Novel Agents and Emerging Strategies for Targeting the B-Cell Receptor Pathway in CLL
Dimitar G. Efremov1, Adrian Wiestner2 and Luca Laurenti3
1Molecular
Hematology, International Centre for Genetic Engineering &
Biotechnology, Campus "A. Buzzati-Traverso", Rome, 00016, Italy
2Hematology Branch, National Heart, Lung, Blood Institute, National Institutes of Health, Bethesda, MD, USA
3Department of Hematology, Catholic University Hospital "A. Gemelli", Rome, 00168, Italy
2Hematology Branch, National Heart, Lung, Blood Institute, National Institutes of Health, Bethesda, MD, USA
3Department of Hematology, Catholic University Hospital "A. Gemelli", Rome, 00168, Italy
Correspondence
to:
Dimitar G. Efremov, MD, PhD. Molecular Hematology Section, ICGEB,
Campus "Adriano Buzzati-Traverso", Via E. Ramarini
32, I-00016 Monterotondo Scalo (Rome). Italy. Ttel:
+39-06-90091300. E-mail: efremov@icgeb.org
Published: October 9, 2012
Received: September 26, 2012
Accepted: September 28, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012067, DOI 10.4084/MJHID.2012.067
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Chronic
lymphocytic leukemia (CLL) is a disease of malignant CD5+ B lymphocytes
that are characterized by frequent expression of autoreactive B-cell
receptors (BCRs) and marked dependence on microenvironmental signals
for proliferation and survival. Among the latter, signals propagated
through the BCR are believed to play a key role in leukemia initiation,
maintenance and evolution. Drugs that can disrupt these signals have
recently emerged as potential therapeutic agents in CLL and several of
them are currently being evaluated in clinical trials. Particularly
promising clinical responses have been obtained with inhibitors of the
kinases SYK, BTK, and PI3Kδ, which function by blocking BCR signal
transduction. In addition, recent studies focusing on the phosphatase
PTPN22, which is involved in the pathogenesis of multiple autoimmune
diseases and is markedly overexpressed in CLL cells, suggest that it
may be possible in the future to develop strategies that will
selectively reprogram BCR survival signals into signals that induce
leukemic cell death. This review focuses on the biological basis behind
these strategies and highlights some of the most promising
BCR-targeting agents in ongoing preclinical and clinical studies.
Introduction
The B-cell receptor (BCR) signaling pathway has recently emerged as a major therapeutic target in CLL.[1-3] Signals propagated through the BCR have been shown to increase leukemic cell survival in vitro[4-6] and there is growing evidence that such signals are continuously delivered to the leukemic cells in vivo.[7,8] This evidence particularly refers to data obtained from gene expression profiling studies, which have shown that freshly isolated CLL cells express high levels of genes that can be induced in normal B cells by BCR engagement.[9] Such BCR target genes are especially enriched in CLL cells isolated from lymph nodes, which is an important site of antigen encounter.[10] In addition, the kinase SYK, which is activated immediately downstream of the BCR, is frequently phosphorylated in freshly isolated CLL cells,[11,12] and this is also more prominent in CLL cells isolated from lymph nodes than peripheral blood.[10] Lastly, surface IgM expression is downmodulated on freshly isolated CLL cells but recovers after a few days in culture, further indicating that the leukemic BCRs are continuously triggered by antigen in vivo.[13] Altogether, these findings suggest that the BCR pathway is aberrantly or excessively activated in CLL cells and may therefore represent a promising target for therapeutic intervention.
BCR Signals Generated in CLL Cells
Normal B lymphocytes receive two types of signals from their BCRs. The first type is triggered by binding of the BCR to external antigen, which results in the assembly and activation of a signaling complex that propagates the signal to the interior of the cell[14] (Figure 1A). This BCR signal can induce a variety of cellular responses, including proliferation, survival, differentiation, anergy and apoptosis. The final outcome depends on several factors, such as the nature of the antigen, the availability of co-stimulatory signals and the stage of B cell differentiation.[15] The second type of signal occurs in the absence of an external ligand and has been termed tonic BCR signal. The role of this signal in normal B cell biology has still not been fully established, but recent studies suggest that it is important for the survival of mature B cells and for normal B cell development and maturation.[16,17]
In recent years, evidence has emerged that both types of signals are excessively or aberrantly generated in CLL cells. For example, the kinases LYN and SYK, which are activated immediately downstream of the BCR, often shown increased basal activity in unstimulated CLL cells.[11,12,18-21] This increased basal activity is maintained during prolonged in vitro culture, suggesting that it is independent of an external antigen. Increased basal activity has also been reported for other signaling molecules that are located further downstream along the BCR pathway, such as the kinases PI3K,[22,23] BTK,[24] PKC[25] and ERK,[26] and the transcription factors NF-kB and NF-AT.[27-30] Importantly, inhibition or downregulation of many of these signaling molecules induces apoptosis in unstimulated CLL cells. Together, these findings suggest that CLL cells have an elevated tonic or basal BCR signaling activity, which contributes to their increased apoptosis resistance.
The mechanism that generates the elevated basal BCR signaling activity in CLL cells was only very recently revealed.[31] In their seminal paper, Dühren-von Minden et al investigated a large series of CLL BCRs (n=17) and showed that all of them are capable of inducing an autonomous signal when introduced in a B cell line that does not express a functional BCR. Additional experiments demonstrated that this signal is dependent on an interaction between the heavy-chain complementarity-determining region 3 (HCDR3) of one BCR and an intrinsic motif in the framework region 2 (FR2) of another. Thus, the interaction between the HCDR3 of one BCR and the FR2 of another leads to aggregation of neighboring BCRs, which in turn generates a cell autonomous signal that is independent of an external ligand (Figure 1B).
The cell autonomous BCR signal appears to be different from the tonic BCR signal of normal B cells, as the latter occurs in the absence of any kind of ligand and is believed to be generated by transient and stochastic interactions between the BCR and the LYN and SYK kinases (Figure 1C).[16] The cell autonomous BCR signal is also different from the extrinsic antigen-dependent BCR signal in several important aspects. For example, the cell autonomous BCR signal does not seem to result in full SYK activation, considering that phosphorylation of the activation loop tyrosines (YY525/526) of SYK is usually not detected in unstimulated CLL cells, whereas it is readily detected in most CLL samples stimulated with anti-IgM antibodies, which are used to mimic external antigen.[32] Phosphorylation of the activation loop tyrosines in SYK is required for sustained PLCγ2, AKT and ERK activation and SYK-mediated B cell proliferation.[32] In addition, most CLL cells are in a quiescent state when isolated from the peripheral blood, but can be activated and induced to enter the G1 phase of the cell cycle by BCR engagement.[6,33,34] Together, these data suggest that CLL cell proliferation is driven by extrinsic antigen-dependent BCR signals, whereas the role of the cell autonomous BCR signal in the pathogenesis of CLL could be to maintain the viability of the leukemic precursors until they encounter the factors that promote their expansion and subsequent malignant transformation.
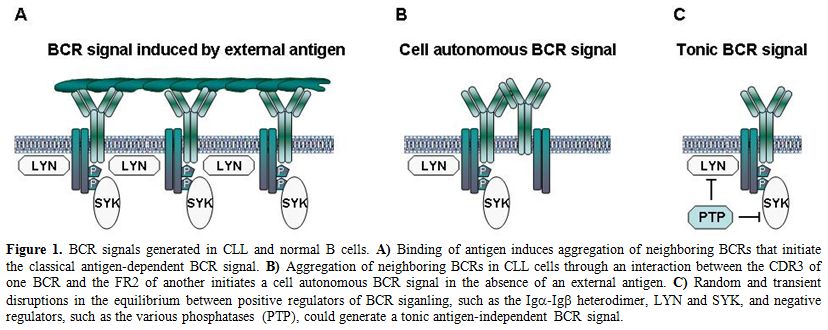
Figure 1. BCR signals generated in CLL and normal B cells. A) Binding of antigen induces aggregation of neighboring BCRs that initiate the classical antigen-dependent BCR signal. B) Aggregation of neighboring BCRs in CLL cells through an interaction between the CDR3 of one BCR and the FR2 of another initiates a cell autonomous BCR signal in the absence of an external antigen. C) Random and transient disruptions in the equilibrium between positive regulators of BCR siganling, such as the Igα-Igß heterodimer, LYN and SYK, and negative regulators, such as the various phosphatases (PTP), could generate a tonic antigen-independent BCR signal.
The nature and identity of the antigens that drive CLL cell proliferation has still not been established, although the restricted variable region gene repertoire and the expression of stereotyped BCRs in over 30% of the cases suggest that the leukemic cells in many cases recognize similar or identical antigens.[35,36] In cases with unmutated IGHV genes (U-CLL), the leukemic BCRs often bind to autoantigens that are generated or exposed on apoptotic cells.[37-41] Whether or not these autoantigens drive the expansion of the malignant cells is still unclear, since U-CLL BCRs are frequently polyreactive and can also bind to microbial antigens, such as Streptococcus pneumoniae polysaccharides[37] or the cytomegalovirus phosphoprotein pUL32.[42] Thus, it still remains possible that the driving force for CLL cell proliferation is a chronic infectious agent, as is the case with certain indolent lymphomas that arise in the setting of chronic bacterial or viral infections, such as H. Pylori-associated gastric MALT lymphomas[43,44] or hepatitis C virus (HCV)-associated splenic marginal zone and lymphoplasmacytoid lymphomas.[45,46] Alternatively, the driving force could be autoantigens associated with apoptotic blebs, which co-localize with DNA- or RNA-containing complexes and can therefore provide necessary co-stimulatory signals by triggering Toll-like receptors.[47]
Features of the BCR Pathway in CLL Cells
CLL cells have several distinct BCR signaling features that distinguish them from normal B cells. These include the previously described cell autonomous BCR signal, the low-level surface IgM expression and the heterogeneous signaling responses induced by BCR engagement in vitro. The latter are particularly evident with respect to BCR proximal signaling events, such as induction of SYK and PLCγ2 phosphorylation and intracellular Ca2+ mobilization, which are weak or absent in a substantial proportion of cases.[48-50] These impaired signaling responses are more common in cases with favorable prognostic features, such as those that express IGHV-mutated BCRs or do not express ZAP-70.[51,52] However, even in cases that appear to transduce BCR signals relatively efficiently, activation of downstream pathways can be variable and incomplete when compared to normal B cells. For example, despite efficient activation of AKT and ERK, anti-IgM induced activation of JNK, p38MAPK and NF-kB is often negligible or absent.[5]
Heterogeneous responses are also observed when the same cells are stimulated with different types of BCR crosslinking agents. For example, stimulation with soluble anti-IgM antibodies induces apoptosis in most CLL samples, whereas stimulation with the same antibodies immobilized on solid structures usually increases the survival of the leukemic cells.[5,6,53] These data suggest that different types of antigens can induce different responses in CLL cells and indicate that the variability in the clinical course could be in part determined by the antigen specificity of the malignant B cells.
Another distinct feature of CLL cells is the aberrant expression of multiple molecules that are involved in BCR signal transduction. Most of these regulatory molecules are expressed at higher levels in CLL than normal B cells, but some show reduced expression. For example, the kinases LYN,[18] LCK,[54] SYK,[20] ZAP-70,[9] BTK[24] and PKCß,[55] the adaptor protein TCL1[56] and the phosphatase PTPN22[57] are all overexpressed in CLL cells, whereas the adaptor protein SHC[58] and the phosphatase PHLPP1[59] are expressed at markedly reduced levels. The functional significance of these abnormalities is still not completely clear, although at least for some of them there is evidence for a positive effect on the activity of downstream signaling pathways that regulate CLL cell survival. This particularly refers to the reduced PHLPP1 and increased TCL1 and PTPN22 expression, which result in enhanced AKT activation in anti-IgM-stimulated CLL cells.[56,57,59] AKT is a key signaling molecule that regulates the survival of BCR-stimulated CLL cells by inducing the expression of several important antiapoptotic proteins, including MCL-1, BCL-xL and XIAP.[60] Reduced expression of PHLPP1 could additionally increase the survival of BCR-stimulated CLL cells by allowing for enhanced activation of ERK,[59] which regulates the activity of the proapoptotic protein BIM.[61] Finally, overexpression of PTPN22 can have a positive effect on CLL cell survival not only by enhancing AKT activation, but also by inhibiting signaling molecules that activate proapoptotic pathways, such as p38MAPK.[57] Together, these findings indicate that CLL cells have a very delicately fine-tuned signaling machinery that allows them to amplify weak antiapoptotic BCR signals while at the same time blocking signals that could have a negative impact on their survival. Such a machinery could be particularly advantageous to CLL cells that express autoreactive BCRs, as it could prevent their elimination by immunological tolerance mechanisms and may even allow for their positive selection by autoantigen.
Potential Targets Along the BCR Pathway in CLL
LYN. Transduction of the BCR signal is a complex process that involves multiple kinases, phosphatases and adaptor proteins, which could represent potential therapeutic targets (Figure 2). The signal is initially propagated by SRC-family kinases, such as LYN, FYN or BLK, which phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) in the Igα and Igß chains of the BCR. The phosphorylated ITAMs recruit the kinase SYK, which then becomes activated through SRC-family kinase-dependent phosphorylation at Y352 and trans-autophosphorylation at YY525/526. LYN not only phosphorylates and activates SYK, but it also activates phosphatases that inhibit BCR signal transduction. Thus, LYN functions as both a positive and negative regulator of BCR signaling.
As mentioned earlier, LYN is overexpressed, constitutively active and abnormally distributed in CLL cells.[18] Targeting of LYN with SRC-family kinase inhibitors, such as PP2 and Dasatinib, induces leukemic cell apoptosis.[18,62,63] However, it is still uncertain whether the cytotoxic effect of these compounds is due to inhibition of LYN or inhibition of related kinases. For example, BTK and ABL are also inhibited by Dasatinib, and both of these molecules appear to be important for CLL cell survival.[64-66] Silencing of LYN by RNA interference should clarify this issue, but so far this approach has not proven very effective presumably because of the significant overexpression and long half-life of LYN in CLL cells.[18,67]
Clinical experience with LYN inhibitors. Dasatinib is an oral pan-SRC kinase and ABL inhibitor that is currently approved for the treatment of CML (Table 1). A phase 2 trial in patients with relapsed or refractory CLL treated with a daily dose of 140 mg for up to 24 months was recently published.[68] Modest clinical activity was observed. Partial responses by NCI-WG criteria were achieved in 3 of the 15 enrolled patients (20%). Among the remaining 12 patients, 5 had nodal responses by physical exam, and one additional patient had a nodal and lymphocyte response but with severe myelosuppression. Primary toxicity was myelosuppression, with grade 3/4 neutropenia occurring in 67% of patients and grade 3/4 thrombocytopenia in 40%. Pharmacodynamic studies indicated apoptosis in peripheral blood CLL cells within 3 to 6 hours after dasatinib administration, which interestingly was associated with downregulation of SYK mRNA.
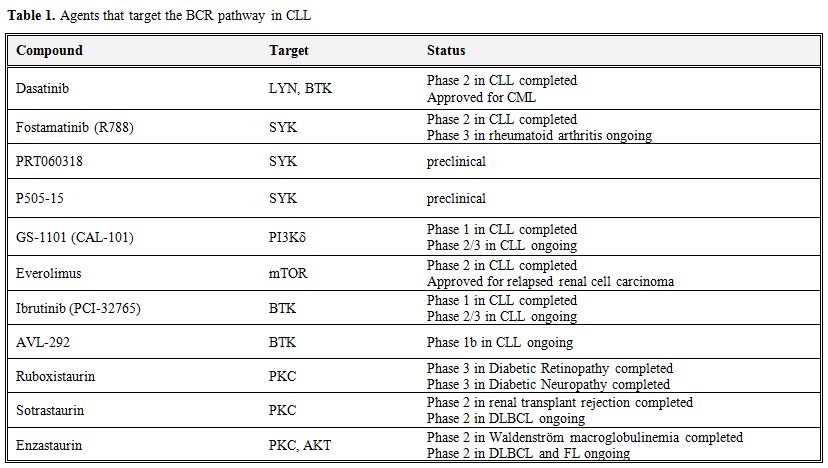
Table 1. Agents that target the BCR pathway in CLL.
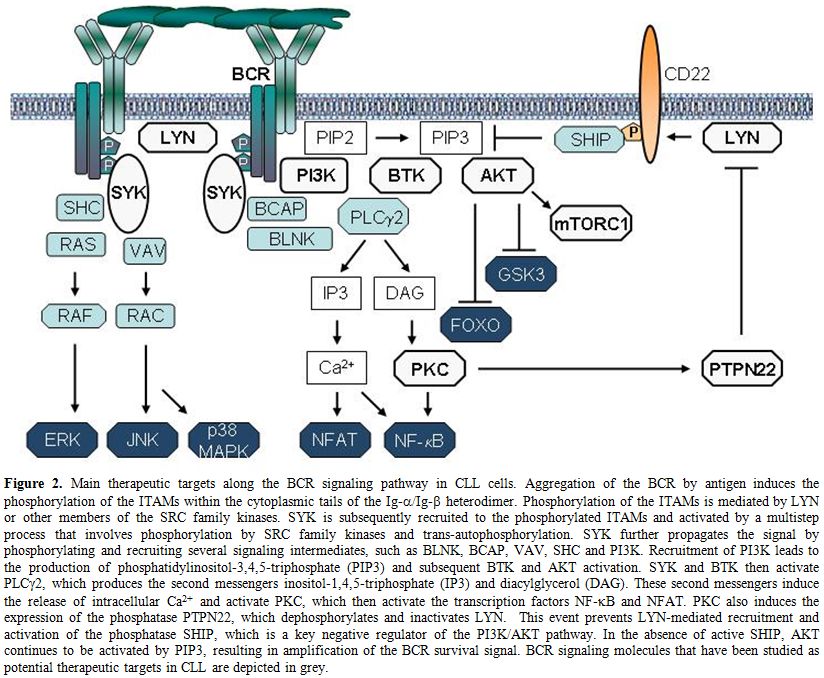
Figure 2. Main therapeutic targets along the BCR signaling pathway in CLL cells. Aggregation of the BCR by antigen induces the phosphorylation of the ITAMs within the cytoplasmic tails of the Ig-α/Ig-ß heterodimer. Phosphorylation of the ITAMs is mediated by LYN or other members of the SRC family kinases. SYK is subsequently recruited to the phosphorylated ITAMs and activated by a multistep process that involves phosphorylation by SRC family kinases and trans-autophosphorylation. SYK further propagates the signal by phosphorylating and recruiting several signaling intermediates, such as BLNK, BCAP, VAV, SHC and PI3K. Recruitment of PI3K leads to the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) and subsequent BTK and AKT activation. SYK and BTK then activate PLCγ2, which produces the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These second messengers induce the release of intracellular Ca2+ and activate PKC, which then activate the transcription factors NF-kB and NFAT. PKC also induces the expression of the phosphatase PTPN22, which dephosphorylates and inactivates LYN. This event prevents LYN-mediated recruitment and activation of the phosphatase SHIP, which is a key negative regulator of the PI3K/AKT pathway. In the absence of active SHIP, AKT continues to be activated by PIP3, resulting in amplification of the BCR survival signal. BCR signaling molecules that have been studied as potential therapeutic targets in CLL are depicted in grey.
SYK. SYK is currently one of the most attractive targets for therapeutic intervention along the BCR pathway in CLL. This kinase propagates the BCR signal by phosphorylating the adaptor proteins BLNK, BCAP and SHC, and activating key signaling intermediates, such as PI3K, BTK and PLCγ2 (Figure 2). In addition to its essential role in transducing the BCR signal, SYK is also involved in integrin, chemokine and Fc-receptor signaling.[69]
SYK is constitutively phosphorylated on the activating Y352 residue in CLL cells,[12] presumably as a consequence of the cell autonomous BCR signal.[31] Inhibition or silencing of SYK decreases the basal activity of downstream signaling molecules, providing additional evidence that constitutively active SYK molecules are present in CLL cells.[12,21] Inhibition of SYK also induces moderate apoptosis in unstimulated CLL cells, further suggesting that the cell autonomous BCR signal increases leukemic cell survival.[12,19-21] In addition, treatment of CLL cells with the SYK inhibitor fostamatinib completely blocks transduction of extrinsic antigen-dependent BCR signals.[12] Fostamatinib also inhibits integrin signaling, antagonizes the protective effect of stromal cells, and reduces migration of CLL cells to chemokines and their adhesion to stromal components.[19,70]
Many of the in vitro effects of fostamatinib have been recapitulated in vivo using the Eµ-TCL1 transgenic mouse model of CLL.[71] In this model, fostamatinib treatment results in inhibition of BCR signaling, an initial transient lymphocytosis, reduced proliferation and survival of the malignant B cells, and prolonged survival of the treated animals.
Clinical experience with SYK inhibitors. Fostamatinib, also called R788, is an oral prodrug that is converted in vivo into the active compound R406.[72] The latter is a potent inhibitor of SYK, with an IC50 in vitro of 41 nM. R406 is not entirely specific for SYK, as it also inhibits FLT3, JAK, LCK, VEGFR2 and RET at similar or slightly higher concentrations.
R406 is the first BCR signaling agent that was shown to be active in CLL.[73] This compound was initially developed as potential treatment for allergy and rheumatoid arthritis, because of its capacity to inhibit signaling through FcεRI and Fcg receptors.[74] Early trials of fostamatinib in patients with rheumatoid arthritis showed clinical activity and potent anti-inflammatory effects.[75,76] Subsequently, fostamatinib was tested in a phase 1/2 trial of relapsed or refractory NHL and CLL.[77] In the phase 1 part of the trial a dose of 200mg p.o. bid was established. Dose-limiting toxicities were neutropenia, thrombocytopenia, and diarrhea. In the Phase 2 study, 68 patients were enrolled into 3 groups according to histology, including 23 patients with diffuse large B cell lymphoma (DLBCL), 21 patient with follicular lymphoma and 24 patients with other NHL, among which 11 had SLL/CLL. The highest response rate was observed in SLL/CLL, where 6 patients (55%) achieved a partial response (PR). Objective responses were also seen in 22% of DLBCL [1 complete response (CR), 4 PRs], 10% of follicular lymphoma (2 PRs) and 11% of mantle cell lymphoma patients (1 PR). In the phase 2 portion of the trial the most common adverse events were reversible cytopenias, fatigue, diarrhea, and hypertension. All patients with CLL/SLL exhibited a transient initial lymphocytosis that has since been observed with all other agents that target the BCR pathway. This effect is believed to be caused by disruption of adhesion and retention signals delivered through the BCR, chemokine receptors and/or integrins, resulting in mobilization of CLL cells from lymph nodes and marrow into the blood.[19,70]
Follow-up studies with fostamatinib in CLL have not been performed to date because the company that is developing this agent is pursuing a registration strategy in rheumatoid arthritis. Clinical trials are proceeding in diffuse large B cell lymphoma, but it remains unclear whether fostamatinib will be available for future studies in CLL. However, newer more potent and more specific SYK inhibitors with promising pre-clinical activity in CLL have recently been developed and are expected to enter into clinical trials in the near future.[78,79]
PI3Kδ. PI3K is a key downstream mediator of BCR signaling.[80] CLL cells generally express high levels of active PI3K[22,23] and sustained activation of this pathway increases their proliferative capacity and survival.[60,81]
Several PI3K isoforms exist, some of which are ubiquitously expressed whereas others are cell type specific. PI3Kδ is the predominantly expressed isoform in leukocytes. It consists of two components, a regulatory p85 and a catalytic p110δ subunit. Upon crosslinking of the BCR, p85 binds via its SH2 domains to phospho-tyrosine motifs on CD19 and BCAP, which are a co-receptor and adaptor protein that are phosphorylated by LYN and SYK, respectively.[80] Binding of p85 activates the catalytic p110 subunit, which phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 then recruits the cytoplasmic kinases BTK and AKT to the cellular membrane. AKT is a key PI3K effector and following its translocation to the cellular membrane is activated by PDK1 and the mammalian target of rapamycin complex 2 (mTORC2). Active AKT then phosphorylates a number of downstream targets that are important for B-cell proliferation and survival, including the GSK3 kinase, the FOXO transcription factors and the mTORC1 inhibitor TSC2 (Figure 2). In addition to transducing signals from the BCR, PI3Kδ also plays a role in the transduction of co-stimulatory signals that originate from other receptors, such as BAFF, CD40, and Toll-like receptors.
Treatment of CLL cells with the PI3Kδ inhibitor CAL-101 (recently renamed to GS-1101) induces apoptosis in unstimulated CLL cells and inhibits survival signals generated by triggering of the BCR.[23,82,83] In addition, CAL-101 inhibits AKT and ERK activation and abrogates the protective effects of CD40L, BAFF, fibronectin, nurse-like cells and stromal cells in vitro.[23,83] CAL-101 also down-regulates secretion of chemokines and reduces CLL cell migration beneath marrow stromal cells, consistent with clinical data showing marked reductions in circulating CCL3, CCL4, and CXCL13 levels and a transient lymphocytosis during CAL-101 treatment.[83]
Clinical experience with PI3Kδ inhibitors. CAL-101 is an orally bioavailable, potent and selective inhibitor of p110δ. In vitro, the selectivity of CAL-101 to p110δ has been reported to be IC50 of 2.5nM compared to 820, 565 and 89nM for p110α, β, and γ, respectively.[82] In addition CAL-101 was found to be 400-4000 fold more selective against the related kinases C2β, hVPS34, DNA-PK, and mTOR, whereas no activity was observed against a panel of 402 diverse kinases at 10µM.[82]
Clinical results with CAL-101 have not yet been published, but data presented at conferences suggest promising clinical activity. At the American Society of Hematology (ASH) 2010 scientific meeting, Furman et al reported the results from a phase 1 study in 37 patients with relapsed or refractory CLL.[84] The investigated CAL-101 dose levels were 50 mg, 100 mg, 150 mg, 200 mg and 350 mg BID, and 300 mg QD. A significant decrease in lymphadenopathy was observed, with 91% of patients having at least a 50% reduction in lymph node size. Similar to the experience with fostamatinib, an increase in lymphocytosis occurred in 60% of the patients, which was maximal during the first 2 cycles and gradually decreased thereafter. Considering traditional response criteria, partial responses were observed in 33% of the patients. Pharmacodynamic studies with peripheral blood CLL cells showed reduction of phospho-AKT to background levels after just one week of treatment.
Updated data on 54 patients were presented at the American Society of Clinical Oncology (ASCO) annual meeting in 2011. Overall response rate (ORR) by IWCLL criteria was 26%. However, 84% of the patients had a reduction in lymphadenopathy by ≥50%, but did not meet the criteria for response by IWCLL because of the increase in circulating lymphocytes.[85] Treatment was well tolerated over prolonged exposure periods exceeding one year. Grade ≥3 adverse events included pneumonia (24%), neutropenia (24%), thrombocytopenia (7%), febrile neutropenia (7%), anemia (6%), and hepatic transaminases elevation (6%). Other side effects were generally mild. Dose response assessments supported the 150 mg BID dose for future phase 2 studies.
CAL-101 is currently studied in various combinations. A recent preliminary report was presented on a phase 1 study of CAL-101 in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL.[86] All three combinations showed a favorable safety profile and more than 80% of patients receiving each regimen met IWCLL criteria for response. The lymphocyte mobilization phenomenon observed with CAL-101 monotherapy was significantly reduced, particularly with bendamustine, likely due to its rapid cytotoxic effects.
mTOR. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase with a critical role in signal transduction pathways linking growth stimuli with cell cycle progression. It is present in two complexes that share mTOR as their catalytic subunit: mTORC1, which is a downstream effector of AKT, and mTORC2, which is an activator of AKT. The defining subunits of the two mTORCs are the regulatory-associated protein of mTOR (RAPTOR) in mTORC1 and the Rapamycin-insensitive companion of mTOR (RICTOR) in mTORC2. AKT activates mTORC1 by phosphorylating the tuberous sclerosis complex 2 (TSC2), which otherwise inhibits mTORC1 activity.
In vitro treatment of CLL cells with Rapamycin (sirolimus), a highly specific inhibitor of mTORC1, inhibits cell cycle progression in CLL cells that have been induced to proliferate with CpG-oligonucleotides and interleukin-2.[87] In addition, rapamycin prevents upregulation of survivin, a pro-survival molecule from the family of inhibitors of apoptosis proteins (IAPs) that is overexpressed in proliferative centers of B-CLL patients in vivo.[87,88] Rapamycin also displayed activity in the Eµ-TCL1 transgenic mouse model of CLL, where it slowed leukemia growth and prolonged the survival of the treated animals.[89]
Clinical experience with mTOR inhibitors. Everolimus (RAD001) is a rapamycin analog that is FDA-approved for the treatment of relapsed renal cell carcinoma. A pilot trial of everolimus in patients with advanced B-CLL demonstrated some degree of activity, but was stopped early because of toxicity concerns.[90]
A larger phase 2 study of oral single-agent everolimus (10 mg/day) was subsequently conducted in patients with recurrent/refractory indolent lymphoid malignancies, including 22 patients with CLL.[91] Four CLL patients (18%) achieved a partial response by NCI Working Group 1996 criteria. Six additional patients (27%) achieved a nodal response with a simultaneous increase in absolute lymphocyte counts, suggesting that this agent also induces the lymphocyte mobilization phenomenon typical of BCR signaling inhibitors. Myelosuppression was more common with everolimus than with other agents that target the BCR pathway. Grade 3 or 4 anemia, neutropenia, and thrombocytopenia were reported in 23%, 32%, and 50% of patients, respectively. Five serious infections occurred, including 2 fatal, supporting the need for antimicrobial prophylaxis and growth factor support in future clinical trials with this drug.
BTK. BTK is a cytoplasmic tyrosine kinase that belongs to the TEC kinase family. It is predominantly expressed in hematopoietic cells, particularly B lymphocytes and myeloid cells, and has an essential role in B cell development and BCR signaling. Mutations in BTK block B cell maturation at the pre B cell stage and cause X linked agammaglobulinemia (Bruton’s agammaglobulinemia), an immunodeficiency disorder characterized by the virtual absence of B cells and recurrent bacterial infections.
BTK is activated upon BCR engagement in two steps. The first step is recruitment to the plasma membrane by PIP3, which is generated by PI3K. Subsequently, BTK is phosphorylated by SRC family kinases at Y551 and autophosphorylated at Y223. BTK then phosphorylates PLCγ2, which produces the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These second messengers induce the release of intracellular Ca++ and activate PKC, which in turn activate the transcription factors NF-kB and NFAT (Figure 2). Activation of NF-kB results in the induction of multiple antiapoptotic genes and increased cell survival.
Downregulation of BTK by RNA interference induces apoptosis in DLBCL cell lines with a chronic active BCR signal,[92] but similar experiments have not yet been reported with primary CLL cells. However, the BTK inhibitor PCI-32765 induces modest apoptosis in primary CLL cells, which is greater than that observed in normal B cells.[24] PCI-32765 also effectively abrogates survival signals provided to CLL cells through various microenvironmental stimuli, including BCR and integrin engagement, soluble factors (CD40L, BAFF, IL-6, IL-4, and TNF-α), and stromal cell contact.[24] In addition, PCI-32765 inhibits proliferation of CLL cells induced by CpG oligonucleotides,[24] presumably by blocking Toll-like receptor signals that are also transduced through BTK.[93]
PCI-32765 has similar effects on CLL cell migration and adhesion as the previously described BCR inhibitors. These include inhibition of migration towards CXCL12 and CXCL13, inhibition of CCL3 and CCL4 secretion and inhibition of α4β1-mediated adhesion to fibronectin and VCAM-1.[94,95] These effects have also been recapitulated in vivo in the Eµ-TCL1 transgenic mouse model, where PCI-32765 causes a transient early increase in lymphocytosis followed by a delay in leukemia progression.[94]
Clinical experience with BTK inhibitors. PCI-32765 (recently renamed to Ibrutinib) is a potent, orally available, and specific inhibitor of BTK, with an IC50 of 0.5 nM.[96] It irreversibly inhibits BTK by covalently binding to its cysteine-481 residue.
The initial phase 1 study with this agent examined dose escalation in various B-cell malignancies.[97] In this study, 47 patients were enrolled, including 15 patients with CLL. Objective responses were seen in all histological subtypes. The ORR in CLL was 60%, with 1 CR and 8 PRs. Treatment was well tolerated and drug related grade 3/4 toxicities were reported in only 9 patients (19%).
In a subsequent phase 1b/2 study, 61 patients with relapsed or refractory CLL were enrolled at 2 different doses (420 mg and 840 mg).[98] As seen with other BCR inhibitors, a characteristic pattern of response occurred in the majority of patients, with an initial transient increase in lymphocytosis that diminished over time. The ORR by IWCLL criteria in the 420mg cohort was 70% at 10.2 months median follow-up, whereas in the 840mg cohort it was 44% but with a shorter median follow-up of 6.5 months. An additional 19% and 35% of patients in these cohorts, respectively, had a nodal PR (>50% reduction in aggregate lymph node size) with residual lymphocytosis. The ORR appeared to be independent of molecular risk features, such as high-risk cytogenetic abnormalities and unmutated IGHV genes. Disease progression occurred in only 8% (5/61) of patients during treatment; 6-month progression free survival (PFS) was 92% in the 420mg cohort and 90% in the 840mg cohort. Grade 3 adverse events considered potentially related to PCI-32765 treatment occurred in 21% of patients.
PCI-32765 is also currently being evaluated in a Phase 1b/2 trial of elderly (>65 years old) previously untreated CLL/SLL patients.[99] Preliminary data show an ORR by IWCLL criteria of 73% (19/26), including 2 CRs, at a median follow-up of 10.7 month. Adverse effects were minimal and estimated 12 month median PFS was 93%, suggesting that PCI-32765 could be an effective and well tolerated therapeutic option for elderly patients with CLL.
Like CAL-101, PCI-32765 is currently being evaluated in combination with chemoimmunotherapy in patients with relapsed or refractory CLL. High OR rates of 90% and 100% were recently reported for the combinations PCI-32765/bendamustine/rituximab and PCI-32765/ofatumumab, respectively.[100,101] Both combinations were well tolerated.
Another selective, irreversible and potent BTK inhibitor with an IC50<0.5nM, called AVL-292, has been developed more recently. Preliminary data from a phase 1b trial in CLL and B-NHL have been reported.[102] The drug was well tolerated and displayed the typical early effects of BCR inhibitors, including the initial rise in absolute lymphocyte counts and the simultaneous reduction in lymphadenopathy.
PTPN22. PTPN22 is a protein tyrosine phosphatase that terminates antigen-receptor signaling in T and B cells by dephosphorylating the activating tyrosine residue of SRC family kinases.[103,104] It is expressed solely in cells of the immune system, including T cells, B cells and dendritic cells. A common polymorphic allele of PTPN22 that carries a substitution of tryptophan for arginine at position 620 (R620W) is a major risk factor for the development of multiple autoimmune diseases, including insulin-dependent diabetes mellitus, rheumatoid arthritis, and systemic lupus erythematosus. Interestingly, the risk allele inhibits more strongly antigen-receptor signaling than the wild-type protein. Reduced BCR and TCR signaling in autoreactive B and T cells could be a mechanism to circumvent autoantigen-induced negative selection, indicating that the risk allele contributes to the development of autoimmunity by blocking proapoptotic stimuli that are responsible for the deletion of autoreactive lymphocytes.[103-105]
PTPN22 is markedly overexpressed in CLL cells and may play a similar role in CLL pathogenesis as has been proposed for autoimmune diseases.[57] In support of this possibility, experiments with primary CLL cells in vitro show that silencing of PTPN22 by RNA interference results in greater cell death following exposure to proapoptotic BCR stimuli, such as soluble anti-IgM.[57] The capacity of PTPN22 to protect CLL cells from proapoptotic BCR stimuli is derived from its dual role in BCR signal transduction. On the one hand, PTPN22 blocks activation of downstream molecules that activate proapoptotic pathways, such as p38MAPK, whereas on the other hand it increases the activity of the AKT kinase, which provides a powerful survival signal to antigen-stimulated CLL cells. Thus, overexpression of PTPN22 results in a net increase in AKT activity despite the overall attenuation of the BCR signal. This selective uncoupling of AKT from other downstream BCR pathways is the result of inhibition of a negative regulatory circuit that involves LYN and the phosphatase SHIP, which is a major negative regulator of the PI3K/AKT pathway (Figure 2). Collectively, these findings suggest that PTPN22 could represent another promising therapeutic target in CLL, as reducing the amount or activity of this phosphatase would be expected to reprogram signals that increase leukemic cell survival into signals that induce leukemic cell death.
Inhibitors of PTPN22 are currently being developed by various research groups and pharmaceutical companies as potential therapeutic agents for the treatment of multiple autoimmune diseases.[106] The main challenge with the development of these compounds is related to their insufficient selectivity and cell permeability, but recent progress in the field suggests that clinically useful compounds may become available for testing in CLL and PTPN22-associated autoimmune diseases in the near future.[107,108]
An alternative approach for targeting PTPN22 would be to reduce its expression. In vitro experiments have shown that PTPN22 can be induced by external stimuli that activate PKC, such as TPA or bone marrow stromal cells. Induction of PTPN22 by co-culture with bone marrow stromal cells could be efficiently blocked by the PKC inhibitors ruboxistaurin and sotrastaurin.[57] The latter are two orally available drugs that are in advanced stages of clinical development for the treatment of various nonmalignant diseases, including diabetic retinopathy, psoriasis, and prevention of renal allograft rejection.[109,110] Importantly, in experiments performed with primary CLL cells in vitro, both ruboxistaurin and sotrastaurin significantly enhanced the cytotoxic effect of soluble anti-IgM despite having no direct cytotoxic effect of their own.[57] These data suggest that PKC inhibitors could be capable of selectively killing CLL cells in vivo by reducing PTPN22 expression, although it remains possible that the enhanced apoptosis induction observed with these agents involves also other mechanisms that are unrelated to PTPN22.
Clinical studies with PKC inhibitors in CLL have not yet been conducted, but phase 2 clinical trials are ongoing with sotrastaurin in DLBCL and with the dual PKC and AKT inhibitor enzastaurin in DLBCL, FL and mantle cell lymphoma. Considering the good tolerability and absence of significant toxic effects of ruboxistaurin and sotrastaurin, clinical studies with these PKC inhibitors in CLL appear warranted.
Conclusion
The recent development of therapeutic agents that target the BCR pathway has generated considerable excitement both among researchers and clinicians dealing with CLL. This excitement is because these therapeutic agents can be given orally, they have minimal side effects, and they are producing very high response rates and durable responses. In addition, novel agents that target the BCR pathway continue to be developed and will hopefully allow even more selective killing of the malignant B cells. A challenge for the future will be to define the optimal drug combinations and identify the most appropriate therapeutic settings that will provide the greatest clinical benefit from this promising class of agents.
The B-cell receptor (BCR) signaling pathway has recently emerged as a major therapeutic target in CLL.[1-3] Signals propagated through the BCR have been shown to increase leukemic cell survival in vitro[4-6] and there is growing evidence that such signals are continuously delivered to the leukemic cells in vivo.[7,8] This evidence particularly refers to data obtained from gene expression profiling studies, which have shown that freshly isolated CLL cells express high levels of genes that can be induced in normal B cells by BCR engagement.[9] Such BCR target genes are especially enriched in CLL cells isolated from lymph nodes, which is an important site of antigen encounter.[10] In addition, the kinase SYK, which is activated immediately downstream of the BCR, is frequently phosphorylated in freshly isolated CLL cells,[11,12] and this is also more prominent in CLL cells isolated from lymph nodes than peripheral blood.[10] Lastly, surface IgM expression is downmodulated on freshly isolated CLL cells but recovers after a few days in culture, further indicating that the leukemic BCRs are continuously triggered by antigen in vivo.[13] Altogether, these findings suggest that the BCR pathway is aberrantly or excessively activated in CLL cells and may therefore represent a promising target for therapeutic intervention.
BCR Signals Generated in CLL Cells
Normal B lymphocytes receive two types of signals from their BCRs. The first type is triggered by binding of the BCR to external antigen, which results in the assembly and activation of a signaling complex that propagates the signal to the interior of the cell[14] (Figure 1A). This BCR signal can induce a variety of cellular responses, including proliferation, survival, differentiation, anergy and apoptosis. The final outcome depends on several factors, such as the nature of the antigen, the availability of co-stimulatory signals and the stage of B cell differentiation.[15] The second type of signal occurs in the absence of an external ligand and has been termed tonic BCR signal. The role of this signal in normal B cell biology has still not been fully established, but recent studies suggest that it is important for the survival of mature B cells and for normal B cell development and maturation.[16,17]
In recent years, evidence has emerged that both types of signals are excessively or aberrantly generated in CLL cells. For example, the kinases LYN and SYK, which are activated immediately downstream of the BCR, often shown increased basal activity in unstimulated CLL cells.[11,12,18-21] This increased basal activity is maintained during prolonged in vitro culture, suggesting that it is independent of an external antigen. Increased basal activity has also been reported for other signaling molecules that are located further downstream along the BCR pathway, such as the kinases PI3K,[22,23] BTK,[24] PKC[25] and ERK,[26] and the transcription factors NF-kB and NF-AT.[27-30] Importantly, inhibition or downregulation of many of these signaling molecules induces apoptosis in unstimulated CLL cells. Together, these findings suggest that CLL cells have an elevated tonic or basal BCR signaling activity, which contributes to their increased apoptosis resistance.
The mechanism that generates the elevated basal BCR signaling activity in CLL cells was only very recently revealed.[31] In their seminal paper, Dühren-von Minden et al investigated a large series of CLL BCRs (n=17) and showed that all of them are capable of inducing an autonomous signal when introduced in a B cell line that does not express a functional BCR. Additional experiments demonstrated that this signal is dependent on an interaction between the heavy-chain complementarity-determining region 3 (HCDR3) of one BCR and an intrinsic motif in the framework region 2 (FR2) of another. Thus, the interaction between the HCDR3 of one BCR and the FR2 of another leads to aggregation of neighboring BCRs, which in turn generates a cell autonomous signal that is independent of an external ligand (Figure 1B).
The cell autonomous BCR signal appears to be different from the tonic BCR signal of normal B cells, as the latter occurs in the absence of any kind of ligand and is believed to be generated by transient and stochastic interactions between the BCR and the LYN and SYK kinases (Figure 1C).[16] The cell autonomous BCR signal is also different from the extrinsic antigen-dependent BCR signal in several important aspects. For example, the cell autonomous BCR signal does not seem to result in full SYK activation, considering that phosphorylation of the activation loop tyrosines (YY525/526) of SYK is usually not detected in unstimulated CLL cells, whereas it is readily detected in most CLL samples stimulated with anti-IgM antibodies, which are used to mimic external antigen.[32] Phosphorylation of the activation loop tyrosines in SYK is required for sustained PLCγ2, AKT and ERK activation and SYK-mediated B cell proliferation.[32] In addition, most CLL cells are in a quiescent state when isolated from the peripheral blood, but can be activated and induced to enter the G1 phase of the cell cycle by BCR engagement.[6,33,34] Together, these data suggest that CLL cell proliferation is driven by extrinsic antigen-dependent BCR signals, whereas the role of the cell autonomous BCR signal in the pathogenesis of CLL could be to maintain the viability of the leukemic precursors until they encounter the factors that promote their expansion and subsequent malignant transformation.

Figure 1. BCR signals generated in CLL and normal B cells. A) Binding of antigen induces aggregation of neighboring BCRs that initiate the classical antigen-dependent BCR signal. B) Aggregation of neighboring BCRs in CLL cells through an interaction between the CDR3 of one BCR and the FR2 of another initiates a cell autonomous BCR signal in the absence of an external antigen. C) Random and transient disruptions in the equilibrium between positive regulators of BCR siganling, such as the Igα-Igß heterodimer, LYN and SYK, and negative regulators, such as the various phosphatases (PTP), could generate a tonic antigen-independent BCR signal.
The nature and identity of the antigens that drive CLL cell proliferation has still not been established, although the restricted variable region gene repertoire and the expression of stereotyped BCRs in over 30% of the cases suggest that the leukemic cells in many cases recognize similar or identical antigens.[35,36] In cases with unmutated IGHV genes (U-CLL), the leukemic BCRs often bind to autoantigens that are generated or exposed on apoptotic cells.[37-41] Whether or not these autoantigens drive the expansion of the malignant cells is still unclear, since U-CLL BCRs are frequently polyreactive and can also bind to microbial antigens, such as Streptococcus pneumoniae polysaccharides[37] or the cytomegalovirus phosphoprotein pUL32.[42] Thus, it still remains possible that the driving force for CLL cell proliferation is a chronic infectious agent, as is the case with certain indolent lymphomas that arise in the setting of chronic bacterial or viral infections, such as H. Pylori-associated gastric MALT lymphomas[43,44] or hepatitis C virus (HCV)-associated splenic marginal zone and lymphoplasmacytoid lymphomas.[45,46] Alternatively, the driving force could be autoantigens associated with apoptotic blebs, which co-localize with DNA- or RNA-containing complexes and can therefore provide necessary co-stimulatory signals by triggering Toll-like receptors.[47]
Features of the BCR Pathway in CLL Cells
CLL cells have several distinct BCR signaling features that distinguish them from normal B cells. These include the previously described cell autonomous BCR signal, the low-level surface IgM expression and the heterogeneous signaling responses induced by BCR engagement in vitro. The latter are particularly evident with respect to BCR proximal signaling events, such as induction of SYK and PLCγ2 phosphorylation and intracellular Ca2+ mobilization, which are weak or absent in a substantial proportion of cases.[48-50] These impaired signaling responses are more common in cases with favorable prognostic features, such as those that express IGHV-mutated BCRs or do not express ZAP-70.[51,52] However, even in cases that appear to transduce BCR signals relatively efficiently, activation of downstream pathways can be variable and incomplete when compared to normal B cells. For example, despite efficient activation of AKT and ERK, anti-IgM induced activation of JNK, p38MAPK and NF-kB is often negligible or absent.[5]
Heterogeneous responses are also observed when the same cells are stimulated with different types of BCR crosslinking agents. For example, stimulation with soluble anti-IgM antibodies induces apoptosis in most CLL samples, whereas stimulation with the same antibodies immobilized on solid structures usually increases the survival of the leukemic cells.[5,6,53] These data suggest that different types of antigens can induce different responses in CLL cells and indicate that the variability in the clinical course could be in part determined by the antigen specificity of the malignant B cells.
Another distinct feature of CLL cells is the aberrant expression of multiple molecules that are involved in BCR signal transduction. Most of these regulatory molecules are expressed at higher levels in CLL than normal B cells, but some show reduced expression. For example, the kinases LYN,[18] LCK,[54] SYK,[20] ZAP-70,[9] BTK[24] and PKCß,[55] the adaptor protein TCL1[56] and the phosphatase PTPN22[57] are all overexpressed in CLL cells, whereas the adaptor protein SHC[58] and the phosphatase PHLPP1[59] are expressed at markedly reduced levels. The functional significance of these abnormalities is still not completely clear, although at least for some of them there is evidence for a positive effect on the activity of downstream signaling pathways that regulate CLL cell survival. This particularly refers to the reduced PHLPP1 and increased TCL1 and PTPN22 expression, which result in enhanced AKT activation in anti-IgM-stimulated CLL cells.[56,57,59] AKT is a key signaling molecule that regulates the survival of BCR-stimulated CLL cells by inducing the expression of several important antiapoptotic proteins, including MCL-1, BCL-xL and XIAP.[60] Reduced expression of PHLPP1 could additionally increase the survival of BCR-stimulated CLL cells by allowing for enhanced activation of ERK,[59] which regulates the activity of the proapoptotic protein BIM.[61] Finally, overexpression of PTPN22 can have a positive effect on CLL cell survival not only by enhancing AKT activation, but also by inhibiting signaling molecules that activate proapoptotic pathways, such as p38MAPK.[57] Together, these findings indicate that CLL cells have a very delicately fine-tuned signaling machinery that allows them to amplify weak antiapoptotic BCR signals while at the same time blocking signals that could have a negative impact on their survival. Such a machinery could be particularly advantageous to CLL cells that express autoreactive BCRs, as it could prevent their elimination by immunological tolerance mechanisms and may even allow for their positive selection by autoantigen.
Potential Targets Along the BCR Pathway in CLL
LYN. Transduction of the BCR signal is a complex process that involves multiple kinases, phosphatases and adaptor proteins, which could represent potential therapeutic targets (Figure 2). The signal is initially propagated by SRC-family kinases, such as LYN, FYN or BLK, which phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) in the Igα and Igß chains of the BCR. The phosphorylated ITAMs recruit the kinase SYK, which then becomes activated through SRC-family kinase-dependent phosphorylation at Y352 and trans-autophosphorylation at YY525/526. LYN not only phosphorylates and activates SYK, but it also activates phosphatases that inhibit BCR signal transduction. Thus, LYN functions as both a positive and negative regulator of BCR signaling.
As mentioned earlier, LYN is overexpressed, constitutively active and abnormally distributed in CLL cells.[18] Targeting of LYN with SRC-family kinase inhibitors, such as PP2 and Dasatinib, induces leukemic cell apoptosis.[18,62,63] However, it is still uncertain whether the cytotoxic effect of these compounds is due to inhibition of LYN or inhibition of related kinases. For example, BTK and ABL are also inhibited by Dasatinib, and both of these molecules appear to be important for CLL cell survival.[64-66] Silencing of LYN by RNA interference should clarify this issue, but so far this approach has not proven very effective presumably because of the significant overexpression and long half-life of LYN in CLL cells.[18,67]
Clinical experience with LYN inhibitors. Dasatinib is an oral pan-SRC kinase and ABL inhibitor that is currently approved for the treatment of CML (Table 1). A phase 2 trial in patients with relapsed or refractory CLL treated with a daily dose of 140 mg for up to 24 months was recently published.[68] Modest clinical activity was observed. Partial responses by NCI-WG criteria were achieved in 3 of the 15 enrolled patients (20%). Among the remaining 12 patients, 5 had nodal responses by physical exam, and one additional patient had a nodal and lymphocyte response but with severe myelosuppression. Primary toxicity was myelosuppression, with grade 3/4 neutropenia occurring in 67% of patients and grade 3/4 thrombocytopenia in 40%. Pharmacodynamic studies indicated apoptosis in peripheral blood CLL cells within 3 to 6 hours after dasatinib administration, which interestingly was associated with downregulation of SYK mRNA.

Table 1. Agents that target the BCR pathway in CLL.

Figure 2. Main therapeutic targets along the BCR signaling pathway in CLL cells. Aggregation of the BCR by antigen induces the phosphorylation of the ITAMs within the cytoplasmic tails of the Ig-α/Ig-ß heterodimer. Phosphorylation of the ITAMs is mediated by LYN or other members of the SRC family kinases. SYK is subsequently recruited to the phosphorylated ITAMs and activated by a multistep process that involves phosphorylation by SRC family kinases and trans-autophosphorylation. SYK further propagates the signal by phosphorylating and recruiting several signaling intermediates, such as BLNK, BCAP, VAV, SHC and PI3K. Recruitment of PI3K leads to the production of phosphatidylinositol-3,4,5-triphosphate (PIP3) and subsequent BTK and AKT activation. SYK and BTK then activate PLCγ2, which produces the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These second messengers induce the release of intracellular Ca2+ and activate PKC, which then activate the transcription factors NF-kB and NFAT. PKC also induces the expression of the phosphatase PTPN22, which dephosphorylates and inactivates LYN. This event prevents LYN-mediated recruitment and activation of the phosphatase SHIP, which is a key negative regulator of the PI3K/AKT pathway. In the absence of active SHIP, AKT continues to be activated by PIP3, resulting in amplification of the BCR survival signal. BCR signaling molecules that have been studied as potential therapeutic targets in CLL are depicted in grey.
SYK. SYK is currently one of the most attractive targets for therapeutic intervention along the BCR pathway in CLL. This kinase propagates the BCR signal by phosphorylating the adaptor proteins BLNK, BCAP and SHC, and activating key signaling intermediates, such as PI3K, BTK and PLCγ2 (Figure 2). In addition to its essential role in transducing the BCR signal, SYK is also involved in integrin, chemokine and Fc-receptor signaling.[69]
SYK is constitutively phosphorylated on the activating Y352 residue in CLL cells,[12] presumably as a consequence of the cell autonomous BCR signal.[31] Inhibition or silencing of SYK decreases the basal activity of downstream signaling molecules, providing additional evidence that constitutively active SYK molecules are present in CLL cells.[12,21] Inhibition of SYK also induces moderate apoptosis in unstimulated CLL cells, further suggesting that the cell autonomous BCR signal increases leukemic cell survival.[12,19-21] In addition, treatment of CLL cells with the SYK inhibitor fostamatinib completely blocks transduction of extrinsic antigen-dependent BCR signals.[12] Fostamatinib also inhibits integrin signaling, antagonizes the protective effect of stromal cells, and reduces migration of CLL cells to chemokines and their adhesion to stromal components.[19,70]
Many of the in vitro effects of fostamatinib have been recapitulated in vivo using the Eµ-TCL1 transgenic mouse model of CLL.[71] In this model, fostamatinib treatment results in inhibition of BCR signaling, an initial transient lymphocytosis, reduced proliferation and survival of the malignant B cells, and prolonged survival of the treated animals.
Clinical experience with SYK inhibitors. Fostamatinib, also called R788, is an oral prodrug that is converted in vivo into the active compound R406.[72] The latter is a potent inhibitor of SYK, with an IC50 in vitro of 41 nM. R406 is not entirely specific for SYK, as it also inhibits FLT3, JAK, LCK, VEGFR2 and RET at similar or slightly higher concentrations.
R406 is the first BCR signaling agent that was shown to be active in CLL.[73] This compound was initially developed as potential treatment for allergy and rheumatoid arthritis, because of its capacity to inhibit signaling through FcεRI and Fcg receptors.[74] Early trials of fostamatinib in patients with rheumatoid arthritis showed clinical activity and potent anti-inflammatory effects.[75,76] Subsequently, fostamatinib was tested in a phase 1/2 trial of relapsed or refractory NHL and CLL.[77] In the phase 1 part of the trial a dose of 200mg p.o. bid was established. Dose-limiting toxicities were neutropenia, thrombocytopenia, and diarrhea. In the Phase 2 study, 68 patients were enrolled into 3 groups according to histology, including 23 patients with diffuse large B cell lymphoma (DLBCL), 21 patient with follicular lymphoma and 24 patients with other NHL, among which 11 had SLL/CLL. The highest response rate was observed in SLL/CLL, where 6 patients (55%) achieved a partial response (PR). Objective responses were also seen in 22% of DLBCL [1 complete response (CR), 4 PRs], 10% of follicular lymphoma (2 PRs) and 11% of mantle cell lymphoma patients (1 PR). In the phase 2 portion of the trial the most common adverse events were reversible cytopenias, fatigue, diarrhea, and hypertension. All patients with CLL/SLL exhibited a transient initial lymphocytosis that has since been observed with all other agents that target the BCR pathway. This effect is believed to be caused by disruption of adhesion and retention signals delivered through the BCR, chemokine receptors and/or integrins, resulting in mobilization of CLL cells from lymph nodes and marrow into the blood.[19,70]
Follow-up studies with fostamatinib in CLL have not been performed to date because the company that is developing this agent is pursuing a registration strategy in rheumatoid arthritis. Clinical trials are proceeding in diffuse large B cell lymphoma, but it remains unclear whether fostamatinib will be available for future studies in CLL. However, newer more potent and more specific SYK inhibitors with promising pre-clinical activity in CLL have recently been developed and are expected to enter into clinical trials in the near future.[78,79]
PI3Kδ. PI3K is a key downstream mediator of BCR signaling.[80] CLL cells generally express high levels of active PI3K[22,23] and sustained activation of this pathway increases their proliferative capacity and survival.[60,81]
Several PI3K isoforms exist, some of which are ubiquitously expressed whereas others are cell type specific. PI3Kδ is the predominantly expressed isoform in leukocytes. It consists of two components, a regulatory p85 and a catalytic p110δ subunit. Upon crosslinking of the BCR, p85 binds via its SH2 domains to phospho-tyrosine motifs on CD19 and BCAP, which are a co-receptor and adaptor protein that are phosphorylated by LYN and SYK, respectively.[80] Binding of p85 activates the catalytic p110 subunit, which phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 then recruits the cytoplasmic kinases BTK and AKT to the cellular membrane. AKT is a key PI3K effector and following its translocation to the cellular membrane is activated by PDK1 and the mammalian target of rapamycin complex 2 (mTORC2). Active AKT then phosphorylates a number of downstream targets that are important for B-cell proliferation and survival, including the GSK3 kinase, the FOXO transcription factors and the mTORC1 inhibitor TSC2 (Figure 2). In addition to transducing signals from the BCR, PI3Kδ also plays a role in the transduction of co-stimulatory signals that originate from other receptors, such as BAFF, CD40, and Toll-like receptors.
Treatment of CLL cells with the PI3Kδ inhibitor CAL-101 (recently renamed to GS-1101) induces apoptosis in unstimulated CLL cells and inhibits survival signals generated by triggering of the BCR.[23,82,83] In addition, CAL-101 inhibits AKT and ERK activation and abrogates the protective effects of CD40L, BAFF, fibronectin, nurse-like cells and stromal cells in vitro.[23,83] CAL-101 also down-regulates secretion of chemokines and reduces CLL cell migration beneath marrow stromal cells, consistent with clinical data showing marked reductions in circulating CCL3, CCL4, and CXCL13 levels and a transient lymphocytosis during CAL-101 treatment.[83]
Clinical experience with PI3Kδ inhibitors. CAL-101 is an orally bioavailable, potent and selective inhibitor of p110δ. In vitro, the selectivity of CAL-101 to p110δ has been reported to be IC50 of 2.5nM compared to 820, 565 and 89nM for p110α, β, and γ, respectively.[82] In addition CAL-101 was found to be 400-4000 fold more selective against the related kinases C2β, hVPS34, DNA-PK, and mTOR, whereas no activity was observed against a panel of 402 diverse kinases at 10µM.[82]
Clinical results with CAL-101 have not yet been published, but data presented at conferences suggest promising clinical activity. At the American Society of Hematology (ASH) 2010 scientific meeting, Furman et al reported the results from a phase 1 study in 37 patients with relapsed or refractory CLL.[84] The investigated CAL-101 dose levels were 50 mg, 100 mg, 150 mg, 200 mg and 350 mg BID, and 300 mg QD. A significant decrease in lymphadenopathy was observed, with 91% of patients having at least a 50% reduction in lymph node size. Similar to the experience with fostamatinib, an increase in lymphocytosis occurred in 60% of the patients, which was maximal during the first 2 cycles and gradually decreased thereafter. Considering traditional response criteria, partial responses were observed in 33% of the patients. Pharmacodynamic studies with peripheral blood CLL cells showed reduction of phospho-AKT to background levels after just one week of treatment.
Updated data on 54 patients were presented at the American Society of Clinical Oncology (ASCO) annual meeting in 2011. Overall response rate (ORR) by IWCLL criteria was 26%. However, 84% of the patients had a reduction in lymphadenopathy by ≥50%, but did not meet the criteria for response by IWCLL because of the increase in circulating lymphocytes.[85] Treatment was well tolerated over prolonged exposure periods exceeding one year. Grade ≥3 adverse events included pneumonia (24%), neutropenia (24%), thrombocytopenia (7%), febrile neutropenia (7%), anemia (6%), and hepatic transaminases elevation (6%). Other side effects were generally mild. Dose response assessments supported the 150 mg BID dose for future phase 2 studies.
CAL-101 is currently studied in various combinations. A recent preliminary report was presented on a phase 1 study of CAL-101 in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL.[86] All three combinations showed a favorable safety profile and more than 80% of patients receiving each regimen met IWCLL criteria for response. The lymphocyte mobilization phenomenon observed with CAL-101 monotherapy was significantly reduced, particularly with bendamustine, likely due to its rapid cytotoxic effects.
mTOR. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase with a critical role in signal transduction pathways linking growth stimuli with cell cycle progression. It is present in two complexes that share mTOR as their catalytic subunit: mTORC1, which is a downstream effector of AKT, and mTORC2, which is an activator of AKT. The defining subunits of the two mTORCs are the regulatory-associated protein of mTOR (RAPTOR) in mTORC1 and the Rapamycin-insensitive companion of mTOR (RICTOR) in mTORC2. AKT activates mTORC1 by phosphorylating the tuberous sclerosis complex 2 (TSC2), which otherwise inhibits mTORC1 activity.
In vitro treatment of CLL cells with Rapamycin (sirolimus), a highly specific inhibitor of mTORC1, inhibits cell cycle progression in CLL cells that have been induced to proliferate with CpG-oligonucleotides and interleukin-2.[87] In addition, rapamycin prevents upregulation of survivin, a pro-survival molecule from the family of inhibitors of apoptosis proteins (IAPs) that is overexpressed in proliferative centers of B-CLL patients in vivo.[87,88] Rapamycin also displayed activity in the Eµ-TCL1 transgenic mouse model of CLL, where it slowed leukemia growth and prolonged the survival of the treated animals.[89]
Clinical experience with mTOR inhibitors. Everolimus (RAD001) is a rapamycin analog that is FDA-approved for the treatment of relapsed renal cell carcinoma. A pilot trial of everolimus in patients with advanced B-CLL demonstrated some degree of activity, but was stopped early because of toxicity concerns.[90]
A larger phase 2 study of oral single-agent everolimus (10 mg/day) was subsequently conducted in patients with recurrent/refractory indolent lymphoid malignancies, including 22 patients with CLL.[91] Four CLL patients (18%) achieved a partial response by NCI Working Group 1996 criteria. Six additional patients (27%) achieved a nodal response with a simultaneous increase in absolute lymphocyte counts, suggesting that this agent also induces the lymphocyte mobilization phenomenon typical of BCR signaling inhibitors. Myelosuppression was more common with everolimus than with other agents that target the BCR pathway. Grade 3 or 4 anemia, neutropenia, and thrombocytopenia were reported in 23%, 32%, and 50% of patients, respectively. Five serious infections occurred, including 2 fatal, supporting the need for antimicrobial prophylaxis and growth factor support in future clinical trials with this drug.
BTK. BTK is a cytoplasmic tyrosine kinase that belongs to the TEC kinase family. It is predominantly expressed in hematopoietic cells, particularly B lymphocytes and myeloid cells, and has an essential role in B cell development and BCR signaling. Mutations in BTK block B cell maturation at the pre B cell stage and cause X linked agammaglobulinemia (Bruton’s agammaglobulinemia), an immunodeficiency disorder characterized by the virtual absence of B cells and recurrent bacterial infections.
BTK is activated upon BCR engagement in two steps. The first step is recruitment to the plasma membrane by PIP3, which is generated by PI3K. Subsequently, BTK is phosphorylated by SRC family kinases at Y551 and autophosphorylated at Y223. BTK then phosphorylates PLCγ2, which produces the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These second messengers induce the release of intracellular Ca++ and activate PKC, which in turn activate the transcription factors NF-kB and NFAT (Figure 2). Activation of NF-kB results in the induction of multiple antiapoptotic genes and increased cell survival.
Downregulation of BTK by RNA interference induces apoptosis in DLBCL cell lines with a chronic active BCR signal,[92] but similar experiments have not yet been reported with primary CLL cells. However, the BTK inhibitor PCI-32765 induces modest apoptosis in primary CLL cells, which is greater than that observed in normal B cells.[24] PCI-32765 also effectively abrogates survival signals provided to CLL cells through various microenvironmental stimuli, including BCR and integrin engagement, soluble factors (CD40L, BAFF, IL-6, IL-4, and TNF-α), and stromal cell contact.[24] In addition, PCI-32765 inhibits proliferation of CLL cells induced by CpG oligonucleotides,[24] presumably by blocking Toll-like receptor signals that are also transduced through BTK.[93]
PCI-32765 has similar effects on CLL cell migration and adhesion as the previously described BCR inhibitors. These include inhibition of migration towards CXCL12 and CXCL13, inhibition of CCL3 and CCL4 secretion and inhibition of α4β1-mediated adhesion to fibronectin and VCAM-1.[94,95] These effects have also been recapitulated in vivo in the Eµ-TCL1 transgenic mouse model, where PCI-32765 causes a transient early increase in lymphocytosis followed by a delay in leukemia progression.[94]
Clinical experience with BTK inhibitors. PCI-32765 (recently renamed to Ibrutinib) is a potent, orally available, and specific inhibitor of BTK, with an IC50 of 0.5 nM.[96] It irreversibly inhibits BTK by covalently binding to its cysteine-481 residue.
The initial phase 1 study with this agent examined dose escalation in various B-cell malignancies.[97] In this study, 47 patients were enrolled, including 15 patients with CLL. Objective responses were seen in all histological subtypes. The ORR in CLL was 60%, with 1 CR and 8 PRs. Treatment was well tolerated and drug related grade 3/4 toxicities were reported in only 9 patients (19%).
In a subsequent phase 1b/2 study, 61 patients with relapsed or refractory CLL were enrolled at 2 different doses (420 mg and 840 mg).[98] As seen with other BCR inhibitors, a characteristic pattern of response occurred in the majority of patients, with an initial transient increase in lymphocytosis that diminished over time. The ORR by IWCLL criteria in the 420mg cohort was 70% at 10.2 months median follow-up, whereas in the 840mg cohort it was 44% but with a shorter median follow-up of 6.5 months. An additional 19% and 35% of patients in these cohorts, respectively, had a nodal PR (>50% reduction in aggregate lymph node size) with residual lymphocytosis. The ORR appeared to be independent of molecular risk features, such as high-risk cytogenetic abnormalities and unmutated IGHV genes. Disease progression occurred in only 8% (5/61) of patients during treatment; 6-month progression free survival (PFS) was 92% in the 420mg cohort and 90% in the 840mg cohort. Grade 3 adverse events considered potentially related to PCI-32765 treatment occurred in 21% of patients.
PCI-32765 is also currently being evaluated in a Phase 1b/2 trial of elderly (>65 years old) previously untreated CLL/SLL patients.[99] Preliminary data show an ORR by IWCLL criteria of 73% (19/26), including 2 CRs, at a median follow-up of 10.7 month. Adverse effects were minimal and estimated 12 month median PFS was 93%, suggesting that PCI-32765 could be an effective and well tolerated therapeutic option for elderly patients with CLL.
Like CAL-101, PCI-32765 is currently being evaluated in combination with chemoimmunotherapy in patients with relapsed or refractory CLL. High OR rates of 90% and 100% were recently reported for the combinations PCI-32765/bendamustine/rituximab and PCI-32765/ofatumumab, respectively.[100,101] Both combinations were well tolerated.
Another selective, irreversible and potent BTK inhibitor with an IC50<0.5nM, called AVL-292, has been developed more recently. Preliminary data from a phase 1b trial in CLL and B-NHL have been reported.[102] The drug was well tolerated and displayed the typical early effects of BCR inhibitors, including the initial rise in absolute lymphocyte counts and the simultaneous reduction in lymphadenopathy.
PTPN22. PTPN22 is a protein tyrosine phosphatase that terminates antigen-receptor signaling in T and B cells by dephosphorylating the activating tyrosine residue of SRC family kinases.[103,104] It is expressed solely in cells of the immune system, including T cells, B cells and dendritic cells. A common polymorphic allele of PTPN22 that carries a substitution of tryptophan for arginine at position 620 (R620W) is a major risk factor for the development of multiple autoimmune diseases, including insulin-dependent diabetes mellitus, rheumatoid arthritis, and systemic lupus erythematosus. Interestingly, the risk allele inhibits more strongly antigen-receptor signaling than the wild-type protein. Reduced BCR and TCR signaling in autoreactive B and T cells could be a mechanism to circumvent autoantigen-induced negative selection, indicating that the risk allele contributes to the development of autoimmunity by blocking proapoptotic stimuli that are responsible for the deletion of autoreactive lymphocytes.[103-105]
PTPN22 is markedly overexpressed in CLL cells and may play a similar role in CLL pathogenesis as has been proposed for autoimmune diseases.[57] In support of this possibility, experiments with primary CLL cells in vitro show that silencing of PTPN22 by RNA interference results in greater cell death following exposure to proapoptotic BCR stimuli, such as soluble anti-IgM.[57] The capacity of PTPN22 to protect CLL cells from proapoptotic BCR stimuli is derived from its dual role in BCR signal transduction. On the one hand, PTPN22 blocks activation of downstream molecules that activate proapoptotic pathways, such as p38MAPK, whereas on the other hand it increases the activity of the AKT kinase, which provides a powerful survival signal to antigen-stimulated CLL cells. Thus, overexpression of PTPN22 results in a net increase in AKT activity despite the overall attenuation of the BCR signal. This selective uncoupling of AKT from other downstream BCR pathways is the result of inhibition of a negative regulatory circuit that involves LYN and the phosphatase SHIP, which is a major negative regulator of the PI3K/AKT pathway (Figure 2). Collectively, these findings suggest that PTPN22 could represent another promising therapeutic target in CLL, as reducing the amount or activity of this phosphatase would be expected to reprogram signals that increase leukemic cell survival into signals that induce leukemic cell death.
Inhibitors of PTPN22 are currently being developed by various research groups and pharmaceutical companies as potential therapeutic agents for the treatment of multiple autoimmune diseases.[106] The main challenge with the development of these compounds is related to their insufficient selectivity and cell permeability, but recent progress in the field suggests that clinically useful compounds may become available for testing in CLL and PTPN22-associated autoimmune diseases in the near future.[107,108]
An alternative approach for targeting PTPN22 would be to reduce its expression. In vitro experiments have shown that PTPN22 can be induced by external stimuli that activate PKC, such as TPA or bone marrow stromal cells. Induction of PTPN22 by co-culture with bone marrow stromal cells could be efficiently blocked by the PKC inhibitors ruboxistaurin and sotrastaurin.[57] The latter are two orally available drugs that are in advanced stages of clinical development for the treatment of various nonmalignant diseases, including diabetic retinopathy, psoriasis, and prevention of renal allograft rejection.[109,110] Importantly, in experiments performed with primary CLL cells in vitro, both ruboxistaurin and sotrastaurin significantly enhanced the cytotoxic effect of soluble anti-IgM despite having no direct cytotoxic effect of their own.[57] These data suggest that PKC inhibitors could be capable of selectively killing CLL cells in vivo by reducing PTPN22 expression, although it remains possible that the enhanced apoptosis induction observed with these agents involves also other mechanisms that are unrelated to PTPN22.
Clinical studies with PKC inhibitors in CLL have not yet been conducted, but phase 2 clinical trials are ongoing with sotrastaurin in DLBCL and with the dual PKC and AKT inhibitor enzastaurin in DLBCL, FL and mantle cell lymphoma. Considering the good tolerability and absence of significant toxic effects of ruboxistaurin and sotrastaurin, clinical studies with these PKC inhibitors in CLL appear warranted.
Conclusion
The recent development of therapeutic agents that target the BCR pathway has generated considerable excitement both among researchers and clinicians dealing with CLL. This excitement is because these therapeutic agents can be given orally, they have minimal side effects, and they are producing very high response rates and durable responses. In addition, novel agents that target the BCR pathway continue to be developed and will hopefully allow even more selective killing of the malignant B cells. A challenge for the future will be to define the optimal drug combinations and identify the most appropriate therapeutic settings that will provide the greatest clinical benefit from this promising class of agents.
References
- Efremov DG, Laurenti L. The Syk kinase as a
therapeutic target in leukemia and lymphoma. Expert Opin Investig
Drugs. 2011;20(5):623-36.
http://dx.doi.org/10.1517/13543784.2011.570329
PMid:21438742

- Woyach JA, Johnson AJ, Byrd JC. The B-cell
receptor signaling pathway as a therapeutic target in CLL. Blood.
2012;120(6):1175-84.
http://dx.doi.org/10.1182/blood-2012-02-362624
PMid:22715122

- Wiestner A. Emerging role of kinase
targeted strategies in chronic lymphocytic leukemia. Blood. 2012 Aug 8.
[Epub ahead of print]
http://dx.doi.org/10.1182/blood-2012-05-423194

- Bernal A, Pastore RD, Asgary Z, Keller SA,
Cesarman E, Liou HC, Schattner EJ. Survival of leukemic B cells
promoted by engagement of the antigen receptor. Blood.
2001;98(10):3050-7.
http://dx.doi.org/10.1182/blood.V98.10.3050
PMid:11698290

- Petlickovski A, Laurenti L, Li X, Marietti
S, Chiusolo P, Sica S, Leone G, Efremov DG. Sustained signaling through
the B-cell receptor induces Mcl-1 and promotes survival of chronic
lymphocytic leukemia B cells. Blood. 2005;105(12):4820-7.
http://dx.doi.org/10.1182/blood-2004-07-2669 PMid:15728130

- Deglesne PA, Chevallier N, Letestu R,
Baran-Marszak F, Beitar T, Salanoubat C, Sanhes L, Nataf J, Roger C,
Varin-Blank N, Ajchenbaum-Cymbalista F. Survival response to B-cell
receptor ligation is restricted to progressive chronic lymphocytic
leukemia cells irrespective of Zap70 expression. Cancer Res.
2006;66(14):7158-66.
http://dx.doi.org/10.1158/0008-5472.CAN-06-0085 PMid:16849562

- Efremov DG, Gobessi S, Longo PG. Signaling
pathways activated by antigen-receptor engagement in chronic
lymphocytic leukemia B-cells. Autoimmun Rev. 2007;7(2):102-8.
http://dx.doi.org/10.1016/j.autrev.2007.02.021
PMid:18035318

- Stevenson FK, Krysov S, Davies AJ, Steele
AJ, Packham G. B-cell receptor signaling in chronic lymphocytic
leukemia. Blood. 2011;118(16):4313-20.
http://dx.doi.org/10.1182/blood-2011-06-338855
PMid:21816833

- Rosenwald A, Alizadeh AA, Widhopf G, Simon
R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein
D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ,
Brown PO, Staudt LM. Relation of gene expression phenotype to
immunoglobulin mutation genotype in B cell chronic lymphocytic
leukemia. J Exp Med. 2001;194(11):1639-47.
http://dx.doi.org/10.1084/jem.194.11.1639 PMid:11733578
PMCid:2193523

- Herishanu Y, Pérez-Galán P, Liu D,
Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E,
Stennett L, Raghavachari N, Liu P, McCoy JP, Raffeld M,
Stetler-Stevenson M, Yuan C, Sherry R, Arthur DC, Maric I, White T,
Marti GE, Munson P, Wilson WH, Wiestner A. The lymph node
microenvironment promotes B-cell receptor signaling, NF-kappaB
activation, and tumor proliferation in chronic lymphocytic leukemia.
Blood. 2011;117(2):563-74.
http://dx.doi.org/10.1182/blood-2010-05-284984 PMid:20940416
PMCid:3031480

- Gobessi S, Laurenti L, Longo PG, Sica S,
Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite
absent or inefficient tyrosine kinase activation in chronic lymphocytic
leukemia and lymphoma B cells. Blood. 2007;109(5):2032-9.
http://dx.doi.org/10.1182/blood-2006-03-011759
PMid:17038529

- Gobessi S, Laurenti L, Longo PG, Carsetti
L, Berno V, Sica S, Leone G, Efremov DG. Inhibition of constitutive and
BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in
chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686-97.
http://dx.doi.org/10.1038/leu.2008.346 PMid:19092849

- Mockridge CI, Potter KN, Wheatley I,
Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated
signaling in the two subsets of CLL defined by VH-gene mutational
status. Blood. 2007;109(10):4424-31.
http://dx.doi.org/10.1182/blood-2006-11-056648
PMid:17255355

- Pierce SK, Liu W. The tipping points in
the initiation of B cell signalling: how small changes make big
differences. Nat Rev Immunol. 2010;10(11):767-77.
http://dx.doi.org/10.1038/nri2853 PMid:20935671 PMCid:3406597

- Niiro H, Clark EA. Regulation of B-cell
fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945-56.
http://dx.doi.org/10.1038/nri955 PMid:12461567

- Monroe JG. ITAM-mediated tonic signalling
through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6(4):283-94.
http://dx.doi.org/10.1038/nri1808 PMid:16557260

- Kraus M, Alimzhanov MB, Rajewsky N,
Rajewsky K. Survival of resting mature B lymphocytes depends on BCR
signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787-800.
http://dx.doi.org/10.1016/j.cell.2004.05.014 PMid:15186779

- Contri A, Brunati AM, Trentin L, Cabrelle
A, Miorin M, Cesaro L, Pinna LA, Zambello R, Semenzato G, Donella-Deana
A. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine
kinase, a putative contribution to defective apoptosis. J Clin Invest.
2005;115(2):369-78. PMid:15650771 PMCid:544036

- Quiroga MP, Balakrishnan K, Kurtova AV,
Sivina M, Keating MJ, Wierda WG, Gandhi V, Burger JA. B-cell antigen
receptor signaling enhances chronic lymphocytic leukemia cell migration
and survival: specific targeting with a novel spleen tyrosine kinase
inhibitor, R406. Blood. 2009;114(5):1029-37.
http://dx.doi.org/10.1182/blood-2009-03-212837
PMid:19491390

- Buchner M, Fuchs S, Prinz G, Pfeifer D,
Bartholomé K, Burger M, Chevalier N, Vallat L, Timmer J, Gribben JG,
Jumaa H, Veelken H, Dierks C, Zirlik K. Spleen tyrosine kinase is
overexpressed and represents a potential therapeutic target in chronic
lymphocytic leukemia. Cancer Res. 2009;69(13):5424-32.
http://dx.doi.org/10.1158/0008-5472.CAN-08-4252
PMid:19549911

- Baudot AD, Jeandel PY, Mouska X, Maurer U,
Tartare-Deckert S, Raynaud SD, Cassuto JP, Ticchioni M, Deckert M. The
tyrosine kinase Syk regulates the survival of chronic lymphocytic
leukemia B cells through PKCdelta and proteasome-dependent regulation
of Mcl-1 expression. Oncogene. 2009;28(37):3261-73.
http://dx.doi.org/10.1038/onc.2009.179 PMid:19581935

- Ringshausen I, Schneller F, Bogner C, Hipp
S, Duyster J, Peschel C, Decker T. Constitutively activated
phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of
apoptosis in B-CLL: association with protein kinase Cdelta. Blood.
2002;100(10):3741-8. http://dx.doi.org/10.1182/blood-2002-02-0539
PMid:12393602

- Herman SE, Gordon AL, Wagner AJ, Heerema
NA, Zhao W, Flynn JM, Jones J, Andritsos L, Puri KD, Lannutti BJ, Giese
NA, Zhang X, Wei L, Byrd JC, Johnson AJ. Phosphatidylinositol
3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in
chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic
cellular survival signals. Blood. 2010;116(12):2078-88.
http://dx.doi.org/10.1182/blood-2010-02-271171 PMid:20522708
PMCid:2951855

- Herman SE, Gordon AL, Hertlein E,
Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ,
Hamdy A, Johnson AJ, Byrd JC. Bruton tyrosine kinase represents a
promising therapeutic target for treatment of chronic lymphocytic
leukemia and is effectively targeted by PCI-32765. Blood.
2011;117(23):6287-96.
http://dx.doi.org/10.1182/blood-2011-01-328484 PMid:21422473
PMCid:3122947

- Barragán M, Bellosillo B, Campŕs C,
Colomer D, Pons G, Gil J. Involvement of protein kinase C and
phosphatidylinositol 3-kinase pathways in the survival of B-cell
chronic lymphocytic leukemia cells. Blood. 2002;99(8):2969-76.
http://dx.doi.org/10.1182/blood.V99.8.2969
PMid:11929788

- Muzio M, Apollonio B, Scielzo C,
Frenquelli M, Vandoni I, Boussiotis V, Caligaris-Cappio F, Ghia P.
Constitutive activation of distinct BCR-signaling pathways in a subset
of CLL patients: a molecular signature of anergy. Blood.
2008;112(1):188-95.
http://dx.doi.org/10.1182/blood-2007-09-111344
PMid:18292287

- Schuh K, Avots A, Tony HP, Serfling E,
Kneitz C. Nuclear NF-ATp is a hallmark of unstimulated B cells from
B-CLL patients. Leuk Lymphoma. 1996;23(5-6):583-92.
http://dx.doi.org/10.3109/10428199609054868 PMid:9031090

- Furman RR, Asgary Z, Mascarenhas JO, Liou
HC, Schattner EJ. Modulation of NF-kappa B activity and apoptosis in
chronic lymphocytic leukemia B cells. J Immunol. 2000;164(4):2200-6.
PMid:10657675

- Hewamana S, Alghazal S, Lin TT, Clement M,
Jenkins C, Guzman ML, Jordan CT, Neelakantan S, Crooks PA, Burnett AK,
Pratt G, Fegan C, Rowntree C, Brennan P, Pepper C. The NF-kappaB
subunit Rel A is associated with in vitro survival and clinical disease
progression in chronic lymphocytic leukemia and represents a promising
therapeutic target. Blood. 2008;111(9):4681-9.
http://dx.doi.org/10.1182/blood-2007-11-125278
PMid:18227347

- Le Roy C, Deglesne PA, Chevallier N,
Beitar T, Eclache V, Quettier M, Boubaya M, Letestu R, Lévy V,
Ajchenbaum-Cymbalista F, Varin-Blank N. The degree of BCR and NFAT
activation predicts clinical outcomes in chronic lymphocytic leukemia.
Blood. 2012;120(2):356-65.
http://dx.doi.org/10.1182/blood-2011-12-397158
PMid:22613791

- Dühren-von Minden M, Übelhart R, Schneider
D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Köhler
F, Wardemann H, Zirlik K, Veelken H, Jumaa H. Chronic lymphocytic
leukaemia is driven by antigen-independent cell-autonomous signalling.
Nature. 2012;489(7415):309-12. http://dx.doi.org/10.1038/nature11309
PMid:22885698

- Carsetti L, Laurenti L, Gobessi S, Longo
PG, Leone G, Efremov DG. Phosphorylation of the activation loop
tyrosines is required for sustained Syk signaling and growth
factor-independent B-cell proliferation. Cell Signal.
2009;21(7):1187-94.
http://dx.doi.org/10.1016/j.cellsig.2009.03.007
PMid:19296913

- Vallat LD, Park Y, Li C, Gribben JG.
Temporal genetic program following B-cell receptor cross-linking:
altered balance between proliferation and death in healthy and
malignant B cells. Blood. 2007;109(9):3989-97.
http://dx.doi.org/10.1182/blood-2006-09-045377 PMid:17234734
PMCid:1874586

- Krysov S, Dias S, Paterson A, Mockridge
CI, Potter KN, Smith KA, Ashton-Key M, Stevenson FK, Packham G. Surface
IgM stimulation induces MEK1/2-dependent MYC expression in chronic
lymphocytic leukemia cells. Blood. 2012;119(1):170-9.
http://dx.doi.org/10.1182/blood-2011-07-370403
PMid:22086413

- Messmer BT, Albesiano E, Efremov DG,
Ghiotto F, Allen SL, Kolitz J, Foa R, Damle RN, Fais F, Messmer D, Rai
KR, Ferrarini M, Chiorazzi N. Multiple distinct sets of stereotyped
antigen receptors indicate a role for antigen in promoting chronic
lymphocytic leukemia. J Exp Med. 2004;200(4):519-25.
http://dx.doi.org/10.1084/jem.20040544 PMid:15314077
PMCid:2211936

- Agathangelidis A, Darzentas N,
Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol
EJ, Tresoldi C, Chu CC, Cahill N, Giudicelli V, Tichy B, Pedersen LB,
Foroni L, Bonello L, Janus A, Smedby K, Anagnostopoulos A, Merle-Beral
H, Laoutaris N, Juliusson G, di Celle PF, Pospisilova S, Jurlander J,
Geisler C, Tsaftaris A, Lefranc MP, Langerak AW, Oscier DG, Chiorazzi
N, Belessi C, Davi F, Rosenquist R, Ghia P, Stamatopoulos K.
Stereotyped B-cell receptors in one-third of chronic lymphocytic
leukemia: a molecular classification with implications for targeted
therapies. Blood. 2012;119(19):4467-75.
http://dx.doi.org/10.1182/blood-2011-11-393694
PMid:22415752

- Lanemo Myhrinder A, Hellqvist E, Sidorova
E, Söderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Bäckman E,
Söderberg O, Rosenquist R, Hörkkö S, Rosén A. A new perspective:
molecular motifs on oxidized LDL, apoptotic cells, and bacteria are
targets for chronic lymphocytic leukemia antibodies. Blood.
2008;111(7):3838-48.
http://dx.doi.org/10.1182/blood-2007-11-125450 PMid:18223168

- Chu CC, Catera R, Hatzi K, Yan XJ, Zhang
L, Wang XB, Fales HM, Allen SL, Kolitz JE, Rai KR, Chiorazzi N. Chronic
lymphocytic leukemia antibodies with a common stereotypic rearrangement
recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112(13):5122-9.
http://dx.doi.org/10.1182/blood-2008-06-162024 PMid:18812466
PMCid:2597608

- Catera R, Silverman GJ, Hatzi K, Seiler T,
Didier S, Zhang L, Hervé M, Meffre E, Oscier DG, Vlassara H, Scofield
RH, Chen Y, Allen SL, Kolitz J, Rai KR, Chu CC, Chiorazzi N. Chronic
lymphocytic leukemia cells recognize conserved epitopes associated with
apoptosis and oxidation. Mol Med. 2008;14(11-12):665-74.
http://dx.doi.org/10.2119/2008-00102.Catera PMid:19009014
PMCid:2582860

- Chu CC, Catera R, Zhang L, Didier S,
Agagnina BM, Damle RN, Kaufman MS, Kolitz JE, Allen SL, Rai KR,
Chiorazzi N. Many chronic lymphocytic leukemia antibodies recognize
apoptotic cells with exposed nonmuscle myosin heavy chain IIA:
implications for patient outcome and cell of origin. Blood.
2010;115(19):3907-15.
http://dx.doi.org/10.1182/blood-2009-09-244251 PMid:20110421
PMCid:2869555

- Binder M, Léchenne B, Ummanni R, Scharf C,
Balabanov S, Trusch M, Schlüter H, Braren I, Spillner E, Trepel M.
Stereotypical chronic lymphocytic leukemia B-cell receptors recognize
survival promoting antigens on stromal cells. PLoS One.
2010;5(12):e15992. http://dx.doi.org/10.1371/journal.pone.0015992
PMid:21209908 PMCid:3012720

- Steininger C, Widhopf GF 2nd, Ghia EM,
Morello CS, Vanura K, Sanders R, Spector D, Guiney D, Jäger U, Kipps
TJ. Recombinant antibodies encoded by IGHV1-69 react with pUL32, a
phosphoprotein of cytomegalovirus and B-cell superantigen. Blood.
2012;119(10):2293-301.
http://dx.doi.org/10.1182/blood-2011-08-374058
PMid:22234695

- Wotherspoon AC, Doglioni C, Diss TC, Pan
L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade
B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after
eradication of Helicobacter pylori. Lancet. 1993;342(8871):575-7.
http://dx.doi.org/10.1016/0140-6736(93)91409-F

- Bende RJ, Aarts WM, Riedl RG, de Jong D,
Pals ST, van Noesel CJ. Among B cell non-Hodgkin's lymphomas, MALT
lymphomas express a unique antibody repertoire with frequent rheumatoid
factor reactivity. J Exp Med. 2005;201(8):1229-41.
http://dx.doi.org/10.1084/jem.20050068 PMid:15837810
PMCid:2213160

- Ivanovski M, Silvestri F, Pozzato G, Anand
S, Mazzaro C, Burrone OR, Efremov DG. Somatic hypermutation, clonal
diversity, and preferential expression of the VH 51p1/VL kv325
immunoglobulin gene combination in hepatitis C virus-associated
immunocytomas. Blood. 1998;91(7):2433-42. PMid:9516143

- Hermine O, Lefrčre F, Bronowicki JP,
Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub
P, Brechot C, Varet B, Troussard X. Regression of splenic lymphoma with
villous lymphocytes after treatment of hepatitis C virus infection. N
Engl J Med. 2002;347(2):89-94.
http://dx.doi.org/10.1056/NEJMoa013376 PMid:12110736

- Efremov DG, Bomben B, Gobessi S, Valter
Gattei V. TLR9 signaling defines distinct prognostic subsets in CLL.
Front Biosci. 2013;18:371-386

- Hivroz C, Gény B, Brouet JC,
Grillot-Courvalin C. Altered signal transduction secondary to surface
IgM cross-linking on B-chronic lymphocytic leukemia cells. Differential
activation of the phosphatidylinositol-specific phospholipase C. J
Immunol. 1990;144(6):2351-8. PMid:2107258

- Lankester AC, van Schijndel GM, van der
Schoot CE, van Oers MH, van Noesel CJ, van Lier RA. Antigen receptor
nonresponsiveness in chronic lymphocytic leukemia B cells. Blood.
1995;86(3):1090-7. PMid:7620163

- Semichon M, Merle-Béral H, Lang V, Bismuth
G. Normal Syk protein level but abnormal tyrosine phosphorylation in
B-CLL cells. Leukemia. 1997;11(11):1921-8.
http://dx.doi.org/10.1038/sj.leu.2400832 PMid:9369427

- Chen L, Widhopf G, Huynh L, Rassenti L,
Rai KR, Weiss A, Kipps TJ. Expression of ZAP-70 is associated with
increased B-cell receptor signaling in chronic lymphocytic leukemia.
Blood. 2002;100(13):4609-14.
http://dx.doi.org/10.1182/blood-2002-06-1683 PMid:12393534

- Lanham S, Hamblin T, Oscier D, Ibbotson R,
Stevenson F, Packham G. Differential signaling via surface IgM is
associated with VH gene mutational status and CD38 expression in
chronic lymphocytic leukemia. Blood. 2003;101(3):1087-93.
http://dx.doi.org/10.1182/blood-2002-06-1822 PMid:12393552

- Damle RN, Temburni S, Banapour T, Paul S,
Mongini PK, Allen SL, Kolitz JE, Rai KR, Chiorazzi N. T-cell
independent, B-cell receptor-mediated induction of telomerase activity
differs among IGHV mutation-based subgroups of chronic lymphocytic
leukemia patients. Blood. 2012; doi: 10.1182/blood-2012-02-409110
http://dx.doi.org/10.1182/blood-2012-02-409110

- Majolini MB, D'Elios MM, Galieni P,
Boncristiano M, Lauria F, Del Prete G, Telford JL, Baldari CT.
Expression of the T-cell-specific tyrosine kinase Lck in normal B-1
cells and in chronic lymphocytic leukemia B cells. Blood.
1998;91(9):3390-6. PMid:9558397

- Abrams ST, Lakum T, Lin K, Jones GM,
Treweeke AT, Farahani M, Hughes M, Zuzel M, Slupsky JR. B-cell receptor
signaling in chronic lymphocytic leukemia cells is regulated by
overexpressed active protein kinase CbetaII. Blood.
2007;109(3):1193-201.
http://dx.doi.org/10.1182/blood-2006-03-012021
PMid:17003377

- Herling M, Patel KA, Weit N, Lilienthal N,
Hallek M, Keating MJ, Jones D. High TCL1 levels are a marker of B-cell
receptor pathway responsiveness and adverse outcome in chronic
lymphocytic leukemia. Blood. 2009;114(21):4675-86.
http://dx.doi.org/10.1182/blood-2009-03-208256 PMid:19770358
PMCid:2780304

- Negro R, Gobessi S, Longo PG, He Y, Zhang
ZY, Laurenti L, Efremov DG. Overexpression of the
autoimmunity-associated phosphatase PTPN22 promotes survival of
antigen-stimulated CLL cells by selectively activating AKT. Blood.
2012;119(26):6278-87.
http://dx.doi.org/10.1182/blood-2012-01-403162
PMid:22569400

- Capitani N, Lucherini OM, Sozzi E, Ferro
M, Giommoni N, Finetti F, De Falco G, Cencini E, Raspadori D, Pelicci
PG, Lauria F, Forconi F, Baldari CT. Impaired expression of p66Shc, a
novel regulator of B-cell survival, in chronic lymphocytic leukemia.
Blood. 2010;115(18):3726-36.
http://dx.doi.org/10.1182/blood-2009-08-239244
PMid:20061561

- Suljagic M, Laurenti L, Tarnani M, Alam M,
Malek SN, Efremov DG. Reduced expression of the tumor suppressor PHLPP1
enhances the antiapoptotic B-cell receptor signal in chronic
lymphocytic leukemia B-cells. Leukemia. 2010;24(12):2063-71.
http://dx.doi.org/10.1038/leu.2010.201 PMid:20861921

- Longo PG, Laurenti L, Gobessi S, Sica S,
Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in
mediating antiapoptotic signals downstream of the B-cell receptor in
chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846-55.
http://dx.doi.org/10.1182/blood-2007-05-089037
PMid:17928528

- Paterson A, Mockridge CI, Adams JE, Krysov
S, Potter KN, Duncombe AS, Cook SJ, Stevenson FK, Packham G. Mechanisms
and clinical significance of BIM phosphorylation in chronic lymphocytic
leukemia. Blood. 2012;119(7):1726-36.
http://dx.doi.org/10.1182/blood-2011-07-367417
PMid:22160382

- Veldurthy A, Patz M, Hagist S, Pallasch
CP, Wendtner CM, Hallek M, Krause G. The kinase inhibitor dasatinib
induces apoptosis in chronic lymphocytic leukemia cells in vitro with
preference for a subgroup of patients with unmutated IgVH genes. Blood.
2008;112(4):1443-52.
http://dx.doi.org/10.1182/blood-2007-11-123984
PMid:18550857

- Song Z, Lu P, Furman RR, Leonard JP,
Martin P, Tyrell L, Lee FY, Knowles DM, Coleman M, Wang YL. Activities
of SYK and PLCgamma2 predict apoptotic response of CLL cells to SRC
tyrosine kinase inhibitor dasatinib. Clin Cancer Res.
2010;16(2):587-99.
http://dx.doi.org/10.1158/1078-0432.CCR-09-1519
PMid:20068106

- Hantschel O, Rix U, Schmidt U,
Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL,
Ellmeier W, Valent P, Superti-Furga G. The Btk tyrosine kinase is a
major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S
A. 2007;104(33):13283-8. http://dx.doi.org/10.1073/pnas.0702654104
PMid:17684099 PMCid:1940229

- Hallaert DY, Jaspers A, van Noesel CJ, van
Oers MH, Kater AP, Eldering E. c-Abl kinase inhibitors overcome
CD40-mediated drug resistance in CLL: implications for therapeutic
targeting of chemoresistant niches. Blood. 2008;112(13):5141-9.
http://dx.doi.org/10.1182/blood-2008-03-146704
PMid:18796631

- Allen JC, Talab F, Zuzel M, Lin K, Slupsky
JR. c-Abl regulates Mcl-1 gene expression in chronic lymphocytic
leukemia cells. Blood. 2011;117(8):2414-22.
http://dx.doi.org/10.1182/blood-2010-08-301176
PMid:21220745

- Trentin L, Frasson M, Donella-Deana A,
Frezzato F, Pagano MA, Tibaldi E, Gattazzo C, Zambello R, Semenzato G,
Brunati AM. Geldanamycin-induced Lyn dissociation from aberrant
Hsp90-stabilized cytosolic complex is an early event in apoptotic
mechanisms in B-chronic lymphocytic leukemia. Blood.
2008;112(12):4665-74.
http://dx.doi.org/10.1182/blood-2008-02-139139 PMid:18768392

- Amrein PC, Attar EC, Takvorian T, Hochberg
EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand P,
Hasserjian RP, Werner L, Neuberg D, Brown JR. Phase II study of
dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin
Cancer Res. 2011;17(9):2977-86.
http://dx.doi.org/10.1158/1078-0432.CCR-10-2879 PMid:21402714
PMCid:3108904

- Mócsai A, Ruland J, Tybulewicz VL. The SYK
tyrosine kinase: a crucial player in diverse biological functions. Nat
Rev Immunol. 2010;10(6):387-402.
http://dx.doi.org/10.1038/nri2765 PMid:20467426

- Buchner M, Baer C, Prinz G, Dierks C,
Burger M, Zenz T, Stilgenbauer S, Jumaa H, Veelken H, Zirlik K. Spleen
tyrosine kinase inhibition prevents chemokine- and integrin-mediated
stromal protective effects in chronic lymphocytic leukemia. Blood.
2010;115(22):4497-506.
http://dx.doi.org/10.1182/blood-2009-07-233692
PMid:20335218

- Suljagic M, Longo PG, Bennardo S, Perlas
E, Leone G, Laurenti L, Efremov DG. The Syk inhibitor fostamatinib
disodium (R788) inhibits tumor growth in the Eμ- TCL1 transgenic mouse
model of CLL by blocking antigen-dependent B-cell receptor signaling.
Blood. 2010;116(23):4894-905.
http://dx.doi.org/10.1182/blood-2010-03-275180
PMid:20716772

- Braselmann S, Taylor V, Zhao H, Wang S,
Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, Wong BR, Lovell
S, Sun T, Park G, Argade A, Jurcevic S, Pine P, Singh R, Grossbard EB,
Payan DG, Masuda ES. R406, an orally available spleen tyrosine kinase
inhibitor blocks fc receptor signaling and reduces immune
complex-mediated inflammation. J Pharmacol Exp Ther.
2006;319(3):998-1008. http://dx.doi.org/10.1124/jpet.106.109058
PMid:16946104

- Gobessi S, Laurenti L, Longo P, Carsetti L, Sica S, Leone G, Efremov DG. Constitutive Activation of the Protein Tyrosine Kinase Syk in Chronic Lymphocytic Leukemia B-Cells. Blood (ASH Annual Meeting Abstracts), Nov 2007; 110: 1123.
- Singh R, Masuda ES, Payan DG. Discovery
and development of spleen tyrosine kinase (SYK) inhibitors. J Med Chem.
2012;55(8):3614-43. http://dx.doi.org/10.1021/jm201271b
PMid:22257213

- Weinblatt ME, Kavanaugh A, Burgos-Vargas
R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, Murphy FT,
Musser TK, Straniero N, Vicente-Gonzales AV, Grossbard E. Treatment of
rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week,
randomized, placebo-controlled trial. Arthritis Rheum.
2008;58(11):3309-18. http://dx.doi.org/10.1002/art.23992
PMid:18975322

- Weinblatt ME, Kavanaugh A, Genovese MC,
Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase
(Syk) inhibitor for rheumatoid arthritis. N Engl J Med.
2010;363(14):1303-12. http://dx.doi.org/10.1056/NEJMoa1000500
PMid:20879879

- Friedberg JW, Sharman J, Sweetenham J,
Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R,
Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA.
Inhibition of Syk with fostamatinib disodium has significant clinical
activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia.
Blood. 2010;115(13):2578-85.
http://dx.doi.org/10.1182/blood-2009-08-236471 PMid:19965662
PMCid:2852362

- Cheng S, Coffey G, Zhang XH, Shaknovich R,
Song Z, Lu P, Pandey A, Melnick AM, Sinha U, Wang YL. SYK inhibition
and response prediction in diffuse large B-cell lymphoma. Blood.
2011;118(24):6342-52.
http://dx.doi.org/10.1182/blood-2011-02-333773
PMid:22025527

- Hoellenriegel J, Coffey GP, Sinha U,
Pandey A, Sivina M, Ferrajoli A, Ravandi F, Wierda WG, O'Brien S,
Keating MJ, Burger JA. Selective, novel spleen tyrosine kinase (Syk)
inhibitors suppress chronic lymphocytic leukemia B-cell activation and
migration. Leukemia. 2012;26(7):1576-83.
http://dx.doi.org/10.1038/leu.2012.24 PMid:22362000

- So L, Fruman DA. PI3K signalling in B- and
T-lymphocytes: new developments and therapeutic advances. Biochem J.
2012;442(3):465-81. http://dx.doi.org/10.1042/BJ20112092
PMid:22364281

- Longo PG, Laurenti L, Gobessi S,
Petlickovski A, Pelosi M, Chiusolo P, Sica S, Leone G, Efremov DG. The
Akt signaling pathway determines the different proliferative capacity
of chronic lymphocytic leukemia B-cells from patients with progressive
and stable disease. Leukemia. 2007;21(1):110-20.
http://dx.doi.org/10.1038/sj.leu.2404417 PMid:17024114

- Lannutti BJ, Meadows SA, Herman SE,
Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM,
Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA. CAL-101, a
p110delta selective phosphatidylinositol-3-kinase inhibitor for the
treatment of B-cell malignancies, inhibits PI3K signaling and cellular
viability. Blood. 2011;117(2):591-4.
http://dx.doi.org/10.1182/blood-2010-03-275305
PMid:20959606

- Hoellenriegel J, Meadows SA, Sivina M,
Wierda WG, Kantarjian H, Keating MJ, Giese N, O'Brien S, Yu A, Miller
LL, Lannutti BJ, Burger JA. The phosphoinositide 3'-kinase delta
inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine
networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603-12.
http://dx.doi.org/10.1182/blood-2011-05-352492
PMid:21803855

- Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Wagner-Johnston ND, Flinn IW, Kahl BS, Spurgeon SE, Lannutti B, Giese NA, Webb HK, Ulrich RG, Peterman S, Holes LM, Yuet AS. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, Demonstrates Clinical Activity and Pharmacodynamic Effects In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Blood (ASH Annual Meeting Abstracts) 2010; 116:55
- Coutre SE, Byrd JC, Furman RR, et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110d, in patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2011;29(suppl):abstr 6631.
- Sharman J, de Vos S, Leonard JP, Furman RR, Coutre SE, Flinn IW, Schreeder MT, Barrientos JC, Wagner-Johnston ND, Boyd T, Fowler NH, Holes L, Lannutti B, Johnson D, Jahn TM, Miller LL. A Phase 1 Study of the Selective Phosphatidylinositol 3-Kinase-Delta (PI3K) Inhibitor, CAL-101 (GS-1101), in Combination with Rituximab and/or Bendamustine in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). Blood (ASH Annual Meeting Abstracts), 2011; 118:1787.
- Decker T, Hipp S, Ringshausen I, Bogner C,
Oelsner M, Schneller F, Peschel C. Rapamycin-induced G1 arrest in
cycling B-CLL cells is associated with reduced expression of cyclin D3,
cyclin E, cyclin A, and survivin. Blood. 2003;101(1):278-85.
http://dx.doi.org/10.1182/blood-2002-01-0189 PMid:12393642

- Granziero L, Ghia P, Circosta P, Gottardi
D, Strola G, Geuna M, Montagna L, Piccoli P, Chilosi M,
Caligaris-Cappio F. Survivin is expressed on CD40 stimulation and
interfaces proliferation and apoptosis in B-cell chronic lymphocytic
leukemia. Blood. 2001;97(9):2777-83.
http://dx.doi.org/10.1182/blood.V97.9.2777 PMid:11313271

- Zanesi N, Aqeilan R, Drusco A, Kaou M,
Sevignani C, Costinean S, Bortesi L, La Rocca G, Koldovsky P, Volinia
S, Mancini R, Calin G, Scott CP, Pekarsky Y, Croce CM. Effect of
rapamycin on mouse chronic lymphocytic leukemia and the development of
nonhematopoietic malignancies in Emu-TCL1 transgenic mice. Cancer Res.
2006;66(2):915-20.
http://dx.doi.org/10.1158/0008-5472.CAN-05-3426
PMid:16424025

- Decker T, Sandherr M, Goetze K, Oelsner M,
Ringshausen I, Peschel C. A pilot trial of the mTOR (mammalian target
of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann
Hematol. 2009;88(3):221-7.
http://dx.doi.org/10.1007/s00277-008-0582-9 PMid:18704419

- Zent CS, LaPlant BR, Johnston PB, Call TG,
Habermann TM, Micallef IN, Witzig TE. The treatment of
recurrent/refractory chronic lymphocytic leukemia/small lymphocytic
lymphoma (CLL) with everolimus results in clinical responses and
mobilization of CLL cells into the circulation. Cancer.
2010;116(9):2201-7. PMid:20166206 PMCid:2861142

- Davis RE, Ngo VN, Lenz G, Tolar P, Young
RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer
AL, Wright G, Xiao W, Powell J, Jiang JK, Thomas CJ, Rosenwald A, Ott
G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Johnson NA, Rimsza LM,
Campo E, Jaffe ES, Wilson WH, Delabie J, Smeland EB, Fisher RI, Braziel
RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pierce SK, Staudt LM.
Chronic active B-cell-receptor signalling in diffuse large B-cell
lymphoma. Nature. 2010;463(7277):88-92.
http://dx.doi.org/10.1038/nature08638 PMid:20054396 PMCid:2845535

- Doyle SL, Jefferies CA, Feighery C,
O'Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton's
tyrosine kinase. J Biol Chem. 2007;282(51):36953-60.
http://dx.doi.org/10.1074/jbc.M707682200 PMid:17932028

- Ponader S, Chen SS, Buggy JJ, Balakrishnan
K, Gandhi V, Wierda WG, Keating MJ, O'Brien S, Chiorazzi N, Burger JA.
The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic
lymphocytic leukemia cell survival and tissue homing in vitro and in
vivo. Blood. 2012;119(5):1182-9. http://dx.doi.org/10.1182/blood-2011-10-386417
PMid:22180443

- de Rooij MF, Kuil A, Geest CR, Eldering E,
Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK
inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled
adhesion and migration in chronic lymphocytic leukemia. Blood.
2012;119(11):2590-4.
http://dx.doi.org/10.1182/blood-2011-11-390989
PMid:22279054

- Pan Z, Scheerens H, Li SJ, Schultz BE,
Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus
PG, Jeffery DA, Spoerke JM, Honigberg LA, Young PR, Dalrymple SA,
Palmer JT. Discovery of selective irreversible inhibitors for Bruton's
tyrosine kinase. ChemMedChem. 2007;2(1):58-61.
http://dx.doi.org/10.1002/cmdc.200600221 PMid:17154430

- Fowler N, Sharman JP, Smith SM, Boyd T, Grant B, Kolibaba KS, Furman RR, Buggy J, Loury D, Hamdy A, Advani R. The Btk Inhibitor, PCI-32765, Induces Durable Responses with Minimal Toxicity In Patients with Relapsed/Refractory B-Cell Malignancies: Results From a Phase I Study. Blood (ASH Annual Meeting Abstracts), Nov 2010; 116: 964.
- O'Brien S, Burger JA, Blum KA, Furman RR, Coutre SE, Sharman J, Flinn IW, Grant B, Heerema NA, Johnson AJ, Navarro T, Holmgren E, Hedrick E, Byrd JC. The Bruton's Tyrosine Kinase (BTK) Inhibitor PCI-32765 Induces Durable Responses in Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Follow-up of a Phase Ib/II Study. Blood (ASH Annual Meeting Abstracts), Nov 2011; 118: 983
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Sharman JP, Flinn IW, Grant BW, Heerema NA, Johnson AJ, Navarro T, James DF, Hedrick E, O'Brien SM. The Bruton's tyrosine kinase (BTK) inhibitor PCI-32765 (P) in treatment-naive (TN) chronic lymphocytic leukemia (CLL) patients (pts): Interim results of a phase Ib/II study. J Clin Oncol 30, 2012 (suppl; abstr 6507)
- O'Brien SM, Barrientos JC, Flinn IW, Barr PM, Burger JA, Navarro T, James DF, Hedrick E, Friedberg JW, Brown JR. Combination of the Bruton's tyrosine kinase (BTK) inhibitor PCI-32765 with bendamustine (B)/rituximab (R) (BR) in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): Interim results of a phase Ib/II study. J Clin Oncol 30, 2012 (suppl; abstr 6515^)
- Jaglowski SM, Jones JA, Flynn JM, Andritsos LA, Maddocks KJ, Blum KA, Grever MR, Geyer SM, Woyach JA, Johnson AJ, Heerema NA, Molnar E, Stefanos M, Devlin S, Navarro T, James DF, Lowe AM, Hedrick E, Byrd JC. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. J Clin Oncol 30, 2012 (suppl; abstr 6508)
- Brown JR, Sharman JP, Harb WA, Kelly KR, Schreeder MS, Sweetenham JW, Barr PM, Foran JM, Gabrilove JL, Kipps TJ, Ma S, O'Brien SM, Evans E, Lounsbury H, Silver BA, Singh J, Stiede K, Westlin W, Witowski S, Mahadevan D. Phase Ib trial of AVL-292, a covalent inhibitor of Bruton's tyrosine kinase (Btk), in chronic lymphocytic leukemia (CLL) and B-non-Hodgkin lymphoma (B-NHL). J Clin Oncol 30, 2012 (suppl; abstr 8032) http://dx.doi.org/10.1158/1538-7445.AM2012-1745
- Vang T, Miletic AV, Arimura Y, Tautz L,
Rickert RC, Mustelin T. Protein tyrosine phosphatases in autoimmunity.
Annu Rev Immunol. 2008;26:29-55.
http://dx.doi.org/10.1146/annurev.immunol.26.021607.090418
PMid:18303998

- Rhee I, Veillette A. Protein tyrosine
phosphatases in lymphocyte activation and autoimmunity. Nat Immunol.
2012;13(5):439-47. http://dx.doi.org/10.1038/ni.2246
PMid:22513334

- Vang T, Liu WH, Delacroix L, Wu S, Vasile
S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J, Tasken K,
Tremblay ML, Lie BA, Page R, Mustelin T, Rahmouni S, Rickert RC, Tautz
L. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem
Biol. 2012;8(5):437-46. http://dx.doi.org/10.1038/nchembio.916
PMid:22426112

- Barr AJ. Protein tyrosine phosphatases as
drug targets: strategies and challenges of inhibitor development.
Future Med Chem. 2010;2(10):1563-76.
http://dx.doi.org/10.4155/fmc.10.241 PMid:21426149

- Stanford SM, Krishnamurthy D, Falk MD,
Messina R, Debnath B, Li S, Liu T, Kazemi R, Dahl R, He Y, Yu X, Chan
AC, Zhang ZY, Barrios AM, Woods VL Jr, Neamati N, Bottini N. Discovery
of a novel series of inhibitors of lymphoid tyrosine phosphatase with
activity in human T cells. J Med Chem. 2011;54(6):1640-54.
http://dx.doi.org/10.1021/jm101202j PMid:21341673 PMCid:3086468

- He Y, Zeng LF, Yu ZH, He R, Liu S, Zhang
ZY. Bicyclic benzofuran and indole-based salicylic acids as protein
tyrosine phosphatase inhibitors. Bioorg Med Chem. 2012;20(6):1940-6.
http://dx.doi.org/10.1016/j.bmc.2011.11.004 PMid:22133902

- Aiello LP, Vignati L, Sheetz MJ, Zhi X,
Girach A, Davis MD, Wolka AM, Shahri N, Milton RC; PKC-DRS and PKC-DRS2
Study Groups. Oral protein kinase c β inhibition using ruboxistaurin:
efficacy, safety, and causes of vision loss among 813 patients (1,392
eyes) with diabetic retinopathy in the Protein Kinase C β
Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C β
Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31(10):2084-94.
http://dx.doi.org/10.1097/IAE.0b013e3182111669
PMid:21862954

- Friman S, Arns W, Nashan B, Vincenti F,
Banas B, Budde K, Cibrik D, Chan L, Klempnauer J, Mulgaonkar S,
Nicholson M, Wahlberg J, Wissing KM, Abrams K, Witte S, Woodle ES.
Sotrastaurin, a novel small molecule inhibiting protein-kinase C:
randomized phase II study in renal transplant recipients. Am J
Transplant. 2011;11(7):1444-55.
http://dx.doi.org/10.1111/j.1600-6143.2011.03538.x PMid:21564523
