Advances in the Treatment of Malaria
Francesco Castelli1,2, Lina Rachele Tomasoni2 and Alberto Matteelli2
1Chair of Infectious Diseases, University of Brescia, Italy.
2University Division of Infectious and Tropical Diseases, University of Brescia and Spedali Civili General Hospital, Brescia (Italy).
2University Division of Infectious and Tropical Diseases, University of Brescia and Spedali Civili General Hospital, Brescia (Italy).
Correspondence
to:
Prof. Francesco Castelli, Director, University Division of Infectious
and Tropical Diseases, University of Brescia and Spedali Civili General
Hospital, Brescia (Italy) Piazza Spedali Civili 1, 25123 – Brescia
(Italy). Tel.:+39.030.3995664. Fax:+39.030.3702403 Email: castelli@med.unibs.it
Published: October 3, 2012
Received: August 27, 2012
Accepted: September 24, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012064, DOI 10.4084/MJHID.2012.064
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Malaria
still claims a heavy toll of deaths and disabilities even at the
beginning of the third millennium. The inappropriate sequential use of
drug monotherapy in the past has facilitated the spread of
drug-resistant P. falciparum, and to a lesser extend P. vivax, strains
in most of the malaria endemic areas, rendering most anti-malarial
ineffective. In the last decade, a new combination strategy based on
artemisinin derivatives (ACT) has become the standard of treatment for
most P. falciparum malaria infections. This strategy could prevent the
selection of resistant strains by rapidly decreasing the parasitic
burden (by the artemisinin derivative, mostly artesunate) and exposing
the residual parasite to effective concentrations of the partner drug.
The widespread use of this strategy is somehow constrained by cost and
by the inappropriate use of artemisinin, with possible impact on
resistance, as already sporadically observed in South East Asia.
Parenteral artesunate has now become the standard of care for severe
malaria, even if quinine still retains its value in case artesunate is
not immediately available. The appropriateness of pre-referral use of
suppository artesunate is under close monitoring, while waiting for an
effective anti-malarial vaccine to be made available.
Introduction and Historical Outline
The correct management of clinical malaria cases is a complex issue that has to take into account different targets that may be differently prioritized according to the various clinical and epidemiological situations:
a. to prevent progression of uncomplicated malaria patients to severe life threatening complications (P. falciparum but also P. vivax);
b. to prevent mortality of patients with severe malaria (P. falciparum but also P. vivax and P. knowlesi);
c. to prevent relapses when appropriate (P. vivax, P. ovale);
d. to limit the spreading of the infection/disease in the population;
e. to limit as much as possible the emergence of plasmodium resistant strains.
Considering the complex biological cycle of malaria plasmodia, the ideal drug to meet the clinical targets should have the following properties:
- to act rapidly against the replicating blood erythrocytic asexual forms, primarily schizonts, that are responsible for the clinical manifestation of the disease (parasitological cure);
- to act against liver hypnozoites, when appropriate (radical cure).
In endemic areas, furthermore, the ideal drug to meet the epidemiological targets should have the following properties:
- to act against the sexual forms (gametocytes) that are responsible for the transmission of the infection in the population via the vector mosquitoes; this gametocidal effect is time-sensitive because the appearance of sexual forms is delayed of several days from the clinical malaria attack;
- to avoid selecting plasmodia resistant strains (high resistance barrier).
After quinine selective therapeutical value against malaria plasmodia was suggested by Francesco Torti in Italy (1712), no major advance in malaria chemotherapy occurred until the first decades of the XX century. At that time, and until recently, treatment of malaria access was based on the use of single drug regimens that were subsequently made available according to the emergence of resistance to the previously used molecules. Pamaquine and chloroquine were discovered in Germany in 1924 and 1934 respectively, followed by proguanil (England, 1944), pyrimethamine (England, 1952), primaquine (USA, 1956), sulphonamides (1960-66), mefloquine (USA, 1971-75) and halofantrine (1989).[1]
The value of monotherapy had been questioned since the early ’60, when P. falciparum with decreased sensitivity to chloroquine appeared in South-East Asia and Colombia and then quickly spread to virtually all P. falciparum endemic areas. Thereafter, the same occurred to all antimalarial drugs acting against P. falciparum and, to a lesser extent, against P. vivax.
At the end of the 20th century, the strong anti-parasite efficacy of the long-known Chinese malarial remedy artemisinine and its derivatives was scientifically demonstrated, both on blood asexual and sexual forms (gametocytes). Furthermore, in line with other major infectious diseases such as tuberculosis and HIV infection, the value of combination treatment to lessen the chance of a natural resistant strain to emerge was clearly established and new combination treatment tested (atovaquone-proguanil, chlorproguanil-dapsone).
Artemisinin based combination treatments (ACT) underwent extensive randomized clinical trials that proved their superiority in fever and parasitological clearance times and clinical outcomes.[2]
At present, therefore, artemisinin-based treatment complies with most of the properties of the “ideal antimalarial drug” listed above and represents the standard of care of both complicated and uncomplicated malaria.[3]
Drug resistance of malaria plasmodia
According to WHO, resistance to malaria drug is defines as “the ability of a parasite strain to survive and/or multiply despite the proper administration and absorption of an antimalarial drug in the dose normally recommended”.[3]
This is particularly worrying for P. falciparum, both because of its higher propensity to develop resistance and because of its intrinsic higher virulence and morbidity and mortality burden. Resistance (or lower sensitivity) to antimalarial drugs has also been observed in P. vivax, while it is extremely rare (if present at all) in the other species of Plasmodia.
The spread of resistance is classically a two-step process. First, a mutant clone spontaneously emerges in the replicating parasite population. This clone is usually less fit that the wild-type sensitive ones and tends to disappear, unless it is confronted to selective drug pressure able to kill sensitive parasites but not blood circulating resistant asexual forms that subsequently evolve to gametocytes with possible spread in the population (second step). This phenomenon is usually more likely to happen first in low transmission settings where most parasite-carrying patients are symptomatic and therefore subject to treatment.[3] This is probably the reason why chloroquine and pyrimetamine resistance strains first appeared in South-East Asia in the early sixties before spreading to the African continent,[4] also favoured by the suggested higher predisposition to mutation of Asian P. falciparum strains.[3]
The probability of a genetic resistance mutation to occur is a function of many factors, including (but not limited to) (i) the number of number of replicating parasites and (ii) the drug concentration to which they are exposed. It is then easy to understand why the therapeutic use of single drugs with long half-life (such as chloroquine or even mefloquine) and long decreasing concentration tails has facilitated the selection of resistant plasmodia strains.
Similarly to other infectious diseases where resistance is a major challenge (tuberculosis, HIV infection, etc.) a combination a strategy to limit resistance has been proposed in the ‘90s using a rapidly acting and potent drug able to achieve a fast reduction of parasitic burden (limiting the intrinsic probability of genetic mutation) with few residual parasites exposed to high concentration of the long-acting partner drug (thus limiting the selective potential of low drug concentration).[5] The rapid acting component of these combinations, thereafter called Artemisinin Combination Therapies (ACT), have been identified to be artemisinin and its derivatives, that are now considered the cornerstone of malaria treatment.
Unfortunately, as a possible consequence con drug misuse both in combination and monotherapy, resistance to artemisinin derivatives has been reported, once again in South East Asia,[6] forcing the implementation of a Global Plan for Artemisinin Resistance Containment (GPARC) by the World Health Organization.[7]
Artemisinin and artemisinin-based combination therapy (ACT)
Artemisinin is a sesquiterpene trioxane lactone extracted from the plant Artemisia annua obtained by the Chinese program named ‘Project 523’ in the 1970’s.[8] Its derivates (artesunate, artemether, and arteether) act with a mechanism, still largely unknown[9] that makes them almost a perfect P. falciparum killer. It’s effective on a broader age range of the parasite than do other antimalarial drugs (with considerable effect on ring stages and early immature gametocyte stages, but not on extra-erythrocytic forms - sporozoites, liver schizontes or merozoites).[10] Artemisinin and its derivatives act very fast via the common active metabolite dihydroartemisinin with a very high killing rate: the parasite reduction ratio (PRR), representing the fractional reduction per each asexual life cycle (48 hours long), is in the order of 104. Such activity profile would predict a radical cure (eradication of all parasite from the body) in 7-8 days even when baseline parasite burden is >1012 (100,000/µl or 2% parasitemia).[11] Non clinical observations[12] remark good and fast absorption regardless the administration route mode (Tmax 0.5-1 hour after oral assumption, while intramuscular injection leads to slower absorption and longer sustained plasma levels after repeated administrations with possible increased toxicity). Tissue distribution is good, with high brain penetration and selective carrier-mediated entry into infected erythrocytes where drug concentration is 100-fold greater than in uninfected erythrocytes. Artemisinin and its derivates are bio-transformed by cytocrome P450 into the active metabolite dihydroartemisinin, with the exception of artesunate, which is an ester of the latter and is converted by esterases. However, Achilles’ heel of artemisinin and its derivates is their very short half-life, ranging from 2 to 5 hours, while artesunate's and artemether's half life are <1 hour and 2-4 hours respectively.[13] Artemisinin has a time-dependent pharmacokinetic profile with decreased plasma drug level after five consecutive days of oral administration.[14] Consequently, when artemisin derivatives are used alone, they require long (> 5 days) course of treatment to be fully effective, raising the problem of poor compliance in normal clinical setting. Artemisinin monotherapy is therefore burdened with by high failure rate with recrudescence risk ranging between 25 and 50%.[15,16]
To overcome this problem, artemisinin drugs are now used in combination with other antimalarial drugs with a longer half-life: artemisinin combination therapy (ACT). The ACTs take advantage of the strong and fast initial activity of the artemisinin derivative and of the favorable pharmacokinetic properties of the companion drug that, after a short course treatment, continues to act on low level parasitemia until radical cure.[11,17] With a 3-days course of artemisinin, as now recommended by WHO,[3] a 90% reduction of parasite burden is obtained. Ideally the partner drug should be selected among still well-fitting anti-malarial drugs (ensuring at least a 80% cure rate by itself) and with a half-life not as long to expose replicating parasites to sub-therapeutical drug level that may favour the emergence of resistant parasites. For this reason, the choice of the companion drug might be different in Sub-Saharan Africa and in East Asia.
Currently the ACTs are the most potent weapon in treating falciparum malaria and, from the public health perspective, to limit the spread of drug-resistant strains.[18]
In fact, as for treatment of tuberculosis, leprosy, HIV and many cancers, combining drugs with different mode of action and resistance mechanism, reduces the probability of selecting resistance to both drugs: it has been calculated that it could happen in 1 over 1024 parasites, so once over 10,000 years, being 1020 the cumulative total parasite burden in humans each year.[5]
To delay the emergence of P. falciparum resistance to artemisinin derivatives, monotherapy is to be absolutely avoided both in paediatric and adult populations.[19] However, in 2005, WHO issued a warning about the risk of onset and spread of artemisinin resistance from the Greater Mekong sub-region, where evidence of a slower parasite clearance were emerging. A recent study from Cambodia[6] has confirmed the spread of artemisinin resistance, previously reported for the western border zone of Thailand[20] where artesunate–mefloquine combination has been the first-line treatment for P. falciparum malaria since 1994.
Low toxicity is generally attributed to artemisinins. Animal (on rats) studies have suggested toxicity on the haematopoietic system with reticulocytes reversible decrease, but clinical observations point out to a lower toxicity in malaria patients compared to healthy volunteers.[21] Cardiotoxicity could be related to QTc prolongation that has been reported at significant level after high intramuscular doses of the oil-based artemether and artemotil in toxicological studies conducted in beagle dogs.[22] However, in humans, QTc interval was unaffected by bolus intravenous therapeutic artesunate doses (2.4 mg/kg).[23]
Fatal neurotoxicity, associated with administration of artemether and arteether intramuscularly or artelinic acid orally, has been demonstrated in animals but only for long drug exposure that is not comparable to that obtained with therapeutical courses as recommended for humans.[24]
Recently, cases of late haemolysis after parenteral treatment with artesunate have been reported.[25] This phenomenon, whose underlying mechanism is still largely unknown, had also been reported in vitro in the ‘80s[26] and in the animal model,[27] and it is more pronounced at high dose of artemisinin derivatives and requires longer follow-up of the patients.
Uncomplicated P. falciparum malaria
At the end of the nineties, the World Health Organization (WHO) has promoted a series of clinical trials testing the efficacy of artemisinin-based combinations using various partner drugs (ACTs) in various continents to treat uncomplicated malaria patients.
A large bulk of clear evidence of the superiority of ACTs in achieving both parasitological (parasite clearance time) or clinical (fever clearance time, survival) end points[28,2] has been accumulated in the following years.
Based on these convincing data, WHO now recommends the use of five common ACT combinations (Table 1) as first treatment of uncomplicated P. falciparum malaria in endemic areas. With the exception of artesunate–sulfadoxine–pyrimethamine, the recommended combinations are now available as fixed-dose treatments, which are preferable because of improved ease of use and adherence. Since the few residual parasites surviving the potent and fast effect of the artemisinin component of the ACT are thereafter confronted to the action of the long-acting partner drug, their local sensitivity pattern to the latter is of paramount importance to select the appropriate ACT combination in different geographical settings. Nationally recommended guidelines should carefully consider local resistance patterns to select the ACT combination that has the best chances to remain active for as long as possible.
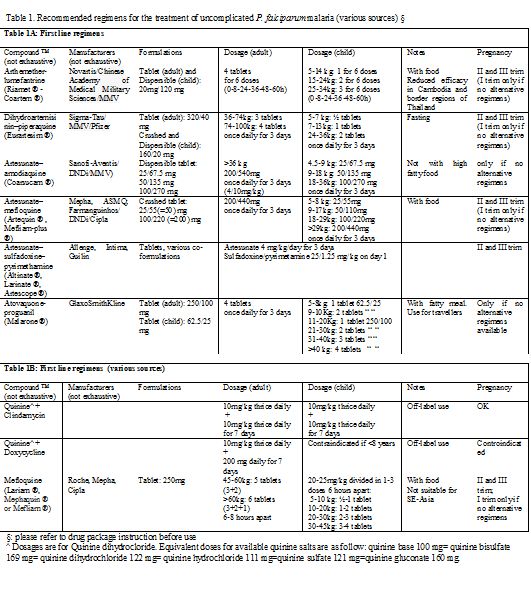
Table 1. Recommended regimens for the treatment of uncomplicated P. falciparum malaria (various sources) §
Even if relatively limited data exist regarding the pharmacokinetic properties of these drugs in pediatric population,[29] ACTs are proposed as first line treatment in children too. To solve problems of swallowability, palatability and dosing, pediatric formulations have been recently developed (syrup, powder for suspension, dispersible tablets and granules) with some evidence of an efficacy comparable to conventional co-formulations (about 98% cure rate) and of a better gastrointestinal tolerability, leading to improved management.[30] However, the evaluation of efficacy, safety and tolerability of administration of pediatric ACTs is still under study.[31]
Evidence of embryo toxicity and lethality in animal studies[32] justifies WHO prudence: for pregnant women ACTs are currently proposed as first-line treatment only in the second and third trimester; during early pregnancy, the use of an ACT is allowed only if the recommended treatments (a seven-day course of quinine plus clindamycin or quinine monotherapy if clindamycin is not available) is not available or has failed, being artesunate plus clindamycin the second-line treatment.[3] Accidental exposures to artemisins in first trimester of pregnancy is being monitored[33] but further studies are needed.
Provided that it has not be used for prophylaxis, atovaquone-proguanil (table 1) is also considered among of the first-line options for travellers returning to non endemic area[34,3] and areas with confirmed artemisinin resistance. Atovaquone acts on the mitochondrial membrane potential,[35] while proguanil interacts with parasite DNA synthesis, inhibiting plasmodial dihydrofolate reductase. Their synergistic action brings to a 98% cure rate[36] and performs better than mefloquine.[37] Although the limited data available suggest that the risk of birth defects associated with atovaquone-proguanil exposure do not exceed 3-times the one observed in the general population,[38] the drug is not currently recommended for use during pregnancy.
When ACT and atovaquone-proguanil are not available or contraindicated, a second line option could be oral quinine plus clindamycin or doxycycline (the latter not to be administered in pregnancy and in children below 8 years) but a 5-7 days treatment course is required with risk of poor adherence mainly linked to quinine-related cinchonism (deafness, ringing in the ear, nausea).
Even if no more recommended by WHO in monotherapy,[39,3] mefloquine, a 4-quinoline methanol, is still considered an acceptable option to treat imported uncomplicated P. falciparum malaria in some western countries guidelines[40,41] but not in others, mainly in relation with a higher rate of neurological adverse effects observed at treatment dosages.[42,43]
Treatment of falciparum uncomplicated malaria with chloroquine is accepted only in patients returning from those limited areas where P. falciparum remains sensible to this drug (Haiti, Dominican Republic, Middle East and Central America - north of Panama Canal), if ACTs are not available.
Uncomplicated non P. falciparum Malaria
It’s important to underline that, when P. falciparum can’t be excluded (co-infection cases; mixed species malaria), any case of uncomplicated malaria coming from areas where resistance is reported should be managed as a falciparum malaria, the more so considering that ACTs and atovaquone-proguanil are effective on blood stages of non-falciparum Plasmodium species.[18,44-47]
However, when P. falciparum infection is safely excluded, chloroquine remains the standard of care for P. malariae, P. ovale and for P. vivax malaria. A total dose of 25mg/kg is recommended (10 mg/kg at T0, followed by 5 mg/kg after 6, 24 and 48 h or, alternatively, 10mg/kg on first and second day and then 5 mg/kg on third day).[3] However, P. vivax is showing decreasing sensitivity in some specific areas. Since first P. vivax chloroquine resistance report in 1989[48] monitoring activity has shown resistant strains mainly in South-East Asia[49] but also in East Africa[50] and Central and South America[51] even if the risk of treatment failure of this drug, as of primaquine, still remains mainly unknown. The experience with P. falciparum resistance allows a reasonable expectation of a deteriorating situation. Chloroquine is well tolerated and safe also in pregnant women and children.
As P. ovale and P. vivax imply a latent hepatic stage (hypnozoites), radical cure to avoid subsequent relapses requires an adjunctive course with a hypnozoites killing drug. Currently, the only molecules with significant activity against this parasite stage are the 8-amino quinoline (buloquine, primaquine, tafenoquine)[52] whose mechanism of action is not well understood but is probably focused on damage to parasite mitochondrial membrane and interference with the parasite's DNA structure.[53] Only primaquine is currently on the market since when it was licensed by FDA in 1952 as anti-malarial drug. The other 8-amino quinoline drugs are still under investigation and seem to possess better pharmacokinetic characteristics (tefenoquine has a longer half-life)[54] and a safer profile (buloquine has a less oxidative toxicity)[55] than primaquine. In fact, the main safety concern in primaquine use is the risk of severe intravascular haemolysis in patients with glucose-6-phosphate dehydrogenase deficiency (G6PDH), which can be life threatening for patients with Mediterranean B variant of the X-chromosome gene. Glucose-6-phosphate dehydrogenase activity is then to be mandatorily assessed before primaquine administration. The drug is contraindicated in cases of severe deficiency (WHO class I and II; ≤ 10% residual enzyme activity);[56] in mild-to-moderate G6PDH deficiency (WHO class III; 10-60% residual activity) primaquine 0.75 mg base/kg body weight may be safely administered once weekly for 8 weeks;[3] in patients without G6PDH deficiency (WHO class IV and V; > 60% enzyme activity) the conventional daily drug adult dosage is 0,25 mg/Kg body weight up to 15 mg/day for 14 days[57] to be taken with food. The efficacy of such low primaquine doses (< 5 mg/kg total dose) in preventing P. vivax relapses is however geographically variable.[58] The Centers for Disease Control and Prevention (CDC) and other Authors currently recommend to increase the adult dosage to 0.5 mg/kg of body weight daily (maximum 30 mg divided in 2 doses) for 14 days when treating Asian P. vivax strains.[41,59,60] Primaquine is contraindicated in pregnant women irrespective of their G6PDH status because the fetus G6PDH status can’t be assessed with certainty and the risk of severe hemolysis and hydrops fetalis may not be ruled out. On the opposite, lactating women can receive the drug if both the mother’s and the child’s G6PDH activity is adequate. Although data are lacking, there is no evidence suggesting that children of any age with normal G6PDH activity do not tolerate the drug. However, some public health authorities suggest caution under various age limits, ranging between 1 month and 4 years.[59]
When primaquine displays a synergistic effect against blood stages when combined with chloroquine. However, the use of an ACT regimen (with exclusion of artesunate plus sulfadoxine-pyrimethamine) seems to be more appropriate in those areas with P. vivax chloroquine resistance where G6PDH activity testing is not easily available.[3,61] Also in this case, however, only a primaquine course guarantees a radical cure from hypnozoites.
Plasmodium knowlesi is the newcomer among human malaria agents. It is microscopically undistinguishable from P. malariae and may even be misdiagnosed as P. vivax or P. falciparum (early trophozoites).[62] Uncomplicated P. knowlesi infection may be cured by chloroquine as other non falciparum malaria. However no official guideline to treat P. knowlesi infection is currently available and there is evidence that other drugs, including mefloquine, quinine, atovaquone/proguanil and sulphadoxine-pyrimethamine may be active against P. knowlesi.[63]
Severe Malaria
Severe malaria, as defined by clinical or laboratory criteria as shown in Table 2 or by high parasitemia (≥ 2% in non immune patients; ≥ 5% in patients in endemic areas), is usually caused by Plasmodium falciparum infection. However, an increasing body of evidence indicates that other Plasmodium species, in particular by P. vivax, may induce severe forms of the infection.[64,65] Case-fatality ratio is high (around 10%)[66] especially among children and even after adoption of intravenous recommended anti-malarial regimens. Patients can deteriorate very quickly with greatest risk of death in the first 24 hours, especially in case of pediatric patients,[67] so that a pre-referral treatment is recommended when appropriate intravenous therapy is likely to be delayed for more than 6 hours.[68]
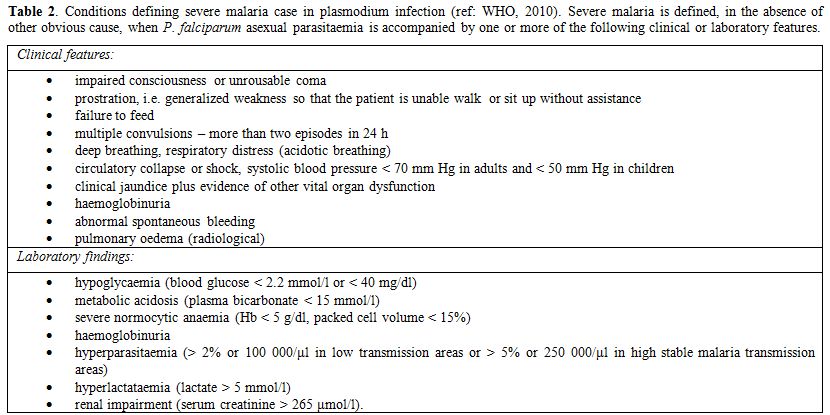
Table 2. Conditions defining severe malaria case in plasmodium infection (ref: WHO, 2010). Severe malaria is defined, in the absence of other obvious cause, when P. falciparum asexual parasitaemia is accompanied by one or more of the following clinical or laboratory features.
Severe malaria should be regarded as a medical emergency and possibly managed in intensive care units (ICU) in order to assure adequate monitoring and treatment of organic dysfunctions.[69-71]
The mainstay of severe malaria therapy, irrespective of the responsible plasmodium species, is a prompt, parenteral, effective anti-malarial treatment with the primary goal of preventing death and disabilities and, only secondarily, recrudescences. Since 2006 WHO recommends intravenous artesunate as first line regimen, preferred to intravenous quinine whenever possible. After multicenter trials have established significant superiority of artesunate over quinine both in South-East Asia[72] and in children in Africa,[73] systematic reviews[74] have demonstrated its effectiveness in reducing case fatality rates regardless of age and geographic differences (RR 0.71). For this reason i.v. artesunate is now considered the standard of care even in the absence of an international drug regulatory authority registration and even against the risk of reduced availability outside Asia. Of notice, the non-GMP (Good Manufacturing Practices) i.v. artesunate produced by Guilin Pharmaceutical Company Ltd., Shanghai, China (the one used in the SEQUAMAT and AQUAMAT trials) has recently been prequalified by WHO.[75] Considerable efforts are currently being made to make a GMP i.v. Artesunate formulation available for clinical use in western countries. In the U.S. the Walter Reed Army Institute of Research (WRAIR) has undergone Phase I trials[76,77] of a formulation currently approved by FDA as an investigational drug, that may be directly requested to CDC.[78] However, also pharmaceutical companies (e.g. Sigma-Tau, Italy) are investing in GMP-standard i.v. artesunate production programmes and on 2007 the European Medicines Agency has assigned the Orphan Medicinal Drug Designation to the drug.[79]
In Europe, only French, Netherlands and Belgian National Health Agencies have temporarily authorized the use of i.v. artesunate within a named patient programme.[80] Malacef ® is the Guilin i.v. artesunate, imported and distributed after quality control by a Dutch company (ACE Pharmaceuticals). Where there is no official authorization, however the use of non –GMP i.v. artesunate may still pose problems under the legal point of view. To overcome this obstacle, a treatment combining market authorized i.v quinine and WHO recommended i.v. prequalified artesunate has been used with satisfactory efficacy and safety profile.[81,82]
The absence of randomized controlled trials to support i.v. artesunate superiority in imported severe malaria cases in non-endemic areas still causes some perplexity.[83] However, both in US by CDC and in Europe by TropNetEurope, a close efficacy and safety monitoring is carried out.[84,85]
If SEAQUAMAT has shown a better safety profile of artesunate when compared with quinine with statistically (p=0.009) significant reduction of hypoglycemia, systematic analysis of randomized trials[74] has pointed out a higher non-statistically significant rate of neurological problems at discharge in patients, especially children, treated with artesunate. This fact may be related to the increased survival of cerebral malaria cases and anyway neurologic sequelae where not permanent. Both SEAQUAMAT and AQUAMAT, however, failed to capture another safety concern that is now emerging by observational studies reporting post-treatment haemolysis, mainly in imported severe malaria cases.[84-87] Patients should be carefully monitored for at least one month after treatment because haemolytic anemia can appear longer after artesunate clearance (median elimination T ˝ is 0.25 [0.11 – 1.82] hours).[88] The precise mechanism underlying such phenomenon are unclear at the moment, nor risk factors are known. As a precautional measure, it could then be prudent to limit the use of i.v. artesunate to the shorter possible necessary (however keeping a minimum of 24 i.v. infusion) in order to exploit its high parasite clearance activity during the first 24 hours and to avoid long unnecessary i.v. treatments. The intra-venous treatment should be followed by a full course of an effective oral anti-malarial treatment: WHO suggests effective ACT (artesunate plus amodiaquine or artemether plus lumefantrine or dihydroartemisinin plus piperaquine) or artesunate (plus clindamycin or doxycycline) or quinine (plus clindamycin or doxycycline) and does not recommend the use of mefloquine because of the increased risk of neuropsychiatric events after cerebral malaria.[3]
Where intravenous parenteral treatment with artesunate is not immediately, i.v. quinine should be used (Table 3). In remote settings, far from health care facilities that could ensure intravenous treatment, pre-referral intra muscular quinine, artemether or artesunate and, even easier to administrate, rectal artesunate are currently recommended by WHO for children.[68] In particular, a placebo controlled trial has shown superiority (p=0.0013) of rectal artesunate over placebo to prevent death or permanent disability (RR 0.49).[89] Even if the trial has been the subject of controversial debate as to its methodological approach,[90] it has the great merit of remarking the urgency of immediate treatment in cases of severe malaria. Some concern may arise as to the risk that encouraging the use of rectal artesunate in monotherapy could impact on resistance pattern. Anyway evaluation of efficacy and appropriateness of this strategy is ongoing.[91]
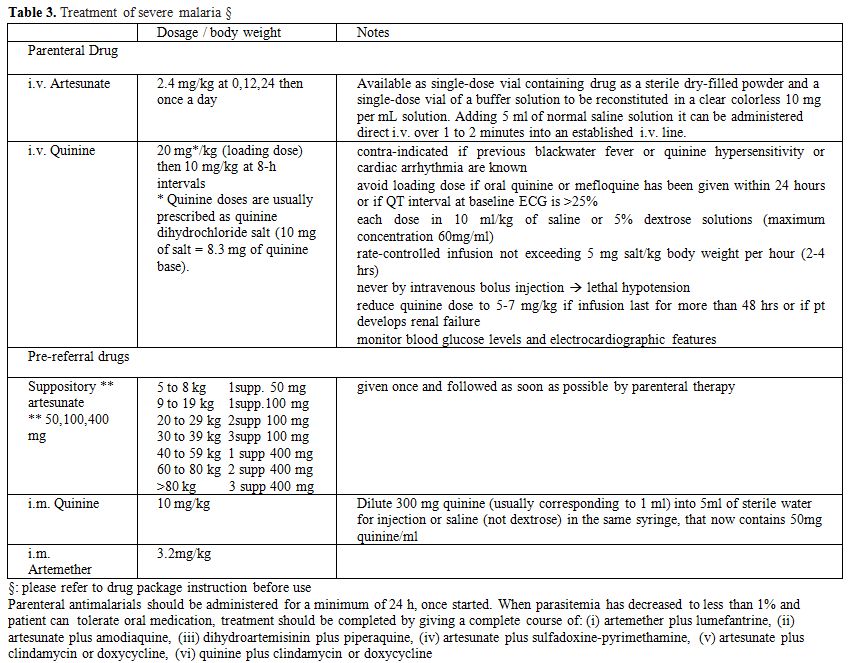
Table 3. Treatment of severe malaria §
The correct management of clinical malaria cases is a complex issue that has to take into account different targets that may be differently prioritized according to the various clinical and epidemiological situations:
a. to prevent progression of uncomplicated malaria patients to severe life threatening complications (P. falciparum but also P. vivax);
b. to prevent mortality of patients with severe malaria (P. falciparum but also P. vivax and P. knowlesi);
c. to prevent relapses when appropriate (P. vivax, P. ovale);
d. to limit the spreading of the infection/disease in the population;
e. to limit as much as possible the emergence of plasmodium resistant strains.
Considering the complex biological cycle of malaria plasmodia, the ideal drug to meet the clinical targets should have the following properties:
- to act rapidly against the replicating blood erythrocytic asexual forms, primarily schizonts, that are responsible for the clinical manifestation of the disease (parasitological cure);
- to act against liver hypnozoites, when appropriate (radical cure).
In endemic areas, furthermore, the ideal drug to meet the epidemiological targets should have the following properties:
- to act against the sexual forms (gametocytes) that are responsible for the transmission of the infection in the population via the vector mosquitoes; this gametocidal effect is time-sensitive because the appearance of sexual forms is delayed of several days from the clinical malaria attack;
- to avoid selecting plasmodia resistant strains (high resistance barrier).
After quinine selective therapeutical value against malaria plasmodia was suggested by Francesco Torti in Italy (1712), no major advance in malaria chemotherapy occurred until the first decades of the XX century. At that time, and until recently, treatment of malaria access was based on the use of single drug regimens that were subsequently made available according to the emergence of resistance to the previously used molecules. Pamaquine and chloroquine were discovered in Germany in 1924 and 1934 respectively, followed by proguanil (England, 1944), pyrimethamine (England, 1952), primaquine (USA, 1956), sulphonamides (1960-66), mefloquine (USA, 1971-75) and halofantrine (1989).[1]
The value of monotherapy had been questioned since the early ’60, when P. falciparum with decreased sensitivity to chloroquine appeared in South-East Asia and Colombia and then quickly spread to virtually all P. falciparum endemic areas. Thereafter, the same occurred to all antimalarial drugs acting against P. falciparum and, to a lesser extent, against P. vivax.
At the end of the 20th century, the strong anti-parasite efficacy of the long-known Chinese malarial remedy artemisinine and its derivatives was scientifically demonstrated, both on blood asexual and sexual forms (gametocytes). Furthermore, in line with other major infectious diseases such as tuberculosis and HIV infection, the value of combination treatment to lessen the chance of a natural resistant strain to emerge was clearly established and new combination treatment tested (atovaquone-proguanil, chlorproguanil-dapsone).
Artemisinin based combination treatments (ACT) underwent extensive randomized clinical trials that proved their superiority in fever and parasitological clearance times and clinical outcomes.[2]
At present, therefore, artemisinin-based treatment complies with most of the properties of the “ideal antimalarial drug” listed above and represents the standard of care of both complicated and uncomplicated malaria.[3]
Drug resistance of malaria plasmodia
According to WHO, resistance to malaria drug is defines as “the ability of a parasite strain to survive and/or multiply despite the proper administration and absorption of an antimalarial drug in the dose normally recommended”.[3]
This is particularly worrying for P. falciparum, both because of its higher propensity to develop resistance and because of its intrinsic higher virulence and morbidity and mortality burden. Resistance (or lower sensitivity) to antimalarial drugs has also been observed in P. vivax, while it is extremely rare (if present at all) in the other species of Plasmodia.
The spread of resistance is classically a two-step process. First, a mutant clone spontaneously emerges in the replicating parasite population. This clone is usually less fit that the wild-type sensitive ones and tends to disappear, unless it is confronted to selective drug pressure able to kill sensitive parasites but not blood circulating resistant asexual forms that subsequently evolve to gametocytes with possible spread in the population (second step). This phenomenon is usually more likely to happen first in low transmission settings where most parasite-carrying patients are symptomatic and therefore subject to treatment.[3] This is probably the reason why chloroquine and pyrimetamine resistance strains first appeared in South-East Asia in the early sixties before spreading to the African continent,[4] also favoured by the suggested higher predisposition to mutation of Asian P. falciparum strains.[3]
The probability of a genetic resistance mutation to occur is a function of many factors, including (but not limited to) (i) the number of number of replicating parasites and (ii) the drug concentration to which they are exposed. It is then easy to understand why the therapeutic use of single drugs with long half-life (such as chloroquine or even mefloquine) and long decreasing concentration tails has facilitated the selection of resistant plasmodia strains.
Similarly to other infectious diseases where resistance is a major challenge (tuberculosis, HIV infection, etc.) a combination a strategy to limit resistance has been proposed in the ‘90s using a rapidly acting and potent drug able to achieve a fast reduction of parasitic burden (limiting the intrinsic probability of genetic mutation) with few residual parasites exposed to high concentration of the long-acting partner drug (thus limiting the selective potential of low drug concentration).[5] The rapid acting component of these combinations, thereafter called Artemisinin Combination Therapies (ACT), have been identified to be artemisinin and its derivatives, that are now considered the cornerstone of malaria treatment.
Unfortunately, as a possible consequence con drug misuse both in combination and monotherapy, resistance to artemisinin derivatives has been reported, once again in South East Asia,[6] forcing the implementation of a Global Plan for Artemisinin Resistance Containment (GPARC) by the World Health Organization.[7]
Artemisinin and artemisinin-based combination therapy (ACT)
Artemisinin is a sesquiterpene trioxane lactone extracted from the plant Artemisia annua obtained by the Chinese program named ‘Project 523’ in the 1970’s.[8] Its derivates (artesunate, artemether, and arteether) act with a mechanism, still largely unknown[9] that makes them almost a perfect P. falciparum killer. It’s effective on a broader age range of the parasite than do other antimalarial drugs (with considerable effect on ring stages and early immature gametocyte stages, but not on extra-erythrocytic forms - sporozoites, liver schizontes or merozoites).[10] Artemisinin and its derivatives act very fast via the common active metabolite dihydroartemisinin with a very high killing rate: the parasite reduction ratio (PRR), representing the fractional reduction per each asexual life cycle (48 hours long), is in the order of 104. Such activity profile would predict a radical cure (eradication of all parasite from the body) in 7-8 days even when baseline parasite burden is >1012 (100,000/µl or 2% parasitemia).[11] Non clinical observations[12] remark good and fast absorption regardless the administration route mode (Tmax 0.5-1 hour after oral assumption, while intramuscular injection leads to slower absorption and longer sustained plasma levels after repeated administrations with possible increased toxicity). Tissue distribution is good, with high brain penetration and selective carrier-mediated entry into infected erythrocytes where drug concentration is 100-fold greater than in uninfected erythrocytes. Artemisinin and its derivates are bio-transformed by cytocrome P450 into the active metabolite dihydroartemisinin, with the exception of artesunate, which is an ester of the latter and is converted by esterases. However, Achilles’ heel of artemisinin and its derivates is their very short half-life, ranging from 2 to 5 hours, while artesunate's and artemether's half life are <1 hour and 2-4 hours respectively.[13] Artemisinin has a time-dependent pharmacokinetic profile with decreased plasma drug level after five consecutive days of oral administration.[14] Consequently, when artemisin derivatives are used alone, they require long (> 5 days) course of treatment to be fully effective, raising the problem of poor compliance in normal clinical setting. Artemisinin monotherapy is therefore burdened with by high failure rate with recrudescence risk ranging between 25 and 50%.[15,16]
To overcome this problem, artemisinin drugs are now used in combination with other antimalarial drugs with a longer half-life: artemisinin combination therapy (ACT). The ACTs take advantage of the strong and fast initial activity of the artemisinin derivative and of the favorable pharmacokinetic properties of the companion drug that, after a short course treatment, continues to act on low level parasitemia until radical cure.[11,17] With a 3-days course of artemisinin, as now recommended by WHO,[3] a 90% reduction of parasite burden is obtained. Ideally the partner drug should be selected among still well-fitting anti-malarial drugs (ensuring at least a 80% cure rate by itself) and with a half-life not as long to expose replicating parasites to sub-therapeutical drug level that may favour the emergence of resistant parasites. For this reason, the choice of the companion drug might be different in Sub-Saharan Africa and in East Asia.
Currently the ACTs are the most potent weapon in treating falciparum malaria and, from the public health perspective, to limit the spread of drug-resistant strains.[18]
In fact, as for treatment of tuberculosis, leprosy, HIV and many cancers, combining drugs with different mode of action and resistance mechanism, reduces the probability of selecting resistance to both drugs: it has been calculated that it could happen in 1 over 1024 parasites, so once over 10,000 years, being 1020 the cumulative total parasite burden in humans each year.[5]
To delay the emergence of P. falciparum resistance to artemisinin derivatives, monotherapy is to be absolutely avoided both in paediatric and adult populations.[19] However, in 2005, WHO issued a warning about the risk of onset and spread of artemisinin resistance from the Greater Mekong sub-region, where evidence of a slower parasite clearance were emerging. A recent study from Cambodia[6] has confirmed the spread of artemisinin resistance, previously reported for the western border zone of Thailand[20] where artesunate–mefloquine combination has been the first-line treatment for P. falciparum malaria since 1994.
Low toxicity is generally attributed to artemisinins. Animal (on rats) studies have suggested toxicity on the haematopoietic system with reticulocytes reversible decrease, but clinical observations point out to a lower toxicity in malaria patients compared to healthy volunteers.[21] Cardiotoxicity could be related to QTc prolongation that has been reported at significant level after high intramuscular doses of the oil-based artemether and artemotil in toxicological studies conducted in beagle dogs.[22] However, in humans, QTc interval was unaffected by bolus intravenous therapeutic artesunate doses (2.4 mg/kg).[23]
Fatal neurotoxicity, associated with administration of artemether and arteether intramuscularly or artelinic acid orally, has been demonstrated in animals but only for long drug exposure that is not comparable to that obtained with therapeutical courses as recommended for humans.[24]
Recently, cases of late haemolysis after parenteral treatment with artesunate have been reported.[25] This phenomenon, whose underlying mechanism is still largely unknown, had also been reported in vitro in the ‘80s[26] and in the animal model,[27] and it is more pronounced at high dose of artemisinin derivatives and requires longer follow-up of the patients.
Uncomplicated P. falciparum malaria
At the end of the nineties, the World Health Organization (WHO) has promoted a series of clinical trials testing the efficacy of artemisinin-based combinations using various partner drugs (ACTs) in various continents to treat uncomplicated malaria patients.
A large bulk of clear evidence of the superiority of ACTs in achieving both parasitological (parasite clearance time) or clinical (fever clearance time, survival) end points[28,2] has been accumulated in the following years.
Based on these convincing data, WHO now recommends the use of five common ACT combinations (Table 1) as first treatment of uncomplicated P. falciparum malaria in endemic areas. With the exception of artesunate–sulfadoxine–pyrimethamine, the recommended combinations are now available as fixed-dose treatments, which are preferable because of improved ease of use and adherence. Since the few residual parasites surviving the potent and fast effect of the artemisinin component of the ACT are thereafter confronted to the action of the long-acting partner drug, their local sensitivity pattern to the latter is of paramount importance to select the appropriate ACT combination in different geographical settings. Nationally recommended guidelines should carefully consider local resistance patterns to select the ACT combination that has the best chances to remain active for as long as possible.

Table 1. Recommended regimens for the treatment of uncomplicated P. falciparum malaria (various sources) §
Even if relatively limited data exist regarding the pharmacokinetic properties of these drugs in pediatric population,[29] ACTs are proposed as first line treatment in children too. To solve problems of swallowability, palatability and dosing, pediatric formulations have been recently developed (syrup, powder for suspension, dispersible tablets and granules) with some evidence of an efficacy comparable to conventional co-formulations (about 98% cure rate) and of a better gastrointestinal tolerability, leading to improved management.[30] However, the evaluation of efficacy, safety and tolerability of administration of pediatric ACTs is still under study.[31]
Evidence of embryo toxicity and lethality in animal studies[32] justifies WHO prudence: for pregnant women ACTs are currently proposed as first-line treatment only in the second and third trimester; during early pregnancy, the use of an ACT is allowed only if the recommended treatments (a seven-day course of quinine plus clindamycin or quinine monotherapy if clindamycin is not available) is not available or has failed, being artesunate plus clindamycin the second-line treatment.[3] Accidental exposures to artemisins in first trimester of pregnancy is being monitored[33] but further studies are needed.
Provided that it has not be used for prophylaxis, atovaquone-proguanil (table 1) is also considered among of the first-line options for travellers returning to non endemic area[34,3] and areas with confirmed artemisinin resistance. Atovaquone acts on the mitochondrial membrane potential,[35] while proguanil interacts with parasite DNA synthesis, inhibiting plasmodial dihydrofolate reductase. Their synergistic action brings to a 98% cure rate[36] and performs better than mefloquine.[37] Although the limited data available suggest that the risk of birth defects associated with atovaquone-proguanil exposure do not exceed 3-times the one observed in the general population,[38] the drug is not currently recommended for use during pregnancy.
When ACT and atovaquone-proguanil are not available or contraindicated, a second line option could be oral quinine plus clindamycin or doxycycline (the latter not to be administered in pregnancy and in children below 8 years) but a 5-7 days treatment course is required with risk of poor adherence mainly linked to quinine-related cinchonism (deafness, ringing in the ear, nausea).
Even if no more recommended by WHO in monotherapy,[39,3] mefloquine, a 4-quinoline methanol, is still considered an acceptable option to treat imported uncomplicated P. falciparum malaria in some western countries guidelines[40,41] but not in others, mainly in relation with a higher rate of neurological adverse effects observed at treatment dosages.[42,43]
Treatment of falciparum uncomplicated malaria with chloroquine is accepted only in patients returning from those limited areas where P. falciparum remains sensible to this drug (Haiti, Dominican Republic, Middle East and Central America - north of Panama Canal), if ACTs are not available.
Uncomplicated non P. falciparum Malaria
It’s important to underline that, when P. falciparum can’t be excluded (co-infection cases; mixed species malaria), any case of uncomplicated malaria coming from areas where resistance is reported should be managed as a falciparum malaria, the more so considering that ACTs and atovaquone-proguanil are effective on blood stages of non-falciparum Plasmodium species.[18,44-47]
However, when P. falciparum infection is safely excluded, chloroquine remains the standard of care for P. malariae, P. ovale and for P. vivax malaria. A total dose of 25mg/kg is recommended (10 mg/kg at T0, followed by 5 mg/kg after 6, 24 and 48 h or, alternatively, 10mg/kg on first and second day and then 5 mg/kg on third day).[3] However, P. vivax is showing decreasing sensitivity in some specific areas. Since first P. vivax chloroquine resistance report in 1989[48] monitoring activity has shown resistant strains mainly in South-East Asia[49] but also in East Africa[50] and Central and South America[51] even if the risk of treatment failure of this drug, as of primaquine, still remains mainly unknown. The experience with P. falciparum resistance allows a reasonable expectation of a deteriorating situation. Chloroquine is well tolerated and safe also in pregnant women and children.
As P. ovale and P. vivax imply a latent hepatic stage (hypnozoites), radical cure to avoid subsequent relapses requires an adjunctive course with a hypnozoites killing drug. Currently, the only molecules with significant activity against this parasite stage are the 8-amino quinoline (buloquine, primaquine, tafenoquine)[52] whose mechanism of action is not well understood but is probably focused on damage to parasite mitochondrial membrane and interference with the parasite's DNA structure.[53] Only primaquine is currently on the market since when it was licensed by FDA in 1952 as anti-malarial drug. The other 8-amino quinoline drugs are still under investigation and seem to possess better pharmacokinetic characteristics (tefenoquine has a longer half-life)[54] and a safer profile (buloquine has a less oxidative toxicity)[55] than primaquine. In fact, the main safety concern in primaquine use is the risk of severe intravascular haemolysis in patients with glucose-6-phosphate dehydrogenase deficiency (G6PDH), which can be life threatening for patients with Mediterranean B variant of the X-chromosome gene. Glucose-6-phosphate dehydrogenase activity is then to be mandatorily assessed before primaquine administration. The drug is contraindicated in cases of severe deficiency (WHO class I and II; ≤ 10% residual enzyme activity);[56] in mild-to-moderate G6PDH deficiency (WHO class III; 10-60% residual activity) primaquine 0.75 mg base/kg body weight may be safely administered once weekly for 8 weeks;[3] in patients without G6PDH deficiency (WHO class IV and V; > 60% enzyme activity) the conventional daily drug adult dosage is 0,25 mg/Kg body weight up to 15 mg/day for 14 days[57] to be taken with food. The efficacy of such low primaquine doses (< 5 mg/kg total dose) in preventing P. vivax relapses is however geographically variable.[58] The Centers for Disease Control and Prevention (CDC) and other Authors currently recommend to increase the adult dosage to 0.5 mg/kg of body weight daily (maximum 30 mg divided in 2 doses) for 14 days when treating Asian P. vivax strains.[41,59,60] Primaquine is contraindicated in pregnant women irrespective of their G6PDH status because the fetus G6PDH status can’t be assessed with certainty and the risk of severe hemolysis and hydrops fetalis may not be ruled out. On the opposite, lactating women can receive the drug if both the mother’s and the child’s G6PDH activity is adequate. Although data are lacking, there is no evidence suggesting that children of any age with normal G6PDH activity do not tolerate the drug. However, some public health authorities suggest caution under various age limits, ranging between 1 month and 4 years.[59]
When primaquine displays a synergistic effect against blood stages when combined with chloroquine. However, the use of an ACT regimen (with exclusion of artesunate plus sulfadoxine-pyrimethamine) seems to be more appropriate in those areas with P. vivax chloroquine resistance where G6PDH activity testing is not easily available.[3,61] Also in this case, however, only a primaquine course guarantees a radical cure from hypnozoites.
Plasmodium knowlesi is the newcomer among human malaria agents. It is microscopically undistinguishable from P. malariae and may even be misdiagnosed as P. vivax or P. falciparum (early trophozoites).[62] Uncomplicated P. knowlesi infection may be cured by chloroquine as other non falciparum malaria. However no official guideline to treat P. knowlesi infection is currently available and there is evidence that other drugs, including mefloquine, quinine, atovaquone/proguanil and sulphadoxine-pyrimethamine may be active against P. knowlesi.[63]
Severe Malaria
Severe malaria, as defined by clinical or laboratory criteria as shown in Table 2 or by high parasitemia (≥ 2% in non immune patients; ≥ 5% in patients in endemic areas), is usually caused by Plasmodium falciparum infection. However, an increasing body of evidence indicates that other Plasmodium species, in particular by P. vivax, may induce severe forms of the infection.[64,65] Case-fatality ratio is high (around 10%)[66] especially among children and even after adoption of intravenous recommended anti-malarial regimens. Patients can deteriorate very quickly with greatest risk of death in the first 24 hours, especially in case of pediatric patients,[67] so that a pre-referral treatment is recommended when appropriate intravenous therapy is likely to be delayed for more than 6 hours.[68]

Table 2. Conditions defining severe malaria case in plasmodium infection (ref: WHO, 2010). Severe malaria is defined, in the absence of other obvious cause, when P. falciparum asexual parasitaemia is accompanied by one or more of the following clinical or laboratory features.
Severe malaria should be regarded as a medical emergency and possibly managed in intensive care units (ICU) in order to assure adequate monitoring and treatment of organic dysfunctions.[69-71]
The mainstay of severe malaria therapy, irrespective of the responsible plasmodium species, is a prompt, parenteral, effective anti-malarial treatment with the primary goal of preventing death and disabilities and, only secondarily, recrudescences. Since 2006 WHO recommends intravenous artesunate as first line regimen, preferred to intravenous quinine whenever possible. After multicenter trials have established significant superiority of artesunate over quinine both in South-East Asia[72] and in children in Africa,[73] systematic reviews[74] have demonstrated its effectiveness in reducing case fatality rates regardless of age and geographic differences (RR 0.71). For this reason i.v. artesunate is now considered the standard of care even in the absence of an international drug regulatory authority registration and even against the risk of reduced availability outside Asia. Of notice, the non-GMP (Good Manufacturing Practices) i.v. artesunate produced by Guilin Pharmaceutical Company Ltd., Shanghai, China (the one used in the SEQUAMAT and AQUAMAT trials) has recently been prequalified by WHO.[75] Considerable efforts are currently being made to make a GMP i.v. Artesunate formulation available for clinical use in western countries. In the U.S. the Walter Reed Army Institute of Research (WRAIR) has undergone Phase I trials[76,77] of a formulation currently approved by FDA as an investigational drug, that may be directly requested to CDC.[78] However, also pharmaceutical companies (e.g. Sigma-Tau, Italy) are investing in GMP-standard i.v. artesunate production programmes and on 2007 the European Medicines Agency has assigned the Orphan Medicinal Drug Designation to the drug.[79]
In Europe, only French, Netherlands and Belgian National Health Agencies have temporarily authorized the use of i.v. artesunate within a named patient programme.[80] Malacef ® is the Guilin i.v. artesunate, imported and distributed after quality control by a Dutch company (ACE Pharmaceuticals). Where there is no official authorization, however the use of non –GMP i.v. artesunate may still pose problems under the legal point of view. To overcome this obstacle, a treatment combining market authorized i.v quinine and WHO recommended i.v. prequalified artesunate has been used with satisfactory efficacy and safety profile.[81,82]
The absence of randomized controlled trials to support i.v. artesunate superiority in imported severe malaria cases in non-endemic areas still causes some perplexity.[83] However, both in US by CDC and in Europe by TropNetEurope, a close efficacy and safety monitoring is carried out.[84,85]
If SEAQUAMAT has shown a better safety profile of artesunate when compared with quinine with statistically (p=0.009) significant reduction of hypoglycemia, systematic analysis of randomized trials[74] has pointed out a higher non-statistically significant rate of neurological problems at discharge in patients, especially children, treated with artesunate. This fact may be related to the increased survival of cerebral malaria cases and anyway neurologic sequelae where not permanent. Both SEAQUAMAT and AQUAMAT, however, failed to capture another safety concern that is now emerging by observational studies reporting post-treatment haemolysis, mainly in imported severe malaria cases.[84-87] Patients should be carefully monitored for at least one month after treatment because haemolytic anemia can appear longer after artesunate clearance (median elimination T ˝ is 0.25 [0.11 – 1.82] hours).[88] The precise mechanism underlying such phenomenon are unclear at the moment, nor risk factors are known. As a precautional measure, it could then be prudent to limit the use of i.v. artesunate to the shorter possible necessary (however keeping a minimum of 24 i.v. infusion) in order to exploit its high parasite clearance activity during the first 24 hours and to avoid long unnecessary i.v. treatments. The intra-venous treatment should be followed by a full course of an effective oral anti-malarial treatment: WHO suggests effective ACT (artesunate plus amodiaquine or artemether plus lumefantrine or dihydroartemisinin plus piperaquine) or artesunate (plus clindamycin or doxycycline) or quinine (plus clindamycin or doxycycline) and does not recommend the use of mefloquine because of the increased risk of neuropsychiatric events after cerebral malaria.[3]
Where intravenous parenteral treatment with artesunate is not immediately, i.v. quinine should be used (Table 3). In remote settings, far from health care facilities that could ensure intravenous treatment, pre-referral intra muscular quinine, artemether or artesunate and, even easier to administrate, rectal artesunate are currently recommended by WHO for children.[68] In particular, a placebo controlled trial has shown superiority (p=0.0013) of rectal artesunate over placebo to prevent death or permanent disability (RR 0.49).[89] Even if the trial has been the subject of controversial debate as to its methodological approach,[90] it has the great merit of remarking the urgency of immediate treatment in cases of severe malaria. Some concern may arise as to the risk that encouraging the use of rectal artesunate in monotherapy could impact on resistance pattern. Anyway evaluation of efficacy and appropriateness of this strategy is ongoing.[91]

Table 3. Treatment of severe malaria §
References
- Gilles H.M. Historical outline in:
Essential Malariology (Warrell D.A. and Gilles H.M. editors), Arnold
International Students Edition, 2002: 1-7
- International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 2004; 363: 9–17 http://dx.doi.org/10.1016/S0140-6736(03)15162-8

- WHO. Guidelines for the Treatment of Malaria: Second Edition. 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html
- Roper C, Pearce R, Nair S, Sharp B, Nosten
F, Anderson T. Intercontinental sspread of pyrimethamine-resistant
malaria. Science, 2004; 305: 1124 http://dx.doi.org/10.1126/science.1098876 PMid:15326348

- Nosten F and White NJ. Artemisinin-Based
Combination Treatment of falciparum Malaria. Am J Trop Med Hyg, 2007,
77 (Suppl 6): 181–192 PMid:18165491

- Phyo AP, Nkhoma S, Stepniewska K, Ashley
EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM,
Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of
artemisinin-resistant malaria on the western border of Thailand: a
longitudinal study. Lancet, 2012; 379: 1960-1966 http://dx.doi.org/10.1016/S0140-6736(12)60484-X

- WHO. Update on artemisinin resistance - April 2012 http://www.who.int/malaria/publications/atoz/arupdate042012.pdf
- Zhang JF. A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin), Yang Cheng Evening News Publishing Company, 2005
- O’Neill PM, Barton VE and Ward SA. The
Molecular Mechanism of Action of Artemisinin. The Debate Continues.
Molecules. 2010; 15: 1705-1721 http://dx.doi.org/10.3390/molecules15031705 PMid:20336009

- Skinner TS, Manning LS, Johnston WA, Davis
TM. In vitro Stage-specific Sensitivity of Plasmodium falciparum to
Quinine and Artemisinin Drugs. Int J Parasitol, 1996; 26: 519-525 http://dx.doi.org/10.1016/0020-7519(96)89380-5

- White NJ. Assessment of the
pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob.
Agents Chemother, 1997; 41: 1413-22 PMid:9210658 PMCid:163932

- Artemisinin Derivatives: Summary of Nonclinical Safety Data Introductory remarks http://apps.who.int/prequal/info_applicants/Guidelines/Nonclinical_Overview_Artemisinin-Derivatives.pdf
- Morris CA, Duparc S, Borghini-Fuhrer I,
Jung D, Shin CS, Fleckenstein L. Review of the clinical
pharmacokinetics of artesunate and its active metabolite
dihydroartemisinin following intravenous, intramuscular, oral or rectal
administration. Malar J, 2011; 10: 263 http://dx.doi.org/10.1186/1475-2875-10-263 PMid:21914160 PMCid:3180444

- Xing J, Bai KH, Liu T, Wang RL, Zhang LF,
Zhang SQ. The multiple-dosing pharmacokinetics of artemether,
artesunate, and their metabolite dihydroartemisinin in rats.
Xenobiotica. 2011, 41: 252-8 http://dx.doi.org/10.3109/00498254.2010.542257 PMid:21175296

- Nguyen DS, Dao BH, Nguyen PD, Nguyen VH,
Le NB, Mai VS, Meshnick SR Treatment of malaria in Vietnam with oral
artemisinin. Am J Trop Med Hyg, 1993; 48: 398–402 PMid:8470777

- Giao PT, Binh TQ, Kager PA, Long HP, Van
Thang N, Van Nam N, de Vries PJ. Artemisinin for treatment of
uncomplicated falciparum malaria: is there a place for monotherapy? Am
J Trop Med Hyg, 2001; 65: 690–695 PMid:11791958

- White NJ. The parasite clearance curve. Malar J, 2011; 10: 278 http://dx.doi.org/10.1186/1475-2875-10-278 PMid:21939506 PMCid:3195204

- Sinclair D, Zani B, Donegan S, Olliaro P,
Garner P. Artemisinin-based combination therapy for treating
uncomplicated malaria. Cochrane Database of Systematic Reviews 2009,
Issue 3. Art. No.: CD007483. DOI: 10.1002/14651858.CD007483. pub2

- Maude RJ, Woodrow CJ, White LJ.
Artemisinin antimalarials: preserving the “Magic Bullet”. Drug Develop
Res, 2010; 71: 12-19 PMid:21399699 PMCid:3048293

- Dondorp AM, Nosten F, Yi P, Das D, Phyo
AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P,
Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S,
Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin
resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361:
455–67 http://dx.doi.org/10.1056/NEJMoa0808859 PMid:19641202

- Clark RL. Effects of artemisinins on
reticulocyte count and relationship to possible embryotoxicity in
confirmed and unconfirmed malarial patients. Birth Defects Res A Clin
Mol Teratol, 2012 94: 61-75. http://dx.doi.org/10.1002/bdra.22868 PMid:22125126

- Classen W, Altmann B, Gretener P, Souppart
C, Skelton-Stroud P, Krinke G. Differential effects of orally versus
parenterally administered qinghaosu derivative artemether in dogs. Exp
Toxicol Pathol, 1999; 51: 507–516 http://dx.doi.org/10.1016/S0940-2993(99)80128-6

- Maude RJ, Plewes K, Faiz MA, Hanson J,
Charunwatthana P, Lee SJ, Tärning J, Yunus EB, Hoque MG, Hasan MU,
Hossain A, Lindegardh N, Day NP, White NJ, Dondorp AM. Does Artesunate
Prolong the Electrocardiograph QT Interval in Patients with Severe
Malaria? Am J Trop Med Hyg, 2009, 80:126–132. PMid:19141850
PMCid:2843440

- Li Q, Hickman M. Toxicokinetic and
toxicodynamic (TK/TD) evaluation to determine and predict
theneurotoxicity of artemisinins. Toxicology. 2011; 279:1-9 http://dx.doi.org/10.1016/j.tox.2010.09.005

- Rolling T, Schmiedel S, Wichmann D,
Wittkopf D, Burchard GD, Cramer JP. Post-treatment haemolysis in severe
imported malaria after intravenous artesunate: case report of three
patients with hyperparasitaemia. Malar J, 2012; 1:169 http://dx.doi.org/10.1186/1475-2875-11-169 PMid:22594446

- Gu H, Warhurst D, Peters W. Hemolysis induced by artemisinin and its derivatives. Acta Pharmacol Sinica, 1986; 3: 269-272

- Omotuyi, IO, Nwangwu SC, Okugbo OT, Okoye
OT, Ojieh GC, Wogu D M. Hepatotoxic and hemolytic effects of acute
exposure of rats to artesunate overdose. Afr J Biochem Res, 2008; 2:
107-110

- von Seidlein L, Milligan P, Pinder M, et
al. Efficacy of artesunate plus pyrimethamine-sulphadoxine for
uncomplicated malaria in Gambian children: a double-blind, randomised,
controlled trial. Lancet 2000; 355: 352–57. http://dx.doi.org/10.1016/S0140-6736(99)10237-X

- Mercer AE, Sarr Sallah M. The
pharmacokinetic evaluation of artemisinin drugs for the treatment of
malaria in paediatric populations. Expert Opin Drug Metab Toxicol,
2011; 7: 427-39. http://dx.doi.org/10.1517/17425255.2011.557064

- Kurth F, Bélard S, Adegnika AA, Gaye O,
Kremsner PG, Ramharter M. Do pediatric drug formulations of artemisinin
combination therapies improve the treatment of children with malaria? A
systematic review and meta-analysis. Lancet Infect Dis, 2010; 10:
125-32 http://dx.doi.org/10.1016/S1473-3099(09)70327-5

- Bélard S, Kurth F, Ramharter M. Paediatric
Formulations of Artemisinin-Combination Therapies for Treating
Uncomplicated Malaria in Children. Cochrane Database of Systematic
Reviews 2012, Issue 1. Art. No.: CD009568. http://dx.doi.org/10.1002/14651858.CD009568

- Clark RL, Lerman SA, Cox EM, Gristwood WE,
White TE. Developmental toxicity of artesunate in the rat: comparison
to other artemisinins, comparison of embryotoxicity and kinetics by
oral and intravenous routes, and relationship to maternal reticulocyte
count. Birth Defects Res B Dev Reprod Toxicol, 2008, 83: 397–406 http://dx.doi.org/10.1002/bdrb.20165 PMid:18702118

- Manyando C, Kayentao K, D'Alessandro U,
Okafor HU, Juma E, Hamed K. A systematic review of the safety and
efficacy of artemether-lumefantrine against uncomplicated Plasmodium
falciparum malaria during pregnancy. Malar J, 2012; 11: 141 http://dx.doi.org/10.1186/1475-2875-11-141 PMid:22548983 PMCid:3405476

- Malvy D, Djossou F, Vatan R, Pistone T,
Etienne G, Longy-Boursier M, Le Bras M. Experience with the combination
atovaquone-proguanil in the treatment of uncomplicated Plasmodium
falciparum malaria--report of 112 cases, Med Trop, 2002; 62: 229-231

- Srivastava IK, Rottenberg H, Vaidya AB.
Atovaquone, a broad spectrum antiparasitic drug, collapses
mitochondrial membrane potential in a malarial parasite. J Biol Chem,
1997; 272: 3961–3966 http://dx.doi.org/10.1074/jbc.272.7.3961 PMid:9020100

- Looareesuwan S, Chulay JD, Canfield CJ,
Hutchinson DB. Malarone (Atovaquone and Proguanil Hydrochloride): a
review of its clinical development for treatment of malaria. Am J Trop
Med Hyg, 1999; 60: 533–541 PMid:10348225

- Osei-Akoto A, Orton LC, Owusu-Ofori S.
Atovaquone-proguanil for treating uncomplicated malaria. Cochrane
Database of Systematic Reviews 2005 (4) art. N. CD004529. http://dx.doi.org/10.1002/14651858.CD004529.pub2 PMid:16235366

- Pasternak B, Hviid A. Atovaquone-Proguanil
Use in Early Pregnancy and the Risk of Birth Defects. Arch Intern Med
2011, 171: 259-60 http://dx.doi.org/10.1001/archinternmed.2010.521 PMid:21325117

- Bukirwa H, Orton LC. Artesunate plus
mefloquine versus mefloquine for treating uncomplicated malaria.
Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No.:
CD004531. http://dx.doi.org/10.1002/14651858.CD004531.pub2 PMid:16235367

- Societe Francaise de Medicine d’Urgence. Prise en charge et prevention du paludisme d’importation ŕ Plasmodium falciparum. Revision 2007 de la Conference de Consensus 1999. http://www.sfmu.org/documents/consensus/rbpc_paludisme-court.pdf
- Centers for Diseases Control and Prevention (CDC). Artesunate is available to treat severe malaria in the United States http://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html accessed August 21st, 2012
- Lalloo DG, Shingadia D, Pasvol G, Chiodini
PL, Whitty CJ, Beeching NJ, Hill DR, Warrell DA, Bannister BA; HPA
Advisory Committee on Malaria Prevention in UK Travellers. UK malaria
treatment guidelines. J Infect, 2007; 54: 111-121 http://dx.doi.org/10.1016/j.jinf.2006.12.003 PMid:17215045

- Public Health Agency of Canada. Canadian Recommendations for the Prevention and treatment of Malaria Among International Travellers. CCDR 2008; 34S3:1-45
- Looareesuwan S, Wilairatana P,
Glanarongran R, Indravijit KA, Supeeranontha L, Chinnapha S, Scott TR,
Chulay JD. Atovaquone and proguanil hydrochloride followed by
primaquine for treatment of Plasmodium vivax malaria in Thailand. Trans
R Soc Trop Med Hyg, 1999; 93: 637-40 http://dx.doi.org/10.1016/S0035-9203(99)90079-2

- Lacy MD, Maguire JD, Barcus MJ, Ling J,
Bangs MJ, Gramzinski R, Basri H, Sismadi P, Miller GB, Chulay JD,
Fryauff DJ, Hoffman SL, Baird JK. Atovaquone/Proguanil Therapy for
plasmodium falciparum and Plasmodium vivax Malaria in Indonesians Who
Lack Clinical Immunity. Clin Infect Dis, 2002; 35:e92-5. http://dx.doi.org/10.1086/343750 PMid:12384852

- Douglas NM, Anstey NM, Angus BJ, Nosten F,
Price RN. Artemisinin combination therapy for vivax malaria. Lancet Inf
Dis, 2010; 10: 405-416 http://dx.doi.org/10.1016/S1473-3099(10)70079-7

- Mombo-Ngoma G, Kleine C, Basra A, Würbel
H, Diop DA, Capan M, Adegnika AA, Kurth F, Mordmüller B, Joanny F,
Kremsner PG, Ramharter M, Bélard S. Prospective evaluation of
artemether-lumefantrine for the treatment of non-falciparum and mixed
species malaria in Gabon. Malar J, 2012, 11: 120 http://dx.doi.org/10.1186/1475-2875-11-120 PMid:22515681 PMCid:3393621

- Rieckmann KH, Davis DR, Hutton DC: Plasmodium vivax resistant to chloroquine? Lancet, 1989, 2: 1183-1184 http://dx.doi.org/10.1016/S0140-6736(89)91792-3

- Congpuong K, Satimai W, Sujariyakul A,
Intanakom S, Harnpitakpong W, Pranuth Y, Cholpol S, Bualombai P. In
vivo sensitivity monitoring of chloroquine for the treatment of
uncomplicated vivax malaria in four bordered provinces of Thailand
during 2009-2010. J Vector Borne Dis 2011, 48:190-6 PMid:22297279

- Teka H, Petros B, Yamuah L, Tesfaye G,
Elhassan I, Muchohi S, Kokwaro G, Aseffa A, Engers H.
Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia.
Malar J, 2008, 7: 220 http://dx.doi.org/10.1186/1475-2875-7-220 PMid:18959774 PMCid:2584068

- Ruebush TK, Zegarra J, Cairo J, Andersen
EM, Green M, Pillai DR, Marquiňo W, Huilca M, Arévalo E, Garcia C,
Solary L, Kain K. Chloroquine-resistant Plasmodium vivax malaria in
Peru. Am J Trop Med Hyg, 2003; vol. 69: 548-552 PMid:14695094

- Tekwani BL, Walker LA. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr Opin Infect Dis, 2006, 19: 623-31 http://dx.doi.org/10.1097/QCO.0b013e328010b848 PMid:17075340

- Basso LGM, Rodrigues RZ, Naal RMZG,
Costa-Filho AJ Effects of the antimalarial drug primaquine on the
dynamic structure of lipid model membranes. BBA-Biomembranes, 2011;
1808: 55-64 http://dx.doi.org/10.1016/j.bbamem.2010.08.009, accessed August 22, 2012

- Crockett M, Kain KC. Tafenoquine: a promising new antimalarial agent. Expert Opin Investig Drug, 2007; 16: 705-15 http://dx.doi.org/10.1517/13543784.16.5.705 PMid:17461742

- Krudsood S, Wilairatana P, Tangpukdee N,
Chalermrut K, Srivilairit S, Thanachartwet V, Muangnoicharoen S,
Luplertlop N, Brittenham GM, Looareesuwan S. Safety and tolerability of
elubaquine (bulaquine, CDRI 80/53) for treatment of Plasmodium vivax
malaria in Thailand. Korean J Parasitol, 2006; 44: 221-8. http://dx.doi.org/10.3347/kjp.2006.44.3.221 PMid:16969059 PMCid:2532664

- WHO working group. Glucose-6-phosphate
dehydrogenase deficiency. Bull World Health Organ 1989; 67: 601–11
PMid:2633878 PMCid:2491315

- Galappaththy GNL, Omari AAA, Tharyan P.
Primaquine for preventing relapses in people with Plasmodium vivax
malaria. Cochrane Database of Systematic Reviews 2007, Issue 1. Art.
No.: CD004389. http://dx.doi.org/10.1002/14651858.CD004389.pub2 PMid:17253504

- John GK, Douglas NM, von Seidlein L,
Nosten F, Baird KJ, White NJ, Price RN. Primaquine radical cure of
Plasmodium vivax: a critical review of the literature. Malar J, 2012;
11: 280 http://dx.doi.org/10.1186/1475-2875-11-280 PMid:22900786

- Hill DR, Baird JK, Parise ME, Lewis LS,
Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on
malaria chemoprophylaxis I . Am J Trop Med Hyg, 2006; 75: 402-415
PMid:16968913

- Fernando D, Rodrigo C, Rajapakse S.
Primaquine in vivax malaria: an update and review on management issues.
Malar J, 2011, 10: 351 http://dx.doi.org/10.1186/1475-2875-10-351 PMid:22152065 PMCid:3306765

- Sinclair D, Gogtay N, Brand F, Olliaro P.
Artemisinin-based combination therapy for treating uncomplicated
Plasmodium vivax malaria. Cochrane Database Syst Rev. 2011 Jul
6;(7):CD008492. Review.

- Sermwittayawong N, Singh B, Nishibuchi M,
Sawangjaroen N, Vuddhakul V. Human Plasmodium knowlesi infection in
Ranong province, southwestern border of Thailand. Malar J, 201; 11: 36

- Daneshvar C, Davis TM, Cox-Singh J,
Rafa'ee MZ, Zakaria SK, Divis PC, Singh B. Clinical and parasitological
response to oral chloroquine and primaquine in uncomplicated human
Plasmodium knowlesi infections. Malar J, 2010; 9: 238 http://dx.doi.org/10.1186/1475-2875-9-238 PMid:20723228 PMCid:2933701

- Singh H, Parakh A, Basu S, Rath B. . Plasmodium vivax malaria: Is it actually benign? J Infect Pub Health, 2011; 4: 91-95 http://dx.doi.org/10.1016/j.jiph.2011.03.002 PMid:21663878

- Lacerda MVG, Fragoso SCP, Alecrim MGC,
Alexandre MAA, Magalhaes BML, Siqueira AM, Ferreira LCL, Araujo JR,
Mourau MPG, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C,
del Portillo H, Ordi J, Alonso PL, Bassat Q. Post-mortem
characterization of patients with clinical diagnosis of Plasmodium
vivax malaria: to what extent does this parasite kill? Clin Infect Dis,
2012; http://dx.doi.org/10.1093/cid/cis615 PMid:22772803

- von Seidlein L, Olaosebikan R, Hendriksen
IC, Lee SJ, Adedoyin OT, Agbenyega T, Nguah SB, Bojang K, Deen JL,
Evans J, Fanello CI, Gomes E, Pedro AJ, Kahabuka C, Karema C, Kivaya E,
Maitland K, Mokuolu OA, Mtove G, Mwanga-Amumpaire J, Nadjm B, Nansumba
M, Ngum WP, Onyamboko MA, Reyburn H, Sakulthaew T, Silamut K, Tshefu
AK, Umulisa N, Gesase S, Day NP, White NJ, Dondorp AM. Predicting the
clinical outcome of severe falciparum malaria in african children:
findings from a large randomized trial. Clin Infect Dis, 2012; 54:
1080-90. http://dx.doi.org/10.1093/cid/cis034 PMid:22412067 PMCid:3309889

- Marsh K, Forster D, Waruiru C, Mwangi I,
Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol
G, Snow R. Indicators of life-threatening malaria in African children.
New Engl J Med, 1995; 332: 1399-404 http://dx.doi.org/10.1056/NEJM199505253322102 PMid:7723795

- WHO. Guidelines for the Treatment of Malaria: Second Edition. Rev. 1. April 2011 http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html
- Pasvol G, Phil D. Management of Severe Malaria: Interventions and Controversies. Infect Dis Clin N Am. 2005;19: 211–240 http://dx.doi.org/10.1016/j.idc.2004.10.007 PMid:15701555

- Pralay KS, Ahluwalia G, Vijayan V, Talwar A. Critical care aspects of malaria. J Intensive Care Med 2010 25: 93-103 http://dx.doi.org/10.1177/0885066609356052 PMid:20018606

- Santos LC, Abreu CF, Xerinda SM, Tavares
M, Lucas R, Sarmento AC.Severe imported malaria in an intensive care
unit : a review of 59 cases. Malar J, 2012, 11: 96 http://dx.doi.org/10.1186/1475-2875-11-96 PMid:22458840 PMCid:3350412

- Dondorp A, Nosten F, Stepniewska K, Day N,
White N; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT)
group. Artesunate versus quinine for treatment of severe falciparum
malaria: a randomized trial. Lancet 2005; 366: 717-25 http://dx.doi.org/10.1016/S0140-6736(05)67176-0

- Dondorp AM, Fanello CI, Hendriksen IC,
Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N,
Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S,
Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M,
Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB,
Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska
K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L,
Day NP, White NJ; AQUAMAT group. Artesunate versus quinine in treatment
of severe falciparum malaria in African children (AQUAMAT): an
open-label, randomized trial. Lancet 2010; 376:1647-57 http://dx.doi.org/10.1016/S0140-6736(10)61924-1

- Sinclair D, Donegan S, Isba R, Lalloo DG.
Artesunate versus quinine for treating severe malaria. Cochrane
Database of Systematic Reviews 2012, Issue 6. Art. No.: CD005967. http://dx.doi.org/10.1002/14651858.CD005967.pub4 PMid:22696354

- WHO. WHO List of prequalified medicinal products. 2011. 16-5-2011 http://apps.who.int/prequal/query/productregistry.aspx
- Li Q, Cantilena LR, Leary KJ, Saviolakis
GA, Miller RS, Melendez V, Weina PJ. Pharmacokinetic profiles of
artesunate after single intravenous doses at 0.5, 1, 2, 4, and 8 mg/kg
in healthy volunteers: a phase I study. Am J Trop Med Hyg, 2009,
81:615–621 http://dx.doi.org/10.4269/ajtmh.2009.09-0150 PMid:19815876

- Miller RS, Li Q, Cantilena LR, Leary KJ,
Saviolakis GA, Melendez V, Smith B, Weina PJ. Pharmacokinetic profiles
of artesunate following multiple intravenous doses of 2, 4, and 8 mg/kg
in healthy volunteers: Phase 1b study Malar J. 2012; 11: 255 http://dx.doi.org/10.1186/1475-2875-11-255 PMid:22853818

- Centers for Diseases Control and Prevention (CDC). Treatment guidelines - Treatment of malaria – 4/2011. http://www.cdc.gov/malaria/resources/pdf/clinicalguidance.pdf
- European Medicine Agency (EMA). Public summary of opinion on orphan designation. Artesunate for the treatment of malaria http://www.emea.europa.eu/pdfs/human/comp/opinion/48693207en.pdf, accessed August 21st, 2012
- AFSSAPS – Protocole d’Utilisation Therapeutique et de recueil d’informations. ATU nominative MALACEF artesunate. Version 2- dec 2011. http://ansm.sante.fr/var/ansm_site/storage/original/application/4482d1364ea7ea8dd45fe9335c5072ee.pdf, accessed August 21st, 2012
- Richter J, Abbasi-Boroudjeni N, Koch S,
Müller-Stöver I, Häussinger D. [Parenteral combined quinine-artesunate
therapy in life-threatening malaria]. [Article in German] Dtsch Med
Wochenschr. 2009 Jan;134:187-90 http://dx.doi.org/10.1055/s-0028-1123976 PMid:19180405

- Bartoloni A, Tomasoni L, Bartalesi F,
Galli L, Sani S, Veloci S, Zammarchi L, Pini A, Castelli F Combined
intravenous treatment with artesunate and quinine for severe malaria in
Italy. Am J Trop Med Hyg, 2010; 83: 274-6 http://dx.doi.org/10.4269/ajtmh.2010.10-0128 PMid:20682867 PMCid:2911170

- Cramer JP, López-Vélez R, Burchard GD,
Grobusch MP, de Vries PJ Treatment of imported severe malaria with
artesunate instead of quinine - more evidence needed? Malar J, 2011;
10: 256 http://dx.doi.org/10.1186/1475-2875-10-256 PMid:21899729 PMCid:3224352

- Zoller T, Junghanss T, Kapaun A, Gjorup I,
Richter J, Hugo-Persson M, Mřrch K, Foroutan B, Suttorp N, Yürek S,
Flick. Intravenous artesunate for severe malaria in travelers, Europe.
Emerg Infect Dis, 2011; 17: 771-7 http://dx.doi.org/10.3201/eid1705.101229 PMid:21529383 PMCid:3321768

- Kreeftmeijer-Vegter AR, van Genderen PJ,
Visser LG, Bierman WF, Clerinx J, van Veldhuizen CK, de Vries PJ.
Treatment outcome of intravenous artesunate in patients with severe
malaria in the Netherlands and Belgium. Malar J, 2012; 11: 102 http://dx.doi.org/10.1186/1475-2875-11-102 PMid:22462806 PMCid:3364861

- Itoda I, Yasunami T, Kikuchi K, Yamaura H,
Totsuka K, Yoshinaga K, Teramura M, Mizoguchi H, Hatabu T, Kano
S.Severe falciparum malaria with prolonged hemolytic anemia after
successful treatment with intravenous artesunate [article in japanese].
Kansenshogaku Zasshi. 2002; 76: 600-3 PMid:12325318

- Caramello P et al. Severe malaria,
artesunate and Haemolysis. 2012 J Antimicrob Chemother
doi:10.1093/jac/dks139 Advance Access publication 19 April 2012

- Byakika-Kibwika P et al. Pharmacokinetics
and pharmacodynamics of intravenous artesunate during severe malaria
treatment in Ugandan adults. Malar J, 2012; 11: 132 http://dx.doi.org/10.1186/1475-2875-11-132 PMid:22540954

- Gomes MF, Faiz MA, Gyapong JO, Warsame M,
Agbenyega T, Babiker A, Baiden F, Yunus EB, Binka F, Clerk C, Folb P,
Hassan R, Hossain MA, Kimbute O, Kitua A, Krishna S, Makasi C, Mensah
N, Mrango Z, Olliaro P, Peto R, Peto TJ, Rahman MR, Ribeiro I, Samad R,
White NJ; Study 13 Research Group. Pre-referral rectal artesunate to
prevent death and disability in severe malaria: A placebo-controlled
trial. Lancet, 2009; 373: 557-566 http://dx.doi.org/10.1016/S0140-6736(08)61734-1

- Karim FH and Zulfiqarali GP. Pre-referral rectal artesunate in severe malaria: flawed trial. Trials, 2011; 12: 188 http://dx.doi.org/10.1186/1745-6215-12-188 PMid:21824389 PMCid:3171715

- Norrie J, Okebe JU, Eisenhut M.
Pre-referral rectal artesunate for severe malaria. Cochrane Database of
Systematic Reviews 2012, Issue 7. Art. No.: CD009964. DOI:
10.1002/14651858.CD009964 http://dx.doi.org/10.1002/14651858.CD009964
