A Fascioliasis Case: a not Rare Cause of Hypereosinophilia in Developing Countries, Present in Developed too
Ismail Necati Hakyemez*, Gülali Aktaş**, Haluk Savli**, Abdülkadir Küçükbayrak*, Safiye Gürel*** and Tekin Taş****
Correspondence
to: Gülali Aktaş. Department of Internal Medicine, Abant Izzet Baysal University Hospital, Bolu, Turkey. E-mail: draliaktas@yahoo.com
Published: May 08, 2012
Received: April 29, 2012
Accepted: May 05, 2012
Mediterr J Hematol Infect Dis 2012, 4(1): e2012029, DOI 10.4084/MJHID.2012.029
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Fascioliasis
is a worlwide parasitic zoonosis, endemic in south-east mediterranean
area, but uncommon in other areas. Clinical signs are usually
non-specific. A 32 year old male patient was admitted to our hospital
with complaints of abdominal pain, diarrhea, fatigue, nausea, lost of
appetite, itching, cough, night sweats and weight loss. Complete blood
count revealed hypereosinophilia. The abdominal ultrasound scan was
normal. But computed tomography scan revealed irregular nodular lesions
in periportal area of the liver. Based on these clinical and
radiological signs and continuous hypereosinophilia, the patient was
serologically investigated for Fasciola hepatica infection. F. hepatica
indirect hemagglutination test in serum was positive at a titer of
1/1280. Single dose Triclabendasole 10mg/kg was administered and
repeated two weeks later. Clinical and laboratory signs were completely
resolved after treatment. Serological tests for fascioliasis
should be included in all patients with hypereosinophilia and
abnormal liver CT.
Introduction
Fasciola hepatica is an acquired zoonotic liver trematode which is common in developing countries. It is uncommon in developed regions. The parasite rarely infects human. People typically become infected by eating raw watercress or other fresh aquatic vegetation that contain F. hepatica metacercarias. Acute infection is characterized with intermittant fever, abdominal pain, weight loss, anemia, urticaria, hypereosinophilia, hepatomegaly and elevation in hepatic function tests. Triclabendazole, first line treatment option, is quite effective in treatment of the infection.[1] The disease may be complicated as biliary colic, cholestasis or cholangitis in untreated patients.[2] Detection of F. hepatica eggs in biliary, duodenal or in faecal samples is diagnostic but usually the eggs are undetectable during the acute period. Radiological imaging and serological tests are useful in diagnosis in this period.[3]
We report a hypereosinophilic patient infected with F. hepatica in this paper. The diagnosis is made in the basis of radiological and serological findings.
Case Report
A 32 years old male was admitted to our hospital with complaints of abdominal pain, diarrhea, fatigue, nausea, loss of appetite, cough, night sweats and weight loss. Lasting for 2 months except for mild diarrhea lasting for 3 days. His history was unremarkable for travelling, animal husbandry, hunting, drugs and agriculture. There were no similar symptoms in his family and neighbours. His axillary body temperature was 37.2°C, blood pressure was 120/80 mmHg, heart rate was 82/min, and respiration rate was 22/min. Physical examination revealed urticarial lesions on his back.
His laboratory tests were as follows: leukocytes 7800/mm3, eosinophils 3900/mm3 (%49), platelets 293000/mm3, hemoglobin 13,8 g/dL, hematocrit %42.6, C-Reactive Protein (CRP) 35 mg/dl. Peripheric blood smear revealed eosinophilia as high as 46% of white blood cells. Routine biochemical tests, urine analysis, and serum IgE were in normal range. Hepatitis B virus surface antigen (HBsAg), anti-hepatitis C virus antibodies (Anti-HCV), anti-human immunodeficiency virus antibodies (Anti-HIV), venereal disease research laboratory (VDRL), Epstein-Barr virus virus capsid antigen (EBV-VCA) IgM, cytomegalovirus (CMV) IgM, anti-rubella IgM, Rose-Bengal, Wright agglutination, and Grubel-Widal tests were negative. The chest radiogram was also normal. Nonpruritic skin lesions on his back were diagnosed as dermographic urticaria after consulting with a dermatologist (Figure 1). Desloratadin and hydroxyzine were administered. The skin lesions were not resolved with these drugs. The patient was hospitalized to investigate the possible infectious causes of hypereosinophilia.
The bacteriological cultures of urine, blood, and sputum and tuberculosis culture of the sputum were negative.
No bacteria were seen in Gram and Ehrlich-Ziehl-Neelsen stainings of the sputum. There was no parasite in stool examination performed on five consecutive days. Mebendazole 200 mg daily was given for possible helmintic infestation for five days. As well as, no clinical or laboratory response was achieved. Anti-nuclear antibody, anti-double-stranded DNA, perinuclear antineutrophil cytoplasmic antibody (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) tests were negative. Abdominal ultrasound (US) was normal. The electrocardiogram revealed an extension in PR duration and echocardiography revealed pleural effusion in the neighbourhood of the right ventricle. Computed tomography (CT) scan of the thorax was normal. However, multipl hypodense lesions were detected in periportal areas of the liver in upper abdominal sections of the CT scan. The largest lesion’s size reached 3x4 cm, the others had approximately 1-1.5 cm diameters. There was no enhancement on lesions (Figure 2).
Serum of the patient was sent to The Refik Saydam Hygiene Center Presidency for serological tests of Toxacara canis and F. hepatica. Although serology for T. canis was negative, F. hepatica indirect hemagglutination (IHA) test was positive at a titer of 1/1280. His history was reviewed repeated and we figured out that he had eaten raw watercress on riverside 3 months before his hospital admission. Triclabendazole (Egaten®) 10mg/kg single dose was started and repeated after 2 weeks. His complaints resolved in 3 weeks of treatment. The hemogram and peripheric blood smear revealed normal eosinophil count, then. CRP level in serum was reduced to normal range too.
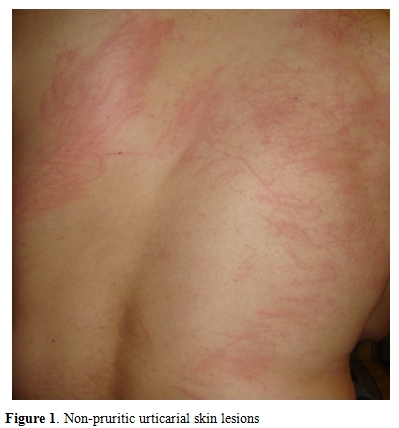
Figure 1. Non-pruritic urticarial skin lesions.
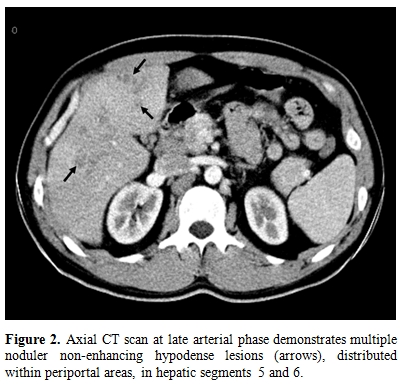
Figure 2.Axial CT scan at late arterial phase demonstrates multiple noduler non-enhancing hypodense lesions (arrows), distributed within periportal areas, in hepatic segments 5 and 6.
Discussion
We report a patient admitted with non-spesific clinical findings, hypereosinophilia and urticarial lesions. Although abdominal ultrasound was normal, CT scan revealed hepatic lesions, which were characteristic for F. hepatica. F. hepatica IHA test was positive at a titer of 1/1280. Clinical and laboratory signs resolved after triclabendazole treatment.
Eosinophil count between 500-1500/mm3 is considered as mild, 1500-5000/mm3 is considered as moderate and more than 5000/mm3 considered as severe eosinophilia. Hypereosinophilia term is characterized by a marked increase in count more than 1500/mm3 in the bloodstream. The most common causes of hypereosinophilia are parasitic infections in rural and allergic diseases in urban areas of Turkey.[4,5] Our patient had eosinophil count of 3900/mm3, consistent with hypereosinophilia.
Zoonotic infections and their reservoirs are important public health problems, especially in developing countries. Infections have been reported from all continents except Antarctica, with the highest rates of infection in Bolivia, Peru, Egypt, Iran, Portugal, and France.[1] F. hepatica is an endemic parasite in Turkey and causes major economic losses in livestock industry.[6] 238 cases were reported from Turkey between the years of 1935 and 2004.[7] According to the data of World Health Organization (WHO), 2.4 million people are infected with Fasciola hepatica and 180 million people live at risk to infection in over 61 countries.[8] Fascioliasis has been recognized as an emerging infection in international travellers and migrants, causing significant diagnostic and therapeutic problems.[9] A single case of fascioliasis may indicate either a familial or local outbreak.[10] Climate and environmental conditions, spreading of reservoirs and intermediate hosts, and eating behaviours such as ingestion of raw watercress on the river side are the factors that causing Fascioliasis.[11,12] We think that our patient had been infected by F. hepatica due to consumption of raw watercress on the river side.
The disease has two clinical phases with different signs. Acute phase is the hepatic invasion period while chronic phase is characterized with invasion of biliary tract and with signs of cholestasis and cholangitis. Urticarial lesions, prolonged fever, abdominal pain localized in right upper quadrant, night sweats, weight loss, hepatomegaly and marked eosinophilia are characteristic features of acute phase. But these sings usually are missed by physicians. Furthermore, diagnosis of acute phase is difficult because it mimics other causes of hepatic abscess. Although eosinophilia are also common in chronic phase, clinical course is mild at this stage. Patients usually are admitted to hospital with biliary colic, jaundice and epigastric pain in chronic phase.[13] The burden of disease caused by chronic subclinical infection is largely unknown. Anemia should be recognized as an important component of the burden of disease from fascioliasis.[14] The patient in our case had complaints such as abdominal pain, fatigue, loss of appetite, nausea, night sweats and weight loss, that consisted with acute hepatic phase of fascioliasis.
Diagnosis of F. hepatica infection may delay due to low incidence of the disease in developed countries and to large differential diagnosis list including viral hepatitis, liver abscess, malignancy, cholecystitis, cholangitis, ruptured hydatic cyst, and parasites such as clonorchiasis and ascariaisis. Diagnosis can be made by observing F. hepatica eggs in bile and stool but diagnosis can not be excluded even the eggs are absent in these samples. Serologic tests are useful during acute infection because symptoms develop 1 to 2 months before eggs are detectable in the stool. The specificity of the indirect hemagglutination test (IHA) using purified adult F. hepatica antigen F1 is 96.9% for serological diagnosis of Fascioliasis.[15] Enzyme-linked immunosorbent assay (ELISA) test could be used both as an individual serodiagnostic test for human fascioliasis when backed up by a compatible clinical history together with a second diagnostic technique for other cross-reactive helminth infections, and in large-scale epidemiological studies of human fascioliasis worldwide. Coproantigen ELISA is a more convenient test than faecal egg counts and holds promise as a diagnostic tool for natural fluke infections but further evaluation of interpretation criteria may be needed.[16,17] In our case, even we could not observe parasitic eggs in faecal sample on five consecutive days the IHA test was positive at a titer of 1/1280, two weeks later.
Ultrasound and computed tomography are useful in radiological diagnosis of Fascioliasis. Radiological findings can often demonstrate characteristic changes, and thereby, assist in the diagnosis of fascioliasis. It is important to differentiate fascioliasis lesions from other focal liver lesions by imaging. US findings are nonspesific in the early phase. In serious infection, hypoechoic lesions with irregular distribution in the liver parenchyma can be observed. Parenchymal lesions regress with the beginning of the ductal phase. Sometimes US can demonstrate mobile flukes in the dilated bile ducts and gallbladder.[18] F. hepatica infection should be considered if CT reveals hypodense nodules without enhancement and tunnel-like linear hypodense lesions in liver.[19] The combination of upper abdominal pain, marked eosinophilia and hypodense lesion in CT imaging is highly indicative of acute fascioliasis. Abdominal ultrasound of our patient was normal, but CT revealed multiple periportal hypodense lesions in the liver.
Fascioliasis is a preventable disease with simple public education in terms of not to ingest uncooked raw watercress or other aquatic vegetation. Treatment response is usually quite enough. Triclabendazole, most frequently prescribed agent in treatment of Fascioliasis, is effective against the adult and immature forms of the parasite. 10mg/kg single dose is administered after meal. Treatment should be repeated 2 weeks later. Triclabendazole is well tolerated by the patients and the efficacy rate is 80-90% in treatment of the disease. Nausea, vomitting, and abdominal pain are the most common side effects of the drug. Even radiological response takes a longer time, clinical and laboratory response should be achieved in several weeks.[20] We administered triclabendazole in a single 10mg/kg dose and repeated it 2 weeks later. We did not observe any side effects associated with the drug and achieved clinical and laboratory response after 3 week-treatment.
Conclusions
Diagnosis of Fascioliasis is difficult to suppose in non-endemic regions however considering the worldwide diffusion of Fascioliasis, in presence of hypereosynophilia with clinical radiological sign suggesting liver involvement serological tests for Fascioliasis should be everywhere performed.
Fasciola hepatica is an acquired zoonotic liver trematode which is common in developing countries. It is uncommon in developed regions. The parasite rarely infects human. People typically become infected by eating raw watercress or other fresh aquatic vegetation that contain F. hepatica metacercarias. Acute infection is characterized with intermittant fever, abdominal pain, weight loss, anemia, urticaria, hypereosinophilia, hepatomegaly and elevation in hepatic function tests. Triclabendazole, first line treatment option, is quite effective in treatment of the infection.[1] The disease may be complicated as biliary colic, cholestasis or cholangitis in untreated patients.[2] Detection of F. hepatica eggs in biliary, duodenal or in faecal samples is diagnostic but usually the eggs are undetectable during the acute period. Radiological imaging and serological tests are useful in diagnosis in this period.[3]
We report a hypereosinophilic patient infected with F. hepatica in this paper. The diagnosis is made in the basis of radiological and serological findings.
Case Report
A 32 years old male was admitted to our hospital with complaints of abdominal pain, diarrhea, fatigue, nausea, loss of appetite, cough, night sweats and weight loss. Lasting for 2 months except for mild diarrhea lasting for 3 days. His history was unremarkable for travelling, animal husbandry, hunting, drugs and agriculture. There were no similar symptoms in his family and neighbours. His axillary body temperature was 37.2°C, blood pressure was 120/80 mmHg, heart rate was 82/min, and respiration rate was 22/min. Physical examination revealed urticarial lesions on his back.
His laboratory tests were as follows: leukocytes 7800/mm3, eosinophils 3900/mm3 (%49), platelets 293000/mm3, hemoglobin 13,8 g/dL, hematocrit %42.6, C-Reactive Protein (CRP) 35 mg/dl. Peripheric blood smear revealed eosinophilia as high as 46% of white blood cells. Routine biochemical tests, urine analysis, and serum IgE were in normal range. Hepatitis B virus surface antigen (HBsAg), anti-hepatitis C virus antibodies (Anti-HCV), anti-human immunodeficiency virus antibodies (Anti-HIV), venereal disease research laboratory (VDRL), Epstein-Barr virus virus capsid antigen (EBV-VCA) IgM, cytomegalovirus (CMV) IgM, anti-rubella IgM, Rose-Bengal, Wright agglutination, and Grubel-Widal tests were negative. The chest radiogram was also normal. Nonpruritic skin lesions on his back were diagnosed as dermographic urticaria after consulting with a dermatologist (Figure 1). Desloratadin and hydroxyzine were administered. The skin lesions were not resolved with these drugs. The patient was hospitalized to investigate the possible infectious causes of hypereosinophilia.
The bacteriological cultures of urine, blood, and sputum and tuberculosis culture of the sputum were negative.
No bacteria were seen in Gram and Ehrlich-Ziehl-Neelsen stainings of the sputum. There was no parasite in stool examination performed on five consecutive days. Mebendazole 200 mg daily was given for possible helmintic infestation for five days. As well as, no clinical or laboratory response was achieved. Anti-nuclear antibody, anti-double-stranded DNA, perinuclear antineutrophil cytoplasmic antibody (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) tests were negative. Abdominal ultrasound (US) was normal. The electrocardiogram revealed an extension in PR duration and echocardiography revealed pleural effusion in the neighbourhood of the right ventricle. Computed tomography (CT) scan of the thorax was normal. However, multipl hypodense lesions were detected in periportal areas of the liver in upper abdominal sections of the CT scan. The largest lesion’s size reached 3x4 cm, the others had approximately 1-1.5 cm diameters. There was no enhancement on lesions (Figure 2).
Serum of the patient was sent to The Refik Saydam Hygiene Center Presidency for serological tests of Toxacara canis and F. hepatica. Although serology for T. canis was negative, F. hepatica indirect hemagglutination (IHA) test was positive at a titer of 1/1280. His history was reviewed repeated and we figured out that he had eaten raw watercress on riverside 3 months before his hospital admission. Triclabendazole (Egaten®) 10mg/kg single dose was started and repeated after 2 weeks. His complaints resolved in 3 weeks of treatment. The hemogram and peripheric blood smear revealed normal eosinophil count, then. CRP level in serum was reduced to normal range too.

Figure 1. Non-pruritic urticarial skin lesions.

Figure 2.Axial CT scan at late arterial phase demonstrates multiple noduler non-enhancing hypodense lesions (arrows), distributed within periportal areas, in hepatic segments 5 and 6.
Discussion
We report a patient admitted with non-spesific clinical findings, hypereosinophilia and urticarial lesions. Although abdominal ultrasound was normal, CT scan revealed hepatic lesions, which were characteristic for F. hepatica. F. hepatica IHA test was positive at a titer of 1/1280. Clinical and laboratory signs resolved after triclabendazole treatment.
Eosinophil count between 500-1500/mm3 is considered as mild, 1500-5000/mm3 is considered as moderate and more than 5000/mm3 considered as severe eosinophilia. Hypereosinophilia term is characterized by a marked increase in count more than 1500/mm3 in the bloodstream. The most common causes of hypereosinophilia are parasitic infections in rural and allergic diseases in urban areas of Turkey.[4,5] Our patient had eosinophil count of 3900/mm3, consistent with hypereosinophilia.
Zoonotic infections and their reservoirs are important public health problems, especially in developing countries. Infections have been reported from all continents except Antarctica, with the highest rates of infection in Bolivia, Peru, Egypt, Iran, Portugal, and France.[1] F. hepatica is an endemic parasite in Turkey and causes major economic losses in livestock industry.[6] 238 cases were reported from Turkey between the years of 1935 and 2004.[7] According to the data of World Health Organization (WHO), 2.4 million people are infected with Fasciola hepatica and 180 million people live at risk to infection in over 61 countries.[8] Fascioliasis has been recognized as an emerging infection in international travellers and migrants, causing significant diagnostic and therapeutic problems.[9] A single case of fascioliasis may indicate either a familial or local outbreak.[10] Climate and environmental conditions, spreading of reservoirs and intermediate hosts, and eating behaviours such as ingestion of raw watercress on the river side are the factors that causing Fascioliasis.[11,12] We think that our patient had been infected by F. hepatica due to consumption of raw watercress on the river side.
The disease has two clinical phases with different signs. Acute phase is the hepatic invasion period while chronic phase is characterized with invasion of biliary tract and with signs of cholestasis and cholangitis. Urticarial lesions, prolonged fever, abdominal pain localized in right upper quadrant, night sweats, weight loss, hepatomegaly and marked eosinophilia are characteristic features of acute phase. But these sings usually are missed by physicians. Furthermore, diagnosis of acute phase is difficult because it mimics other causes of hepatic abscess. Although eosinophilia are also common in chronic phase, clinical course is mild at this stage. Patients usually are admitted to hospital with biliary colic, jaundice and epigastric pain in chronic phase.[13] The burden of disease caused by chronic subclinical infection is largely unknown. Anemia should be recognized as an important component of the burden of disease from fascioliasis.[14] The patient in our case had complaints such as abdominal pain, fatigue, loss of appetite, nausea, night sweats and weight loss, that consisted with acute hepatic phase of fascioliasis.
Diagnosis of F. hepatica infection may delay due to low incidence of the disease in developed countries and to large differential diagnosis list including viral hepatitis, liver abscess, malignancy, cholecystitis, cholangitis, ruptured hydatic cyst, and parasites such as clonorchiasis and ascariaisis. Diagnosis can be made by observing F. hepatica eggs in bile and stool but diagnosis can not be excluded even the eggs are absent in these samples. Serologic tests are useful during acute infection because symptoms develop 1 to 2 months before eggs are detectable in the stool. The specificity of the indirect hemagglutination test (IHA) using purified adult F. hepatica antigen F1 is 96.9% for serological diagnosis of Fascioliasis.[15] Enzyme-linked immunosorbent assay (ELISA) test could be used both as an individual serodiagnostic test for human fascioliasis when backed up by a compatible clinical history together with a second diagnostic technique for other cross-reactive helminth infections, and in large-scale epidemiological studies of human fascioliasis worldwide. Coproantigen ELISA is a more convenient test than faecal egg counts and holds promise as a diagnostic tool for natural fluke infections but further evaluation of interpretation criteria may be needed.[16,17] In our case, even we could not observe parasitic eggs in faecal sample on five consecutive days the IHA test was positive at a titer of 1/1280, two weeks later.
Ultrasound and computed tomography are useful in radiological diagnosis of Fascioliasis. Radiological findings can often demonstrate characteristic changes, and thereby, assist in the diagnosis of fascioliasis. It is important to differentiate fascioliasis lesions from other focal liver lesions by imaging. US findings are nonspesific in the early phase. In serious infection, hypoechoic lesions with irregular distribution in the liver parenchyma can be observed. Parenchymal lesions regress with the beginning of the ductal phase. Sometimes US can demonstrate mobile flukes in the dilated bile ducts and gallbladder.[18] F. hepatica infection should be considered if CT reveals hypodense nodules without enhancement and tunnel-like linear hypodense lesions in liver.[19] The combination of upper abdominal pain, marked eosinophilia and hypodense lesion in CT imaging is highly indicative of acute fascioliasis. Abdominal ultrasound of our patient was normal, but CT revealed multiple periportal hypodense lesions in the liver.
Fascioliasis is a preventable disease with simple public education in terms of not to ingest uncooked raw watercress or other aquatic vegetation. Treatment response is usually quite enough. Triclabendazole, most frequently prescribed agent in treatment of Fascioliasis, is effective against the adult and immature forms of the parasite. 10mg/kg single dose is administered after meal. Treatment should be repeated 2 weeks later. Triclabendazole is well tolerated by the patients and the efficacy rate is 80-90% in treatment of the disease. Nausea, vomitting, and abdominal pain are the most common side effects of the drug. Even radiological response takes a longer time, clinical and laboratory response should be achieved in several weeks.[20] We administered triclabendazole in a single 10mg/kg dose and repeated it 2 weeks later. We did not observe any side effects associated with the drug and achieved clinical and laboratory response after 3 week-treatment.
Conclusions
Diagnosis of Fascioliasis is difficult to suppose in non-endemic regions however considering the worldwide diffusion of Fascioliasis, in presence of hypereosynophilia with clinical radiological sign suggesting liver involvement serological tests for Fascioliasis should be everywhere performed.
References
- Maguire JH. Trematodes (Schistosomes and Other Flukes). In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia: Churchill Livingstone, 2010: 3595-3605. http://dx.doi.org/10.1016/B978-0-443-06839-3.00289-7
- Gulsen MT, Savas MC, Koruk M, Kadayifci A,
Demirci F, 2006. Fascioliasis: a report of five cases presenting with
common bile duct obstruction. Neth J Med 2006; 64: 17-9 PMid:16421437

- Elliott DE. Intestinal infections by parasitic worms. In: Feldman M, Friedman LS, and Brand LJ, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 9th ed. Philadelphia: Elsevier, 2010: 1921-39. http://dx.doi.org/10.1016/B978-1-4160-6189-2.00110-4
- Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126(1):39-44. http://dx.doi.org/10.1016/j.jaci.2010.04.011 PMid:20538328 PMCid:2902584

- Demirci M, Korkmaz M, Sakru N, Kaya S,
Kuman A. Diagnostic importance of serological methods and eosinophilia
in tissue parasites. J Health Popul Nutr 2002; 20: 352-355.
PMid:12659417

- Hamamcı B, Çetinkaya Ü, Yaman O, Kaya M,
Yazar S. Investigation of Fasciola hepatica Antibodies in Foreign High
School Students in Kayseri. Türk Hijyen ve Deneysel Biyoloji Dergisi
2010; 67 (3): 121-6.

- Yilmaz H, Godekmerdan A. Human fasciolosis in Van province, Turkey. Acta Trop 2004; 92: 161-2. http://dx.doi.org/10.1016/j.actatropica.2004.04.009 PMid:15350869

- World Health Organization. Report of the WHO Informal Meeting on use of triclabendazole in fascioliasis control. Geneva: WHO, 2007.
- Rowan SE, Levi ME, Youngwerth JM, Brauer B,
Everson GT, Johnson S. The Variable Presentations and Broadening
Geographic Distribution of Hepatic Fascioliasis. Clin Gastroenterol
Hepatol. 2012 Feb 25. [Epub ahead of print]).

- Karahocagil MK, Akdeniz H, Sunnetcioglu M,
Cicek M, Mete R, Akman N, Ceylan E, Karsen H, Yapici K. A familial
outbreak of fascioliasis in Eastern Anatolia: a report with review of
literature. Acta Trop 2011; 118(3): 177-83. http://dx.doi.org/10.1016/j.actatropica.2008.08.013 PMid:18930014

- Fica A, Dabanch J, Farias C, Castro M,
Jercic MI, Weitzel T. Acute fascioliasis--clinical and epidemiological
features of four patients in Chile. Clin Microbiol Infect 2012; 18(1):
91-6. http://dx.doi.org/10.1111/j.1469-0691.2011.03575.x PMid:21668579

- Valero MA, Perez-Crespo I, Khoubbane M,
Artigas P, Panova M, Ortiz P, Maco V, Espinoza JR, Mas-Coma S. Fasciola
hepatica phenotypic characterization in Andean human endemic areas:
Valley versus altiplanic patterns analysed in liver flukes from sheep
from Cajamarca and Mantaro, Peru. Infect Genet Evol 2012; 12(2):
403-10. http://dx.doi.org/10.1016/j.meegid.2012.01.009 PMid:22285769

- Aksoy DY, Kerimoglu U, Oto A, Erguven S,
Arslan S, Unal S, et al. Infection with Fasciola hepatica. Clin
Microbiol Infect 2005; 11: 859-61. http://dx.doi.org/10.1111/j.1469-0691.2005.01254.x PMid:16216098

- Lopez M, White AC Jr, Cabada MM. Burden of
Fasciola hepatica Infection among Children from Paucartambo in Cusco,
Peru. Am J Trop Med Hyg 2012; 86(3): 481-5. http://dx.doi.org/10.4269/ajtmh.2012.11-0448 PMid:22403322

- Azab M el-S, el Zayat EA. Evaluation of
purified antigens in haemagglutination test (IHA) for determination of
cross reactivities in diagnosis of fascioliasis and schistosomiasis. J
Egypt Soc Parasitol 1996; 26(3): 677-85. PMid:8918041

- Adela Valero M, Victoria Periago M,
Pérez-Crespo I, Rodríguez E, Jesús Perteguer M, Gárate T,
González-Barberá EM, Mas-Coma S. Assessing the validity of an ELISA
test for the serological diagnosis of human fascioliasis in different
epidemiological situations. Trop Med Int Health. 2012 Mar 13.. [Epub
ahead of print]. http://dx.doi.org/10.1111/j.1365-3156.2012.02964.x

- Gordon DK, Zadoks RN, Stevenson H,
Sargison ND, Skuce PJ. On farm evaluation of the coproantigen ELISA and
coproantigen reduction test in Scottish sheep naturally infected with
Fasciola hepatica. Vet Parasitol. 2012 Feb 21. [Epub ahead of print]). http://dx.doi.org/10.1016/j.vetpar.2012.02.009

- Dusak A, Onur MR, Cicek M, Firat U, Ren T,
Dogra VS. Radiological Imaging Features of Fasciola hepatica Infection
- A Pictorial Review. J Clin Imaging Sci. 2012; 2: 2. http://dx.doi.org/10.4103/2156-7514.92372 PMid:22347685 PMCid:3279695

- Koç Z, Ulusan Ş, Tokmak N. Hepatobiliary
fascioliasis: imaging characteristics with a new finding. Diagn Interv
Radiol 2009; 15: 247-51. PMid:19908182

- Kaya M, Beştaş R, Cetin S. Clinical
presentation and management of Fasciola hepatica infection:
single-center experience. World J Gastroenterol 2011; 17(44): 4899-904.
http://dx.doi.org/10.3748/wjg.v17.i44.4899 PMid:22171131 PMCid:3235633
