A
Study on the Expression of BCR-ABL Transcript in Mixed Phenotype Acute
Leukemia (MPAL) Cases Using the Reverse Transcriptase Polymerase
Reaction Assay (RT-PCR) and its Correlation with Hematological
Remission Status Post Initial Induction Therapy
Prateek Bhatia1, Jogeshwar Binota2, Neelam Varma3, Deepak Bansal4, Amita Trehan4, Ram Kumar Marwaha5, Pankaj Malhotra5 and Subhash Varma6
1Assistant Professor-Pediatrics, 2Senior Laboratory Technician, 3Professor and Head -Hematology, 4Additional Professor, 5Professor – Pediatric Hemato-oncology and 5Additional Professor, 6Professor & Head – Internal Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh
Correspondence
to: Prof. Neelam Varma, Professor & Head. Department of Hematology, PGIMER, Chandigarh. Phone: +91-01722755125. Email: varmaneelam@yahoo.com
Published: May 3, 2012
Received: February 18, 2011
Accepted: March 20, 2011
Mediterr J Hematol Infect Dis 2012, 4(1): e20120 , DOI 10.4084/MJHID.2012.024
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Introduction:
The MPAL comprise 2-5% of all acute leukemia. The present WHO 2008
classification has separated two groups in MPAL based on t(9;22)
positivity and MLL rearrangement. Aims & Objectives: The aim of the
present pilot study is to note the frequency of BCR-ABL transcript in
MPAL cases using the RT-PCR assay and to correlate the status with
hematological remission post induction. Materials & Methods: A
total of 10 MPAL cases classified on Flow-cytometry based on the
current WHO 2008 criteria were enrolled. In all the cases Bone marrow
or peripheral blood sample in EDTA was processed for molecular studies
and the RT-PCR reaction carried out using primers specific to the t
(9;22) and t(4;11) translocation. The post induction check marrow
slides were also reviewed. Results: Out of the total 10 MPAL cases,
7/10 (70%) were adult and 3/10 (30%) pediatric cases. A total of 4/10
(40%) cases showed positivity for the t(9;22) transcript and none for t
(4;11). Of the 4 positive cases, 3/10(30%) were adult cases and
1/10(10%) pediatric case. The BCR-ABL transcript type in adult cases
was b3a2 (p210) in 2/3 (66%) and e1a2 (p190) in 1/3 (33.3%) case. The
single pediatric case was positive for b3a2 transcript. Discussion
& Conclusion: All the 4 positive MPAL cases presented with high TLC
and low platelet count (p<0.05). The positive cases also showed
hematological remission at post induction check marrow (blasts<5%).
This could partly be explained due to good response to the imatinib
added to the treatment protocol.
Introduction
Acute leukemia with a mixed phenotype is a rare disease and comprises 2–5% of all acute leukemias. Immunophenotyping for B/T/Myeloid markers is necessary for detection of Mixed Phenotype acute leukemias. The WHO 2008 classification identifies two subtypes of MPAL with characteristic genetic lesion as separate entities:- i) MPAL with t(9;22) and ii) MPAL with MLL rearrangements. The incidence of MPAL with t(9;22) and MLL rearrangement in adults is 28-30% and 2-3% and in children 3-5% and 10-15% respectively[1]. The aim of the present study was to note the frequency of BCR-ABL positivity in MPAL cases using the Reverse Transcriptase Polymerase Reaction Assay (RT-PCR) and to correlate BCR-ABL positivity with status of hematological remission at 1st check marrow.
Materials and Methods
The study was a prospective study carried out over a period of one year from July 2010-June 2011. Inclusion criteria included: (i) All cases of Acute leukemia coming for Bone marrow examination to Department of Hematology, PGIMER, Chandigarh and diagnosed as Mixed Phenotype Acute Leukemia according to WHO 2008 criteria on Bone marrow examination and Immunophenotyping. (ii) All MPAL Cases receiving treatment in the Institute and having complete follow-up details of 1st check marrow. Cases of Acute leukemia with aberrant B/T/Myeloid marker expression on immunophenotyping, known cases of Chronic Myeloid leukemia (CML) in Blast crisis and cases with incomplete follow-up details/not receiving treatment in the institute were excluded from the study.
Methodology
RNA was extracted from 1-2ml Bone Marrow aspirate in EDTA or 3-5ml Peripheral blood (if blasts > 20% and TLC> 50X109 /L) using the commercial kit (Qiagen Miniamp RNA Blood kit) according to manufacturer’s instructions. The RNA quality was checked in each case by absorbance at 260nm in a spectrophotometer and by running a 1% formaldehyde gel for 18s and 28s RNA bands. This was followed by cDNA synthesis according to the commercial kit protocol (Fermantas cDNA kit). The quality of cDNA was checked using primers for β-Actin housekeeping gene. Reverse Transcriptase polymerase chain reaction was carried out using primers specific for p210 (b3a2 and b2a2)[2] adapted from the study of Jones et al and the protocol was standardized in our laboratory in 2004 for detection of p210 transcript in CML cases. A separate Multiplex RT-PCR was done for detection of p190 and MLL-AF4 transcript as adapted from study by Pakakasama et al.[3] This Multiplex RT-PCR is routinely used in our laboratory as a screening PCR for detection of common chimeric fusion transcripts in ALL cases. The PCR conditions for both the PCR are outlined below and the primer sequences and the product base size are highlighted in the table 1. The BCR-ABL PCR for p210 transcripts (b3a2 and b2a2) was carried out in following conditions: Pre-Denaturation- 94c-4 mts –one cycle; Denaturation-94c-1 mt, Annealing-63c-2 mt and Extension- 72c- 3 mt for 32 cycles. This was followed by one cycle of final Extension at 72c-10 mt. The Multiplex RT-PCR for p190 (e1a2) transcript and the MLL-AF4 transcript was carried out as follows: The PCR was carried out in a final volume of 25 ul with 1 ul cDNA, 1x PCR buffer, 200 uM dNTP, 1.5 mM MgCl2, 120 nM of each primer pair and 1 unit of Tag polymerase. After initial denaturation step at 940C for 3 min, the 35 cycles of PCR condition including 940C for 45 s, 630C for 1 min, and 720C for 1.5 min were performed. The final extension for 7 min at 720C was set to ensure a complete extension of all PCR products. The PCR products were then run on agarose gel and stained with Ethidium Bromide and visualized under UV-Gel doc for b3a2 (385bp), b2a2 (310bp) and e1a2 (521bp) bands. Appropriate positive and negative controls were included in each run.
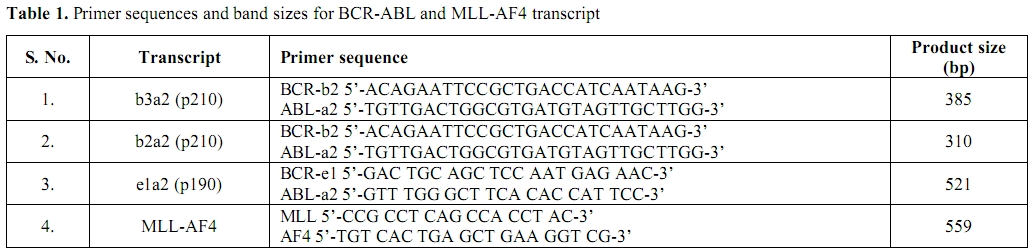
Table 1. Primer sequences and band sizes for BCR-ABL and MLL-AF4 transcript
Review of Geimsa stained Bone marrow and peripheral blood slides at 1st check marrow (Day 14-Pediatric cases & Day 28-Adult cases) for Remission status (Standard Remission criteria- Hb>10g/dl; TLC 4-12X 109/L; Platelet 150-450 x 109/L; Bone marrow blasts<5%; No Auer Rod; No blasts in peripheral blood) was performed and relevant data analyzed. Cytogenetic samples were taken in all cases.
Ethical Justification
The blood sample used in the study is withdrawn as a part of routine diagnostic work-up of the patient and no additional sample pricks were performed. Prior informed consent was taken from all patients/guardians before withdrawl of sample.
Statistical Analysis
The X2 and the Fischer exact test were used to correlate the clinical and laboratory features in the two groups and a p value of ≤0.05 was taken as significant.
Results
The total frequency of MPAL was 6% (10/170) of all acute leukemia cases that underwent routine diagnostic evaluation during the study period. Out of the 10 MPAL cases, 7/10 (70%) were adult and 3/10 (30%) were Pediatric cases. The age in adult cases ranged from 26-60 years (Mean age 31.5 yrs) and M: F ratio was 1.3:1. The age in Pediatric cases ranged from 7 months – 7 years (Mean age 3.7 years) and all were Male children. The clinical and Hematological data of the MPAL cases is outlined in table 2.
On Immunophenotyping, 60% (6/10) were B/Myeloid and 40% (4/10) were T/Myeloid. The incidence of BCR-ABL positivity in MPAL cases was 40% (4/10). All four MPAL cases positive for BCR-ABL transcript presented with High TLC and Low platelet count (p value ≤0.05). 3/4 (75%) BCR-ABL positive MPAL’s had B/Myeloid phenotype while 1/4 (25%) had T/Myeloid phenotype. Molecular break-point was p210 (b3a2 type) in 3/4 (75%) cases and p190 in 1/4 (25%) case. The Immunophenotyping, molecular and remission data in MPAL cases is detailed in table 3. Cytogenetics revealed satisfactory metaphases in 6/10 cases. Philadelphia positive metaphases were noted in all four BCR-ABL positive MPAL cases with a 100% correlation. However, no additional cytogenetic abnormality could be identified in any of the cases.
Figure 1 and 2 show RT-PCR agarose gels for Housekeeping gene (β-actin) and BCR-ABL transcript positive MPAL cases. None of the MPAL cases showed positivity for MLL-AF4 transcript.
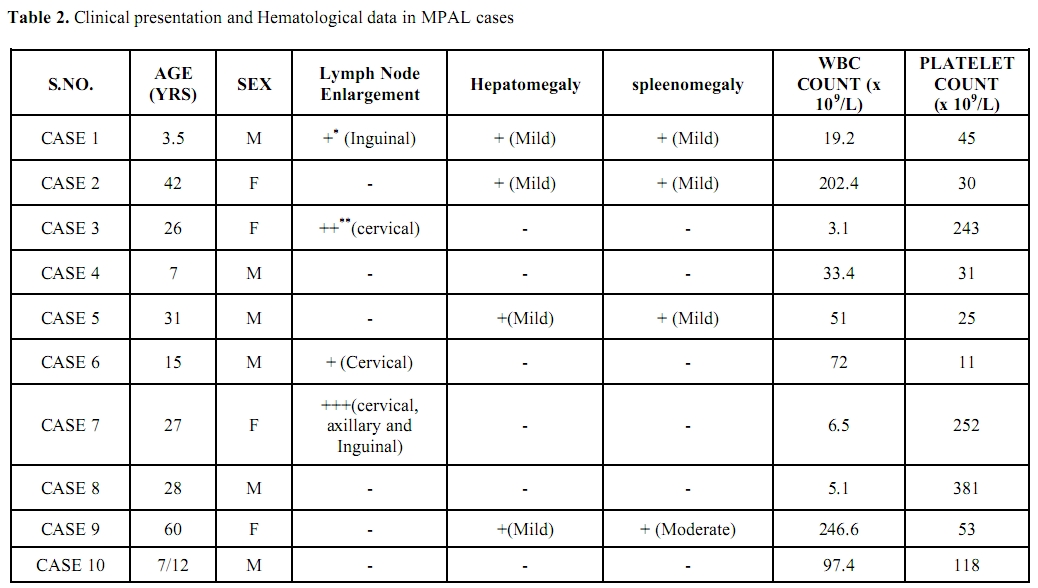
Table 2. Clinical presentation and Hematological data in MPAL cases
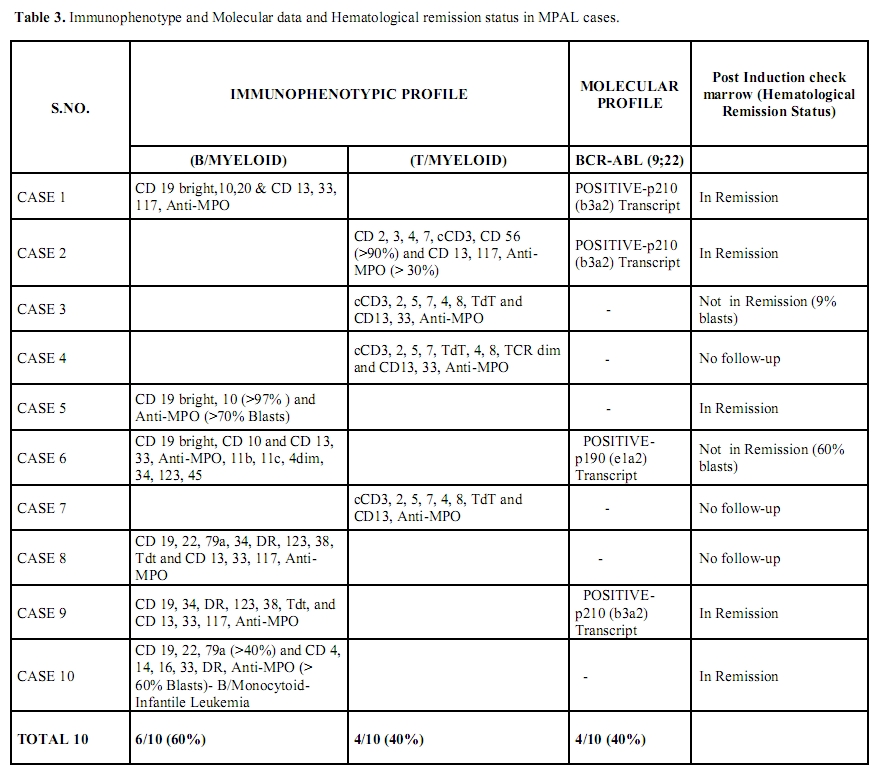
Table 3. Immunophenotype and Molecular data and Hematological remission status in MPAL cases.
Figure 1. Beta Actin band positivity as marker of internal cDNA quality in all 4 positive MPAL cases (Two per case)
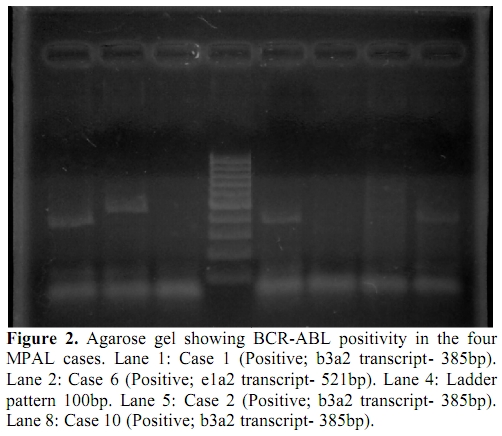
Figure 2. Agarose gel showing BCR-ABL positivity in the four MPAL cases. Lane 1: Case 1 (Positive; b3a2 transcript- 385bp). Lane 2: Case 6 (Positive; e1a2 transcript- 521bp). Lane 4: Ladder pattern 100bp. Lane 5: Case 2 (Positive; b3a2 transcript- 385bp). Lane 8: Case 10 (Positive; b3a2 transcript- 385bp).
Discussion
In the present study all the MPAL cases positive for BCR-ABL transcript had high WBC count and low platelet count (p<0.05). Many studies have also shown BCR-ABL positive MPAL’s presenting with high WBC count[4,5], but present study also shows relation of BCR-ABL positivity with low platelet count. There were 4 cases of T/Myeloid MPAL,of which one showed BCR-ABL positivity (Figure 3). Mastutes et al[6] in their study had also found BCR-ABL positivity in 13% (2/15) cases with T/Myeloid phenotype.
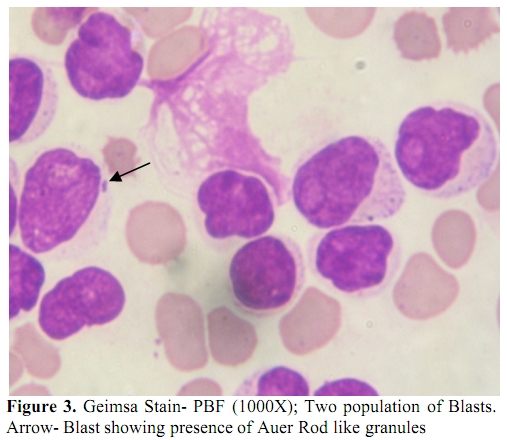
Figure 3. Geimsa Stain- PBF (1000X); Two population of Blasts. Arrow- Blast showing presence of Auer Rod like granules.
The incidence of MPAL cases was 6% and the BCR-ABL positivity in present study was 40% which is quiet comparable to few other studies from the subcontinent and west. Studies from Asian sub-continent by Xu et al[7], Lee et al[8] and Mi et al[9] found incidence of MPAL as 4.6%, 2.1% and 3.4% and BCR-ABL positivity as 25.0%, 36.8% and 16.7% respectively. Studies from west by Owaidah et al[10], Carbonell et al[11], Legrand et al[12], Killick et al[13] and Weir et al[14] found the incidence of MPAL as 3.4%, 4.0%, 8.0%, 3.6% and 1.3% and BCR-ABL positivity as 9.1%, 30.8%, 35.0%, 38.1% and 18.8% respectively.
Check marrow is performed at Day 14 in Pediatric Acute leukemia cases and Day 28 in adult cases. The Complete Hematological Remission rate (CHR) at first check marrow after induction therapy was 50% (5/10) in MPAL cases; 30% cases had no follow-up details while 20% were not in CHR. Of the four BCR-ABL positive cases 75% (3/4) were in CHR and all these were positive for b3a2 transcript type (p<0.05%). Only one BCR-ABL positive MPAL (p190 transcript) showed 60% blasts in Bone marrow at 1st check marrow. However, the case number and positivity for p190 transcript is low and the follow-up data is limited to derive any conclusive statement regarding prognostic difference between the two transcript types in MPAL cases. The MPAL cases are treated with ALL/AML standard induction protocol based on predominant Blast immunophenotype. In addition Imatinib is added to the treatment regimen if the MPAL case is BCR-ABL positive on RT-PCR. All the above four BCR-ABL positive MPAL cases received Imatinib along with standard induction regimen drugs. The good response in three cases could be attributed to the possible favorable effect of Imatinib addition to the standard induction regimen, which is quiet well described in literature. However long term follow up data in more number of cases is needed to further substantiate the findings. None of our MPAL case was positive for MLL-AF4 transcript. However we had not looked for other MLL rearrangements in these cases.
Conclusions
Immunophenotyping of all acute leukemia cases is necessary with a complete panel of lineage specific markers to detect presence of Mixed Phenotype Acute leukemias. RT-PCR for BCR-ABL is also mandatory in all MPAL cases as the overall prognosis in these patients may improve with addition of Imatinib (Gleevac /STI571) therapy to the ALL treatment regimen.
Acute leukemia with a mixed phenotype is a rare disease and comprises 2–5% of all acute leukemias. Immunophenotyping for B/T/Myeloid markers is necessary for detection of Mixed Phenotype acute leukemias. The WHO 2008 classification identifies two subtypes of MPAL with characteristic genetic lesion as separate entities:- i) MPAL with t(9;22) and ii) MPAL with MLL rearrangements. The incidence of MPAL with t(9;22) and MLL rearrangement in adults is 28-30% and 2-3% and in children 3-5% and 10-15% respectively[1]. The aim of the present study was to note the frequency of BCR-ABL positivity in MPAL cases using the Reverse Transcriptase Polymerase Reaction Assay (RT-PCR) and to correlate BCR-ABL positivity with status of hematological remission at 1st check marrow.
Materials and Methods
The study was a prospective study carried out over a period of one year from July 2010-June 2011. Inclusion criteria included: (i) All cases of Acute leukemia coming for Bone marrow examination to Department of Hematology, PGIMER, Chandigarh and diagnosed as Mixed Phenotype Acute Leukemia according to WHO 2008 criteria on Bone marrow examination and Immunophenotyping. (ii) All MPAL Cases receiving treatment in the Institute and having complete follow-up details of 1st check marrow. Cases of Acute leukemia with aberrant B/T/Myeloid marker expression on immunophenotyping, known cases of Chronic Myeloid leukemia (CML) in Blast crisis and cases with incomplete follow-up details/not receiving treatment in the institute were excluded from the study.
Methodology
RNA was extracted from 1-2ml Bone Marrow aspirate in EDTA or 3-5ml Peripheral blood (if blasts > 20% and TLC> 50X109 /L) using the commercial kit (Qiagen Miniamp RNA Blood kit) according to manufacturer’s instructions. The RNA quality was checked in each case by absorbance at 260nm in a spectrophotometer and by running a 1% formaldehyde gel for 18s and 28s RNA bands. This was followed by cDNA synthesis according to the commercial kit protocol (Fermantas cDNA kit). The quality of cDNA was checked using primers for β-Actin housekeeping gene. Reverse Transcriptase polymerase chain reaction was carried out using primers specific for p210 (b3a2 and b2a2)[2] adapted from the study of Jones et al and the protocol was standardized in our laboratory in 2004 for detection of p210 transcript in CML cases. A separate Multiplex RT-PCR was done for detection of p190 and MLL-AF4 transcript as adapted from study by Pakakasama et al.[3] This Multiplex RT-PCR is routinely used in our laboratory as a screening PCR for detection of common chimeric fusion transcripts in ALL cases. The PCR conditions for both the PCR are outlined below and the primer sequences and the product base size are highlighted in the table 1. The BCR-ABL PCR for p210 transcripts (b3a2 and b2a2) was carried out in following conditions: Pre-Denaturation- 94c-4 mts –one cycle; Denaturation-94c-1 mt, Annealing-63c-2 mt and Extension- 72c- 3 mt for 32 cycles. This was followed by one cycle of final Extension at 72c-10 mt. The Multiplex RT-PCR for p190 (e1a2) transcript and the MLL-AF4 transcript was carried out as follows: The PCR was carried out in a final volume of 25 ul with 1 ul cDNA, 1x PCR buffer, 200 uM dNTP, 1.5 mM MgCl2, 120 nM of each primer pair and 1 unit of Tag polymerase. After initial denaturation step at 940C for 3 min, the 35 cycles of PCR condition including 940C for 45 s, 630C for 1 min, and 720C for 1.5 min were performed. The final extension for 7 min at 720C was set to ensure a complete extension of all PCR products. The PCR products were then run on agarose gel and stained with Ethidium Bromide and visualized under UV-Gel doc for b3a2 (385bp), b2a2 (310bp) and e1a2 (521bp) bands. Appropriate positive and negative controls were included in each run.

Table 1. Primer sequences and band sizes for BCR-ABL and MLL-AF4 transcript
Review of Geimsa stained Bone marrow and peripheral blood slides at 1st check marrow (Day 14-Pediatric cases & Day 28-Adult cases) for Remission status (Standard Remission criteria- Hb>10g/dl; TLC 4-12X 109/L; Platelet 150-450 x 109/L; Bone marrow blasts<5%; No Auer Rod; No blasts in peripheral blood) was performed and relevant data analyzed. Cytogenetic samples were taken in all cases.
Ethical Justification
The blood sample used in the study is withdrawn as a part of routine diagnostic work-up of the patient and no additional sample pricks were performed. Prior informed consent was taken from all patients/guardians before withdrawl of sample.
Statistical Analysis
The X2 and the Fischer exact test were used to correlate the clinical and laboratory features in the two groups and a p value of ≤0.05 was taken as significant.
Results
The total frequency of MPAL was 6% (10/170) of all acute leukemia cases that underwent routine diagnostic evaluation during the study period. Out of the 10 MPAL cases, 7/10 (70%) were adult and 3/10 (30%) were Pediatric cases. The age in adult cases ranged from 26-60 years (Mean age 31.5 yrs) and M: F ratio was 1.3:1. The age in Pediatric cases ranged from 7 months – 7 years (Mean age 3.7 years) and all were Male children. The clinical and Hematological data of the MPAL cases is outlined in table 2.
On Immunophenotyping, 60% (6/10) were B/Myeloid and 40% (4/10) were T/Myeloid. The incidence of BCR-ABL positivity in MPAL cases was 40% (4/10). All four MPAL cases positive for BCR-ABL transcript presented with High TLC and Low platelet count (p value ≤0.05). 3/4 (75%) BCR-ABL positive MPAL’s had B/Myeloid phenotype while 1/4 (25%) had T/Myeloid phenotype. Molecular break-point was p210 (b3a2 type) in 3/4 (75%) cases and p190 in 1/4 (25%) case. The Immunophenotyping, molecular and remission data in MPAL cases is detailed in table 3. Cytogenetics revealed satisfactory metaphases in 6/10 cases. Philadelphia positive metaphases were noted in all four BCR-ABL positive MPAL cases with a 100% correlation. However, no additional cytogenetic abnormality could be identified in any of the cases.
Figure 1 and 2 show RT-PCR agarose gels for Housekeeping gene (β-actin) and BCR-ABL transcript positive MPAL cases. None of the MPAL cases showed positivity for MLL-AF4 transcript.

Table 2. Clinical presentation and Hematological data in MPAL cases

Table 3. Immunophenotype and Molecular data and Hematological remission status in MPAL cases.

Figure 1. Beta Actin band positivity as marker of internal cDNA quality in all 4 positive MPAL cases (Two per case)

Figure 2. Agarose gel showing BCR-ABL positivity in the four MPAL cases. Lane 1: Case 1 (Positive; b3a2 transcript- 385bp). Lane 2: Case 6 (Positive; e1a2 transcript- 521bp). Lane 4: Ladder pattern 100bp. Lane 5: Case 2 (Positive; b3a2 transcript- 385bp). Lane 8: Case 10 (Positive; b3a2 transcript- 385bp).
Discussion
In the present study all the MPAL cases positive for BCR-ABL transcript had high WBC count and low platelet count (p<0.05). Many studies have also shown BCR-ABL positive MPAL’s presenting with high WBC count[4,5], but present study also shows relation of BCR-ABL positivity with low platelet count. There were 4 cases of T/Myeloid MPAL,of which one showed BCR-ABL positivity (Figure 3). Mastutes et al[6] in their study had also found BCR-ABL positivity in 13% (2/15) cases with T/Myeloid phenotype.

Figure 3. Geimsa Stain- PBF (1000X); Two population of Blasts. Arrow- Blast showing presence of Auer Rod like granules.
The incidence of MPAL cases was 6% and the BCR-ABL positivity in present study was 40% which is quiet comparable to few other studies from the subcontinent and west. Studies from Asian sub-continent by Xu et al[7], Lee et al[8] and Mi et al[9] found incidence of MPAL as 4.6%, 2.1% and 3.4% and BCR-ABL positivity as 25.0%, 36.8% and 16.7% respectively. Studies from west by Owaidah et al[10], Carbonell et al[11], Legrand et al[12], Killick et al[13] and Weir et al[14] found the incidence of MPAL as 3.4%, 4.0%, 8.0%, 3.6% and 1.3% and BCR-ABL positivity as 9.1%, 30.8%, 35.0%, 38.1% and 18.8% respectively.
Check marrow is performed at Day 14 in Pediatric Acute leukemia cases and Day 28 in adult cases. The Complete Hematological Remission rate (CHR) at first check marrow after induction therapy was 50% (5/10) in MPAL cases; 30% cases had no follow-up details while 20% were not in CHR. Of the four BCR-ABL positive cases 75% (3/4) were in CHR and all these were positive for b3a2 transcript type (p<0.05%). Only one BCR-ABL positive MPAL (p190 transcript) showed 60% blasts in Bone marrow at 1st check marrow. However, the case number and positivity for p190 transcript is low and the follow-up data is limited to derive any conclusive statement regarding prognostic difference between the two transcript types in MPAL cases. The MPAL cases are treated with ALL/AML standard induction protocol based on predominant Blast immunophenotype. In addition Imatinib is added to the treatment regimen if the MPAL case is BCR-ABL positive on RT-PCR. All the above four BCR-ABL positive MPAL cases received Imatinib along with standard induction regimen drugs. The good response in three cases could be attributed to the possible favorable effect of Imatinib addition to the standard induction regimen, which is quiet well described in literature. However long term follow up data in more number of cases is needed to further substantiate the findings. None of our MPAL case was positive for MLL-AF4 transcript. However we had not looked for other MLL rearrangements in these cases.
Conclusions
Immunophenotyping of all acute leukemia cases is necessary with a complete panel of lineage specific markers to detect presence of Mixed Phenotype Acute leukemias. RT-PCR for BCR-ABL is also mandatory in all MPAL cases as the overall prognosis in these patients may improve with addition of Imatinib (Gleevac /STI571) therapy to the ALL treatment regimen.
References
- OK Weinberg, DA Arber.Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia 2010; 24: 1844–1851. http://dx.doi.org/10.1038/leu.2010.202 PMid:20844566

- Carol D. Jones, Cecilia Yeung, and James L.
Zehnder. Comprehensive Validation of a Real-Time Quantitative bcr-abl
Assay for Clinical Laboratory Use. Am J Clin Pathol 2003; 120: 42-48. http://dx.doi.org/10.1309/60A9C8WGEGHRNXEE

- S. Pakakasama, S. Kajanachumpol, S.
Kanjanapongkul et al. Simple multiplex RT-PCR for identifying common
fusion transcripts in childhood acute leukemia. Int. Jnl. Lab. Hem.
2008, 30; 286–291. http://dx.doi.org/10.1111/j.1751-553X.2007.00954.x PMid:18665825

- Mikulic M, Batinic D, Sucic M et al.
Biological features and outcome of Biphenotypic acute leukemia: a case
series. Hematol Oncol Stem cell Ther. 2008; 1: 225-230.

- Wang Y, Gu M, Mi Y et al. Clinical
characteristics and outcomes of mixed phenotype acute leukemia with
Philadelphia chromosome positive and/or bcr-abl positive in adult. Int
J Hematology 2011; 94: 552-555. http://dx.doi.org/10.1007/s12185-011-0953-1 PMid:22015493

- Estella Matutes, Winfried F. Pickl, Mars
van't Veer et al. Mixed-phenotype acute leukemia: clinical and
laboratory features and outcome in 100 patients defined according to
the WHO 2008 Classification. Blood 2011; 17: 3163-3171. http://dx.doi.org/10.1182/blood-2010-10-314682 PMid:21228332

- Xiao-Qian Xu, Jian-Min Wang, Shu-Qing Lüˇ
et al. Clinical and biological characteristics of adult biphenotypic
acute leukemia in comparison with that of acute myeloid leukemia and
acute lymphoblastic leukemia: a case series of a Chinese population.
Hematologica 2009; 94: 919-927. http://dx.doi.org/10.3324/haematol.2008.003202 PMid:19454497 PMCid:2704302

- Lee JH, Min YH, Chung CW et al. Korean
Society of Hematology AML/MDS Working Party. Prognostic implications of
the immunophenotype in Biphenotypic acute leukemia. Leuk Lymphoma 2008;
49: 700-709. http://dx.doi.org/10.1080/10428190701843247

- Mi Y, Bian S, Meng Q et al. Study on the
clinical characteristics of Biphenotypic acute leukemia. Zhonghua Xue
Ye Xue Za Zhi 2000; 21: 352-354. PMid:11877003

- Owaidah TM, Al Beihany A, Iqbal MA et al.
Cytogenetics, molecular and ultrastructural characteristics of
biphenotypic acute leukemia identified by the EGIL scoring system.
Leukemia 2006; 20: 620-626. http://dx.doi.org/10.1038/sj.leu.2404128 PMid:16437134

- Carbonell F, Swansbury J, Min T et al.
Cytogenetic findings in acute Biphenotypic leukaemia. Leukemia 1996;
10: 1283-1287. PMid:8709632

- Legrand O, Perrot JY, Simonin G et al.
Adult Biphenotypic acute leukemia: an entity with poor prognosis which
is related to unfavorable cytogenetics and P-glycoprotein
over-expression. Br J Haematol 1998; 100: 147-155. http://dx.doi.org/10.1046/j.1365-2141.1998.00523.x PMid:9450804

- Killick
S, Matutes E, Powles RL et al. Outcome of Biphenotypic acute leukemia.
Haematologica 1999; 84: 699-706. PMid:10457405

- Weir EG, Ali Ansari-Lari M, Batista DA et
al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia
2007; 21: 2264-2270. http://dx.doi.org/10.1038/sj.leu.2404848 PMid:17611554
