Tuberculosis After Gastrectomy, Plasmatic Concentration of Antitubercular Drugs
De Socio Giuseppe Vittorio1, D’Avolio Antonio2, Sgrelli Alessio1, Baietto Lorena2, Lisa Malincarne1, Di Perri Giovanni2 and Baldelli Franco1
1Department of Infectious Diseases “Santa Maria della Misericordia” Hospital, University of Perugia, Perugia Italy
2Laboratory of Clinical Pharmacology and Pharmacogenetic, Department of Infectious Diseases, University of Turin, Amedeo di Savoia Hospital, Turin, Italy
2Laboratory of Clinical Pharmacology and Pharmacogenetic, Department of Infectious Diseases, University of Turin, Amedeo di Savoia Hospital, Turin, Italy
Correspondence
to:
Giuseppe Vittorio L De Socio, MD PhD. Clinica di Malattie Infettive,
UniversitÓ degli Studi di Perugia, Ospedale "Santa Maria della
Misericordia", piazzale Menghini, 1 – 06129 Perugia, Italy. Tel:
+39-075-5784321, Fax: +39-075-5784346. E-mail: giuseppedesocio@yahoo.it
Published: January 24, 2012
Received: October 14, 2011
Accepted: December 27, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e2012007, DOI 10.4084/MJHID.2012.007
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
We
report pharmacokinetic data on two gastrectomized, patients affected by
tuberculosis. Drugs plasmatic concentrations were measured after seven
days of oral therapy by a validated high performance liquid
chromatography-mass spectrometry (HPLC-MS) method and the area under
the concentration-time-curve (AUC) over 24 hours (AUC0-24) was
calculated. A sub-therapeutic level of isoniazid was found in a patient
with total gastrectomy with a Cmax of 0,395 mg\L and AUC0-24 level of
4.75 hr*mg/L. The level of the other antitubercular drugs was adequate.
These findings support the need to monitor anti tubercular drug levels to facilitate early detection of therapeutic failure, above all in patients treated with isoniazid and with potential problems on oral drugs absorption.
These findings support the need to monitor anti tubercular drug levels to facilitate early detection of therapeutic failure, above all in patients treated with isoniazid and with potential problems on oral drugs absorption.
Introduction.
Little is known of antitubercular treatment in gastrectomized patients. By a MEDLINE search we found no evidence in the literature regarding the antitubercular drugs absorption after gastrectomy. Total and partial gastric resection alters the anatomy and secretory activity of the gastrointestinal tract. It might be expected that the consequences of such changes should affect the pharmacokinetics, especially concerning the absorption of orally administered drugs that are primarily absorbed in the stomach or duodenum leading to sub-therapeutic drugs level.
Case Studies and Methods.
We report here the case of two consecutive gastrectomized patients affected by tuberculosis. To estimate the degree to which absorption is impaired, the drug levels were measured in duplicate plasma samples. The patients were fasting at the time of drugs intake. Plasma sample levels were measured by a validated high performance liquid chromatography-mass spectrometry (HPLC-MS) method with the following lower limit of quantification (LOQ) and detection (LOD): 0.015 mg/L (LOQ) and 0.007 mg/L (LOD) for isoniazid; 0.195 mg/L (LOQ) and 0.048 mg/L (LOD) for pyrazinamide; 0.019 mg/L (LOQ) and 0.008 mg/L (LOD) for rifampin; 0.020 mg/L (LOQ) and 0.010 mg/L (LOD) for ethambutol. Non-compartmental pharmacokinetic analysis of the data was performed using Kinetica version 5.0 software (Thermo Fisher Scientific). The area under the concentration-time curve (AUC) over 24 hours (AUC0-24) in plasma was calculated by the linear-log trapezoidal rule, using the same Ctrough concentration for time 0 and 24 and applying a limited sampling model.[1]
Case 1.
A 68-year-old, HIV negative was affected by tubercular (TB) epididymitis. His previous medical history was remarkable for a total gastrectomy with Roux-en-Y gastric bypass procedure performed five years before, due to gastric cancer. His renal and liver function was normal. His weight was 65Kg. He was treated with standard oral anti TB therapy including isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 1200 mg daily and pyrazinamide 2000 mg daily, vitamin B6 300 mg daily. His chronic medications were: enalapril 20 mg daily, amlodipine 5 mg daily, ticlopidine 500 mg daily. Therefore plasma concentrations of anti TB drugs were measured after seven days of drug intake in the hospital. Plasma samples were collected before the drug intake to measure Ctrough and after 2 hours to measure Cmax (peak plasma concentration). We found very low Cmax and AUC0-24 levels of isoniazid (0,395 mg\L and 4.75 hr*mg/L respectively), not attributable to patient’s anti-hypertensive and anti-tubercular drug-drug interaction. The results of plasma concentrations, AUC0-24 and the therapeutic expected range are shown in the following table. The patient obtained slow clinical improvement.
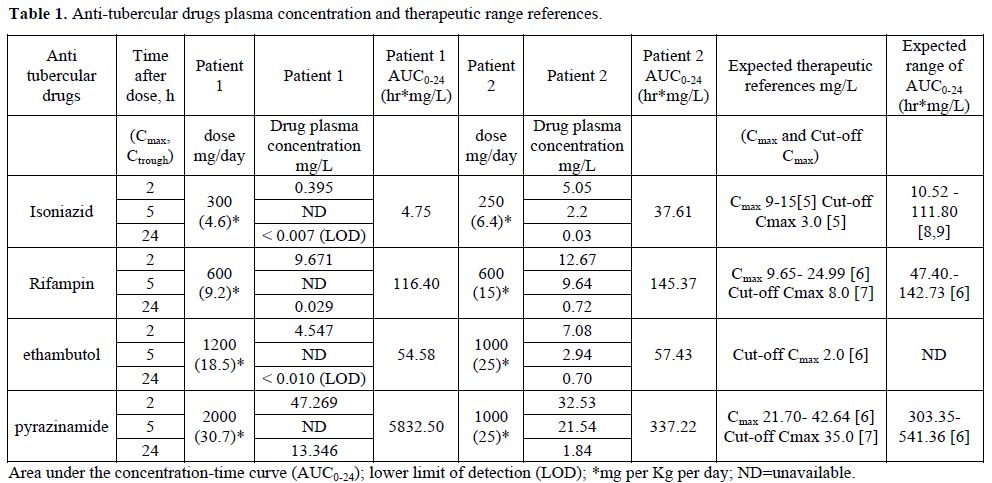
Table 1. Anti-tubercular drugs plasma concentration and therapeutic range references.
Case 2.
A 65-year-old HIV negative woman was affected by pulmonary tuberculosis. She underwent a partial gastrectomy for a gastric ulcer 15 years earlier. Her weight was 39 Kg; she was treated with standard anti TB therapy including isoniazid 250 mg daily, rifampin 600 mg daily, ethambutol 1000 mg daily and pyrazinamide 1000 mg daily, vitamin B6 300 mg daily. The drugs were administered intra venous (iv) in the first 15 days and oral subsequently (pyrazinamide and vitamin B6 were always administered orally). No other drugs were taken by this patient. We studied the plasmatic drugs concentration in the course of iv and oral therapy. Plasma samples were collected before the daily drug intake to measure Ctrough and after 2 hours and 5 hours to measure Cmax. The results of plasma concentrations, AUC0-24 during oral therapy are shown in the table. During iv therapy the measured AUC0-24 was very similar from the one reported in the course of oral treatment: isoniazid 26.70 hr*mg/L, rifampin 154.87 hr*mg/L, ethambutol 32.48 hr*mg/L. The patient obtained adequate clinical response.
Discussion.
To our knowledge, data regarding the first line antitubercular drugs absorption after gastrectomy are scantily. Overall the measured plasmatic antitubercular drugs in gastrectomized patients were generally adequate. We observed that a patient undergoing a complete gastric resection followed by a Roux-en-Y gastric bypass procedure (case 1) had poor isoniazid plasmatic level. In the second case with the patient who had undergone a partial gastric resection, the absorption of isoniazid was right and comparable to iv administration. Rifampin, ethambutol and pyrazinamide in both patients always had suitable level.
Obviously, a single case study has several limitations principally linked to individual variability, thus we can’t draw conclusions. In addition, in case 1 we have calculated the AUC0-24 from two determinations only according to a validated limited sampling model,[1] and assuming Cmax appropriate after two hours of drugs intake. Even considering these limitations, we believe the observation is important at least for two reasons: firstly, it is known that gastrectomy patients have greater risk of having tuberculosis in later life and preventive therapy is mainly based on isoniazid administration;[2,3] secondly, low serum concentration of antitubercular drugs have been associated with treatment failure, relapse and drug resistance.[4]
Although the clinical significance of low concentration should be defined, it may be necessary to optimise drug dosages by TDM, especially in patients with an inadequate clinical response.
These findings support the need to monitor anti tubercular drug levels to facilitate early detection of therapeutic failure above all in patients with potential problems on oral drugs absorption.
Little is known of antitubercular treatment in gastrectomized patients. By a MEDLINE search we found no evidence in the literature regarding the antitubercular drugs absorption after gastrectomy. Total and partial gastric resection alters the anatomy and secretory activity of the gastrointestinal tract. It might be expected that the consequences of such changes should affect the pharmacokinetics, especially concerning the absorption of orally administered drugs that are primarily absorbed in the stomach or duodenum leading to sub-therapeutic drugs level.
Case Studies and Methods.
We report here the case of two consecutive gastrectomized patients affected by tuberculosis. To estimate the degree to which absorption is impaired, the drug levels were measured in duplicate plasma samples. The patients were fasting at the time of drugs intake. Plasma sample levels were measured by a validated high performance liquid chromatography-mass spectrometry (HPLC-MS) method with the following lower limit of quantification (LOQ) and detection (LOD): 0.015 mg/L (LOQ) and 0.007 mg/L (LOD) for isoniazid; 0.195 mg/L (LOQ) and 0.048 mg/L (LOD) for pyrazinamide; 0.019 mg/L (LOQ) and 0.008 mg/L (LOD) for rifampin; 0.020 mg/L (LOQ) and 0.010 mg/L (LOD) for ethambutol. Non-compartmental pharmacokinetic analysis of the data was performed using Kinetica version 5.0 software (Thermo Fisher Scientific). The area under the concentration-time curve (AUC) over 24 hours (AUC0-24) in plasma was calculated by the linear-log trapezoidal rule, using the same Ctrough concentration for time 0 and 24 and applying a limited sampling model.[1]
Case 1.
A 68-year-old, HIV negative was affected by tubercular (TB) epididymitis. His previous medical history was remarkable for a total gastrectomy with Roux-en-Y gastric bypass procedure performed five years before, due to gastric cancer. His renal and liver function was normal. His weight was 65Kg. He was treated with standard oral anti TB therapy including isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 1200 mg daily and pyrazinamide 2000 mg daily, vitamin B6 300 mg daily. His chronic medications were: enalapril 20 mg daily, amlodipine 5 mg daily, ticlopidine 500 mg daily. Therefore plasma concentrations of anti TB drugs were measured after seven days of drug intake in the hospital. Plasma samples were collected before the drug intake to measure Ctrough and after 2 hours to measure Cmax (peak plasma concentration). We found very low Cmax and AUC0-24 levels of isoniazid (0,395 mg\L and 4.75 hr*mg/L respectively), not attributable to patient’s anti-hypertensive and anti-tubercular drug-drug interaction. The results of plasma concentrations, AUC0-24 and the therapeutic expected range are shown in the following table. The patient obtained slow clinical improvement.

Table 1. Anti-tubercular drugs plasma concentration and therapeutic range references.
Case 2.
A 65-year-old HIV negative woman was affected by pulmonary tuberculosis. She underwent a partial gastrectomy for a gastric ulcer 15 years earlier. Her weight was 39 Kg; she was treated with standard anti TB therapy including isoniazid 250 mg daily, rifampin 600 mg daily, ethambutol 1000 mg daily and pyrazinamide 1000 mg daily, vitamin B6 300 mg daily. The drugs were administered intra venous (iv) in the first 15 days and oral subsequently (pyrazinamide and vitamin B6 were always administered orally). No other drugs were taken by this patient. We studied the plasmatic drugs concentration in the course of iv and oral therapy. Plasma samples were collected before the daily drug intake to measure Ctrough and after 2 hours and 5 hours to measure Cmax. The results of plasma concentrations, AUC0-24 during oral therapy are shown in the table. During iv therapy the measured AUC0-24 was very similar from the one reported in the course of oral treatment: isoniazid 26.70 hr*mg/L, rifampin 154.87 hr*mg/L, ethambutol 32.48 hr*mg/L. The patient obtained adequate clinical response.
Discussion.
To our knowledge, data regarding the first line antitubercular drugs absorption after gastrectomy are scantily. Overall the measured plasmatic antitubercular drugs in gastrectomized patients were generally adequate. We observed that a patient undergoing a complete gastric resection followed by a Roux-en-Y gastric bypass procedure (case 1) had poor isoniazid plasmatic level. In the second case with the patient who had undergone a partial gastric resection, the absorption of isoniazid was right and comparable to iv administration. Rifampin, ethambutol and pyrazinamide in both patients always had suitable level.
Obviously, a single case study has several limitations principally linked to individual variability, thus we can’t draw conclusions. In addition, in case 1 we have calculated the AUC0-24 from two determinations only according to a validated limited sampling model,[1] and assuming Cmax appropriate after two hours of drugs intake. Even considering these limitations, we believe the observation is important at least for two reasons: firstly, it is known that gastrectomy patients have greater risk of having tuberculosis in later life and preventive therapy is mainly based on isoniazid administration;[2,3] secondly, low serum concentration of antitubercular drugs have been associated with treatment failure, relapse and drug resistance.[4]
Although the clinical significance of low concentration should be defined, it may be necessary to optimise drug dosages by TDM, especially in patients with an inadequate clinical response.
These findings support the need to monitor anti tubercular drug levels to facilitate early detection of therapeutic failure above all in patients with potential problems on oral drugs absorption.
References
- Kayano
Y, Horiuchi I, Mori YI, Ishida K, Saito T, Taguchi M, Hashimoto Y. A
simulation study to evaluate limited sampling strategies to estimate
area under the curve of drug oncentration versus time following
repetitive oral dosing: limited sampling model versus naive trapezoidal
method. Biol Pharm Bull. 2009;32:1486-90.
http://dx.doi.org/10.1248/bpb.32.1486

- Steiger Z, Nickel WO, Shannon GJ, Nedwicki
EG, Higgins RF.Pulmonary tuberculosis after gastric resection. Am J
Surg. 1976;131:668-71.
http://dx.doi.org/10.1016/0002-9610(76)90174-4

- Snider DE Jr. Tuberculosis and gastrectomy. Chest. 1985;87:414-5 http://dx.doi.org/10.1378/chest.87.4.414 PMid:3979126

- Um SW, Lee SW, Kwon SY, Yoon HI, Park KU,
Song J, et al. Low serum concentrations of anti-tuberculosis drugs and
determinants of their serum levels. Int J Tuberc Lung Dis.
2007;11:972-8. PMid:17705974

- Kimerling ME, Phillips P, Patterson P, Hall
M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in
non-HIV-infected tuberculosis patients. Chest. 1998;113:1178-83
http://dx.doi.org/10.1378/chest.113.5.1178 PMid:9596291

- Peloquin CA, Jaresko GS, Yong CL, Keung AC,
Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of
isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother.
1997;41:2670-9. PMid:9420037 PMCid:164187

- Chideya S, Winston CA, Peloquin CA,
Bradford WZ, Hopewell PC, Wells CD et al. Isoniazid, rifampin,
ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes
among a predominantly HIV-infected cohort of adults with tuberculosis
from Botswana. Clin Infect Dis. 2009;48:1685-94.
http://dx.doi.org/10.1086/599040 PMid:19432554

- Donald PR, Parkin DP, Seifart HI, Schaaf
HS, van Helden PD, Werely CJ et al. The influence of dose and
N-acetyltransferase-2 (NAT2) genotype and phenotype on the
pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin
Pharmacol. 2007;63:633-9. http://dx.doi.org/10.1007/s00228-007-0305-5
PMid:17505821

- Weiner M, Burman W, Vernon A, Benator D,
Peloquin CA, Khan A et al. Tuberculosis Trials Consortium. Low
isoniazid concentrations and outcome of tuberculosis treatment with
once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med.
2003;167:1341-7. http://dx.doi.org/10.1164/rccm.200208-951OC
PMid:12531776
