Migration and Malaria in Europe
Begoña Monge-Maillo and Rogelio López-Vélez
Tropical Medicine.
Infectious Diseases Department. Hospital Ramón y Cajal, Madrid, Spain
Correspondence
to:
Rogelio López-Vélez. Tropical Medicine. Infectious Diseases Department.
Hospital Ramón y Cajal. Carretera de Colmenar 9,1. Madrid 28034, Spain.
E-mail: rlopezvelez.hrc@salud.madrid.org
Published: March 10, 2012
Received: January 10, 2011
Accepted: February 6, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e2012014, DOI: 10.4084/MJHID.2012.014
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
The
proportion of imported malaria cases due to immigrants in Europe has
increased during the lasts decades, with higher rates associated with
settled immigrants who travel to visit friends and relatives (VFRs) in
their country of origin. Cases are mainly due to P. falciparum and
Sub-Saharan Africa is the most common origin. Clinically, malaria in
immigrants is characterised by a mild clinical presentation including
asymptomatic or delayed malaria cases and low parasitic levels. These
characteristics may be explained by a semi-immunity acquired after long
periods of time exposed to stable malaria transmission. Malaria cases
among immigrants, even asymptomatic patients with sub-microscopic
parasitemia, could increase the risk of transmission and cause the
reintroduction of malaria in certain areas that have adequate vectors
and climate conditions. Moreover, imported malaria cases in immigrants
can also play an important role in the non-vector transmission out of
endemic areas, through blood transfusions, organ transplantation or
congenital transmission or occupational exposures. Consequently,
outside of endemic areas, malaria screening should be carried out among
recently arrived immigrants coming from malaria endemic countries. The
aim of screening is to reduce the risk of clinical malaria in the
individual as well as to prevent autochthonous transmission of malaria
in areas where it has been eradicated.
Introduction.
Malaria continues to present one of the major challenges to global public health with an estimated 225 million clinical cases and more than 700000 deaths in 2009, mostly in children under 5 years old from sub-Saharan Africa.[1] In Europe, malaria is eradicated in almost all countries of the World Health Organization European Region except for Azerbaijan, Georgia, Kyrgyzstan, Tajikistan and Turkey.[1]
In 2010 there were 47.3 million foreign-born people in the European Union (EU), corresponding to 9.4% of the total population. The majority of them, 31.4 million, were born in non- EU countries, while 16 million were born in another EU Member State. Data about those coming from endemic malarial countries is scarce. Estimates indicate that more than 5 million African immigrants could be living in Europe. Among them, about two thirds are from North Africa (Algeria, Morocco and Tunisia) and the rest are from Sub-Saharan Africa (SSA), mostly from West Africa (Ghana, Nigeria and Senegal). About 4 million come from South East Asia and nearly 2.2 million from Latin America.[2]
Imported malaria is defined as an infection acquired in a malaria endemic area but diagnosed in a non-endemic country. The malaria Programme of the WHO European Region annually collects data of malaria cases from 51 countries in the region. It reported an eight-fold increase in the number of imported malaria cases between 1972 and 1988 (from 1500 to 12000 cases), followed by a more gradual rise in 2000 (15500 cases). Most of the cases were imported to Western Europe, with France, the United Kingdom (UK), Germany and Italy accounting for more than 70% of all cases.[3] Although, in the last decade data from the World Health Organization (Figure 1) has shown a progressive decrease in the global incidence of imported malaria in most European countries,[4] despite a slight rise in cases reported from 2008.[5] Several published studies have corroborated such decreases in certain European countries such as the Netherlands[6] and the UK[7] which could be explained by a reduction in the global malaria transmission in SSA countries.
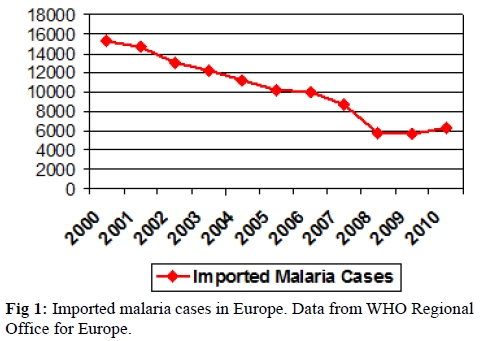
Figure 1. Imported malaria cases in Europe. Data from WHO Regional Office for Europe.
Today, the profile of immigrants is changing with higher rates of immigrants from southern (malaria-endemic) areas moving to northern (malaria free) industrialised areas of the world.[3] The proportion of imported malaria cases due to immigrants in Europe has increased during the last few years from 14% in research published more than 10 years ago, to 86% in more recent studies.[8] On pooling the reports, nearly 43% of malaria cases registered in key European centres occurred in non-nationals. The rates of malaria are much higher in settled immigrants who travel to visit friends and relatives (VFRs) in their country of origin. They can account for up to 70% of the cases in several reports.[9,10]
Methods.
A literature review was conducted using MEDLINE, EMBASE, Web of Science and the Cochrane Library database. The review included case studies, reviews and expert opinion. Search was based on published articles on imported infectious diseases in general and specifically malaria. Only those including data relating to immigrants (adult and paediatric) and based in European countries were included. A table with the most relevant characteristics of imported malaria among immigrants in Europe was created. Data referring to visiting friends and relatives (VFRs) and to refugees was not included. The selection criterion for including articles was based on the subjective opinion of the authors who considered article relevancy to the current research.
Imported malaria in immigrants and in immigrant-travellers (VFRs).
Most of the imported malaria cases among immigrants in Europe are due to P. falciparum and SSA, in particular West Africa, is the most common origin.[11-15] The incidence of infection by P. ovale and mixed infections is very similar to the incidence found in West Africa, which is probably due to the very high number of immigrants from these areas. Data from several of the selected published reports about imported malaria among immigrants in different European countries is summarised in Table 1.
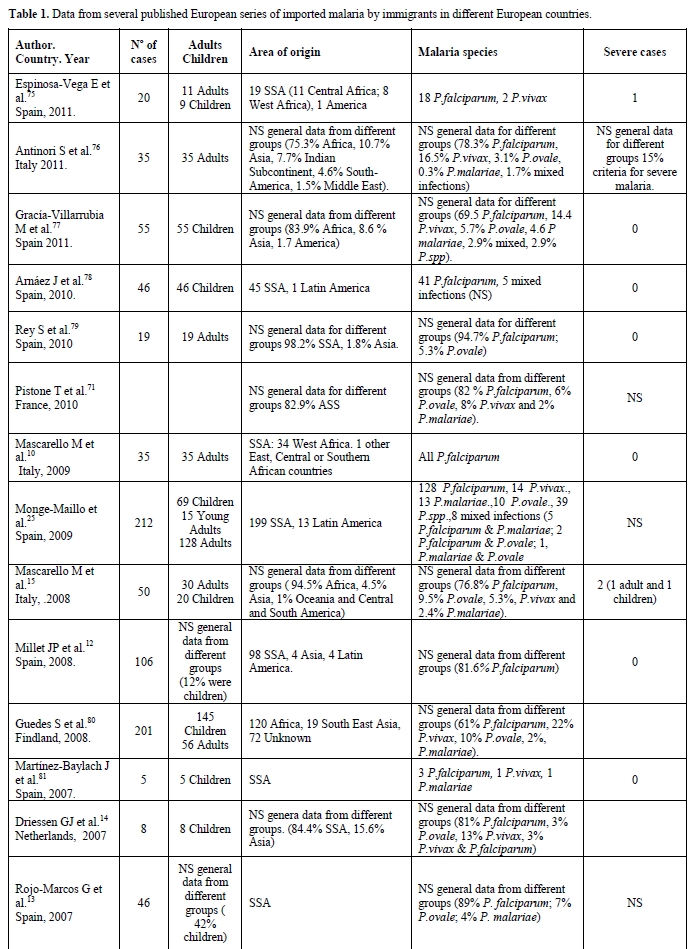
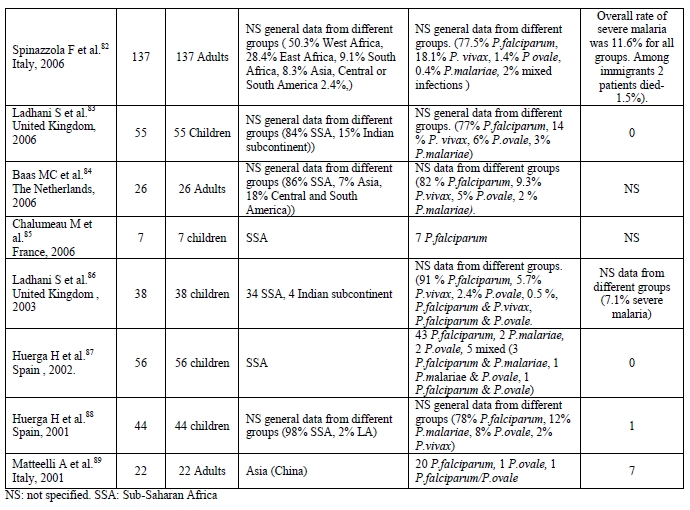
Table 1. Data from several published European series of imported malaria by immigrants in different European countries.
Children account for around 15-20% of all imported malaria cases in Europe and are increasing, as more children travel. Similarly as in adults, P. falciparum is the most frequently identified species and SSA is the most common origin.[8-16] When comparing clinical malaria among adults and children, the latter are less likely to complain of chills, arthralgia/myalgia or headaches. Instead they are more likely to present with non-specific symptoms or with gastrointestinal symptoms. They also have hepatomegaly and jaundice more often than adults.[16] In general, children seem to have a higher risk of severe malaria than adults worldwide. For imported cases, the risk factors for developing severe malaria include young age (< 5 years), delayed diagnosis (probably due to cultural and language barriers that make it difficult to access health care systems) and non-immunity to malaria.
There is a specific group of immigrants who, once settled in the host country, travel to their countries of origin to visit friends and relatives (VFRs). They have been described as a special risk group for certain travel-related illnesses, especially infectious diseases, when compared to other types of travellers. This is because they usually travel to high-risk destinations, for longer periods of time and usually do not seek pre-travel advice.[17-19] This is especially significant in the case of malaria, where returned VFRs make up the largest proportion of malaria cases reported in many developed countries[15] mainly due to P. falciparum (75.8%) and almost exclusively in patients from SSA.[12,20,21] In a Spanish report, they accounted for 37% of malaria cases notified during the study period.[12] Among UK surveillance data of imported malaria from 1978 to 2006, 64.5% of the cases were VFRs.[21] The GeoSentinell report published in 2006 found that immigrant VFRs who travelled to SSA had more than eight times the odds of receiving a diagnosis of malaria than a tourist who visited this region. Immigrant VFRs also had more than twice the odds of receiving a diagnosis of malaria after travel to Asia and more than three times the odds after travel to Latin America when compared with tourist travellers.[20]
The higher risk VFRs have of acquiring malaria is due to diverse factors. First of all they travel to high malaria endemic areas. Among the different reports published about VFRs, the most common origin was SSA.[12,20,21] Moreover, they usually travel during the Northern Hemisphere’s summer months, which coincide with the rainy season in West Africa and with monsoon season in the Indian subcontinent. VFRs are also more likely to be travelling to rural areas and destinations not developed for tourism, often with a poor health infrastructure. They also usually stay at their destination for long periods of time.[22]
VFRs commonly believe they are immune to malaria,[17] but such immunity seems to disappear some years after arriving in Europe. This makes them as vulnerable as other travellers to developing symptomatic and even severe malaria when travelling “home”. Moreover, VFRs are more likely to travel with small children or while pregnant when compared with other types of travellers. In both these cases, VFRs are groups with a higher risk of contracting severe malaria. The low perception of risk makes them rarely seek pre-travel advice. When advice is sought, it is more likely to be from a general practitioner who may not be up to date on pre-travel recommendations. This means that anti-malarial prophylaxis is used less frequently or taken incorrectly.[22] Cost may also be an impediment to taking antimalarials. This may lead people to purchase the drugs at their destination where the quality of the drug may be substandard. In other cases no pre-travel advice is sought because travel is undertaken at the last minute in order to attend family events such as marriages, funerals or to visit sick relatives. Sometimes language and cultural barriers may prevent VFRs from seeking medical advice.[23]
Semi-Immunity to Malaria Among Immigrants.
Malaria in immigrants is characterised by milder clinical presentation, lower parasitic levels, shorter time for parasite clearance after treatment and shorter fever duration than malaria in travellers.[15,24] Even asymptomatic malaria cases among recently arrived immigrants coming from African malaria endemic countries have been described. In a Spanish series on imported infectious diseases by immigrants, among SSAs 7.1% of malaria cases due to P. falciparum were asymptomatic at the moment of diagnoses.[25] A study conducted in Italy to determine the prevalence of malaria among asymptomatic SSA immigrants assessed by nucleic acid sequence based amplification found a 31.8% prevalence of malaria.[26]
Much more is known about the prevalence of malaria and about the number of asymptomatic cases among refugees. This is probably due to established protocols on screening for infectious diseases that are required of refugees to enter certain host countries. The reported prevalence of asymptomatic malaria in SSA refugees screened post arrival ranges from 2.4% to 31.8%.[27-29] Although studies have evidence showing the role of asymptomatic Plasmodium spp infection in populations resident in Latin America, less is known about imported asymptomatic cases among immigrants or refugees from the region.[30, 31]
Imported malaria cases among immigrant children coming from endemic malaria countries can also be linked to milder symptoms than those in child travellers born in non- endemic areas.[15] Even evidence of asymptomatic cases among immigrant children has been published. Most reported data for newly arrived SSA refugee children who present with malaria shows a prevalence of 6% to 32% with high rates of asymptomatic cases.[32, 33, 34]
Another characteristic of imported malaria among immigrants are the cases of late clinical presentation of P. falciparum infections. Most P. falciparum infections occurred in the first three months after arrival, with the delayed onset of clinical malaria characteristics of other Plasmodium species. Cases of prolonged P. falciparum malaria have been described two,[35] four,[36] or even eight years[37] after living outside of an endemic area. In all of these cases other risk factors such as travel to other malaria endemic areas, blood transfusions, visits to an airport or contact with a person suffering from malaria had been excluded. A case control study performed in France tried to identify the incidence and the factors associated with prolonged P. falciparum infection out of endemic area. They analysed 2680 patients who had travelled or lived in malaria endemic regions and that were diagnosed with a P. falciparum infection. Case patients had an infection diagnosed > 59 days after arrival in France, and control patients had infections detected ≤ 30 days after their arrival. Late infections were detected in 2.3% of the patients with a median diagnoses delay of 5 months (interquartile range 3-9 months). Three independent factors were positively associated with prolonged P. falciparum infections, these were: being a first arrival immigrant, being a pregnant woman, and taking mefloquine prophylaxis.[38] Undoubtedly many more studies are needed to determine which factors can condition a late presentation of clinical malaria. Other cases of late P. falciparum malaria infections have been published, but these studies found other risk factors, such as the recent arrival of a member of the family from a malaria endemic area who could have transported an infected Anopheles in their luggage.[39, 40]
Asymptomatic malaria cases or clinical symptoms long after arrival may be explained by a semi-immunity status in the migrant. This semi-immunity seems to be related to prolonged and intense exposure and is usually acquired in areas with persistent malaria transmission as well as in areas of seasonal transmission.[41,42] Immunity to malaria infection is relatively slow to develop and incomplete, although immunity to death is acquired more quickly and may be important after a single episode. This semi-immunity does not avoid the infection and the establishment of peripheral blood parasitemia, but seems to protect against several malaria infections. So, in general, people from malaria endemic countries may have sub microscopic levels of parasitemia, but not feel ill or may present atypical features of malaria.[43]
The mean time semi-immunity persists after leaving the transmission area has not yet been clearly established. Malaria immunity has been reported to rapidly wane after the end of expose to the parasite, which suggests that continued exposure to malaria antigens seems to be required. Nor is there agreement on what components of immunity to malaria are lost without exposure, generally this loss in identified only by the fact that such people do experience symptomatic infections.[44] However, a study conducted in France gives evidence that malaria among African adult immigrants is less severe (lower parasite density and lower frequency of severe and complicated diseases) and more readily cured than in Europeans, even more than 14 years after living out of an endemic area.[45] An in vitro study also showed that humoral and cellular responses to defined P. falciparum antigens persisted in migrants from West Africa who spent up to 13 years in France without travelling to their country of origin.[46] This is consistent with the results of two observational studies in the highlands of Madagascar (which is a non-malaria endemic area) during the 1987 malaria outbreak. Patients who were more than 40 years old, who had spent their childhood in malaria hyperendemic areas before control programmes for malaria existed, were found to be more protected against clinical P. falciparum infection than younger patients. They also had a stronger humoral and cellular immune response to P. falciparum antigens.[47] The persistence of the semi-immunity is especially relevant in VFRs. If such immunity is lost, immigrants who travel to their country of origin would have the same risk as travellers born in non-endemic countries of suffering severe malaria.
Migration and Risk of Induced and Reintroduced Malaria.
Migration could increase the risk of transmission and reintroduction of malaria in certain areas where it has previously been eradicated. Adequate climate conditions and the presence of malaria vectors in certain countries could help create a local vectorial transmission and a reintroduction of malaria.[48,49] Moreover, asymptomatic patients with sub-microscopic parasitemia are also capable of infecting mosquitoes, and are thus considerate as reservoirs of malaria.[50]
Malaria cases imported by immigrants can play an important role in the non-vectorial transmission out of endemic areas through other mechanisms of transmission such as blood transfusions, organ transplantation, congenital transmission or occupational exposure.[51,52]
Data on the frequency of transfusion transmitted malaria in non-endemic countries shows a rate of about 0.2% and the most frequently associated species are P. falciparum, P. malariae and P. vivax.[53,54] In most of the published cases the donor was foreign born and came from an endemic area. Among five cases reported in the UK, four of the donors came from a SSA country; the fifth case was a UK traveller to Africa. In all cases P. falciparum was the isolated species.[53] Another interesting case of transfusion transmitted P. falciparum malaria occurred in France: the donor also came from SSA with the peculiarity that he had been living in France for four years with no history of fever or illness.[51]
Malaria is an unusual complication of solid-organ transplantation in non-endemic countries. Most cases have been described after renal transplantation[52,55,56] with a few cases reported after heart[57] or liver transplantations.[52,58,59] Any species of Plasmodium may be involved.[60] Although there are cases where the donor was a traveller to an endemic area,[58] in most of the published cases donors are immigrants coming from a country with a high prevalence of malaria. In one case of P. falciparum transmission through a heart transplant in France, the donor came from SSA and had been asymptomatic during the 15 months she lived in the host country.[57] In a published case of a multi-organ donation in Germany P. vivax was transmitted to receptors: the donor was an immigrant coming from SSA who had lived in Germany for the last 18 months and had been asymptomatic during all of that time.[61]
Congenital malaria out of endemic areas is rare and predominantly seen in infants of recently arrived mothers. Most infants develop symptoms at the age of approximately one month. Because the initial clinical presentation of congenital malaria can be very similar to that of neonatal sepsis, physicians must be aware and suspect Plasmodium infection among those born to immigrant mothers.[62]
In Europe, where malaria has been eradicated since 1975, Anopheles spp. mosquito vectors remain prevalent in parts of Southern and Central Europe. In fact, transmission of malaria to local residents has been reported over the last 10 years, and the hypothesis of malaria reintroduction has also recently been confirmed. In Greece, in 2011, at least 33 autochthonous malaria cases due to P. vivax were recorded.[63,64] In Spain another case of autochthonous P. vivax infection was detected.[65] We must also consider global climate change, which could help spread malaria to northern latitudes.[66] However, the real possibility of a European country becoming an endemic malaria area seems quite small. Surveillance and public health measures would probably avoid such situation.
Screening for Malaria Among Immigrants.
The evidence of asymptomatic malarial infections or the late clinical presentation of these infections, which can be reservoir of malaria added to other possible mechanisms of transmission out of endemic area raises the need of systematic screening processes. Screening has been more established for refugees, where approaches to mitigate the risk of malaria in these populations include mass antimalarial treatment pre-departure or on arrival and screen and treat strategies for targeted groups. The US Center for Disease Control (CDC) issued new guidelines in 2010 for refugees arriving from SSA which recommend presumptive treatment either pre-departure or on arrival.[67] Recent guidelines from the Australian Society for Infectious Disease include malaria testing and treatment at both pre-departure and at the post-arrival health assessment.[68] These measures should probably be extrapolated to recently arrived immigrants coming from malaria endemic countries. Screening for malaria among this population could constitute an effective public health measure, especially when a high proportion of immigrants can be asymptomatic.[69]
The effectiveness of strategies based on screening at arrival is heavily dependent on the proportion of refugees and immigrants who receive systematic assessment by a clinic with specific expertise in imported infectious diseases and, the diagnostic method used.[27] In fact, diagnosing asymptomatic patients requires techniques that enable the measurement of infections with a very low density of parasitemia. The sensitivity of a malaria test is the probability that at least one parasitized red blood cell is detected. This is directly related to the volume of blood that the test can screen and the density of parasites in the blood sample.[70] With microscopy techniques the volume of blood examined is 0,06-0,2 μL, while PCR uses several micro-litres, giving theoretical limits of detection of 5-16 parasites/μL by microscopy and 0.002-1 parasites/μL by PCR.[71] A systematic review and meta-analysis of surveys of endemic populations in which P. falciparum prevalence is measured by both microscopy and PCR techniques indicated that the prevalence of infection measured by microscopy was, on average, 50.8% of that measured by PCR.[72] A study conducted in the United States comparing a rapid antigen capture enzyme assay with PCR for the screening in SSA refugees showed that the sensitivity and specificity of the rapid antigen assay were 22% and 66% respectively.[73] In Canada two different antigen detection tests were also evaluated: ICT Malaria (sensitivity 37.5% and specificity 100%) and OptiMAL (sensitivity 29.1% and specificity 95.6%).[27] In both studies microscopy was also analysed without better outcomes. Therefore, PCR is probably by far the most powerful tool for such surveillance.
Moreover, taking into account the long periods of time after arrival that parasitemias seem to persist, we should consider whether screening should be offered not only to recently arrived immigrants but also immigrants settled in the host country for some time.[69] In fact, a mathematical modelling has estimated the maximum duration of P. falciparum infection after interruption of transmission in about 4 years.[74]
Summary.
Malaria imported by immigrants makes up a significant proportion of the cases diagnosed out of endemic areas. They are mostly due to P. falciparum and found among those coming from West Africa. Malaria in immigrants is characterised by a mild clinical presentation, low parasitic levels; short parasite clearance time after treatment and, short fever duration. In fact, a high proportion of immigrants may be asymptomatic or present clinical malaria long after arrival in the host country. Such characteristics seem to be explained by the semi-immunity to malaria acquired after living in endemic areas.
In parallel with migration, immigrants settled in the host country increasingly travel to their country of origin to visit friends and relatives (VFRs). They have been described as a special risk group for certain travel related infectious diseases when compared to other types of travellers. Malaria is one of the most frequent diagnoses among them, mainly due to P. falciparum and almost exclusively in patients from Sub-Saharan Africa (SSA).
Congenital transmission or transmission due to blood transfusion or organ transplantation out of endemic area has been described. Furthermore, adequate climate conditions and the presence of malaria vectors in certain European countries could contribute to local vectorial transmission and a reintroduction of malaria in areas where it had been eradicated.
Consequently, out of endemic areas, strategies to control imported malaria by immigrants should be multifaceted. Firstly, screening for malaria among recently arrived immigrants from malaria endemic countries should be performed. The aim of this is to reduce the risk of clinical malaria in the individual as well as to prevent autochthonous transmission of malaria in areas where it has been eradicated. The evidence of persistent asymptomatic parasitemia suggests that screening time after arrival could also be considered. Secondly, the relevance of imported malaria cases among VFRs highlights the need of preventive health promotion strategies culturally adapted and focused on pre-travel advice.
Malaria continues to present one of the major challenges to global public health with an estimated 225 million clinical cases and more than 700000 deaths in 2009, mostly in children under 5 years old from sub-Saharan Africa.[1] In Europe, malaria is eradicated in almost all countries of the World Health Organization European Region except for Azerbaijan, Georgia, Kyrgyzstan, Tajikistan and Turkey.[1]
In 2010 there were 47.3 million foreign-born people in the European Union (EU), corresponding to 9.4% of the total population. The majority of them, 31.4 million, were born in non- EU countries, while 16 million were born in another EU Member State. Data about those coming from endemic malarial countries is scarce. Estimates indicate that more than 5 million African immigrants could be living in Europe. Among them, about two thirds are from North Africa (Algeria, Morocco and Tunisia) and the rest are from Sub-Saharan Africa (SSA), mostly from West Africa (Ghana, Nigeria and Senegal). About 4 million come from South East Asia and nearly 2.2 million from Latin America.[2]
Imported malaria is defined as an infection acquired in a malaria endemic area but diagnosed in a non-endemic country. The malaria Programme of the WHO European Region annually collects data of malaria cases from 51 countries in the region. It reported an eight-fold increase in the number of imported malaria cases between 1972 and 1988 (from 1500 to 12000 cases), followed by a more gradual rise in 2000 (15500 cases). Most of the cases were imported to Western Europe, with France, the United Kingdom (UK), Germany and Italy accounting for more than 70% of all cases.[3] Although, in the last decade data from the World Health Organization (Figure 1) has shown a progressive decrease in the global incidence of imported malaria in most European countries,[4] despite a slight rise in cases reported from 2008.[5] Several published studies have corroborated such decreases in certain European countries such as the Netherlands[6] and the UK[7] which could be explained by a reduction in the global malaria transmission in SSA countries.

Figure 1. Imported malaria cases in Europe. Data from WHO Regional Office for Europe.
Today, the profile of immigrants is changing with higher rates of immigrants from southern (malaria-endemic) areas moving to northern (malaria free) industrialised areas of the world.[3] The proportion of imported malaria cases due to immigrants in Europe has increased during the last few years from 14% in research published more than 10 years ago, to 86% in more recent studies.[8] On pooling the reports, nearly 43% of malaria cases registered in key European centres occurred in non-nationals. The rates of malaria are much higher in settled immigrants who travel to visit friends and relatives (VFRs) in their country of origin. They can account for up to 70% of the cases in several reports.[9,10]
Methods.
A literature review was conducted using MEDLINE, EMBASE, Web of Science and the Cochrane Library database. The review included case studies, reviews and expert opinion. Search was based on published articles on imported infectious diseases in general and specifically malaria. Only those including data relating to immigrants (adult and paediatric) and based in European countries were included. A table with the most relevant characteristics of imported malaria among immigrants in Europe was created. Data referring to visiting friends and relatives (VFRs) and to refugees was not included. The selection criterion for including articles was based on the subjective opinion of the authors who considered article relevancy to the current research.
Imported malaria in immigrants and in immigrant-travellers (VFRs).
Most of the imported malaria cases among immigrants in Europe are due to P. falciparum and SSA, in particular West Africa, is the most common origin.[11-15] The incidence of infection by P. ovale and mixed infections is very similar to the incidence found in West Africa, which is probably due to the very high number of immigrants from these areas. Data from several of the selected published reports about imported malaria among immigrants in different European countries is summarised in Table 1.


Table 1. Data from several published European series of imported malaria by immigrants in different European countries.
Children account for around 15-20% of all imported malaria cases in Europe and are increasing, as more children travel. Similarly as in adults, P. falciparum is the most frequently identified species and SSA is the most common origin.[8-16] When comparing clinical malaria among adults and children, the latter are less likely to complain of chills, arthralgia/myalgia or headaches. Instead they are more likely to present with non-specific symptoms or with gastrointestinal symptoms. They also have hepatomegaly and jaundice more often than adults.[16] In general, children seem to have a higher risk of severe malaria than adults worldwide. For imported cases, the risk factors for developing severe malaria include young age (< 5 years), delayed diagnosis (probably due to cultural and language barriers that make it difficult to access health care systems) and non-immunity to malaria.
There is a specific group of immigrants who, once settled in the host country, travel to their countries of origin to visit friends and relatives (VFRs). They have been described as a special risk group for certain travel-related illnesses, especially infectious diseases, when compared to other types of travellers. This is because they usually travel to high-risk destinations, for longer periods of time and usually do not seek pre-travel advice.[17-19] This is especially significant in the case of malaria, where returned VFRs make up the largest proportion of malaria cases reported in many developed countries[15] mainly due to P. falciparum (75.8%) and almost exclusively in patients from SSA.[12,20,21] In a Spanish report, they accounted for 37% of malaria cases notified during the study period.[12] Among UK surveillance data of imported malaria from 1978 to 2006, 64.5% of the cases were VFRs.[21] The GeoSentinell report published in 2006 found that immigrant VFRs who travelled to SSA had more than eight times the odds of receiving a diagnosis of malaria than a tourist who visited this region. Immigrant VFRs also had more than twice the odds of receiving a diagnosis of malaria after travel to Asia and more than three times the odds after travel to Latin America when compared with tourist travellers.[20]
The higher risk VFRs have of acquiring malaria is due to diverse factors. First of all they travel to high malaria endemic areas. Among the different reports published about VFRs, the most common origin was SSA.[12,20,21] Moreover, they usually travel during the Northern Hemisphere’s summer months, which coincide with the rainy season in West Africa and with monsoon season in the Indian subcontinent. VFRs are also more likely to be travelling to rural areas and destinations not developed for tourism, often with a poor health infrastructure. They also usually stay at their destination for long periods of time.[22]
VFRs commonly believe they are immune to malaria,[17] but such immunity seems to disappear some years after arriving in Europe. This makes them as vulnerable as other travellers to developing symptomatic and even severe malaria when travelling “home”. Moreover, VFRs are more likely to travel with small children or while pregnant when compared with other types of travellers. In both these cases, VFRs are groups with a higher risk of contracting severe malaria. The low perception of risk makes them rarely seek pre-travel advice. When advice is sought, it is more likely to be from a general practitioner who may not be up to date on pre-travel recommendations. This means that anti-malarial prophylaxis is used less frequently or taken incorrectly.[22] Cost may also be an impediment to taking antimalarials. This may lead people to purchase the drugs at their destination where the quality of the drug may be substandard. In other cases no pre-travel advice is sought because travel is undertaken at the last minute in order to attend family events such as marriages, funerals or to visit sick relatives. Sometimes language and cultural barriers may prevent VFRs from seeking medical advice.[23]
Semi-Immunity to Malaria Among Immigrants.
Malaria in immigrants is characterised by milder clinical presentation, lower parasitic levels, shorter time for parasite clearance after treatment and shorter fever duration than malaria in travellers.[15,24] Even asymptomatic malaria cases among recently arrived immigrants coming from African malaria endemic countries have been described. In a Spanish series on imported infectious diseases by immigrants, among SSAs 7.1% of malaria cases due to P. falciparum were asymptomatic at the moment of diagnoses.[25] A study conducted in Italy to determine the prevalence of malaria among asymptomatic SSA immigrants assessed by nucleic acid sequence based amplification found a 31.8% prevalence of malaria.[26]
Much more is known about the prevalence of malaria and about the number of asymptomatic cases among refugees. This is probably due to established protocols on screening for infectious diseases that are required of refugees to enter certain host countries. The reported prevalence of asymptomatic malaria in SSA refugees screened post arrival ranges from 2.4% to 31.8%.[27-29] Although studies have evidence showing the role of asymptomatic Plasmodium spp infection in populations resident in Latin America, less is known about imported asymptomatic cases among immigrants or refugees from the region.[30, 31]
Imported malaria cases among immigrant children coming from endemic malaria countries can also be linked to milder symptoms than those in child travellers born in non- endemic areas.[15] Even evidence of asymptomatic cases among immigrant children has been published. Most reported data for newly arrived SSA refugee children who present with malaria shows a prevalence of 6% to 32% with high rates of asymptomatic cases.[32, 33, 34]
Another characteristic of imported malaria among immigrants are the cases of late clinical presentation of P. falciparum infections. Most P. falciparum infections occurred in the first three months after arrival, with the delayed onset of clinical malaria characteristics of other Plasmodium species. Cases of prolonged P. falciparum malaria have been described two,[35] four,[36] or even eight years[37] after living outside of an endemic area. In all of these cases other risk factors such as travel to other malaria endemic areas, blood transfusions, visits to an airport or contact with a person suffering from malaria had been excluded. A case control study performed in France tried to identify the incidence and the factors associated with prolonged P. falciparum infection out of endemic area. They analysed 2680 patients who had travelled or lived in malaria endemic regions and that were diagnosed with a P. falciparum infection. Case patients had an infection diagnosed > 59 days after arrival in France, and control patients had infections detected ≤ 30 days after their arrival. Late infections were detected in 2.3% of the patients with a median diagnoses delay of 5 months (interquartile range 3-9 months). Three independent factors were positively associated with prolonged P. falciparum infections, these were: being a first arrival immigrant, being a pregnant woman, and taking mefloquine prophylaxis.[38] Undoubtedly many more studies are needed to determine which factors can condition a late presentation of clinical malaria. Other cases of late P. falciparum malaria infections have been published, but these studies found other risk factors, such as the recent arrival of a member of the family from a malaria endemic area who could have transported an infected Anopheles in their luggage.[39, 40]
Asymptomatic malaria cases or clinical symptoms long after arrival may be explained by a semi-immunity status in the migrant. This semi-immunity seems to be related to prolonged and intense exposure and is usually acquired in areas with persistent malaria transmission as well as in areas of seasonal transmission.[41,42] Immunity to malaria infection is relatively slow to develop and incomplete, although immunity to death is acquired more quickly and may be important after a single episode. This semi-immunity does not avoid the infection and the establishment of peripheral blood parasitemia, but seems to protect against several malaria infections. So, in general, people from malaria endemic countries may have sub microscopic levels of parasitemia, but not feel ill or may present atypical features of malaria.[43]
The mean time semi-immunity persists after leaving the transmission area has not yet been clearly established. Malaria immunity has been reported to rapidly wane after the end of expose to the parasite, which suggests that continued exposure to malaria antigens seems to be required. Nor is there agreement on what components of immunity to malaria are lost without exposure, generally this loss in identified only by the fact that such people do experience symptomatic infections.[44] However, a study conducted in France gives evidence that malaria among African adult immigrants is less severe (lower parasite density and lower frequency of severe and complicated diseases) and more readily cured than in Europeans, even more than 14 years after living out of an endemic area.[45] An in vitro study also showed that humoral and cellular responses to defined P. falciparum antigens persisted in migrants from West Africa who spent up to 13 years in France without travelling to their country of origin.[46] This is consistent with the results of two observational studies in the highlands of Madagascar (which is a non-malaria endemic area) during the 1987 malaria outbreak. Patients who were more than 40 years old, who had spent their childhood in malaria hyperendemic areas before control programmes for malaria existed, were found to be more protected against clinical P. falciparum infection than younger patients. They also had a stronger humoral and cellular immune response to P. falciparum antigens.[47] The persistence of the semi-immunity is especially relevant in VFRs. If such immunity is lost, immigrants who travel to their country of origin would have the same risk as travellers born in non-endemic countries of suffering severe malaria.
Migration and Risk of Induced and Reintroduced Malaria.
Migration could increase the risk of transmission and reintroduction of malaria in certain areas where it has previously been eradicated. Adequate climate conditions and the presence of malaria vectors in certain countries could help create a local vectorial transmission and a reintroduction of malaria.[48,49] Moreover, asymptomatic patients with sub-microscopic parasitemia are also capable of infecting mosquitoes, and are thus considerate as reservoirs of malaria.[50]
Malaria cases imported by immigrants can play an important role in the non-vectorial transmission out of endemic areas through other mechanisms of transmission such as blood transfusions, organ transplantation, congenital transmission or occupational exposure.[51,52]
Data on the frequency of transfusion transmitted malaria in non-endemic countries shows a rate of about 0.2% and the most frequently associated species are P. falciparum, P. malariae and P. vivax.[53,54] In most of the published cases the donor was foreign born and came from an endemic area. Among five cases reported in the UK, four of the donors came from a SSA country; the fifth case was a UK traveller to Africa. In all cases P. falciparum was the isolated species.[53] Another interesting case of transfusion transmitted P. falciparum malaria occurred in France: the donor also came from SSA with the peculiarity that he had been living in France for four years with no history of fever or illness.[51]
Malaria is an unusual complication of solid-organ transplantation in non-endemic countries. Most cases have been described after renal transplantation[52,55,56] with a few cases reported after heart[57] or liver transplantations.[52,58,59] Any species of Plasmodium may be involved.[60] Although there are cases where the donor was a traveller to an endemic area,[58] in most of the published cases donors are immigrants coming from a country with a high prevalence of malaria. In one case of P. falciparum transmission through a heart transplant in France, the donor came from SSA and had been asymptomatic during the 15 months she lived in the host country.[57] In a published case of a multi-organ donation in Germany P. vivax was transmitted to receptors: the donor was an immigrant coming from SSA who had lived in Germany for the last 18 months and had been asymptomatic during all of that time.[61]
Congenital malaria out of endemic areas is rare and predominantly seen in infants of recently arrived mothers. Most infants develop symptoms at the age of approximately one month. Because the initial clinical presentation of congenital malaria can be very similar to that of neonatal sepsis, physicians must be aware and suspect Plasmodium infection among those born to immigrant mothers.[62]
In Europe, where malaria has been eradicated since 1975, Anopheles spp. mosquito vectors remain prevalent in parts of Southern and Central Europe. In fact, transmission of malaria to local residents has been reported over the last 10 years, and the hypothesis of malaria reintroduction has also recently been confirmed. In Greece, in 2011, at least 33 autochthonous malaria cases due to P. vivax were recorded.[63,64] In Spain another case of autochthonous P. vivax infection was detected.[65] We must also consider global climate change, which could help spread malaria to northern latitudes.[66] However, the real possibility of a European country becoming an endemic malaria area seems quite small. Surveillance and public health measures would probably avoid such situation.
Screening for Malaria Among Immigrants.
The evidence of asymptomatic malarial infections or the late clinical presentation of these infections, which can be reservoir of malaria added to other possible mechanisms of transmission out of endemic area raises the need of systematic screening processes. Screening has been more established for refugees, where approaches to mitigate the risk of malaria in these populations include mass antimalarial treatment pre-departure or on arrival and screen and treat strategies for targeted groups. The US Center for Disease Control (CDC) issued new guidelines in 2010 for refugees arriving from SSA which recommend presumptive treatment either pre-departure or on arrival.[67] Recent guidelines from the Australian Society for Infectious Disease include malaria testing and treatment at both pre-departure and at the post-arrival health assessment.[68] These measures should probably be extrapolated to recently arrived immigrants coming from malaria endemic countries. Screening for malaria among this population could constitute an effective public health measure, especially when a high proportion of immigrants can be asymptomatic.[69]
The effectiveness of strategies based on screening at arrival is heavily dependent on the proportion of refugees and immigrants who receive systematic assessment by a clinic with specific expertise in imported infectious diseases and, the diagnostic method used.[27] In fact, diagnosing asymptomatic patients requires techniques that enable the measurement of infections with a very low density of parasitemia. The sensitivity of a malaria test is the probability that at least one parasitized red blood cell is detected. This is directly related to the volume of blood that the test can screen and the density of parasites in the blood sample.[70] With microscopy techniques the volume of blood examined is 0,06-0,2 μL, while PCR uses several micro-litres, giving theoretical limits of detection of 5-16 parasites/μL by microscopy and 0.002-1 parasites/μL by PCR.[71] A systematic review and meta-analysis of surveys of endemic populations in which P. falciparum prevalence is measured by both microscopy and PCR techniques indicated that the prevalence of infection measured by microscopy was, on average, 50.8% of that measured by PCR.[72] A study conducted in the United States comparing a rapid antigen capture enzyme assay with PCR for the screening in SSA refugees showed that the sensitivity and specificity of the rapid antigen assay were 22% and 66% respectively.[73] In Canada two different antigen detection tests were also evaluated: ICT Malaria (sensitivity 37.5% and specificity 100%) and OptiMAL (sensitivity 29.1% and specificity 95.6%).[27] In both studies microscopy was also analysed without better outcomes. Therefore, PCR is probably by far the most powerful tool for such surveillance.
Moreover, taking into account the long periods of time after arrival that parasitemias seem to persist, we should consider whether screening should be offered not only to recently arrived immigrants but also immigrants settled in the host country for some time.[69] In fact, a mathematical modelling has estimated the maximum duration of P. falciparum infection after interruption of transmission in about 4 years.[74]
Summary.
Malaria imported by immigrants makes up a significant proportion of the cases diagnosed out of endemic areas. They are mostly due to P. falciparum and found among those coming from West Africa. Malaria in immigrants is characterised by a mild clinical presentation, low parasitic levels; short parasite clearance time after treatment and, short fever duration. In fact, a high proportion of immigrants may be asymptomatic or present clinical malaria long after arrival in the host country. Such characteristics seem to be explained by the semi-immunity to malaria acquired after living in endemic areas.
In parallel with migration, immigrants settled in the host country increasingly travel to their country of origin to visit friends and relatives (VFRs). They have been described as a special risk group for certain travel related infectious diseases when compared to other types of travellers. Malaria is one of the most frequent diagnoses among them, mainly due to P. falciparum and almost exclusively in patients from Sub-Saharan Africa (SSA).
Congenital transmission or transmission due to blood transfusion or organ transplantation out of endemic area has been described. Furthermore, adequate climate conditions and the presence of malaria vectors in certain European countries could contribute to local vectorial transmission and a reintroduction of malaria in areas where it had been eradicated.
Consequently, out of endemic areas, strategies to control imported malaria by immigrants should be multifaceted. Firstly, screening for malaria among recently arrived immigrants from malaria endemic countries should be performed. The aim of this is to reduce the risk of clinical malaria in the individual as well as to prevent autochthonous transmission of malaria in areas where it has been eradicated. The evidence of persistent asymptomatic parasitemia suggests that screening time after arrival could also be considered. Secondly, the relevance of imported malaria cases among VFRs highlights the need of preventive health promotion strategies culturally adapted and focused on pre-travel advice.
References
- World health organization. World malaria report 2010. Available at: http//www.who.int/en/.
- European Commission. Eurostat. Katya Vasileva. Population and social conditions. 34/2011. Available at: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-SF-11-034/EN/KS-SF-11-034-EN.PDF
- Sabatinelli G, Ejov M, Joergensen P.
Malaria in the WHO European region (1971-1999). Euro Surveill. 2001;6:
61-65. PMid:11679685

- World Health Organization Regional Office for Europe. Centralized information system for infectious diseases (CISID). Available at: http://data.euro.who.int/cisid.
- Odolini S, Parola P, Gkrania-Klotsas E,
Caumes E, Schlagenhauf P, López-Vélez R, Burchard GD, Santos-O'Connor
F, Weld L, von Sonnenburg F, Field V, de Vries P, Jensenius M, Loutan
L, Castelli F. Travel-related imported infections in europe,
EuroTravNet 2009. Clin Microbiol Infect. 2011.

- van Rijckevorsel GG, Sonder GJ, Geskus RB,
Wetsteyn JC, Ligthelm RJ, Visser LG, Keuter M, van Genderen PJ, van den
Hoek A. Declining incidence of imported malaria in the netherlands,
2000-2007. Malar J. 2010; 9: 300. http://dx.doi.org/10.1186/1475-2875-9-300
PMid:21029424 PMCid:2988037

- Behrens RH, Carroll B, Smith V, Alexander
N. Declining incidence of malaria imported into the UK from west
africa. Malar J. 2008; 7: 235. http://dx.doi.org/10.1186/1475-2875-7-235
PMid:19000299 PMCid:2613412

- Jelinek T, Schulte C, Behrens R, Grobusch
MP, Coulaud JP, Bisoffi Z, Matteelli A, Clerinx J, Corachán M, Puente
S, Gjørup I, Harms G, Kollaritsch H, Kotlowski A, Björkmann A, Delmont
JP, Knobloch J, Nielsen LN, Cuadros J, Hatz C, Beran J, Schmid ML,
Schulze M, Lopez-Velez R, Fleischer K, Kapaun A, McWhinney P, Kern P,
Atougia J, Fry G, da Cunha S, Boecken G.Imported falciparum malaria in
Europe: Sentinel surveillance data from the European network on
surveillance of imported infectious diseases. Clin Infect Dis. 2002;
34: 572-576. http://dx.doi.org/10.1086/338235
PMid:11803507

- Romi R, Sabatinelli G, Majori G. Malaria
epidemiological situation in italy and evaluation of malaria incidence
in italian travelers. J Travel Med. 2001; 8: 6-11. http://dx.doi.org/10.2310/7060.2001.5140

- Mascarello M, Gobbi F, Angheben A, Concia
E, Marocco S, Anselmi M, Monteiro G, Rossanese A, Bisoffi Z. Imported
malaria in immigrants to italy: A changing pattern observed in north
eastern italy. J Travel Med. 2009;16: 317-321. http://dx.doi.org/10.1111/j.1708-8305.2009.00321.x
PMid:19796101

- Foca A, Barreca GS, Barbieri V, Matera G,
Liberto MC, De Rosa M. Fourteen-year experience with imported malaria.
Infez Med. 2004; 12: 186-192. PMid:15711132

- Millet JP, Garcia de Olalla P,
Carrillo-Santisteve P, Gascón J, Treviño B, Muñoz J, Gómez I Prat J,
Cabezos J, González Cordón A, Caylà JA. Imported malaria in a
cosmopolitan european city: A mirror image of the world epidemiological
situation. Malar J. 2008; 7: 56. http://dx.doi.org/10.1186/1475-2875-7-56
PMid:18397524 PMCid:2362124

- Rojo-Marcos G, Cuadros-Gonzalez J,
Gete-Garcia L, Prieto-Rios B, Arcos-Pereda P. Imported malaria in a
general hospital in Madrid. Enferm Infecc Microbiol Clin. 2007; 25:
168-171. http://dx.doi.org/10.1157/13099367

- Driessen GJ, Pereira RR, Brabin BJ,
Hartwig NG. Imported malaria in children: A national surveillance in
the Netherlands and a review of European studies. Eur J Public Health.
2008; 18: 184-188. http://dx.doi.org/10.1093/eurpub/ckm101
PMid:17984130

- Mascarello M, Allegranzi B, Angheben A,
Anselmi M, Concia E, Laganà S, Manzoli L, Marocco S, Monteiro G,
Bisoffi Z. Imported malaria in adults and children: Epidemiological and
clinical characteristics of 380 consecutive cases observed in verona,
italy. J Travel Med. 2008; 15: 229-236. http://dx.doi.org/10.1111/j.1708-8305.2008.00204.x
PMid:18666922

- Ladhani S, Aibara RJ, Riordan FA,
Shingadia D. Imported malaria in children: A review of clinical
studies. Lancet Infect Dis. 2007; 7: 349-357. http://dx.doi.org/10.1016/S1473-3099(07)70110-X

- Bacaner N, Stauffer B, Boulware DR, Walker
PF, Keystone JS. Travel medicine considerations for North American
immigrants visiting friends and relatives. JAMA. 2004; 291: 2856-2864. http://dx.doi.org/10.1001/jama.291.23.2856
PMid:15199037

- Angell SY, Behrens RH. Risk assessment and
disease prevention in travelers visiting friends and relatives. Infect
Dis Clin North Am. 2005; 19: 49-65. http://dx.doi.org/10.1016/j.idc.2004.11.001
PMid:15701546

- Angell SY, Cetron MS. Health disparities
among travelers visiting friends and relatives abroad. Ann Intern Med.
2005; 142: 67-72. PMid:15630110

- Leder K, Tong S, Weld L, Kain KC,
Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J;
GeoSentinel Surveillance Network. Illness in travelers visiting friends
and relatives: A review of the GeoSentinel surveillance network.
Clin Infect Dis. 2006; 43: 1185-1193. http://dx.doi.org/10.1086/507893
PMid:17029140

- Smith AD, Bradley DJ, Smith V, Blaze M,
Behrens RH, Chiodini PL, Whitty CJ. Imported malaria and high risk
groups: Observational study using UK surveillance data 1987-2006. BMJ.
2008; 337: a120. http://dx.doi.org/10.1136/bmj.a120 PMid:18599471
PMCid:2453297

- Angell SY, Behrens RH. Risk assessment and
disease prevention in travelers visiting friends and relatives. Infect
Dis Clin North Am. 2005; 19: 49-65. http://dx.doi.org/10.1016/j.idc.2004.11.001
PMid:15701546

- Fulford M, Keystone JS. Health risks
associated with visiting friends and relatives in developing countries.
Curr Infect Dis Rep. 2005; 7: 48-53. http://dx.doi.org/10.1007/s11908-005-0023-z
PMid:15610671

- Muentener P, Schlagenhauf P, Steffen R.
Imported malaria (1985-95): Trends and perspectives. Bull World Health
Organ. 1999; 77: 560-566. PMid:10444879 PMCid:2557700

- Monge-Maillo B, Jiménez BC, Pérez-Molina
JA, Norman F, Navarro M, Pérez-Ayala A, Herrero JM, Zamarrón P,
López-Vélez R. Imported infectious diseases in mobile populations,
spain. Emerg Infect Dis. 2009; 15: 1745-1752. PMid:19891861
PMCid:2857245

- Marangi M, Di Tullio R, Mens PF,
Martinelli D, Fazio V, Angarano G, Schallig HD, Giangaspero A, Scotto
G. Prevalence of plasmodium spp. in malaria asymptomatic african
migrants assessed by nucleic acid sequence based amplification. Malar
J. 2009; 8: 12. http://dx.doi.org/10.1186/1475-2875-8-12
PMid:19138412 PMCid:2634767

- Ndao M, Bandyayera E, Kokoskin E, Gyorkos
TW, MacLean JD, Ward BJ. Comparison of blood smear, antigen detection,
and nested-PCR methods for screening refugees from regions where
malaria is endemic after a malaria outbreak in quebec, canada. J Clin
Microbiol. 2004; 42: 2694-2700. http://dx.doi.org/10.1128/JCM.42.6.2694-2700.2004
PMid:15184454 PMCid:427867

- Cherian S, Fagan JM, Thambiran A, Geddes
J, Burgner D. Severe plasmodium falciparum malaria in refugee children
despite reported predeparture antimalarial treatment. Med J Aust. 2006;
185: 611. PMid:17181503

- Maroushek SR, Aguilar EF, Stauffer W,
Abd-Alla MD. Malaria among refugee children at arrival in the united
states. Pediatr Infect Dis J. 2005; 24: 450-452. http://dx.doi.org/10.1097/01.inf.0000160948.22407.0d
PMid:15876946

- Alves FP, Durlacher RR, Menezes MJ,
Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic
plasmodium vivax and plasmodium falciparum infections in native
amazonian populations. Am J Trop Med Hyg. 2002; 66: 641-648.
PMid:12224567

- Cucunuba ZM, Guerra AP, Rahirant SJ,
Rivera JA, Cortes LJ, Nicholls RS. Asymptomatic plasmodium spp.
infection in Tierralta, Colombia. Mem Inst Oswaldo Cruz. 2008; 103:
668-673. http://dx.doi.org/10.1590/S0074-02762008000700007

- Sheikh M, Pal A, Wang S, MacIntyre CR,
Wood NJ, Isaacs D, Gunasekera H, Raman S, Hale K, Howell A. The
epidemiology of health conditions of newly arrived refugee children: A
review of patients attending a specialist health clinic in Sydney. J
Paediatr Child Health. 2009; 45: 509-513. http://dx.doi.org/10.1111/j.1440-1754.2009.01550.x
PMid:19702607

- Martin JA, Mak DB. Changing faces: A
review of infectious disease screening of refugees by the migrant
health unit, western Australia in 2003 and 2004. Med J Aust. 2006; 185:
607-610. PMid:17181502

- Tiong AC, Patel MS, Gardiner J, Ryan R,
Linton KS, Walker KA, Scopel J, Biggs BA. Health issues in newly
arrived African refugees attending general practice clinics in
Melbourne. Med J Aust. 2006; 185: 602-606. PMid:17181501

- Krajden S, Panisko DM, Tobe B, Yang J,
Keystone JS. Prolonged infection with plasmodium falciparum in a
semi-immune patient. Trans R Soc Trop Med Hyg. 1991; 85: 731-732. http://dx.doi.org/10.1016/0035-9203(91)90434-Z

- Greenwood T, Vikerfors T, Sjoberg M,
Skeppner G, Farnert A. Febrile plasmodium falciparum malaria 4 years
after exposure in a man with sickle cell disease. Clin Infect Dis.
2008; 47: e39-41. http://dx.doi.org/10.1086/590250
PMid:18616395

- Szmitko PE, Kohn ML, Simor AE. Plasmodium
falciparum malaria occurring 8 years after leaving an endemic area.
Diagn Microbiol Infect Dis. 2009; 63: 105-107. http://dx.doi.org/10.1016/j.diagmicrobio.2008.08.017
PMid:18945569

- D'Ortenzio E, Godineau N, Fontanet A,
Houze S, Bouchaud O, Matheron S, Le Bras J. Prolonged plasmodium
falciparum infection in immigrants, Paris. Emerg Infect Dis. 2008; 14:
323-326. http://dx.doi.org/10.3201/eid1402.061475
PMid:18258132 PMCid:2600192

- Revel MP, Datry A, Saint Raimond A, Lenoir
G, Danis M, Gentilini M. Plasmodium falciparum malaria after three
years in a non-endemic area. Trans R Soc Trop Med Hyg. 1988; 82: 832. http://dx.doi.org/10.1016/0035-9203(88)90008-9

- Theunissen C, Janssens P, Demulder A,
Nouboussié D, Van-Esbroeck M, Van-Gompel A, Van-Denende J. Falciparum
malaria in patient 9 years after leaving malaria-endemic area. Emerg
Infect Dis. 2009; 15: 115-116. http://dx.doi.org/10.3201/eid1501.080909
PMid:19116068 PMCid:2660708

- Males S, Gaye O, Garcia A. Long-term
asymptomatic carriage of plasmodium falciparum protects from malaria
attacks: A prospective study among Senegalese children. Clin Infect
Dis. 2008; 46: 516-522. http://dx.doi.org/10.1086/526529
PMid:18199040

- Reyburn H, Mbatia R, Drakeley C, Bruce J,
Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley
EM.Association of transmission intensity and age with clinical
manifestations and case fatality of severe plasmodium falciparum
malaria. JAMA. 2005; 293: 1461-1470. http://dx.doi.org/10.1001/jama.293.12.1461
PMid:15784869

- Struik SS, Riley EM. Does malaria suffer
from lack of memory? Immunol Rev. 2004; 201: 268-290. http://dx.doi.org/10.1111/j.0105-2896.2004.00181.x
PMid:15361247

- Langhorne J, Ndungu FM, Sponaas AM, Marsh
K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;
9: 725-732. http://dx.doi.org/10.1038/ni.f.205
PMid:18563083

- Bouchaud O, Cot M, Kony S, Durand R,
Schiemann R, Ralaimazava P, Coulaud JP, Le Bras J, Deloron P. Do
African immigrants living in France have long-term malarial immunity?
Am J Trop Med Hyg. 2005; 72: 21-25. PMid:15728861

- Chougnet C, Deloron P, Savel J.
Persistence of cellular and humoral response to synthetic peptides from
defined plasmodium falciparum antigens. Ann Trop Med Parasitol. 1991;
85: 357-363. PMid:1720947

- Deloron P, Chougnet C. Is immunity to
malaria really short-lived? Parasitol Today. 1992; 8: 375-378. http://dx.doi.org/10.1016/0169-4758(92)90174-Z

- Lindo JF, Bryce JH, Ducasse MB, Howitt C,
Barrett DM, Lorenzo Morales J, Ord R, Burke M, Chiodini PL, Sutherland
CJ. Plasmodium malariae in Haitian refugees, Jamaica. Emerg Infect Dis.
2007; 13: 931-933. PMid:17553241 PMCid:2792841

- Malaria--great exuma, bahamas, may-june 2006. MMWR.Morbidity and mortality weekly report JID - 7802429. 0922.
- Schneider P, Bousema JT, Gouagna LC,
Otieno S, van de Vegte-Bolmer M, Omar SA, Sauerwein RW. Submicroscopic
plasmodium falciparum gametocyte densities frequently result in
mosquito infection. Am J Trop Med Hyg. 2007; 76: 470-474.
PMid:17360869

- Bruneel F, Thellier M, Eloy O, Mazier D,
Boulard G, Danis M, Bédos JP. Transfusion-transmitted malaria.
Intensive Care Med. 2004; 30: 1851-1852. http://dx.doi.org/10.1007/s00134-004-2366-6
PMid:15258726

- Menichetti F, Bindi ML, Tascini C, Urbani
L, Biancofiore G, Doria R, Esposito M, Mozzo R, Catalano G, Filipponi
F. Fever, mental impairment, acute anemia, and renal failure in patient
undergoing orthotopic liver transplantation: Posttransplantation
malaria. Liver Transpl. 2006; 12: 674-676. http://dx.doi.org/10.1002/lt.20730
PMid:16555320

- Kitchen AD, Barbara JA, Hewitt PE.
Documented cases of post-transfusion malaria occurring in england: A
review in relation to current and proposed donor-selection guidelines.
Vox Sang. 2005; 89: 77-80. http://dx.doi.org/10.1111/j.1423-0410.2005.00661.x
PMid:16101687

- Scuracchio P, Vieira SD, Dourado DA, Bueno
LM, Colella R, Ramos-Sanchez EM, Lima GF, Inoue J, Sanchez MC, Di Santi
SM.Transfusion-transmitted malaria: Case report of asymptomatic donor
harboring plasmodium malariae. Rev Inst Med Trop Sao Paulo. 2011; 53:
55-59. http://dx.doi.org/10.1590/S0036-46652011000100010

- Hung CC, Chang SC, Chen YC, Yen TS, Hsieh
WC. Plasmodium vivax infection in a renal transplant recipient: Report
of a case. J Formos Med Assoc. 1994; 93: 888-889. PMid:7749346

- Einollahi B. Plasmodium falciparum
infection transmitted by living kidney donation: A case report from
Iran. Ann Transplant. 2008; 13: 75-78. PMid:19034228

- Babinet J, Gay F, Bustos D, Dubarry M,
Jaulmes D, Nguyen L, Gentilini M. Transmission of plasmodium falciparum
by heart transplant. BMJ. 1991; 303: 1515-1516. http://dx.doi.org/10.1136/bmj.303.6816.1515
PMid:1782492 PMCid:1671824

- Chiche L, Lesage A, Duhamel C, Salame E,
Malet M, Samba D, Segol P, Treilhaud M. Posttransplant malaria: First
case of transmission of plasmodium falciparum from a white multiorgan
donor to four recipients. Transplantation. 2003; 75: 166-168. http://dx.doi.org/10.1097/00007890-200301150-00031
PMid:12544892

- Crafa F, Gugenheim J, Fabiani P, Di Marzo
L, Militerno G, Iovine L, Goubaux B, Mouiel J. Possible transmission of
malaria by liver transplantation. Transplant Proc. 1991; 23: 2664.
PMid:1926523

- Seth AK, Puri P, Chandra A, Dutta V, Naidu
S, Saha A. Mixed plasmodium falciparum and plasmodium vivax malaria in
orthotopic liver transplant recipient. Transplantation. 2009; 88: 288. http://dx.doi.org/10.1097/TP.0b013e3181acc314
PMid:19623027

- Fischer L, Sterneck M, Claus M,
Costard-Jäckle A, Fleischer B, Herbst H, Rogiers X, Broelsch CE.
Transmission of malaria tertiana by multi-organ donation. Clin
Transplant. 1999; 13: 491-495. http://dx.doi.org/10.1034/j.1399-0012.1999.130609.x
PMid:10617239

- Hagmann S, Khanna K, Niazi M, Purswani M,
Robins EB. Congenital malaria, an important differential diagnosis to
consider when evaluating febrile infants of immigrant mothers. Pediatr
Emerg Care. 2007; 23: 326-329. http://dx.doi.org/10.1097/01.pec.0000270164.78238.7d
PMid:17505278

- European centre for disease prevention and control. epidemiological update: Malaria in Greece, November, 2011. Available at: http://www.ecdc.europa.eu/en/Pages/home.aspx
- Danis K, Baka A, Lenglet A, Van Bortel W,
Terzaki I, Tseroni M, Detsis M, Papanikolaou E, Balaska A, Gewehr S,
Dougas G, Sideroglou T, Economopoulou A, Vakalis N, Tsiodras S, Bonovas
S, Kremastinou J. Autochthonous plasmodium vivax malaria in greece,
2011. Euro Surveill. 2011; 16: 19993.

- Santa-Olalla Peralta P, Vazquez-Torres MC,
Latorre-Fandos E, Mairal-Claver P, Cortina-Solano P, Puy-Azón A, Adiego
Sancho B, Leitmeyer K, Lucientes-Curdi J, Sierra-Moros MJ. First
autochthonous malaria case due to plasmodium vivax since eradication,
Spain, October 2010. Euro Surveill. 2010; 15: 19684.
PMid:20961517

- Rogers
DJ, Randolph SE. The global spread of malaria in a future, warmer
world. Science. 2000; 289: 1763-1766. PMid:10976072

- CDC. Recommendations for Pre-departure Presumptive Treatment and Directed Treatment for Malaria for all Refugees from Sub Saharan Africa to United States. Atlanta, GA: US Department of Health and Human Service, CDC; 2010. Available at http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/malaria-guidelines-overseas.html
- Murray RJ, Davis JS, Burgner DP;
Australasian Society for Infectious Diseases Refugee Health Guidelines
Writing Group, Hansen-Knarhoi M, Krause V, Biggs BA, Lemoh C, Benson J,
Cherian S, Buttery J, Paxton G. The Australasian society for infectious
diseases guidelines for the diagnosis, management and prevention of
infections in recently arrived refugees: An abridged outline. Med J
Aust. 2009; 190: 421-425. PMid:19374613

- Monge-Maillo B, Lopez-Velez R. Is
screening for malaria necessary among asymptomatic refugees and
immigrants coming from endemic countries? Expert Rev Anti Infect Ther.
2011; 9: 521-524. http://dx.doi.org/10.1586/eri.11.37
PMid:21609263

- Ohrt C, O'Meara WP, Remich S, McEvoy P,
Ogutu B, Mtalib R, Odera JS. Pilot assessment of the sensitivity of the
malaria thin film. Malar J. 2008; 7: 22. http://dx.doi.org/10.1186/1475-2875-7-22
PMid:18226243 PMCid:2266769

- Babiker HA, Schneider P. Application of
molecular methods for monitoring transmission stages of malaria
parasites. Biomed Mater. 2008; 3: 034007. http://dx.doi.org/10.1088/1748-6041/3/3/034007
PMid:18708712

- Okell LC, Ghani AC, Lyons E, Drakeley CJ.
Submicroscopic infection in plasmodium falciparum-endemic populations:
A systematic review and meta-analysis. J Infect Dis. 2009; 200:
1509-1517. http://dx.doi.org/10.1086/644781
PMid:19848588

- Stauffer WM, Newberry AM, Cartwright CP,
Rosenblatt JE, Hanson KL, Sloan L, Tsukayama DT, Taylor C, Juni BA.
Evaluation of malaria screening in newly arrived refugees to the united
states by microscopy and rapid antigen capture enzyme assay. Pediatr
Infect Dis J. 2006; 25: 948-950. http://dx.doi.org/10.1097/01.inf.0000235747.28644.6f
PMid:17006296

- Sama W, Killeen G, Smith T. Estimating the
duration of plasmodium falciparum infection from trials of indoor
residual spraying. Am J Trop Med Hyg. 2004; 70: 625-634.
PMid:15211003

- Espinosa-Vega E, Martin-Sanchez AM,
Elcuaz-Romano R, Hernandez-Febles M, Molina-Cabrillana J,
Perez-Arellano JL. Malaria in paradise: Characterization of imported
cases in gran canaria island (1993-2006). J Travel Med. 2011; 18:
165-172. http://dx.doi.org/10.1111/j.1708-8305.2011.00503.x
PMid:21539655

- Antinori S, Cigardi B, Galimberti L,
Orlando G, Schifanella L, Milazzo L, Viola A, Giuliani G, Ridolfo A,
Corbellino M. Diagnosis and therapy for hospitalized imported malaria
in adults in Italy. J Travel Med. 2011; 18: 379-385. http://dx.doi.org/10.1111/j.1708-8305.2011.00554.x
PMid:22017713

- Garcia-Villarrubia M, Millet JP, de Olalla
PG, Gascón J, Fumadó V, i Prat JG, Treviño B, Pinazo MJ, Cabezos J,
Muñoz J, Zarzuela F, Caylà JA. Epidemiology of imported malaria among
children and young adults in Barcelona (1990-2008). Malar J. 2011; 10:
347. http://dx.doi.org/10.1186/1475-2875-10-347
PMid:22118531 PMCid:3250960

- Arnáez J, Roa MA, Albert L, Cogollos R,
Rubio JM, Villares R, Alarabe A, Cervera A, López-Vélez R. Imported
malaria in children: A comparative study between recent immigrants and
immigrant travelers (VFRs). J Travel Med. 2010; 17: 221-227. http://dx.doi.org/10.1111/j.1708-8305.2010.00416.x
PMid:20636594

- Rey S, Zuza I, Martinez-Mondejar B, Rubio
JM, Merino FJ. Imported malaria in an area in southern madrid,
2005-2008. Malar J. 2010; 9: 290. http://dx.doi.org/10.1186/1475-2875-9-290
PMid:20961449 PMCid:2972306

- Guedes S, Siikamaki H, Kantele A,
Lyytikainen O. Imported malaria in finland 1995 to 2008: An overview of
surveillance, travel trends, and antimalarial drug sales. J Travel Med.
2010; 17: 400-404. http://dx.doi.org/10.1111/j.1708-8305.2010.00456.x
PMid:21050321

- Martinez-Baylach J, Cabot Dalmau A, Garcia
Rodriguez L, Sauca G. Imported malaria: Clinical and epidemiological
review of an emerging disease. An Pediatr (Barc). 2007; 67:
199-205.

- Spinazzola F, Nicastri E, Vlassi C, Ghirga
P, De Marco M, Pittalis S, Paglia MG, Ferrari C, Narciso P. Imported
malaria at Italy's national institute for infectious diseases Lazzaro
Spallanzani, 1984-2003. Eur J Clin Microbiol Infect Dis. 2007; 26:
175-179. http://dx.doi.org/10.1007/s10096-007-0266-8
PMid:6756909

- Williams JP, Chitre M, Sharland M.
Increasing plasmodium falciparum malaria in southwest london: A 25 year
observational study. Arch Dis Child. 2002; 86: 428-430. http://dx.doi.org/10.1136/adc.86.6.428
PMid:12023177 PMCid:1763015

- Baas MC, Wetsteyn JC, van Gool T. Patterns
of imported malaria at the academic medical center, amsterdam, the
netherlands. J Travel Med. 2006; 13: 2-7. http://dx.doi.org/10.1111/j.1708-8305.2006.00003.x
PMid:16412103

- Chalumeau M, Holvoet L, Chéron G, Minodier
P, Foix-L'Hélias L, Ovetchkine P, Moulin F, Nouyrigat V, Bréart G,
Gendrel D. Delay in diagnosis of imported plasmodium falciparum malaria
in children. Eur J Clin Microbiol Infect Dis. 2006; 25: 186-189. http://dx.doi.org/10.1007/s10096-006-0105-3
PMid:6756909

- Ladhani S, El Bashir H, Patel VS,
Shingadia D. Childhood malaria in east london. Pediatr Infect Dis J.
2003; 22: 814-819. http://dx.doi.org/10.1097/01.inf.0000086401.13592.79
PMid:14506374

- Huerga H, Lopez-Velez R. Infectious
diseases in sub-saharan african immigrant children in madrid, spain.
Pediatr Infect Dis J. 2002; 21: 830-834. http://dx.doi.org/10.1097/00006454-200209000-00009
PMid:12352804

- Huerga H, Lopez-Velez R. Imported malaria
in immigrant and travelling children in madrid. Eur J Clin Microbiol
Infect Dis. 2001; 20: 591-593. http://dx.doi.org/10.1007/s100960100558
PMid:6756909

- Matteelli A, Volonterio A, Gulletta M,
Galimberti L, Maroccolo S, Gaiera G, Giani G, Rossi M, Dorigoni N,
Bellina L, Orlando G, Bisoffi Z, Castelli F. Malaria in illegal Chinese
immigrants, Italy. Emerg Infect Dis. 2001; 7: 1055-1058. http://dx.doi.org/10.3201/eid0706.010628
PMid:11747743 PMCid:2631896
