Malaria and Hiv in Adults: when the Parasite Runs into the Virus
Emanuele Foc�, Silvia Odolini, Nigritella Brianese and Giampiero Carosi
Institute for Infectious and Tropical Diseases, University of Brescia. Brescia, Italy
Correspondence
to:
Emanuele Foc�, MD. Institute of Infectious and Tropical Diseases,
University of Brescia, School of Medicine, P.le Spedali Civili, 1 25123
Brescia (Italy). Tel: +39.030.3995677; Fax: +39.030.303061. E-mail: emanuelefoca@gmail.com
Published: May 7, 2012
Received: March 28, 2012
Accepted: May 2, 2012
Mediterr J Hematol Infect Dis 2012, 4(1): e2012032, 10.4084/MJHID.2012.032
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Malaria
and HIV/AIDS are among the principal causes of morbidity and mortality
worldwide, particularly in resource-limited settings such as
sub-Saharan Africa. Despite the international community’s efforts to
reduce incidence and prevalence of these diseases, they remain a global
public health problem. Clinical manifestations of malaria may be more
severe in HIV infected patients, which have higher risks of severe
malaria and malaria related death. Co-infected pregnant women, children
and international travelers from non-malaria endemic countries are at
higher risk of clinical complications. However, there is a paucity and
conflicting data regarding malaria and HIV co-infection, particularly
on how HIV infection can modify the response to antimalarial drugs and
about drug-interactions between antiretroviral agents and
artemisinin-based combined regimens. Moreover, consulting HIV-infected
international travelers and physicians specialized in HIV care and
travel medicine should prescribe an adequate chemoprophylaxis in
patients travelling towards malaria endemic areas and pay attention on
interactions between antiretrovirals and antimalarial prophylaxis drugs
in order to prevent clinical complications of this co-infection.
This review aims to evaluate the available international literature on malaria and HIV co-infection in adults providing a critical comprehensive review of nowadays knowledge.
This review aims to evaluate the available international literature on malaria and HIV co-infection in adults providing a critical comprehensive review of nowadays knowledge.
Introduction
Malaria is one of the most important causes of morbidity and mortality in tropical regions, especially in sub-Saharan Africa and South-East Asia.[1] The disease is caused by an infection sustained by a parasite of the genus Plasmodium; five species of Plasmodium (P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi) can infect human and among these species, P. falciparum infection may be fatal.[2]
According to the World Malaria Report 2010, there were 225 million cases of malaria and an estimated 781 000 deaths in 2009. Most deaths occur among children living in Africa where a child dies every 45 seconds for malaria and this disease accounts for approximately 20% of all childhood deaths.[2]
Human Immunodeficiency Virus (HIV) is a retrovirus that can infect immune competent cells causing an impairment of host defense. After the introduction of Highly Active Antiretroviral Therapy (HAART) in 1996, the epidemiology of HIV infection has changed and clinical evolution turned a fatal disease in a treatable chronic infection with an improved quality of life and a reduction of morbidity and mortality.[3] However, nowadays there are still alarming figures of HIV/AIDS infected people worldwide: in 2010 it was estimated that people living with HIV infection were 34 million, 2.7 million people became newly infected in the same year and AIDS-related deaths were 1.8 million, including 250 000 children. Two thirds of HIV infections were in sub-Saharan Africa.[4] Therefore, there is a critical overlap between the two infections especially in sub-Saharan Africa resulting in particular concern for Public Health. The increasing number of people potentially co-infected makes this topic of particular interest in order to correctly understand and control both infections and their particular interactions.[5]
Clinical presentation of malaria in HIV-infected people, even if partially immune to Plasmodium, may be more severe and, although malaria is not the main cause of death among HIV-infected patients,[6] in a systematic review of HIV-1 infection in Africa, malaria was identified as the third cause of HIV-related morbidity.[7] This is more evident in special populations such as HIV-infected pregnant women,[8] as well as in adults male and female with an severely impaired immune system[9] and in HIV-infected travelers.[10]
Moreover, despite the importance of proper treatments against both infections, there is a lack of data regarding treatment issue: conflicting data are presented in literature about the effects of antimalarial drugs (for treatment and chemoprophylaxis) on antiretroviral agents and vice-versa and data about drug-interactions between antimalarial (especially artemisinin-based combinated treatment – ACT) and antiretroviral drugs are missing.[11]
Purpose of this article is to review the current knowledge on clinical tools of malaria and HIV co-infection in adults, focusing on pregnant women and international travellers, and to explore the interactions between antiretrovirals and antimalarial drugs, suggesting future research priorities.
Interactions Between Malaria and HIV Infection.
Sub-Saharan Africa represents the region most heavily affected by both malaria and HIV. In this setting, the overlap of these two infections is common and it would be important to understand their interactions and their correct management in order to limit their clinical burden.[12]
The influences between HIV and malaria are bidirectional and synergistic,[5,13] and the negative effects of this co-infection, for the most part, seems to be due to immunological interactions: HIV replication impairs immune system and consequently malaria control;[14] on the other hand, malaria itself enhances HIV replication by cytokines release and T-cell activation.[15]
Looking at HIV-infection during malaria, it seems that HIV infection worsens the capability to control parasitaemia because of deterioration of immune responses to malaria parasites; several studies reported an association between HIV infection and higher levels of malaria parasitaemia. Whitworth et al. demonstrated that higher levels of parasitaemia were more frequently detected among HIV-positive patients compared to those mono-infected (11.8% vs 6.3%, p<0.0001), moreover they found an association between lower CD4+ T-cell count and higher levels of parasitaemia (p=0.0076), and lower CD4+ T-cell count and risk of clinical manifestations of malaria.[14] French et al. confirmed this data finding that incidence rates of P. falciparum malarial fever were indirectly proportional to CD4+ T-cell count,[16] while Patnaik and colleagues supported the relationship between CD4+ T cell count and malaria parasitaemia in HIV-seropositive subjects finding an adjusted hazard ratio of 1.8 for a first parasitaemia episode, and of 2.5 for a second parasitaemia episode.[17]
The intensity of malaria transmission and the population’s level of acquired immunity may influence the clinical impact of malaria. Two major levels of malaria endemicity are described: i) areas of high (stable) malaria transmission, where most of adults have developed enough immunity that lead to poor clinical manifestations of malaria infection and where the reported incidence of malaria was ≥ 1/1000 per year in 2009;4 ii) areas of low (unstable) malaria transmission, where people have not acquired a significant level of immunity with consequent high level of clinical appearance of the infection. In these areas the transmission is more often seasonal and the reported incidence of malaria was < 1/1000 population per year in 2009.[4] Severe malaria and consequent deaths seem to be higher in unstable transmission areas,[18] but some studies have demonstrated an increase in malaria incidence in regions of stable malaria transmission when it is associated with HIV-infection.[14,16] Whitworth’s study, previously mentioned, was conducted in malaria endemic regions in Uganda, and demonstrated that risk of clinical malaria was 4.0% among HIV-1-positive patients and 1.9% among HIV-negative patients;[14] also French’s trial was conducted among people from Uganda. However, some studies show conflicting data not describing an increase in severity of malaria from areas of stable transmission. Cohen et al. found a statistical significant correlation (p=0.003) between severe malaria and HIV infection among people nonimmune to malaria, instead this association was not found in semi-immune patients (p=0.284).[9] Other studies[19,20] concluded for the lack of association between HIV infection and malaria outcomes, but they suffer form some limitations: patients analysed were predominantly children[19] or only children[20] that, for their characteristic anti-parasite immunity are not comparable with adult population.[16]
HIV-positive patients with malaria presented higher incidence of anaemia,[21] as demonstrated in a study in which co-infected patients showed lower haemoglobin levels compared to subjects with only malaria;[22] in fact, both malaria and HIV may cause anaemia, as well as some drugs (e.g. zidovudine – AZT) used for HIV treatment.[23]
Looking now at the consequences of malaria infection on HIV disease progression, several studies showed an increased HIV-RNA replication in patients with malaria. Hoffman et al found 7-fold higher levels of HIV-1 RNA in patients with malaria than in those with only HIV infection (p <0.0001)[24] and another study showed that HIV-1 RNA concentrations were even 10-fold higher in co-infected patients in respect of HIV mono-infected patients.[5] In a trial conducted in Malawi, Kublin and colleagues demonstrated that viral load returned at baseline levels only after 8-9 weeks of antimalarial therapy.[25] Epidemiological implications of this aspect are that higher HIV-transmission rates are present in co-infected patients.[26]
Based on data presented, we suggest a strict monitoring of co-infected patients in order to improve the outcomes of these two infections, in particular for people most at risk of complications, such as pregnant women, in particular in stable malaria transmission areas where adults’ parasitaemia values are often below threshold of detection.
Malaria and HIV Co-Infection in Pregnant Women.
It is estimated that each year about 24 million pregnant women are infected by P. falciparum, especially in sub-Saharan Africa[8] and about 1 million per year are co-infected with HIV.[27] It is known that co-infected pregnant women are particularly at risk of complications due to these two infections; in fact, women during pregnancy are more likely susceptible to malaria disease than non-pregnant women,[28] and a down regulation of the adaptive immune response was observed with a consequent enhanced placental invasion.[29] High levels of parasitaemia and chronic parasite infection in placental blood can lead to consumption of nutritive blood substances,[30] to a worsening of perinatal outcomes and to increased rates of maternal morbidity.[31] In this context co-infection with HIV represents a further immune system impairment, which can be a concurrent cause of uneffectiveness in parasitaemia control.[12]
Consequences of this co-infection are described in both directions: HIV may promote more severe malaria clinical manifestations and, on the other hand, malaria can favours HIV RNA replication threatening antiretroviral treatment effectiveness.[32]
In co-infected pregnant women, all the adverse pregnancy outcomes are present. HIV impaired malaria outcomes inducing chronic parasitaemia, higher parasite densities and fever, reflecting in more severe clinical malaria manifestations.[12,33] In a study conducted by ter Kuile and colleagues data from several works were summarized: the risk ratio [RR] of malaria parasitaemia in co-infected women were 1.58 during pregnancy, 1.66 at the time of delivery and 1.66 in the placenta in respect of HIV-uninfected women.[34] There is also an higher risk of postpartum maternal anaemia: Ayisi et al. found that the probability to manifest this clinical condition was more than twice higher in women co-infected than in HIV-uninfected women.[35] Malaria and HIV infections were associated with several negative outcomes in newborns to co-infected mothers. Ticconi et al. demonstrated an association between co-infection and an increased risk of stillbirth (OR = 4.74, 95% CI: 1.34-16.78) and preterm delivery (OR = 4.10, 95% CI: 2.17-7.75). The two infections, instead, resulted independently associated with an increased risk of low birth weight (malaria: OR = 10.09, 95% CI: 6.50-15.65; HIV: OR = 3.16, 95% CI: 1.80-5.54) and foetal growth retardation (malaria: OR = 3.98, 95% CI: 2.51-6.30; HIV: OR = 4.07, 95% CI: 2.40-6.92) compared to HIV-uninfected women.[36] Low birth weight (LBW) prevalence appear to be higher in co-infected women (P=0.001) compared to HIV mono-infected women (p=0.09) and malaria infected alone (P=0.006). In addition infant mortality seems to be higher in infants born to co-infected women. A study conducted in Malawi reported significantly higher mortality rates among children born to HIV-seropositive compared to HIV-seronegative women, and in the multivariate model the risk of neonatal mortality was 4.5 greater in co-infected mother compared to mother with only placental malaria and 2.7-7.7 greater in HIV mono-infected mothers.[37]
As previously said, malaria promotes HIV RNA replication: a study showed that women had about 2-fold increase in HIV-1-RNA both in peripheral and placental blood, with a consequent higher possibility of mother-to-child transmission of HIV,[38] even if, at present, a clear association between placental malaria and an increase of mother-to-child transmission of HIV infection still remains uncertain.[12]
An appropriate control of both infections is a priority either for women and for their infants. The main recommendations are: a prompt HAART initiation in pregnant women as soon as the eligibility criteria are met, or an effective antiretroviral prophylaxis if criteria for initiating ART are not present, according to World Health Organization (WHO) specific guidelines.[39] Therefore a proper prophylaxis with co-trimoxazole after the first trimester of pregnancy seems to prevent both opportunistic infections and malaria.[34]
Malaria in HIV-Infected International Travellers.
According to the World Tourism Organization (WTO), an increasing number of international travellers has been reported, from 50 million/year after second World War to 980 million/year during 2011.[40] Approximately 80 million persons from industrialized nations travel to the tropical world each year.[41] Imported malaria mostly occurs in tourists and migrants travelling to their origin countries to visit friends and relatives (VFR)[42] but higher rates of malaria infection have been reported also among HIV-infected people.[43]
This is mainly due to the introduction of HAART and the improved quality of life allowed a steady increase of international travels, especially to tropical areas.[44] A recent report showed that international travel among HIV-infected people was associated with poor adherence to antiretroviral therapy, risky sexual practices and risky exposure to travel-related diseases.[45] Although any travel-related disease may have more severe clinical manifestations in HIV positive travellers compared to HIV-uninfected persons, malaria is certainly one of the most dangerous conditions;[46] therefore HIV-infected patients should be more aware of the necessity for medical counsel prior to travel[47,48] particularly to prevent malaria infection.
Several cases of imported malaria, as well severe cases with fatal outcome, have been reported;[49,50,51,52] however clinical presentation is often similar to HIV-negative patients as recently reported in a retrospective study conducted in Spain.[53] These data suggest that more caution should be taken during the pre-travel counselling.
WHO recommends a viro-immunological parameters check (CD4+ T cell count and HIV viraemia at least) and a clinical examination before travelling.[54] Obviously, for HIV-infected pregnant women and young children travel should be avoided if not strictly necessary as they have higher risks of severe malaria clinical manifestations.
Then, physicians involved in HIV care as well as in travel medicine should consider several factors in pre-travel counselling for HIV-infected travellers such as reason for travel (tourism, VFR, business etc.), travel risk (pre-arranged or organized travel), travel destination, season and malarial epidemic cycle as well as Plasmodium drug sensitivity in the specific area;[10] moreover HIV-infected people travelling to malaria endemic-areas need to start a correct chemoprophylaxis regimen before their travel and counsellors should consider possible drug interactions between antiretroviral and antimalarial drugs.[10]
Therefore the importance of behavioral preventive measures (bed nets, repellents, etc.), adequate chemoprophylaxis and, in selected circumstances, stand-by emergency treatment in case of evocative symptoms should be strongly recommended.
Effects of Co-Trimoxazole Prophylaxis and Antiretroviral Therapy on Malaria.
According to major International Guidelines[55,56] HAART regimens should contain at least three active drugs from two different classes, usually 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs) accompanied with an anchor drug which is either a non-nucleoside reverse transcriptase inhibitors (NNRTI) or a protease inhibitor (PI) with a low-dose of ritonavir (RTV) as “booster”. Sustained virological suppression and CD4+ T cell count recovery is the main objective of HAART.[55] In HIV-infected patients, daily co-trimoxazole should to be prescribed as prophylaxis against major opportunistic infections when CD4+ T cells are less than 200/�l;[57] however several findings demonstrated that daily co-trimoxazole prescription may reduce the occurrence of parasitaemia[58] and clinical malaria either in adults and in children.[59,60,61]
For example, Mermin J and colleagues[58] found that among HIV-infected children the use of co-trimoxazole prophylaxis and insecticide-treated nets might reduce the prevalence of clinical malaria by 97% versus the reduction of 43% with nets alone. Therefore co-trimoxazole prescription in immunocompromised patients, in addition at insecticide-treated nets, seems to be an effective strategy to prevent malaria. However, cross resistance between co-trimoxazole and sulfadoxine-pyrimethamine (SP) has been reported,[62] therefore widely use of co-trimoxazole may promote specific SP resistance; some studies did not find SP related resistances despite co-trimoxazole prescription,[63,64] while others found that co-trimoxazole prophylaxis was effective despite antifolate resistance.[60]
Therefore some authors suggested that a continuation of prophylaxis with co-trimoxazole in malaria endemic areas is beneficial even when patients have been immune restored.[11] Antiretroviral therapy may be effective against malaria infection: Mermin J et al[65] in a prospective cohort study found that HAART containing NRTIs when co-administrated with co-trimoxazole decrease malaria clinical presentation in respect of co-trimoxazole alone; in order to provide an explanation on the possible antimalarial activity of NRTIs, authors hypothesized that HAART probably reduced the frequency of malaria by improving immune function rather than by a direct antimalarial effect. A delayed P. falciparum clearance in HIV-infected patients treated with artemisinin-based regimens has been reported[66] suggesting that immune defences of HIV-infected people may affect effectiveness of antimalarial treatment. Other studies evaluating impact of PIs are ongoing (e.g. NCT00719602) but available studies “in vitro” showed that the PIs lopinavir, saquinavir, indinavir, atazanavir ritonavir have direct effect on inhibition of P. falciparum, at concentration used in clinical practice.[67,68] Anyway, antiretroviral treatment maybe effective against P. falciparum and may reduce malarial clinical manifestation, but, in order to better clarify this hypothesis, its underlying biological mechanisms and the role of antiretroviral drugs or classes need further investigations.
Interactions Between Antiretrovirals and Antimalarial Agents:
Antiretrovirals and antimalarial drugs (treatment). Pharmacological interactions between antiretroviral and antimalarial drugs has been previously extensively reviewed,[69,13] however the knowledge about this critical topic is still debated and data reported are not definitive, particularly due to either the paucity of data about newer drugs and classes of antiretroviral agents and the absence of specific guidelines. Moreover the relevance of “in vitro” studies in common clinical practice is controversial. According to WHO, first-line treatment for uncomplicated malaria in high-endemicity areas consists of a combination of either artemether plus lumefantrine or artesunate plus one of the following drugs: amodiaquine, mefloquine, or sulfadoxine–pyrimethamine, or dihydroartemisinin–piperaquine.[1] Interactions between amodiaquine and efavirenz has been reported reflecting in higher amodiquine concentrations;[70] these evidences may have a clinical impact, as a significant number of HIV-infected patients in sub-Saharan Africa receive an efavirenz-based treatment[57] and WHO contraindicates this co-administration;[27] furthermore, given that these drugs are both potentially hepatotoxic[71,72] patients prescribing this combination have to be strictly monitored. Finally, we know that quinine, halofantrine, and lumefantrine are all antimalarial drugs metabolized through the cytochrome P-450 enzyme system;[73,74] so that, these drugs may potentially interact with the non-nucleoside reverse transcriptase inhibitors (NNRTIs) resulting in reduced bioavailability of the antimalarial drugs.[75] Recently, a cross-sectional study in HIV and malaria co-infected adults showed significant pharmacokinetic interactions among nevirapine, artemether and dihydroartemisinin: these interactions maybe increase the risk of treatment failure of both infections and the risk for development of drug resistance.[76] Drug-interactions with PIs, that are also metabolized through the hepatic cytochrome P-450 enzyme system, and enzyme inhibition by ritonavir may on the contrary increase serum concentrations of these antimalarials.[77] Despite this study, performed in healthy volunteers, ritonavir might boost the bioavailability of either PIs and antimalarial drugs, however this altered pharmacokinetics do not seems clinically relevant. By contrast, a recent study confirmed that LPV/r-based HAART significantly increases lumefantrine exposure without an increase in adverse effects. Artemether and dihydroartemisinin concentrations were also significantly increased by ritonavir boosted lopinavir-based treatment, but to a lesser extent.[78] Data from randomized-controlled trials will be highly valuable in evaluating the clinical significance of all these interactions.
Most of potential clinically significant interactions between antimalarial and antiretrovirals drugs are showed in Table 1.
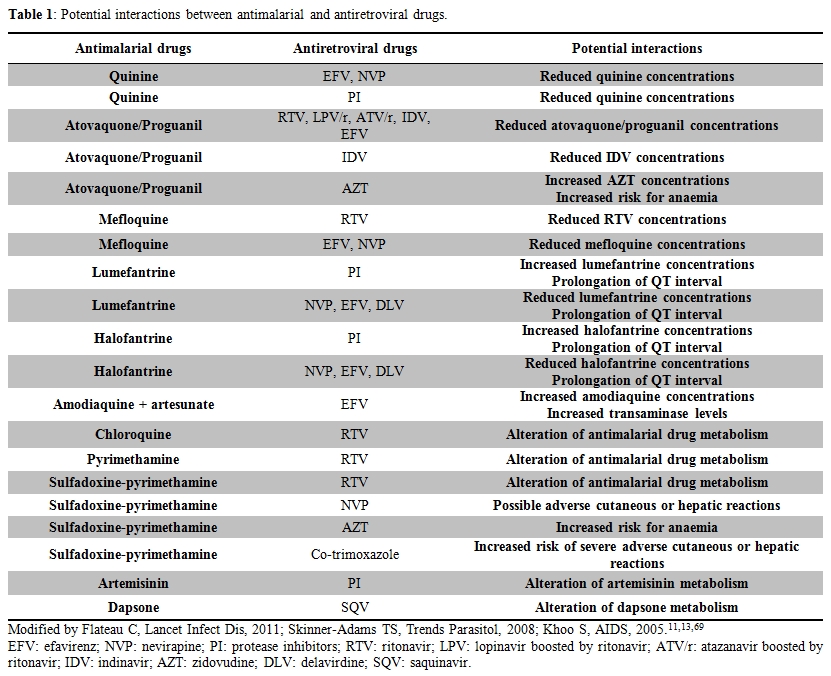
Table 1. Potential interactions between antimalarial and antiretroviral drugs.
Malaria chemoprophylaxis in HIV infected subjects. Despite several reports and reviews on malaria and HIV co-infection, few data are present in literature about the use of malaria chemoprophylaxis in HIV infected travellers.[57] Mefloquine reduces some ritonavir boosted protease inhibitors levels and mefloquine plasma levels could be reduced by efavirenz and nevirapine, but these data are from patients who received mefloquine as treatment, little is known about its use in chemoprophylactic regimen. Atovaquone/proguanil levels could be reduced by indinavir, as well as lopinavir, atazanavir, ritonavir and efavirenz;[79] moreover, atovaquone/proguanil could reduce indinavir levels and increase zidovudine levels causing hematological troubles.[80] No significant data are available on doxycycline interactions.[81] All these considerations should be taken into account before starting chemoprophylaxis in HIV infected travelers taking HAART, and drugs dosages should be arranged in order to avoid drug interactions. If chemoprophylaxis is not administered, atovaquone/proguanil might be a suitable stand-by emergency treatment (SBET) option for HIV-infected subjects receiving NNRTI-based regimens, while mefloquine could be considered as SBET drug in those prescribing PI-based HAART.[82]
Conclusion And Future Research Perspectives.
Malaria and HIV infections are two of the most important infectious diseases worldwide, particularly in sub-Saharan Africa: their overlapping epidemiology as well their impact in clinical practice needs to be continuously updated.
HIV-infected adults, especially if international travelers, even with a restored immunity, are more likely to have severe clinical manifestations of malaria compared to travellers without HIV infection. Moreover co-infection in pregnant women contributes to develop clinical malaria in women, maternal severe anaemia, grater rates of stillbirth and preterm delivery, low birth weight, foetal grow retardation and increased risk of infant mortality.
The wider implementation of co-trimoxazole prophylaxis and antiretroviral therapy could change the impact of malaria in HIV-infected patients.
The benefits of the continuation of co-trimoxazole prophylaxis to prevent malaria, the antimalarial effects of several antiretroviral agents, as well the interactions between antiretroviral and antimalarial drugs, need to be further investigated. The proper malaria prophylaxis and treatment in HIV-infected pregnant women also needs to be defined. Several clinical trials are ongoing to address several of these opened questions: the results of these trials are needed to create specific guidelines for the prevention and management of malaria and HIV co-infection and to help clinicians involved in travel medicine as well in HIV care.
Authors’ Contributions.
All authors conceived the concept and design of the review. EF, SO, and NB wrote and GC critically revised the review. All authors approved the final version for submission.
Conflict of Interest.
EF and GC have received unrestricted educational grants (as speakers or for participation to conferences) from several Pharmaceuticals Companies producing antiretroviral drugs but this did not influence the content of this review. SO and NB declare no conflicts of interest.
Malaria is one of the most important causes of morbidity and mortality in tropical regions, especially in sub-Saharan Africa and South-East Asia.[1] The disease is caused by an infection sustained by a parasite of the genus Plasmodium; five species of Plasmodium (P. falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi) can infect human and among these species, P. falciparum infection may be fatal.[2]
According to the World Malaria Report 2010, there were 225 million cases of malaria and an estimated 781 000 deaths in 2009. Most deaths occur among children living in Africa where a child dies every 45 seconds for malaria and this disease accounts for approximately 20% of all childhood deaths.[2]
Human Immunodeficiency Virus (HIV) is a retrovirus that can infect immune competent cells causing an impairment of host defense. After the introduction of Highly Active Antiretroviral Therapy (HAART) in 1996, the epidemiology of HIV infection has changed and clinical evolution turned a fatal disease in a treatable chronic infection with an improved quality of life and a reduction of morbidity and mortality.[3] However, nowadays there are still alarming figures of HIV/AIDS infected people worldwide: in 2010 it was estimated that people living with HIV infection were 34 million, 2.7 million people became newly infected in the same year and AIDS-related deaths were 1.8 million, including 250 000 children. Two thirds of HIV infections were in sub-Saharan Africa.[4] Therefore, there is a critical overlap between the two infections especially in sub-Saharan Africa resulting in particular concern for Public Health. The increasing number of people potentially co-infected makes this topic of particular interest in order to correctly understand and control both infections and their particular interactions.[5]
Clinical presentation of malaria in HIV-infected people, even if partially immune to Plasmodium, may be more severe and, although malaria is not the main cause of death among HIV-infected patients,[6] in a systematic review of HIV-1 infection in Africa, malaria was identified as the third cause of HIV-related morbidity.[7] This is more evident in special populations such as HIV-infected pregnant women,[8] as well as in adults male and female with an severely impaired immune system[9] and in HIV-infected travelers.[10]
Moreover, despite the importance of proper treatments against both infections, there is a lack of data regarding treatment issue: conflicting data are presented in literature about the effects of antimalarial drugs (for treatment and chemoprophylaxis) on antiretroviral agents and vice-versa and data about drug-interactions between antimalarial (especially artemisinin-based combinated treatment – ACT) and antiretroviral drugs are missing.[11]
Purpose of this article is to review the current knowledge on clinical tools of malaria and HIV co-infection in adults, focusing on pregnant women and international travellers, and to explore the interactions between antiretrovirals and antimalarial drugs, suggesting future research priorities.
Interactions Between Malaria and HIV Infection.
Sub-Saharan Africa represents the region most heavily affected by both malaria and HIV. In this setting, the overlap of these two infections is common and it would be important to understand their interactions and their correct management in order to limit their clinical burden.[12]
The influences between HIV and malaria are bidirectional and synergistic,[5,13] and the negative effects of this co-infection, for the most part, seems to be due to immunological interactions: HIV replication impairs immune system and consequently malaria control;[14] on the other hand, malaria itself enhances HIV replication by cytokines release and T-cell activation.[15]
Looking at HIV-infection during malaria, it seems that HIV infection worsens the capability to control parasitaemia because of deterioration of immune responses to malaria parasites; several studies reported an association between HIV infection and higher levels of malaria parasitaemia. Whitworth et al. demonstrated that higher levels of parasitaemia were more frequently detected among HIV-positive patients compared to those mono-infected (11.8% vs 6.3%, p<0.0001), moreover they found an association between lower CD4+ T-cell count and higher levels of parasitaemia (p=0.0076), and lower CD4+ T-cell count and risk of clinical manifestations of malaria.[14] French et al. confirmed this data finding that incidence rates of P. falciparum malarial fever were indirectly proportional to CD4+ T-cell count,[16] while Patnaik and colleagues supported the relationship between CD4+ T cell count and malaria parasitaemia in HIV-seropositive subjects finding an adjusted hazard ratio of 1.8 for a first parasitaemia episode, and of 2.5 for a second parasitaemia episode.[17]
The intensity of malaria transmission and the population’s level of acquired immunity may influence the clinical impact of malaria. Two major levels of malaria endemicity are described: i) areas of high (stable) malaria transmission, where most of adults have developed enough immunity that lead to poor clinical manifestations of malaria infection and where the reported incidence of malaria was ≥ 1/1000 per year in 2009;4 ii) areas of low (unstable) malaria transmission, where people have not acquired a significant level of immunity with consequent high level of clinical appearance of the infection. In these areas the transmission is more often seasonal and the reported incidence of malaria was < 1/1000 population per year in 2009.[4] Severe malaria and consequent deaths seem to be higher in unstable transmission areas,[18] but some studies have demonstrated an increase in malaria incidence in regions of stable malaria transmission when it is associated with HIV-infection.[14,16] Whitworth’s study, previously mentioned, was conducted in malaria endemic regions in Uganda, and demonstrated that risk of clinical malaria was 4.0% among HIV-1-positive patients and 1.9% among HIV-negative patients;[14] also French’s trial was conducted among people from Uganda. However, some studies show conflicting data not describing an increase in severity of malaria from areas of stable transmission. Cohen et al. found a statistical significant correlation (p=0.003) between severe malaria and HIV infection among people nonimmune to malaria, instead this association was not found in semi-immune patients (p=0.284).[9] Other studies[19,20] concluded for the lack of association between HIV infection and malaria outcomes, but they suffer form some limitations: patients analysed were predominantly children[19] or only children[20] that, for their characteristic anti-parasite immunity are not comparable with adult population.[16]
HIV-positive patients with malaria presented higher incidence of anaemia,[21] as demonstrated in a study in which co-infected patients showed lower haemoglobin levels compared to subjects with only malaria;[22] in fact, both malaria and HIV may cause anaemia, as well as some drugs (e.g. zidovudine – AZT) used for HIV treatment.[23]
Looking now at the consequences of malaria infection on HIV disease progression, several studies showed an increased HIV-RNA replication in patients with malaria. Hoffman et al found 7-fold higher levels of HIV-1 RNA in patients with malaria than in those with only HIV infection (p <0.0001)[24] and another study showed that HIV-1 RNA concentrations were even 10-fold higher in co-infected patients in respect of HIV mono-infected patients.[5] In a trial conducted in Malawi, Kublin and colleagues demonstrated that viral load returned at baseline levels only after 8-9 weeks of antimalarial therapy.[25] Epidemiological implications of this aspect are that higher HIV-transmission rates are present in co-infected patients.[26]
Based on data presented, we suggest a strict monitoring of co-infected patients in order to improve the outcomes of these two infections, in particular for people most at risk of complications, such as pregnant women, in particular in stable malaria transmission areas where adults’ parasitaemia values are often below threshold of detection.
Malaria and HIV Co-Infection in Pregnant Women.
It is estimated that each year about 24 million pregnant women are infected by P. falciparum, especially in sub-Saharan Africa[8] and about 1 million per year are co-infected with HIV.[27] It is known that co-infected pregnant women are particularly at risk of complications due to these two infections; in fact, women during pregnancy are more likely susceptible to malaria disease than non-pregnant women,[28] and a down regulation of the adaptive immune response was observed with a consequent enhanced placental invasion.[29] High levels of parasitaemia and chronic parasite infection in placental blood can lead to consumption of nutritive blood substances,[30] to a worsening of perinatal outcomes and to increased rates of maternal morbidity.[31] In this context co-infection with HIV represents a further immune system impairment, which can be a concurrent cause of uneffectiveness in parasitaemia control.[12]
Consequences of this co-infection are described in both directions: HIV may promote more severe malaria clinical manifestations and, on the other hand, malaria can favours HIV RNA replication threatening antiretroviral treatment effectiveness.[32]
In co-infected pregnant women, all the adverse pregnancy outcomes are present. HIV impaired malaria outcomes inducing chronic parasitaemia, higher parasite densities and fever, reflecting in more severe clinical malaria manifestations.[12,33] In a study conducted by ter Kuile and colleagues data from several works were summarized: the risk ratio [RR] of malaria parasitaemia in co-infected women were 1.58 during pregnancy, 1.66 at the time of delivery and 1.66 in the placenta in respect of HIV-uninfected women.[34] There is also an higher risk of postpartum maternal anaemia: Ayisi et al. found that the probability to manifest this clinical condition was more than twice higher in women co-infected than in HIV-uninfected women.[35] Malaria and HIV infections were associated with several negative outcomes in newborns to co-infected mothers. Ticconi et al. demonstrated an association between co-infection and an increased risk of stillbirth (OR = 4.74, 95% CI: 1.34-16.78) and preterm delivery (OR = 4.10, 95% CI: 2.17-7.75). The two infections, instead, resulted independently associated with an increased risk of low birth weight (malaria: OR = 10.09, 95% CI: 6.50-15.65; HIV: OR = 3.16, 95% CI: 1.80-5.54) and foetal growth retardation (malaria: OR = 3.98, 95% CI: 2.51-6.30; HIV: OR = 4.07, 95% CI: 2.40-6.92) compared to HIV-uninfected women.[36] Low birth weight (LBW) prevalence appear to be higher in co-infected women (P=0.001) compared to HIV mono-infected women (p=0.09) and malaria infected alone (P=0.006). In addition infant mortality seems to be higher in infants born to co-infected women. A study conducted in Malawi reported significantly higher mortality rates among children born to HIV-seropositive compared to HIV-seronegative women, and in the multivariate model the risk of neonatal mortality was 4.5 greater in co-infected mother compared to mother with only placental malaria and 2.7-7.7 greater in HIV mono-infected mothers.[37]
As previously said, malaria promotes HIV RNA replication: a study showed that women had about 2-fold increase in HIV-1-RNA both in peripheral and placental blood, with a consequent higher possibility of mother-to-child transmission of HIV,[38] even if, at present, a clear association between placental malaria and an increase of mother-to-child transmission of HIV infection still remains uncertain.[12]
An appropriate control of both infections is a priority either for women and for their infants. The main recommendations are: a prompt HAART initiation in pregnant women as soon as the eligibility criteria are met, or an effective antiretroviral prophylaxis if criteria for initiating ART are not present, according to World Health Organization (WHO) specific guidelines.[39] Therefore a proper prophylaxis with co-trimoxazole after the first trimester of pregnancy seems to prevent both opportunistic infections and malaria.[34]
Malaria in HIV-Infected International Travellers.
According to the World Tourism Organization (WTO), an increasing number of international travellers has been reported, from 50 million/year after second World War to 980 million/year during 2011.[40] Approximately 80 million persons from industrialized nations travel to the tropical world each year.[41] Imported malaria mostly occurs in tourists and migrants travelling to their origin countries to visit friends and relatives (VFR)[42] but higher rates of malaria infection have been reported also among HIV-infected people.[43]
This is mainly due to the introduction of HAART and the improved quality of life allowed a steady increase of international travels, especially to tropical areas.[44] A recent report showed that international travel among HIV-infected people was associated with poor adherence to antiretroviral therapy, risky sexual practices and risky exposure to travel-related diseases.[45] Although any travel-related disease may have more severe clinical manifestations in HIV positive travellers compared to HIV-uninfected persons, malaria is certainly one of the most dangerous conditions;[46] therefore HIV-infected patients should be more aware of the necessity for medical counsel prior to travel[47,48] particularly to prevent malaria infection.
Several cases of imported malaria, as well severe cases with fatal outcome, have been reported;[49,50,51,52] however clinical presentation is often similar to HIV-negative patients as recently reported in a retrospective study conducted in Spain.[53] These data suggest that more caution should be taken during the pre-travel counselling.
WHO recommends a viro-immunological parameters check (CD4+ T cell count and HIV viraemia at least) and a clinical examination before travelling.[54] Obviously, for HIV-infected pregnant women and young children travel should be avoided if not strictly necessary as they have higher risks of severe malaria clinical manifestations.
Then, physicians involved in HIV care as well as in travel medicine should consider several factors in pre-travel counselling for HIV-infected travellers such as reason for travel (tourism, VFR, business etc.), travel risk (pre-arranged or organized travel), travel destination, season and malarial epidemic cycle as well as Plasmodium drug sensitivity in the specific area;[10] moreover HIV-infected people travelling to malaria endemic-areas need to start a correct chemoprophylaxis regimen before their travel and counsellors should consider possible drug interactions between antiretroviral and antimalarial drugs.[10]
Therefore the importance of behavioral preventive measures (bed nets, repellents, etc.), adequate chemoprophylaxis and, in selected circumstances, stand-by emergency treatment in case of evocative symptoms should be strongly recommended.
Effects of Co-Trimoxazole Prophylaxis and Antiretroviral Therapy on Malaria.
According to major International Guidelines[55,56] HAART regimens should contain at least three active drugs from two different classes, usually 2 nucleos(t)ide reverse transcriptase inhibitors (NRTIs) accompanied with an anchor drug which is either a non-nucleoside reverse transcriptase inhibitors (NNRTI) or a protease inhibitor (PI) with a low-dose of ritonavir (RTV) as “booster”. Sustained virological suppression and CD4+ T cell count recovery is the main objective of HAART.[55] In HIV-infected patients, daily co-trimoxazole should to be prescribed as prophylaxis against major opportunistic infections when CD4+ T cells are less than 200/�l;[57] however several findings demonstrated that daily co-trimoxazole prescription may reduce the occurrence of parasitaemia[58] and clinical malaria either in adults and in children.[59,60,61]
For example, Mermin J and colleagues[58] found that among HIV-infected children the use of co-trimoxazole prophylaxis and insecticide-treated nets might reduce the prevalence of clinical malaria by 97% versus the reduction of 43% with nets alone. Therefore co-trimoxazole prescription in immunocompromised patients, in addition at insecticide-treated nets, seems to be an effective strategy to prevent malaria. However, cross resistance between co-trimoxazole and sulfadoxine-pyrimethamine (SP) has been reported,[62] therefore widely use of co-trimoxazole may promote specific SP resistance; some studies did not find SP related resistances despite co-trimoxazole prescription,[63,64] while others found that co-trimoxazole prophylaxis was effective despite antifolate resistance.[60]
Therefore some authors suggested that a continuation of prophylaxis with co-trimoxazole in malaria endemic areas is beneficial even when patients have been immune restored.[11] Antiretroviral therapy may be effective against malaria infection: Mermin J et al[65] in a prospective cohort study found that HAART containing NRTIs when co-administrated with co-trimoxazole decrease malaria clinical presentation in respect of co-trimoxazole alone; in order to provide an explanation on the possible antimalarial activity of NRTIs, authors hypothesized that HAART probably reduced the frequency of malaria by improving immune function rather than by a direct antimalarial effect. A delayed P. falciparum clearance in HIV-infected patients treated with artemisinin-based regimens has been reported[66] suggesting that immune defences of HIV-infected people may affect effectiveness of antimalarial treatment. Other studies evaluating impact of PIs are ongoing (e.g. NCT00719602) but available studies “in vitro” showed that the PIs lopinavir, saquinavir, indinavir, atazanavir ritonavir have direct effect on inhibition of P. falciparum, at concentration used in clinical practice.[67,68] Anyway, antiretroviral treatment maybe effective against P. falciparum and may reduce malarial clinical manifestation, but, in order to better clarify this hypothesis, its underlying biological mechanisms and the role of antiretroviral drugs or classes need further investigations.
Interactions Between Antiretrovirals and Antimalarial Agents:
Antiretrovirals and antimalarial drugs (treatment). Pharmacological interactions between antiretroviral and antimalarial drugs has been previously extensively reviewed,[69,13] however the knowledge about this critical topic is still debated and data reported are not definitive, particularly due to either the paucity of data about newer drugs and classes of antiretroviral agents and the absence of specific guidelines. Moreover the relevance of “in vitro” studies in common clinical practice is controversial. According to WHO, first-line treatment for uncomplicated malaria in high-endemicity areas consists of a combination of either artemether plus lumefantrine or artesunate plus one of the following drugs: amodiaquine, mefloquine, or sulfadoxine–pyrimethamine, or dihydroartemisinin–piperaquine.[1] Interactions between amodiaquine and efavirenz has been reported reflecting in higher amodiquine concentrations;[70] these evidences may have a clinical impact, as a significant number of HIV-infected patients in sub-Saharan Africa receive an efavirenz-based treatment[57] and WHO contraindicates this co-administration;[27] furthermore, given that these drugs are both potentially hepatotoxic[71,72] patients prescribing this combination have to be strictly monitored. Finally, we know that quinine, halofantrine, and lumefantrine are all antimalarial drugs metabolized through the cytochrome P-450 enzyme system;[73,74] so that, these drugs may potentially interact with the non-nucleoside reverse transcriptase inhibitors (NNRTIs) resulting in reduced bioavailability of the antimalarial drugs.[75] Recently, a cross-sectional study in HIV and malaria co-infected adults showed significant pharmacokinetic interactions among nevirapine, artemether and dihydroartemisinin: these interactions maybe increase the risk of treatment failure of both infections and the risk for development of drug resistance.[76] Drug-interactions with PIs, that are also metabolized through the hepatic cytochrome P-450 enzyme system, and enzyme inhibition by ritonavir may on the contrary increase serum concentrations of these antimalarials.[77] Despite this study, performed in healthy volunteers, ritonavir might boost the bioavailability of either PIs and antimalarial drugs, however this altered pharmacokinetics do not seems clinically relevant. By contrast, a recent study confirmed that LPV/r-based HAART significantly increases lumefantrine exposure without an increase in adverse effects. Artemether and dihydroartemisinin concentrations were also significantly increased by ritonavir boosted lopinavir-based treatment, but to a lesser extent.[78] Data from randomized-controlled trials will be highly valuable in evaluating the clinical significance of all these interactions.
Most of potential clinically significant interactions between antimalarial and antiretrovirals drugs are showed in Table 1.

Table 1. Potential interactions between antimalarial and antiretroviral drugs.
Malaria chemoprophylaxis in HIV infected subjects. Despite several reports and reviews on malaria and HIV co-infection, few data are present in literature about the use of malaria chemoprophylaxis in HIV infected travellers.[57] Mefloquine reduces some ritonavir boosted protease inhibitors levels and mefloquine plasma levels could be reduced by efavirenz and nevirapine, but these data are from patients who received mefloquine as treatment, little is known about its use in chemoprophylactic regimen. Atovaquone/proguanil levels could be reduced by indinavir, as well as lopinavir, atazanavir, ritonavir and efavirenz;[79] moreover, atovaquone/proguanil could reduce indinavir levels and increase zidovudine levels causing hematological troubles.[80] No significant data are available on doxycycline interactions.[81] All these considerations should be taken into account before starting chemoprophylaxis in HIV infected travelers taking HAART, and drugs dosages should be arranged in order to avoid drug interactions. If chemoprophylaxis is not administered, atovaquone/proguanil might be a suitable stand-by emergency treatment (SBET) option for HIV-infected subjects receiving NNRTI-based regimens, while mefloquine could be considered as SBET drug in those prescribing PI-based HAART.[82]
Conclusion And Future Research Perspectives.
Malaria and HIV infections are two of the most important infectious diseases worldwide, particularly in sub-Saharan Africa: their overlapping epidemiology as well their impact in clinical practice needs to be continuously updated.
HIV-infected adults, especially if international travelers, even with a restored immunity, are more likely to have severe clinical manifestations of malaria compared to travellers without HIV infection. Moreover co-infection in pregnant women contributes to develop clinical malaria in women, maternal severe anaemia, grater rates of stillbirth and preterm delivery, low birth weight, foetal grow retardation and increased risk of infant mortality.
The wider implementation of co-trimoxazole prophylaxis and antiretroviral therapy could change the impact of malaria in HIV-infected patients.
The benefits of the continuation of co-trimoxazole prophylaxis to prevent malaria, the antimalarial effects of several antiretroviral agents, as well the interactions between antiretroviral and antimalarial drugs, need to be further investigated. The proper malaria prophylaxis and treatment in HIV-infected pregnant women also needs to be defined. Several clinical trials are ongoing to address several of these opened questions: the results of these trials are needed to create specific guidelines for the prevention and management of malaria and HIV co-infection and to help clinicians involved in travel medicine as well in HIV care.
Authors’ Contributions.
All authors conceived the concept and design of the review. EF, SO, and NB wrote and GC critically revised the review. All authors approved the final version for submission.
Conflict of Interest.
EF and GC have received unrestricted educational grants (as speakers or for participation to conferences) from several Pharmaceuticals Companies producing antiretroviral drugs but this did not influence the content of this review. SO and NB declare no conflicts of interest.
References
- WHO. Guidelines for the treatment of malaria, 2nd edition. Geneva, World Health Organization. 2010. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf
- WHO. World malaria report 2010. Geneva, World Health Organization, 2010. http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf
- Palella FJ jr, Delaney KM, Moorman AC,
Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining
morbidity and mortality among patients with advanced human
immunodeficiency virus infection. HIV Outpatient Study Investigators. N
Engl J Med. 1998; 338(13):853-60 http://dx.doi.org/10.1056/NEJM199803263381301 PMid:9516219

- WHO. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Geneva, World Health Organization, 2011. http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf
- Abu-Raddad LJ, Patnaik P, Kublin JG. Dual
infection with HIV and malaria fuels the spread of both diseases in
sub-Saharan Africa. Science. 2006; 314(5805):1603-6 http://dx.doi.org/10.1126/science.1132338 PMid:17158329

- Rana FS, Hawken MP, Mwachari C, Bhatt SM,
Abdullah F, Ng'ang'a LW, Power C, Githui WA, Porter JD, Lucas SB.
Autopsy study of HIV-1-positive and HIV-1-negative adult medical
patients in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2000;
24(1):23-9 PMid:10877491

- Holmes CB, Losina E, Walensky RP,
Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus
type 1-related opportunistic infections in sub-Saharan Africa. Clin
Infect Dis. 2003; 36(5):652-62 http://dx.doi.org/10.1086/367655 PMid:12594648

- Steketee RW, Nahlen BL, Parise ME, Menendez
C. The burden of malaria in pregnancy in malaria-endemic areas. Am J
Trop Med Hyg. 2001; 64(1−2 suppl):28−35

- Cohen C, Karstaedt A, Frean J, Thomas J,
Govender N, Prentice E, Dini L, Galpin J, Crewe-Brown H. Increased
prevalence of severe malaria in HIV-infected adults in South Africa.
Clin Infect Dis. 2005; 41(11):1631−37 http://dx.doi.org/10.1086/498023 PMid:16267737

- Bhadelia N, Klotman M, Caplivski D. The HIV-positive traveler. Am J Med. 2007; 120(7):574-80 http://dx.doi.org/10.1016/j.amjmed.2007.02.018 PMid:17602926

- Flateau C, Le Loup G, Pialoux G.
Consequences of HIV infection on malaria and therapeutic implications:
a systematic review. Lancet Infect Dis. 2011; 11(7):541-56 http://dx.doi.org/10.1016/S1473-3099(11)70031-7

- Idemyor V. Human immunodeficiency virus
(HIV) and malaria interaction in sub-Saharan Africa: the collision of
two Titans. HIV Clin Trials. 2007; 8(4):246-53 http://dx.doi.org/10.1310/hct0804-246 PMid:17720665

- Skinner-Adams TS, McCarthy JS, Gardiner
DL, Andrews KT. HIV and malaria co-infection: interactions and
consequences of chemotherapy. Trends Parasitol. 2008; 24(6):264-71 http://dx.doi.org/10.1016/j.pt.2008.03.008 PMid:18456554

- Whitworth J, Morgan D, Quigley M, Smith A,
Mayanja B, Eotu H, Omoding N, Okongo M, Malamba S, Ojwiya A. Effect of
HIV-1 and increasing immunosuppression on malaria parasitaemia and
clinical episodes in adults in rural Uganda: a cohort study. Lancet.
2000; 356(9235):1051-6 http://dx.doi.org/10.1016/S0140-6736(00)02727-6

- Xiao L, Owen SM, Rudolph DL, Lal RB, Lal
AA. Plasmodium falciparum antigen-induced human immunodeficiency virus
type 1 replication is mediated through induction of tumor necrosis
factor-alpha. J Infect Dis. 1998; 177(2):437-45 http://dx.doi.org/10.1086/514212 PMid:9466533

- French N, Nakiyingi J, Lugada E, Watera C,
Whitworth JA, Gilks CF. Increasing rates of malarial fever with
deteriorating immune status in HIV-1-infected Ugandan adults. AIDS.
2001; 15(7):899-906 http://dx.doi.org/10.1097/00002030-200105040-00010 PMid:11399962

- Patnaik P, Jere CS, Miller WC, Hoffman IF,
Wirima J, Pendame R, Meshnick SR, Taylor TE, Molyneux ME, Kublin JG.
Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell
count on the incidence of malaria infection in a cohort of adults in
rural Malawi. J Infect Dis. 2005; 192(6):984-91 http://dx.doi.org/10.1086/432730 PMid:16107950

- Grimwade K, French N, Mbatha DD, Zungu DD,
Dedicoat M, Gilks CF. HIV infection as cofactor for severe falciparum
malaria in adults living in a region of unstable malaria transmission
in South Africa. AIDS. 2004; 18(3):547-554 http://dx.doi.org/10.1097/00002030-200402200-00023 PMid:15090809

- Colebunders R, Karstaedt A, Frean J,
Thomas J, Govender N, Prentice E, Dini L, Galpin J, Crewe-Brown H.
Incidence of malaria and efficacy of oral quinine in patients recently
infected with human immunodeficiency virus in Kinshasa, Zaire. J
Infect. 1990; 21(2):167-73 http://dx.doi.org/10.1016/0163-4453(90)91701-E

- Nguyen-Dinh P, Greenberg AE, Mann JM,
Kabote N, Francis H, Colebunders RL, Huong AY, Quinn TC, Davachi F,
Lyamba B, et al. Absence of association between Plasmodium falciparum
malaria and human immunodeficiency virus infection in children in
Kinshasa, Zaire. Bull World Health Organ. 1987; 65:607-13 PMid:3322600
PMCid:2491065

- Diallo AH, Ki-Zerbo G, Sawadogo AB,
Guiguemde TR. Severe malaria and HIV in adult patients in
Bobo-Dioulasso, Burkina Faso. Med Trop. 2004; 64:345-50

- Van Geertruyden JP, Mulenga M, Chalwe V,
Michael N, Moerman F, Mukwamataba D, Colebunders R, D'alessandro U.
Impact of HIV-1 infection on the hematological recovery after clinical
malaria. J Acquir Immune Defic Syndr. 2009; 50(2):200-5 http://dx.doi.org/10.1097/QAI.0b013e3181900159 PMid:19131887

- Brentlinger PE, Behrens CB, Kublin JG.
Challenges in the prevention, diagnosis, and treatment of malaria in
human immunodeficiency virus infected adults in sub-Saharan Africa.
Arch Intern Med. 2007; 167(17):1827-36 http://dx.doi.org/10.1001/archinte.167.17.1827 PMid:17893303

- Hoffman IF, Jere CS, Taylor TE, Munthali
P, Dyer JR, Wirima JJ, Rogerson SJ, Kumwenda N, Eron JJ, Fiscus SA,
Chakraborty H, Taha TE, Cohen MS, Molyneux ME. The effect of Plasmodium
falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;
13(4):487-94 http://dx.doi.org/10.1097/00002030-199903110-00007 PMid:10197377

- Kublin JG, Patnaik P, Jere CS, Miller WC,
Hoffman IF, Chimbiya N, Pendame R, Taylor TE, Molyneux ME. Effect of
Plasmodium falciparum malaria on concentration of HIV-1-RNA in the
blood of adults in rural Malawi: a prospective cohort study. Lancet.
2005; 365(9455):233-40 PMid:15652606

- Lee TH, Sakahara N, Fiebig E, Busch MP,
O'Brien TR, Herman SA. Correlation of HIV-1 from RNA levels in plasma
and heterosexual transmission of HIV-1 from infected transfusion
recipients. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;
12:427–428 http://dx.doi.org/10.1097/00042560-199608010-00015 PMid:8673554

- WHO. Malaria and HIV/AIDS interactions and their implications for public health policy. Report of a technical consultation. 23-25 June 2004. Geneva, World Health Organization, 2004. http://www.who.int/hiv/pub/prev_care/malariahiv.pdf
- Uneke CJ. Impact of placental Plasmodium
falciparum malaria on pregnancy and perinatal outcome in sub-Saharan
Africa: I: introduction to placental malaria. Yale J Biol Med. 2007;
80(2):39-50 PMid:18160989 PMCid:2140183

- Meeusen EN, Bischof RJ, Lee CS.
Comparative T-cell responses during pregnancy in large animals and
humans. Am J Reprod Immunol. 2001; 46(2):169-79 http://dx.doi.org/10.1111/j.8755-8920.2001.460208.x PMid:11506082

- Ismail MR, Ordi J, Menendez C, Ventura PJ,
Aponte JJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL. Placental pathology
in malaria: a histological, immunohistochemical, and quantitative
study. Hum Pathol. 2000; 31(1):85-93 http://dx.doi.org/10.1016/S0046-8177(00)80203-8

- Pell C, Straus L, Andrew EV, Me�aca A,
Pool R. Social and cultural factors affecting uptake of interventions
for malaria in pregnancy in Africa: a systematic review of the
qualitative research. PLoS One. 2011; 6(7):e22452 http://dx.doi.org/10.1371/journal.pone.0022452 PMid:21799859 PMCid:3140529

- Briand V, Badaut C, Cot M. Placental
malaria, maternal HIV infection and infant morbidity. Ann Trop
Paediatr. 2009; 29(2):71-83. http://dx.doi.org/10.1179/146532809X440699 PMid:19460261

- van Ejik AM, Ayisi JG, ter Kuile FO,
Misore AO, Otieno JA, Rosen DH, Kager PA, Steketee RW, Nahlen BL. HIV
increases the risk of malaria in women of all gravidities in Kisumu,
Kenya. AIDS. 2003; 17(13):2002-2003 PMid:12960841

- ter Kuile FO, Parise ME, Verhoeff FH,
Udhayakumar V, Newman RD, van Eijk AM, Rogerson SJ, Steketee RW. The
burden of co-infection with human immunodeficiency virus type 1 and
malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg.
2004; 71(2 Suppl):41-54 PMid:15331818

- Ajisi JC, van Eijk AM, ter Kuile FO,
Kolczak MS, Otieno JA, Misore AO, Kager PA, Steketee RW, Nahlen BL. The
effect of dual infection with HIV and malaria on pregnancy outcome in
western Kenya. AIDS. 2003; 17:585-94 http://dx.doi.org/10.1097/00002030-200303070-00014 PMid:12598779

- Ticconi C, Mapfumo M, Dorrucci M, Naha N,
Tarira E, Pietropolli A, Rezza G. Effect of maternal HIV and malaria
infection on pregnancy and perinatal outcome in Zimbabwe. J Acquir
Immune Defic Syndr. 2003; 34(3):289-94 http://dx.doi.org/10.1097/00126334-200311010-00005

- Bloland PB, Wirima JJ, Steketee RW,
Chilima B, Hightower A, Breman JG. Maternal HIV infection and infant
mortality in Malawi: evidence for increased mortality due to placental
malaria infection. AIDS. 1995; 9(7):721-6 http://dx.doi.org/10.1097/00002030-199507000-00009 PMid:7546417

- Mwapasa V, Rogerson SJ, Molyneux ME,
Abrams ET, Kamwendo DD, Lema VM, Tadesse E, Chaluluka E, Wilson PE,
Meshnick SR. The effect of Plasmodium falciparum malaria on peripheral
and placental HIV-1 RNA concentrations in pregnant Malawian women.
AIDS. 2004; 18(7):1051-9 http://dx.doi.org/10.1097/00002030-200404300-00014 PMid:15096809

- WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommendations for a public health approach. Geneva, World Health Organization, 2010. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf
- United Nations World Tourism Organization (UNWTO), Tourism ighlights 2011 Edition. http://mkt.unwto.org/sites/all/files/docpdf/unwtohighlights11enlr_3.pdf
- Gautret P, Schlagenhauf P, Gaudart J,
Castelli F, Brouqui P, von Sonnenburg F, Loutan L, Parola P;
GeoSentinel Surveillance Network. Multicenter EuroTravNet/GeoSentinel
study of travel-related infectious diseases in Europe. Emerg Infect
Dis. 2009; 15(11):1783-90 PMid:19891866 PMCid:2857260

- Millet JP, Olalla PG, Gasc�n J, Prat JG,
Trevino B, Pinazo MJ, Cabezos J, Munoz J, Zarzuela F, Cayl� JA.
Imported malaria among African immigrants: is there still a
relationship between developed countries and their ex-colonies? Malar
J. 2009; 8:111 PMid:19463171 PMCid:2693516

- Kemper CA, Linett A, Kane C, Deresinsky SC. Frequency of travel of adults infected with HIV. J Travel Med 1995; 2(2):85–8 http://dx.doi.org/10.1111/j.1708-8305.1995.tb00632.x PMid:9815367

- Sherrard AW, McCarthy AE. Travel patterns
and health risks for patients infected with HIV. Travel Med Infect Dis.
2009; 7(5):291-5 http://dx.doi.org/10.1016/j.tmaid.2009.03.006 PMid:19747664

- Salit IE, Sano M, Boggild AK, Kain KC.
Travel patterns and risk behaviour of HIV-positive people traveling
internationally. CMAJ. 2005; 172(7):884-8 http://dx.doi.org/10.1503/cmaj.1040877 PMid:15795409 PMCid:554873

- Chalwe V, Van geertruyden JP, Mukwamataba
D, Menten J, Kamalamba J, Mulenga M, D'Alessandro U. Increased risk for
severe malaria in HIV-1-infected adults, Zambia. Emerg Infect Dis.
2009; 15(5):749-55 http://dx.doi.org/10.3201/eid1505.081009 PMid:19402961 PMCid:2687012

- Franco-Paredes C, Hidron A, Tellez I,
Lesesne J, Del Rio C. HIV infection and travel: pretravel
recommendations and health-related risks. Top HIV Med. 2009; 17(1):2-11
PMid:19270343

- Castelli F, Odolini S, Autino B, Foca E,
Russo R. Malaria prophylaxis: a comprehensive review. Pharmaceuticals.
2010; 3:3212-3239 http://dx.doi.org/10.3390/ph3103212

- Mouala C, Houz� S, Guiguet M, Abboud P,
Pialoux G, Viget N, Costagliola D, Matheron S. Imported malaria in
HIV-infected patients enrolled in the ANRS CO4 FHDH study. J Acquir
Immune Defic Syndr. 2008; 49(1):55-60 http://dx.doi.org/10.1097/QAI.0b013e31817e635b PMid:18667929

- Mouala C, Guiguet M, Houz� S, Damond F,
Pialoux G, Viget N, Costagliola D, Le Bras J, Matheron S; FHDH-ANRS CO4
Clinical Epidemiology Group. Impact of HIV infection on severity of
imported malaria is restricted to patients with CD4 cell counts <
350 cells/microl. AIDS. 2009; 23(15):1997-2004 http://dx.doi.org/10.1097/QAD.0b013e32832f4215 PMid:19654499

- Matteelli A, Casalini C, Bussi G, Saleri
N, Nasta P, Pizzocolo C, Gulletta M, Castelli F. Imported malaria in an
HIV-positive traveler: a case report with a fatal outcome. J Travel
Med. 2005; 12(4):222-4 http://dx.doi.org/10.2310/7060.2005.12409

- Zamidei L, Durval A, Bettocchi D, Luzzio
MG, Bartoloni A, Consales G. Efficacy and safety of quinine-artesunate
in an HIV-positive patient with severe falciparum malaria. Minerva
Anestesiol. 2010 Jan;76(1):66-9 PMid:20125075

- Ram�rez-Olivencia G, Herrero MD, Subirats
M, de Juanes JR, Pe�a JM, Puente S. Imported malaria and HIV infection
in Madrid. Clinical and epidemiological features. Rev Clin Esp. 2012;
212(1):10-7 PMid:22071125

- WHO. International travel and health: 2011. Geneva, World Health Organization, 2011 http://www.who.int/ith/chapters/ith2011chap7.pdf
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. October 14, 2011; 1–167. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- European AIDS Clinical Society (EACS). Guidelines. Version 6.1. November 2011. http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf
- WHO. Antiretroviral therapy for HIV infection in adults and adolescents, recommendations for a public health approach. Geneva, World Health Organization, 2010. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf
- Mermin J, Lule J, Ekwaru JP, Malamba S,
Downing R, Ransom R, Kaharuza F, Culver D, Kizito F, Bunnell R, Kigozi
A, Nakanjako D, Wafula W, Quick R. Effect of co-trimoxazole prophylaxis
on morbidity, mortality, CD4-cell count, and viral load in HIV
infection in rural Uganda. Lancet. 2004; 364(9443):1428-34 http://dx.doi.org/10.1016/S0140-6736(04)17225-5

- Anglaret X, Ch�ne G, Attia A, Toure S,
Lafont S, Combe P, Manlan K, N'Dri-Yoman T, Salamon R. Early
chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected
adults in Abidjan, C�te d'Ivoire: a randomised trial. Cotrimo-CI Study
Group. Lancet. 1999; 353(9163):1463-8 http://dx.doi.org/10.1016/S0140-6736(98)07399-1

- Gasasira AF, Kamya MR, Ochong EO, Vora N,
Achan J, Charlebois E, Ruel T, Kateera F, Meya DN, Havlir D, Rosenthal
PJ, Dorsey G. Effect of trimethoprim-sulphamethoxazole on the risk of
malaria in HIV-infected Ugandan children living in an area of
widespread antifolate resistance. Malar J. 2010; 9:177 http://dx.doi.org/10.1186/1475-2875-9-177 PMid:20573194 PMCid:2903607

- Kamya MR, Gasasira AF, Achan J, Mebrahtu
T, Ruel T, Kekitiinwa A, Charlebois ED, Rosenthal PJ, Havlir D, Dorsey
G. Effects of trimethoprim-sulfamethoxazole and insecticide-treated
bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;
21(15):2059-66 http://dx.doi.org/10.1097/QAD.0b013e3282ef6da1 PMid:17885296

- Iyer JK, Milhous WK, Cortese JF, Kublin
JG, Plowe CV. Plasmodium falciparum cross-resistance between
trimethoprim and pyrimethamine. Lancet. 2001; 358(9287):1066-7 http://dx.doi.org/10.1016/S0140-6736(01)06201-8

- Thera MA, Sehdev PS, Coulibaly D, Traore
K, Garba MN, Cissoko Y, Kone A, Guindo A, Dicko A, Beavogui AH, Djimde
AA, Lyke KE, Diallo DA, Doumbo OK,Plowe CV. Impact of
trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria
infection and disease. J Infect Dis. 2005; 192(10):1823-9 http://dx.doi.org/10.1086/498249 PMid:16235184 PMCid:2740817

- Malamba S, Sandison T, Lule J, Reingold A,
Walker J, Dorsey G, Mermin J. Plasmodium falciparum dihydrofolate
reductase and dihyropteroate synthase mutations and the use of
trimethoprim-sulfamethoxazole prophylaxis among persons infected with
human immunodeficiency virus. Am J Trop Med Hyg. 2010; 82(5):766-71 http://dx.doi.org/10.4269/ajtmh.2010.08-0408 PMid:20439953 PMCid:2861394

- Mermin J, Ekwaru JP, Liechty CA, Were W,
Downing R, Ransom R, Weidle P, Lule J, Coutinho A, Solberg P. Effect of
co-trimoxazole prophylaxis, antiretroviral therapy, and
insecticide-treated bednets on the frequency of malaria in
HIV-1-infected adults in Uganda: a prospective cohort study. Lancet.
2006; 367(9518):1256-61 http://dx.doi.org/10.1016/S0140-6736(06)68541-3

- Birku Y, Mekonnen E, Bj�rkman A, Wolday D.
Delayed clearance of Plasmodium falciparum in patients with human
immunodeficiency virus co-infection treated with artemisinin. Ethiop
Med J. 2002; 40 Suppl 1:17-26 PMid:12802828

- Parikh S, Gut J, Istvan E, Goldberg DE,
Havlir DV, Rosenthal PJ. Antimalarial activity of human
immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents
Chemother. 2005; 49(7):2983-5 http://dx.doi.org/10.1128/AAC.49.7.2983-2985.2005 PMid:15980379 PMCid:1168637

- Skinner-Adams TS, McCarthy JS, Gardiner
DL, Hilton PM, Andrews KT. Antiretrovirals as antimalarial agents. J
Infect Dis. 2004; 190(11):1998-2000 http://dx.doi.org/10.1086/425584 PMid:15529265

- Khoo S, Back D, Winstanley P. The
potential for interactions between antimalarial and antiretroviral
drugs. AIDS. 2005; 19(10):995-1005 http://dx.doi.org/10.1097/01.aids.0000174445.40379.e0 PMid:15958830

- German P, Greenhouse B, Coates C, Dorsey
G, Rosenthal PJ, Charlebois E, Lindegardh N, Havlir D, Aweeka FT.
Hepatotoxicity due to a drug interaction between amodiaquine plus
artesunate and efavirenz. Clin Infect Dis. 2007; 44(6):889-91 http://dx.doi.org/10.1086/511882 PMid:17304470

- Gu�vart E, Agu�mon A. Two cases of
fulminant hepatitis during a curative treatment with an
artesunate-amodiaquine combination. Med Mal Infect. 2009; 39(1):57-60
PMid:19013042

- Mart�n-Carbonero L, N��ez M,
Gonz�lez-Lahoz J, Soriano V. Incidence of liver injury after beginning
antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials.
2003; 4(2):115-20 http://dx.doi.org/10.1310/N4VT-3E9U-4BKN-CRPW

- Lef�vre G, Bindschedler M, Ezzet F,
Schaeffer N, Meyer I, Thomsen MS. Pharmacokinetic interaction trial
between co-artemether and mefloquine. Eur J Pharm Sci. 2000;
10(2):141-51 http://dx.doi.org/10.1016/S0928-0987(00)00060-9

- Svensson US, Ashton M. Identification of
the human cytochrome P450 enzymes involved in the in vitro metabolism
of artemisinin. Br J Clin Pharmacol. 1999; 48(4):528-35 http://dx.doi.org/10.1046/j.1365-2125.1999.00044.x PMCid:2014388

- Plosker GL, Scott LJ. Saquinavir: a review
of its use in boosted regimens for treating HIV infection. Drugs. 2003;
63(12):1299-324 http://dx.doi.org/10.2165/00003495-200363120-00007 PMid:12790697

- Byakika-Kibwika P, Lamorde M, Mayanja-Kizza H, Katabira E, Khoo S, Back D, Lindegardh N, Tarning J, de Vries P, Merry C. Significant Pharmacokinetic Interaction between Nevirapine and Artemether-Lumefantrine in HIV+ Adults: Uganda. 19th Conference on Retroviruses and Opportunistic Infections, Seattle WA. 2012. p. 614
- Khaliq Y, Gallicano K, Tisdale C, Carignan
G, Cooper C, McCarthy A. Pharmacokinetic interaction between mefloquine
and ritonavir in healthy volunteers. Br J Clin Pharmacol. 2001;
51(6):591-600 http://dx.doi.org/10.1046/j.1365-2125.2001.01393.x PMid:11422019 PMCid:2014486

- Kredo T, Mauff K, van der Walt JS, Cohen K, Wiesner L, Smith P, Maartens G, Barnes K. The Interaction between Lopinavir/ritonavir and Artemether-Lumefantrine in HIV+ Patients. 19th Conference on Retroviruses and Opportunistic Infections, Seattle WA. 2012. p. 613
- van Luin M, Van der Ende ME, Richter C,
Visser M, Faraj D, Van der Ven A, Gelinck L, Kroon F, Wit FW, Van
Schaik RH, Kuks PF, Burger DM. Lower atovaquone/proguanil
concentrations in patients taking efavirenz, lopinavir/ritonavir or
atazanavir/ritonavir. AIDS. 2010; 24(8):1223-6 http://dx.doi.org/10.1097/QAD.0b013e3283389129 PMid:20299957

- Committee to advise on Tropical Medicine
and travel (CATMAT). The immunocompromised traveller. An Advisory
Committee Statement (ACS). Canada Communicable Dis. Rep. 2007;
33:1-24. http://origin.phac-aspc.gc.ca/publicat/ccdr-rmtc/07pdf/acs33-04.pdf
- University of Liverpool. Drug Interactions. http://www.hiv-druginteractions.org/
- Cavassini ML, D'Acremont V, Furrer H,
Genton B, Tarr PE. Pharmacotherapy, vaccines and malaria advice for
HIV-infected travellers. Expert Opin Pharmacother. 2005; 6(6):891-913 http://dx.doi.org/10.1517/14656566.6.6.891 PMid:15952919
