Bacillus Cereus Catheter Related Bloodstream Infection in a Patient with Acute Lymphoblastic Leukemia
N Gurler1, L Oksuz1, M Muftuoglu2, FD Sargin2 and SK Besisik2
1Istanbul University, Istanbul Medical Faculty, Department of Medical Microbiology, Capa-Istanbul Turkey
2Istanbul University, Istanbul Medical Faculty, Department of Haematology, Capa-Istanbul Turkey
2Istanbul University, Istanbul Medical Faculty, Department of Haematology, Capa-Istanbul Turkey
Correspondence
to:
Dr Lütfiye Öksüz. Istanbul University, Istanbul Medical Faculty,
Department of Medical Microbiology, Capa-Istanbul Turkey. E-mail: loksuz34@yahoo.com
Published: Jabuary 18, 2012
Received: November 1, 2011
Accepted: December 23, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e2012004 , DOI 10.4084/MJHID.2012.004
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Bacillus
cereus infection is rarely associated with actual infection and for
this reason single positive blood culture is usually regarded as
contamination. However it may cause a number of infections, such
catheter-related bloodstream infections. Significant catheter-related
bloodstream infections (CRBSI) caused by Bacillus spp. are mainly due
to B. cereus and have been predominantly reported in immunocompromised
hosts. Catheter removal is generally advised for management of
infection. In this report, catheter-related bacteremia caused by B.
cereus in a patient with acute lymphoblastıc leukemia (ALL) in Istanbul
Medical Faculty was presented.
Case.
A 44-year old man presented with fatigue, weight loss, epistaxis and high fever. Physical examination was insignificant except for pallorness, Complete blood count revealed total white blood cells at 130,000/mm3 hemoglobin at 7.6 g/dl, platelet 30,000/mm3. He was diagnosed with Pre-B acute lymphoblastic leukemia following bone marrow aspiration, biyopsy and flow cytometric analysis. Cytogenetic analysis showed a karyotype of 45, XY del 9 del 16. The patient achieved complete hematological remission accompanied by disappearance of cytogenetic abnormalities following induction chemotherapy with BFM ALL protocol. The patient was considered to be a candidate for stem cell transplantion because of initial patient characteristics indicating high risk for ALL relapse (Age, complex clonal cytogenetic abnormalities, high total leukocyte count). An HLA-identical unrelated donor, living abroad, was identified. A double-lumen Hickman–catheter (Bard 12.0 Fr, Round Dual Lumen) was inserted by surgical cut-down to access the right subclavian vein which would be necessary for allogeneic stem cell transplantation. Two cycles of consolidation chemotherapy were administered before proceeding to stem cell transplantation. Three weeks later central nervous system (CNS) relapse of ALL occurred, which is treated with four cycles of intrathecal chemoterapy, comprising of methotrexate, cytosine arabinoside and dexamethasone. At the same time a cycle of cytosine arabinoside and cyclophosphamide-based chemotherapy was administered. Stem cell transplantation was planned to be carried out in a month. Three weeks later the patient presented with high fever and headache. Blood cultures were obtained from the catheter and from a peripheral vein and evaluated on the BACTEC 9120 system (Becton Dickinson, USA). Bacillus spp. was isolated from the cathether while blood culture obtained from the peripheral vein remained negative. It was regarded as contaminant so that complete identification of Bacillus species was not carried out. Lumbar puncture was performed and CNS involvement was documented. Lymphoblastic cells were seen on peripheral blood smear which is considered compatible with hematological relapse. Intrathecal chemotherapy, and methotrexate and high-dose cytosine arabinoside-based systemic reinduction chemotherapy were started. One week after chemotherapy he developed chills and high fever as absolute neutrophil count was 100/mm3. The physical examination was noncontributary and blood cultures were collected from the catheter and a peripheral vein. Two blood culture bottles (one from the catheter and one from the peripheral) were positive gram-positive bacilli after three days of incubation at 35°C. The organism produced large, greenish colonies. The bacterium was identified as B.cereus on the basis of Gram staining, colony morphology, motility, lecitinase activity. The bacterial identification was confirmed as B.cereus using VITEK identification system (bioMeriéux, France). The strain was sensitive to imipenem, vancomycin, gentamicin and ciprofloxacin, whereas resistant to penicillin, cephalosporins and co-trimoxazole. He was started on piperacillin-tazobactam at a dose of 4 g/500 mg every 6 h emprically before blood culture result was available. Vancomycin was added to the treatment regimen due to incomplete clinical and laboratory response. Hickmann catheter, which was inserted about ten months ago, was accepted as the focus of infection and, prolonged maintenance of the catheter was thought to be no more possible. High fever subsided completely upon removal. Hematological remission was achieved and allogeneic stem cell transplantation was scheduled to be performed one week later.
Conclusion.
Bacillus species are known to be responsible for several systemic infections, especially in immunocompromised patients. The most commonly reported systemic infection is bacteremia.[2,3] Bacillus bacteremia can be serious and even fatal, in immunocompromised patients, such as neutropenia. The most common feature in true Bacillus bacteremia is the presence of an intravascular catheter. B. cereus produce biofilms, which can play a major role in attachment to catheters.[10] Bacillus species are associated with CRBSIs and they have been well documented, especially among patients with hematological malignancies.[10,22] Some of the reported cases in the literature were shown in the table 1. The table shows that the majority of the patients have abdominal symptoms, but our patients not.
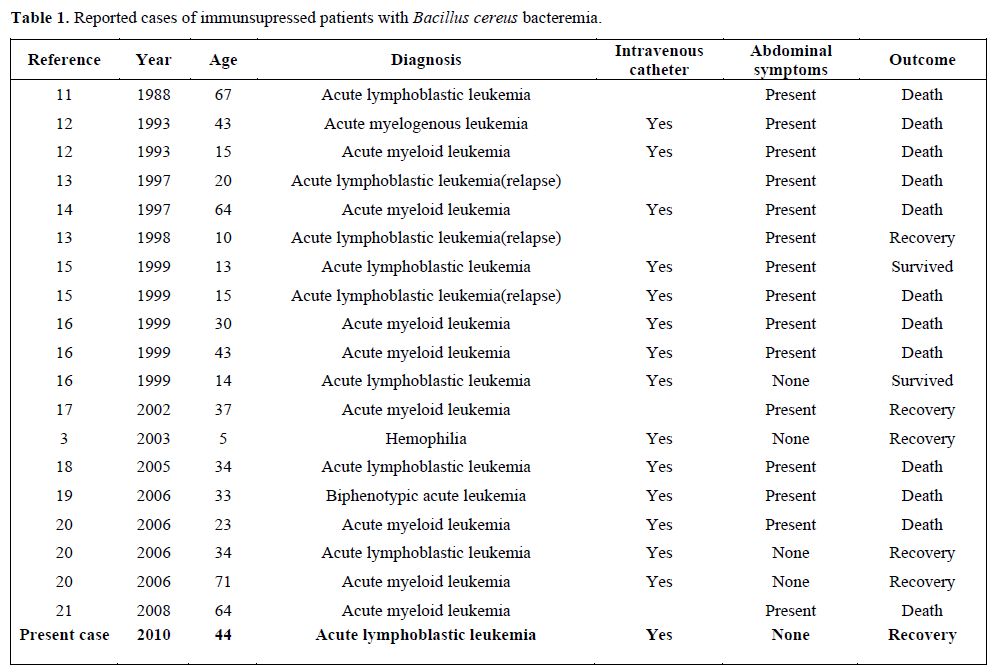
Table 1. Reported cases of immunsupressed patients with Bacillus cereus bacteremia.
The isolation of Bacillus species raises the possibility of contamination on the basis of Bacillus spp. being common contaminants of blood cultures.[8] The ability of Bacillus spp to form a biofilm matrix and the adherence properties account for their relation with central venous catheter (CVC) infections.[4] Catheter-related Bacillus spp infections are difficult to eradicate because of slime formation and biofilm production on the catheter surface. Most published data are based on small case series and data regarding the management of Bacillus spp. infections are still lacking. Recently conducted study, which is currently the largest series reported, has concluded that retention of the catheter beyond 72 hours after the onset of bacteremia was related to a higher incidence of recurrent Bacillus bacteremia.[2] The drug of choice for Bacillus infections is vancomycin.[5] Despite the proper agent and appropriate length of treatment the clinical outcome remains unsatisfactory. This is largely due to inactivity of vancomycin against the organisms dwelling in biofilm layer and slime production,making the organism highly adherent to the catheters. Based on preliminary data and available “Infectious Diseases Society of America (IDSA)” guideline early catheter removal should be the cornerstone of Bacillus bacteremia management.[2,6,7] In compatible with this report, our patient developed intermittent fever spikes under the appropriate antimicrobial therapy even though neutropenia resolved with G-CSF support. The Hickmann catheter was removed on the 10th day of vancomycin therapy. Septicemia-related thrombocytopenia unresponsive to aggressive thrombocyte transfusion hindered early removal of the catheter. Catheter tip culture remained negative, which is attributed to ongoing antibiotherapy consisting of piperacillin-tazobactam and vancomycin.
According to the IDSA guidelines published in 2009,[6] Bacillus CRBSIs should be considered after blood contamination is ruled out on the basis of multiple positive culture results, with at least one blood culture sample obtained from a peripheral vein. It is also suggested that the catheters should be removed if the diagnosis is certain, except for in patients having uncomplicated CRBSI involving long-term catheters because of limited access sites and requiring long-term vascular access for survival.
Bacillus cereus is a growing concern as a cause of life-threatening infections in patients with hematologic malignancies.[9] B.cereus should be suspected in immunosuppressed patients with intravascular catheter. B.cereus septicemia may be fatal in immunocompromised hosts despite broad-spectrum appropriate treatment. Catheter removal is essential for prevention of recurrent bacteremia. Long-term catheter salvage should be reserved for appropriate patient group.
Acknowledgments.
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University. Project number 14413.
A 44-year old man presented with fatigue, weight loss, epistaxis and high fever. Physical examination was insignificant except for pallorness, Complete blood count revealed total white blood cells at 130,000/mm3 hemoglobin at 7.6 g/dl, platelet 30,000/mm3. He was diagnosed with Pre-B acute lymphoblastic leukemia following bone marrow aspiration, biyopsy and flow cytometric analysis. Cytogenetic analysis showed a karyotype of 45, XY del 9 del 16. The patient achieved complete hematological remission accompanied by disappearance of cytogenetic abnormalities following induction chemotherapy with BFM ALL protocol. The patient was considered to be a candidate for stem cell transplantion because of initial patient characteristics indicating high risk for ALL relapse (Age, complex clonal cytogenetic abnormalities, high total leukocyte count). An HLA-identical unrelated donor, living abroad, was identified. A double-lumen Hickman–catheter (Bard 12.0 Fr, Round Dual Lumen) was inserted by surgical cut-down to access the right subclavian vein which would be necessary for allogeneic stem cell transplantation. Two cycles of consolidation chemotherapy were administered before proceeding to stem cell transplantation. Three weeks later central nervous system (CNS) relapse of ALL occurred, which is treated with four cycles of intrathecal chemoterapy, comprising of methotrexate, cytosine arabinoside and dexamethasone. At the same time a cycle of cytosine arabinoside and cyclophosphamide-based chemotherapy was administered. Stem cell transplantation was planned to be carried out in a month. Three weeks later the patient presented with high fever and headache. Blood cultures were obtained from the catheter and from a peripheral vein and evaluated on the BACTEC 9120 system (Becton Dickinson, USA). Bacillus spp. was isolated from the cathether while blood culture obtained from the peripheral vein remained negative. It was regarded as contaminant so that complete identification of Bacillus species was not carried out. Lumbar puncture was performed and CNS involvement was documented. Lymphoblastic cells were seen on peripheral blood smear which is considered compatible with hematological relapse. Intrathecal chemotherapy, and methotrexate and high-dose cytosine arabinoside-based systemic reinduction chemotherapy were started. One week after chemotherapy he developed chills and high fever as absolute neutrophil count was 100/mm3. The physical examination was noncontributary and blood cultures were collected from the catheter and a peripheral vein. Two blood culture bottles (one from the catheter and one from the peripheral) were positive gram-positive bacilli after three days of incubation at 35°C. The organism produced large, greenish colonies. The bacterium was identified as B.cereus on the basis of Gram staining, colony morphology, motility, lecitinase activity. The bacterial identification was confirmed as B.cereus using VITEK identification system (bioMeriéux, France). The strain was sensitive to imipenem, vancomycin, gentamicin and ciprofloxacin, whereas resistant to penicillin, cephalosporins and co-trimoxazole. He was started on piperacillin-tazobactam at a dose of 4 g/500 mg every 6 h emprically before blood culture result was available. Vancomycin was added to the treatment regimen due to incomplete clinical and laboratory response. Hickmann catheter, which was inserted about ten months ago, was accepted as the focus of infection and, prolonged maintenance of the catheter was thought to be no more possible. High fever subsided completely upon removal. Hematological remission was achieved and allogeneic stem cell transplantation was scheduled to be performed one week later.
Conclusion.
Bacillus species are known to be responsible for several systemic infections, especially in immunocompromised patients. The most commonly reported systemic infection is bacteremia.[2,3] Bacillus bacteremia can be serious and even fatal, in immunocompromised patients, such as neutropenia. The most common feature in true Bacillus bacteremia is the presence of an intravascular catheter. B. cereus produce biofilms, which can play a major role in attachment to catheters.[10] Bacillus species are associated with CRBSIs and they have been well documented, especially among patients with hematological malignancies.[10,22] Some of the reported cases in the literature were shown in the table 1. The table shows that the majority of the patients have abdominal symptoms, but our patients not.

Table 1. Reported cases of immunsupressed patients with Bacillus cereus bacteremia.
The isolation of Bacillus species raises the possibility of contamination on the basis of Bacillus spp. being common contaminants of blood cultures.[8] The ability of Bacillus spp to form a biofilm matrix and the adherence properties account for their relation with central venous catheter (CVC) infections.[4] Catheter-related Bacillus spp infections are difficult to eradicate because of slime formation and biofilm production on the catheter surface. Most published data are based on small case series and data regarding the management of Bacillus spp. infections are still lacking. Recently conducted study, which is currently the largest series reported, has concluded that retention of the catheter beyond 72 hours after the onset of bacteremia was related to a higher incidence of recurrent Bacillus bacteremia.[2] The drug of choice for Bacillus infections is vancomycin.[5] Despite the proper agent and appropriate length of treatment the clinical outcome remains unsatisfactory. This is largely due to inactivity of vancomycin against the organisms dwelling in biofilm layer and slime production,making the organism highly adherent to the catheters. Based on preliminary data and available “Infectious Diseases Society of America (IDSA)” guideline early catheter removal should be the cornerstone of Bacillus bacteremia management.[2,6,7] In compatible with this report, our patient developed intermittent fever spikes under the appropriate antimicrobial therapy even though neutropenia resolved with G-CSF support. The Hickmann catheter was removed on the 10th day of vancomycin therapy. Septicemia-related thrombocytopenia unresponsive to aggressive thrombocyte transfusion hindered early removal of the catheter. Catheter tip culture remained negative, which is attributed to ongoing antibiotherapy consisting of piperacillin-tazobactam and vancomycin.
According to the IDSA guidelines published in 2009,[6] Bacillus CRBSIs should be considered after blood contamination is ruled out on the basis of multiple positive culture results, with at least one blood culture sample obtained from a peripheral vein. It is also suggested that the catheters should be removed if the diagnosis is certain, except for in patients having uncomplicated CRBSI involving long-term catheters because of limited access sites and requiring long-term vascular access for survival.
Bacillus cereus is a growing concern as a cause of life-threatening infections in patients with hematologic malignancies.[9] B.cereus should be suspected in immunosuppressed patients with intravascular catheter. B.cereus septicemia may be fatal in immunocompromised hosts despite broad-spectrum appropriate treatment. Catheter removal is essential for prevention of recurrent bacteremia. Long-term catheter salvage should be reserved for appropriate patient group.
Acknowledgments.
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University. Project number 14413.
References
- Leonidou
L, Gogos CA. Catheter-related bloodstream infections: catheter
management according to pathogen. Int J Antimicrob Agents 2010; 36:

- Kassar R, Hachem R, Jiang Y, Chaftari AM,
Raad I. Management of Bacillus bacteremia: the need for catheter
removal. Medicine (Baltimore) 2009; 88: 279-283.

- Srivaths PR, Rozans MK, Kelly E Jr,
Venkateswaran L. Bacillus cereus central line infection in an
immunocompetent child with hemophilia. J Pediatr Hematol Oncol. 2004;
26: 194-196 http://dx.doi.org/10.1097/00043426-200403000-00010 PMid:15125613

- Branda SS, Chu F, Kearns DB, Losick R,
Kolter R. A major protein component of the Bacillus subtilis biofilm
matrix. Mol Microbiol 2006;59: 1229-1238. http://dx.doi.org/10.1111/j.1365-2958.2005.05020.x PMid:16430696

- Cotton DJ, Gill VJ, Marshall DJ, Gress J,
Thaler M, Pizzo PA. Clinical features and therapeutic interventions in
17 cases of Bacillus bacteremia in an immunosuppressed patient
population. J Clin Microbiol 1987; 25: 672-674. PMid:3571476
PMCid:266057

- Mermel LA, Allon M, Bouza E, Craven DE et
al. Clinical practice guidelines for the diagnosis and management of
intravascular catheter-related infection: 2009 update by the Infectious
Diseases Society of America. Clin Infect Dis 2009; 49: 1-45. http://dx.doi.org/10.1086/599376 PMid:19489710

- Park HG, Choi SH. Long-term central venous
catheter salvage in patients with Bacillus bacteremia. Medicine. 2010;
89: 346; 347.

- Weber DJ, Saviteer SM, Rutala WA, Thomann
CA. Clinical significance of Bacillus species isolated from blood
cultures. South Med J 1989; 82: 705–9. http://dx.doi.org/10.1097/00007611-198906000-00008 PMid:2499933

- Inoue D, Nagaj Y, Mori M et al. Fulminant
sepsis caused by Bacillus cereus in patients with hematologic
malignancies: analysis of its prognosis and risk factors. Leuk Lymphoma
2010; 51: 860-869. http://dx.doi.org/10.3109/10428191003713976

- Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microb Rev 2010; 23: 382-398. http://dx.doi.org/10.1128/CMR.00073-09 PMid:20375358 PMCid:2863360

- Funada H, Uatoni C, Machi T, et al.
Bacillus cereus bacteremia in an adult with acute leukemia. Jpn J Clin
Oncol 1988; 18: 69-74. PMid:3127617

- Yoshida H, Moriyama Y, Tatekawa T,
Tominaga N, Teshima H, et al. Two cases of acute myelogenous leukemia
with Bacillus cereus bacteremia resulting in fatal intracranial
hemorrhagie. Rinsho Ketsueki 1993; 34: 1568-72. PMid:8295331

- Arnaout MK, Tamburro RF, Bodner SM, et al.
Bacillus cereus causing fulminant sepsis and hemolysis in two patients
with acute leukemia. J Ped Hematol/Oncol 1999; 21: 431-435.

- Akiyama N, Mitani K, Tanaka Y, Hanazano Y,
et al. Fulminant septisemic syndrome of Bacillus cereus in a leukemic
patient. Int Med 1997; 36: 221-226. http://dx.doi.org/10.2169/internalmedicine.36.221 PMid:9144019

- Gaur AH, Patrick CC, Mc Cullers JA, Flynn PM; et al. Clin Infect Dis 2001; 32: 1456-1462. http://dx.doi.org/10.1086/320154 PMid:11317247

- Musa OM, Douri MA, Khan S, Shafi T, et al.
Fulminant septicaemic syndrome of Bacillus cereus: Three case reports.
J Infect 1999; 39: 154-156. http://dx.doi.org/10.1016/S0163-4453(99)90009-9

- Le Scannf J, Mohammedi I, Thie baut A,
Martin O, Argaud L, Robert D. Necrotizing gastritis due to Bacillus
cereus in an immunocompromised patient. Infection 2006; 34: 98-99.
PMid:16703301

- Cone LA, Dreisbach L, Potts BE, Comess BE,
Burleigh WA. Fatal Bacillus cereus endocarditis masquerading as an
anthrax-like infection in a patient with acute lymphoblastic leukemia:
case report. J Heart Valve Dis 2005; 14: 37-39. PMid:15700434

- Kiyomizu K, Yagi T, Yoshida H, Minami R,
et al. Fulminant septicemia of Bacillus cereus resistant to carbapenem
in a patient with biphenotypic acute leukemia. J Infect Chemother 2008;
14: 361-367. http://dx.doi.org/10.1007/s10156-008-0627-y PMid:18936889

- Ozkocaman V, Ozcelik T, Ali R, Ozkalemkas
F, et al. Bacillus spp. Among hospitalized patients with haematological
malignancies: clinicial features, epidemics and outcomes. J Hosp Infect
2006; 64: 169-176. http://dx.doi.org/10.1016/j.jhin.2006.05.014 PMid:16891037

- Kawatani E, Kishikawa Y, Sankoda C,
Kuwahara N, et al. Bacillus cereus sepsis and subarachnoid hemorrhage
following consolidation chemotherapy for acute myelogenous leukemia.
Rinsho Ketsueki 2009; 50: 300-303. PMid:19404024

- Fekete T. Bacillus species and related
genera other than Bacillus anthracis. Mandell G, Bennet JE, Dolin R
(Eds): Mandell, Douglas and Bennett’s Principle’s and Practice of
Infectious Diseases, 7th edition, Chapter 209, p.2727, Philadelphia,
Elsevier/Churchill Livingstone, 2010.