncol 17:2454-60, 1999.PMid:10561309

- Ricci F, Tdeschi A, Morra E, Montillo M.
Fludarabine in the treatment of chronic lymphocytic leukemia: a review.
Therapeutics and Clinical Risk Management 2009; 5: 187-207.

- Morrison VA, Rai KR, Peterson BL, Kolitz
JE, Elias L, Appelbaum FR, Hines JD, Shepherd L, Larson RA, Schiffer
CA. Therapy-related myeloid leukemias are observed in patients with
chronic lymphocytic leukemia after treatment with fludarabine and
chlorambucil: results of an intergroup study, cancer and leukemia group
B 9011. J Clin Oncol. 2002; 20: 3878-84.doi:10.1200/JCO.2002.08.128
PMid:12228208

- Smith MR, Neuberg D, Flinn IW, Grever MR, Bennett JM, Dewald G, Paietta EM, Litzow MR, Rowe JM, Lucas D, Kitada S, Jelinek DF, Gribben JG, Byrd JC, Reed JC, Hussein MA, Appelbaum FR, Larson RA, Moore DF, and Tallman MS. Increased Incidence of Therapy Related Myeloid Neoplasia (t-MN) After Initial Therapy for CLL with Fludarabine-Cyclophosphamide (FC) Vs Fludarabine (F): Long-Term Follow-up of US Intergroup Study E2997. Blood (ASH Annual Meeting Abstracts) 2010 116: Abstract 924.
- Badoux XC, Keating MJ, Wang X, O'Brien SM,
Ferrajoli A, Faderl S, Burger J, Koller C, Lerner S, Kantarjian H,
Wierda WG. Fludarabine, cyclophosphamide and rituximab
chemoimmunotherapy is highly effective treatment for relapsed patients
with CLL. Blood 2011; 117: 3016-24. doi:10.1182/blood-2010-08-304683
PMid:21245487

- Woyach JA, Ruppert AS, Heerema NA,
Peterson BL, Gribben JG, Morrison VA, Rai KR, Larson RA, Byrd JC.
Chemoimmunotherapy With Fludarabine and Rituximab Produces Extended
Overall Survival and Progression-Free Survival in Chronic Lymphocytic
Leukemia: Long-Term Follow-Up of CALGB Study 9712. J Clin Oncol. 2011;
Feb 14 [Epub ahead of print]

- Gribben JG, Zahrieh D, Stephans K,
Bartlett-Pandite L, Alyea EP, Fisher DC, Freedman AS, Mauch P,
Schlossman R, Sequist LV, Soiffer RJ, Marshall B, Neuberg D, Ritz J,
Nadler LM. Autologous and allogeneic stem cell transplantations for
poor-risk chronic lymphocytic leukemia. Blood 2005; 106:
4389-96.doi:10.1182/blood-2005-05-1778
PMid:16131571 PMCid:1895235

- Dreger P, Döhner H, Greinix H, McClanahan F, Hensel M, Hertenstein B, Dührsen U, Hentrich M, Trümper L, Sonnen R, Emmerich B, Knauf WU, Hopfinger G, Hallek M, Kneba M, Schmitz N and Stilgenbauer S. Early Autologous Stem Cell Transplantation (autoSCT) May Overcome the Adverse Impact of Del 11q- in Poor-Risk Chronic Lymphocytic Leukemia (CLL): Results From the GCLLSG CLL3 Trial. Blood (ASH Annual Meeting Abstracts) 2009; 114: 879.
- Milligan DW, Fernandes S, Dasgupta R,
Davies FE, Matutes E, Fegan CD, McConkey C, Child JA, Cunningham D,
Morgan GJ, Catovsky D. National Cancer Research Institute
Haematological Studies Group. Results of the MRC pilot study show
autografting for younger patients with chronic lymphocytic leukemia is
safe and achieves a high percentage of molecular responses.Blood 2005;
105: 397-404. doi:10.1182/blood-2004-01-0298
PMid:15117764

- Michallet M, Dreger P, Sutton L, Brand R,
Richards S, van Os M, Sobh M, Choquet S, Corront B, Dearden C, Gratwohl
A, Herr W, Catovsky D, Hallek M, de Witte T, Niederwieser D, Leporrier
M, Milligan D. Autologous hematopoietic stem cell transplantation in
chronic lymphocytic leukemia: results of European intergroup randomized
trial comparing autografting versus observation. Blood 2011; 117:
1516-21. doi:10.1182/blood-2010-09-308775
PMid:21106985
