Update on the Role of Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma
P. Tosi,1 M. Imola,1 A. M. Mianulli,1 S. Tomassetti,1 A. Merli,1 A. Molinari,1 S. Mangianti,1 M. Ratta,1 A. Isidori2 and G. Visani2
1 Hematology Unit, Department of Oncology and Hematology, Infermi Hospital Rimini Italy
2 Hematology and Transplant Center AORMN Marche Nord Pesaro Italy
2 Hematology and Transplant Center AORMN Marche Nord Pesaro Italy
Correspondence
to:
Patrizia Tosi, MD. UO Ematologia, Dipartimento di Oncologia ed
Ematologia, Ospedale Infermi, Viale Settembrini, 2, 47100 – Rimini.
Italy.
Published: November 5, 2012
Received: September 4 , 2012
Accepted: September 26, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012069, DOI 10.4084/MJHID.2012.069
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
In poor countries,
tragically, people die unnecessarily. Having changed our understanding
about issues related to poverty, even in the fight against malaria we
must keep in mind a number of issues other than simple lack of economic
resources. In this article we tried to discuss the various aspects that
make malaria a disease closely related to poverty and the effects of
malaria on the same poverty of patients who are affected. If you want
the program to "Rool Back Malaria" to succeed, you must program
interventions that improve the living conditions of populations in
endemic area, individually and as communities. As has become clear that
the discovery of an effective vaccine will not eradicate the disease,
remains a fundamental understanding of mechanisms related to poverty
that cause Malaria remains one of the major killers in the world, to
help communities affected and individuals to prevent, cure properly and
not being afraid of this ancient disease.
Introduction and Hystorical Background
Multiple myeloma (MM) is a clonal B cell disorder characterized by proliferation and accumulation of B lymphocytes and plasma cells in the bone marrow and, more rarely, at extramedullary sites. Its annual incidence is 6/100000 in western countries, thus representing the second most common hematological malignancy after non Hodgkin lymphomas.[1]
For many years the combination of melphalan and prednisone (MP), that was developed in the early sixties by Bergsagel et al,[2] has been considered the gold standard treatment for MM, as different polychemotherapy regimens failed to demonstrate a better efficacy.[3] MP was able to induce a response in over 40% of treated patients; complete responses, however, were achieved in less than 5% of the cases, and overall patients survival did not exceeded 3 years. The first step towards introduction of autologous stem cell transplantation in MM was represented by in vitro studies showing a a dose-response effect of melphalan in MM cells.[4] The potential to overcome resistance to melphalan by using higher doses of the drug was subsequently explored in vivo;[5] 27% previously untreated patients reached a complete response (CR), and this translated into a prolonged survival, even though treatment related mortality was unacceptably high. In order to reduce the duration of profound cytopenia related to the use of high dose melphalan (HDM), autologous stem cell rescue was then introduced in the clinical practice, initially for relapsed/refractory disease, then for newly diagnosed MM.[6,7] The formal demonstration that autologous stem cell transplantation (ASCT) is superior to conventional chemotherapy in terms of response, duration of response and survival, came from two randomized trials, the first one from the Intergroup Francophone du Myeloma (IFM)[8] and the second one from the Medical Research Council (MRC).[9] In order to ameliorate these results, the application of two subsequent ASCTs was then explored by IFM[10] and by the Bologna group;[11] both studies demonstrated an improvement in response rate and event-free survival (EFS); however only the French study was able to show a survival advantage for patients receiving a double ASCT. Further analysis of the IFM trial showed that a second ASCT could result into an increased OS only in patients failing to achieve at least a very good partial response (VGPR)10 after the first ASCT, these data were in agreement with a subanalysis of the Bologna trial showing an improved event-free survival (EFS) after a second ASCT in patients failing to achieve at least a near-CR after the first one.[11]
While the use of a double ASCT is still matter of debate, from late nineties on, a single ASCT has been referred as the standard of care for newly diagnosed MM patients aged <60-65 years with no relevant comorbidities, this in accord with the upper age limit that has been considered appropriate for patients with other kinds of hematological malignancies, even though interesting results were obtained also in older patient populations.[12]
The Role of CR
When MP was the only available therapeutic strategy for MM, the attainment of CR was no matter of concern as only a minority of patients could achieve a minimal residual disease status. The introducion of more aggressive therapeutic programs including ASCT, prompted a better evaluation of minimal residual disease, including also cytofluorimetric[13] and molecular techniques.[14] At present, the International Myeloma Working Group (IMWG) has provided the definition of "stringent CR" including negative serum/urine immunofixation together with a normal serum free-light chain ratio and absence of clonal plasma cells in the bone marrow.[15] Several groups have analyzed the relationship beween CR and patients outcome, and have pointed out that CR is a strong predictor of survival,[16] expecially when extended over several years;[17] for this reason it is now generally recognized that every effort should be made in order to achieve maximal disease eradication through the various phases of the treatment program.[18]
Incorporation of Novel Drugs in Induction Phase
In addition to the clinical benefit offered by ASCT, in recent years the therapeutic results for MM have significantly improved due to the availability of drugs that are active both on neoplastic plasma cells and on bone marrow microenvironment, such as thalidomide, lenalidomide and bortezomib. After testing in patients with advanced, relapsed/refractory disease, these compounds were evaluated in clinical trials in the framework of induction therapy prior to ASCT in newly diagnosed patients in order to increase the depth of response thus improving patients outcome. Thalidomide was the first agent included in induction therapy for newly diagnosed MM patients eligible for ASCT; the drug was used in combination to high-dose dexamethasone (TD) (Table 1) yielding interesting results as compared to conventional chemotherapy in a case-match retrospective analysis[19] or to high-dose dexamethasone in a prospective randomized trial.[20] In a further randomized trial (Total Therapy 2) thalidomide was continuously applied in the various phases of the whole treatment program until patient relapse,[21] again an advantage in terms of CR rate and EFS was observed in patients treated with thalidomide as compared to those not receiving the drug, but OS was similar in the two groups of patients. Subsequent trials were designed aiming at evaluating the combination of TD with doxorubicin;[22] a significant improvement in response rate was observed as compared to conventional chemotherapy (VAD) (Table 1). Bortezomib was tested in combination to dexamethasone (VD) in a phase II study;[23] a VGPR rate of over 30% was achieved after induction and upgraded to over 50% after ASCT (Table 2). A further phase II study was designed aiming at comparing VD to conventional vincristin-doxorubicin-dexmethasone (VAD);[24] again the arm treated with the novel regimen showed a significantly higher response rate (38% VGPR or better vs 15%) that was confirmed after ASCT. The combination of VD with cyclophosphamide (VCD) was able to induce a VGPR or better in over 60% of the patients,[25] similar results were reported using VD+ doxorubicin (PAD).[26] Lenalidomide was studied in a randomized trial in combination to high (RD) vs low (Rd) doses dexamethasone,[27] after 4 courses patients were allowed to undergo ASCT or to proceed with the same therapy; even though response rate was significantly higher in the RD group, survival was the same due to the higher toxicity experienced by the RD group.
A further improvement in the results obtained with novel drugs±steroids±chemotherapy was achieved combining two novel drugs with dexamethasone (Table 2). The combination bortezomib-thalidomide and dexamethasone (VTD) was randomly compared to thalidomide-dexamethasone (TD) as induction therapy prior to ASCT, yielding a significant advantage in terms of response, both CR and VGPR.[28] These data were confirmed by a recent study of the Pethema group.[29] A bortezomib+thalidomide-containing regimen was also used in Total Therapy 3 trial,[30] in the context of a polychemotherapy program involving induction, ASCT, consolidation and maintenance; as compared to Total Therapy 2, in which only TD was used,[21] a significant prolongation of EFS was observed. These results so far indicate that induction therapy in preparation to ASCT should include bortezomib+dexamethasone+an immunomodulating agent, either thalidomide or lenalidomide, that is presently being explored in phase II trials.[31]
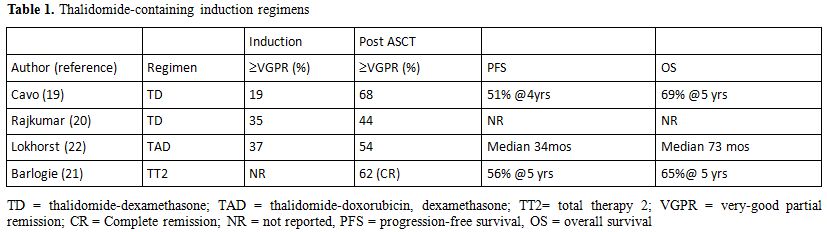
Table 1. Thalidomide-containing induction regimens.
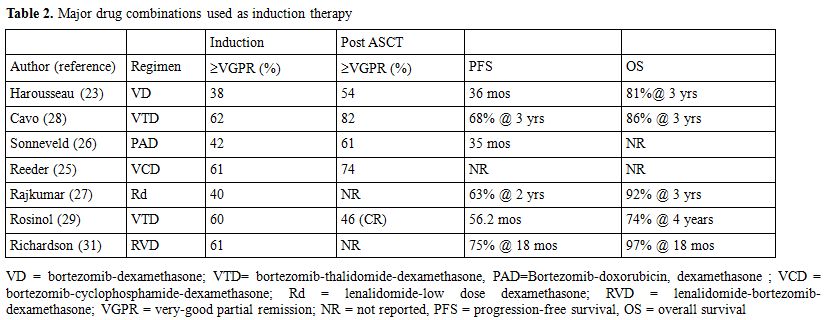
Table 2. Major drug combinations used as induction therapy.
Controversial Issues
Consolidation, Maintenance or Both? The administration of some kind of treatment upon completion of major therapy in order to improve/maintain its efficacy represents the standard of care in several lymphoproliferative neoplasms such as acute lymphoblastic leukemia, low grade lymphoma or mantle cell lymphoma, and for this reason it has been considered an attractive option also for MM. Several groups have addressed the issue of post transplantation treatment, and interesting results have been reported; at present, however, no data can definitely support a treatment over another, and no drug has been formally approved for the therapy of MM at this disease stage.
Consolidation therapy is defined as a short course of treatment administered after ASCT aiming at further reduce tumor load (Table 3). A study from the nordic group[32] has evaluated the efficacy of a short course of Bortezomib, and an increased percentage of CRs was observed. Two different studies analyzed the effects of a short course of Bortezomib-thalidomide-dexamethasone (VTD) administered as consolidation after ASCT, both trials showed that a molecular response can be achieved in up to 60% of the patients.[33-35] Maintenance therapy is defined as long-term treatment aiming at preventing disease recurrence or progression. Alpha interferon has been widely tested after ASCT and despite two reports showing an improved survival, side effects greatly overcome the possible advantage, so that this approach has been definitely abandoned.[36] A limited efficacy was also reported with long term use of steroids.[37] Thalidomide has been studied in six trials[21-22,38-41] (Table 4), in 3 of which the drug was used also in induction phase. Although all the trials showed an advantage in terms of EFS or PFS; an OS advantage for patients treated with thalidomide was observed only in 2 trials. A major concern regarding the use of this drug as maintenance therapy is the high percentage of patients dropping out due to long term side effects, specifically peripheral neuropathy.[38-41] Furthermore, the likelihood of selecting MM clones resistant to thalidomide and responsible for short post-relapse survival should probably be taken into consideration[21-22,41] as well as the limited efficacy of the drugs in case of poor-risk cytogenetic.[41] Due to its favorable toxicity profile, and specifically to the lack of long-term neurological toxicity, lenalidomide has been tested as maintenance therapy in two randomized studies,[42-43] both of them showed a significant advance in TTP, while OS was significantly improved only in one study.[43] Side effects were mainly hematological, a higher percentage of second primary malignancies were observed in Lenalidomide-treated patients,[42,43] however this data need further observation as it is clear that survival benefit outgrows the risk of death from second malignancies.[44] A recent report analyzed the role of bortezomib maintenance after ASCT;[26] patients showed a significant advantage in terms of PFS and OS, even though the potential of neurological toxicity should be taken into consideration.
Despite these interesting results, however, data are not mature to recommend a specific strategy, and the issue of consolidation and/or maintenance treatment remains still debated.
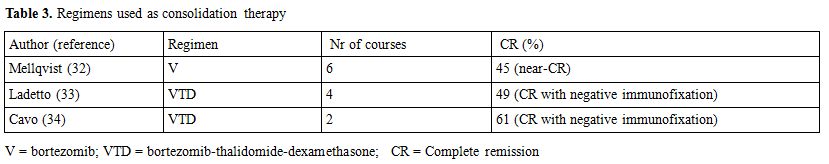
Table 3. Regimens used as consolidation therapy.
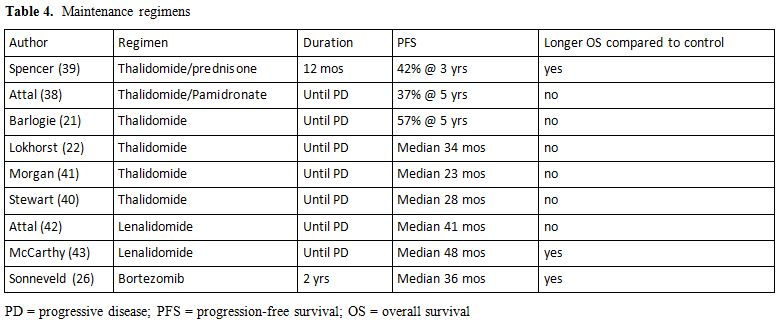
Table 4. Maintenance regimens.
Upfront or Salvage ASCT? Early studies on ASCT in MM were performed in patients with relapsed/refractory disease but, due to the poor result that were obtained,[45] the procedure is now preferentially employed in newly diagnosed patients.[46] Furthermore, a timely-dependant application of ASCT seems to be crucial in determining an optimal response.[47] A randomized study from the French group,[48] however, demonstrated a comparable outcome in terms of survival in patients undergoing early vs deferred ASCT (64.4 vs 64 months OS). These data were obtained when only chemotherapeutic agents were available; it is now evident that new drugs, when applied during induction, are able to determine a deeper response than that obtained with conventional chemotherapy combinations. Several groups have thus designed studies aimed at evaluating efficacy of long term treatment with new drugs as compared to ASCT,[49,50] applying transplant only upon relapse. Results that have been published so far failed to show a difference in patients survival even though early ASCT is related to a shorter duration of treatment and drug exposure. A recent retrospective study has shown that, in patients treated with thalidomide or lenalidomide followed by early stem cell mobilization,[51] comparable results were achieved after early vs late ASCT. Data from further studies are awaited
Is ASCT Feasible in Elderly Patients? Patients aged > 65 years are not considered good candidates to ASCT as their survival is significantly shorter than that observed in younger patients (50% vs 68% at 5 years,[52]). Several reports, however, have identified a “grey zone” represented by patients aged 65-70 in good clinical conditions, that could potentially take advantage from this procedure. In particular, a randomized study conducted in these patients has demonstrated that intermediate dose melphalan (10mg/sqm) with PBSC support results into a significantly prolonged event-free and overall survival as compared to melphalan-prednisone.[53] On the other hand, a later study conducted in older patients (65-75 years) failed to show an advantage of intermediate dose melphalan as compared to MP, and both regimens were inferior to the combination melphalan-prednisone and thalidomide.[54] At present, however, no data can unequivocably establish whether an ASCT program including new drugs can be useful in older patients as it happens in younger ones. At present only one phase II study has been reported, aimed at evaluating the toxicity and the efficacy of bortezomib and lenalidomide included in pre-transplant induction and post transplant consolidation and maintenance in patients aged 65-75 years.[55] The percentage of patients obtaining a CR increased progressively through the various phases of the treatment program (13% after induction, 43% after transplant and 73% during consolidation/maintenance) and hematological and non-hematological toxicities were acceptable. These data indicate that, in selected elderly patients, an ASCT program including new drugs can be safely performed, thus representing a possible therapeutic option.
Is ASCT the Best Treatment for High-Risk Patients? In recent years, many attempts have been made in order to identify patients at high risk of relapse and poor survival, and several parameters have been taken into consideration. The simplest and cheapest one is the International Staging System (ISS) prognostic model,[56] designed by the IMWG, based on beta-2 microglobulin and albumin level; a significantly different survival (62 months, 44 months and 29 months) was shown in stage 1, 2 or 3 patients, respectively. The major pitfall of this risk stratification is that it does not take into account cytogenetic alterations, that are now considered the main parameter affecting patients prognosis. No agreement does still exist on which, among fluorescence-in situ hybridization (FISH), comparative genomic hybridization (CGH) and gene expression profile (GEP) is the best method to use in order to detect chromosomal abnormalities. However, patients showing t (4;14), t (14;16) deletion 17q[57] or 1q abnormalities[57,58] carry a worse prognosis and should be treated differently from patients with no chromosomal abnormality.[59] Very few data however, are presently available concerning the efficacy of different therapeutic regimens in poor risk patients. A bortezomib -containing induction therapy seems to be able to overcome the adverse prognosis carried by t(4;14).[28] This is not the case for thalidomide,[60] especially in maintenance trials[37] while conflicting results were reported regarding lenalidomide-dexamethasone induction.[61] On the other hand, patients with 17q deletion seem not to benefit from Bortezomib followed by ASCT.[62] Dose dense regimens, upfront myeloablative allogeneic stem cell transplantation or novel agents are presently proposed for high risk patients, in the context of clinical trials, aiming at finding a proper therapeutic approach.
Autologous, Allogeneic or Tandem Autologous-Allogeneic SCT? Myeloablative allogeneic bone marrow transplantation (allo-BMT) or, later on, allo-SCT for the treatment of MM was introduced in the early 80s by several Institutions.[63] This procedure allowed to demonstrate that high dose chemo/radiotherapy coupled with the graft versus myeloma (GVM) effect could overcome drug resistance and induce long-lasting complete remission; transplant-related mortality (TRM), however, remained a major issue for many years, with most of the trials reporting mortality rates ranging from 30 to 50%.[64-65] On the other hand, allo-SCT can result into a more frequent molecular CR and decreased probability of relapse as compared to ASCT;[66] therefore it is likely that this procedure is probably the only therapeutic approach which has the potential ability to eradicate the myeloma clone. A decrease in TRM could be achieved using of non-myeloablative preparative regimens (RIC-allo-SCT), aimed at reducing conditioning-related toxicity while sparing GVM effect. A great variety of preparative regimens have been used, either including low dose (2Gy) total body irradiation with fludarabine or intermediate-dose melphalan plus fludarabine; a favorable outcome is more frequently observed in non-heavily pretreated patients and in chemosensitive disease.[67] A tandem strategy of high-dose melphalan and ASCT followed by RIC-allo-SCT has been propsed by several groups, in order to further decrease tumor burden prior to induce GVM effect. A direct comparison of double ASCT versus tandem ASCT followed by RIC-allo-SCT led to controversial results; with the autologous+allogeneic strategy resulting superior according to Bruno and Bjorkstrand[68-69] and inferior according to Moreau and Krishnan.[70-71] A recently published meta analysis concluded that ASCT followed by RIC-allo-SCT is associated with a higher percentage of CR, but TRM is also higher, thus leading to lack of improvement of PFS and OS.[72]
Concluding Remarks
In the last few years the outcome of MM patients has significantly improved with the introduction of novel drugs in the clinical practice. The inclusion of thalidomide, lenalidomide or bortezomib in various combinations, in the different phases of an ASCT program, increases the percentage of patients achieving a CR, thus potentially leading to patients cure. Data are not mature, so far, to establish whether a combination of new drugs, administered for a prolonged period of time, could render ASCT unnecessary. At present, in many US Institutions, both physicians and patients are in favor of a delayed ASCT policy, in order to avoid complications related to the period of myelosuppression related to the procedure. It cannot be taken for granted, however, that patients quality of life is worse in case of a short time myelosuppression as in ASCT, rather than in case ofa prolonged therapy with any of the new drugs that are presently available and whose side effects are well known. At present, at least in Europe, ASCT is still considered the standard of care for young patients with newly diagnosed MM, and the issue is how the results can be further improved. A number of new drugs are presently being tested in MM, at various disease phases. Among them carfilzomib, an irreversible proteasome inhibitor, that after having proven effective in relapsed/refractory disease, has been tested in combination with lenalidomide in newly diagnosed MM patients[73] inducing up to 40% stringently defined CR. Pomalidomide, a thalidomide derivative, has demonstrated to be effective even in lenalidomide or bortazomib-refractory patients.[74] These drugs will be probably included into induction therapy prior to ASCT in order to further improve disease eradication.
Multiple myeloma (MM) is a clonal B cell disorder characterized by proliferation and accumulation of B lymphocytes and plasma cells in the bone marrow and, more rarely, at extramedullary sites. Its annual incidence is 6/100000 in western countries, thus representing the second most common hematological malignancy after non Hodgkin lymphomas.[1]
For many years the combination of melphalan and prednisone (MP), that was developed in the early sixties by Bergsagel et al,[2] has been considered the gold standard treatment for MM, as different polychemotherapy regimens failed to demonstrate a better efficacy.[3] MP was able to induce a response in over 40% of treated patients; complete responses, however, were achieved in less than 5% of the cases, and overall patients survival did not exceeded 3 years. The first step towards introduction of autologous stem cell transplantation in MM was represented by in vitro studies showing a a dose-response effect of melphalan in MM cells.[4] The potential to overcome resistance to melphalan by using higher doses of the drug was subsequently explored in vivo;[5] 27% previously untreated patients reached a complete response (CR), and this translated into a prolonged survival, even though treatment related mortality was unacceptably high. In order to reduce the duration of profound cytopenia related to the use of high dose melphalan (HDM), autologous stem cell rescue was then introduced in the clinical practice, initially for relapsed/refractory disease, then for newly diagnosed MM.[6,7] The formal demonstration that autologous stem cell transplantation (ASCT) is superior to conventional chemotherapy in terms of response, duration of response and survival, came from two randomized trials, the first one from the Intergroup Francophone du Myeloma (IFM)[8] and the second one from the Medical Research Council (MRC).[9] In order to ameliorate these results, the application of two subsequent ASCTs was then explored by IFM[10] and by the Bologna group;[11] both studies demonstrated an improvement in response rate and event-free survival (EFS); however only the French study was able to show a survival advantage for patients receiving a double ASCT. Further analysis of the IFM trial showed that a second ASCT could result into an increased OS only in patients failing to achieve at least a very good partial response (VGPR)10 after the first ASCT, these data were in agreement with a subanalysis of the Bologna trial showing an improved event-free survival (EFS) after a second ASCT in patients failing to achieve at least a near-CR after the first one.[11]
While the use of a double ASCT is still matter of debate, from late nineties on, a single ASCT has been referred as the standard of care for newly diagnosed MM patients aged <60-65 years with no relevant comorbidities, this in accord with the upper age limit that has been considered appropriate for patients with other kinds of hematological malignancies, even though interesting results were obtained also in older patient populations.[12]
The Role of CR
When MP was the only available therapeutic strategy for MM, the attainment of CR was no matter of concern as only a minority of patients could achieve a minimal residual disease status. The introducion of more aggressive therapeutic programs including ASCT, prompted a better evaluation of minimal residual disease, including also cytofluorimetric[13] and molecular techniques.[14] At present, the International Myeloma Working Group (IMWG) has provided the definition of "stringent CR" including negative serum/urine immunofixation together with a normal serum free-light chain ratio and absence of clonal plasma cells in the bone marrow.[15] Several groups have analyzed the relationship beween CR and patients outcome, and have pointed out that CR is a strong predictor of survival,[16] expecially when extended over several years;[17] for this reason it is now generally recognized that every effort should be made in order to achieve maximal disease eradication through the various phases of the treatment program.[18]
Incorporation of Novel Drugs in Induction Phase
In addition to the clinical benefit offered by ASCT, in recent years the therapeutic results for MM have significantly improved due to the availability of drugs that are active both on neoplastic plasma cells and on bone marrow microenvironment, such as thalidomide, lenalidomide and bortezomib. After testing in patients with advanced, relapsed/refractory disease, these compounds were evaluated in clinical trials in the framework of induction therapy prior to ASCT in newly diagnosed patients in order to increase the depth of response thus improving patients outcome. Thalidomide was the first agent included in induction therapy for newly diagnosed MM patients eligible for ASCT; the drug was used in combination to high-dose dexamethasone (TD) (Table 1) yielding interesting results as compared to conventional chemotherapy in a case-match retrospective analysis[19] or to high-dose dexamethasone in a prospective randomized trial.[20] In a further randomized trial (Total Therapy 2) thalidomide was continuously applied in the various phases of the whole treatment program until patient relapse,[21] again an advantage in terms of CR rate and EFS was observed in patients treated with thalidomide as compared to those not receiving the drug, but OS was similar in the two groups of patients. Subsequent trials were designed aiming at evaluating the combination of TD with doxorubicin;[22] a significant improvement in response rate was observed as compared to conventional chemotherapy (VAD) (Table 1). Bortezomib was tested in combination to dexamethasone (VD) in a phase II study;[23] a VGPR rate of over 30% was achieved after induction and upgraded to over 50% after ASCT (Table 2). A further phase II study was designed aiming at comparing VD to conventional vincristin-doxorubicin-dexmethasone (VAD);[24] again the arm treated with the novel regimen showed a significantly higher response rate (38% VGPR or better vs 15%) that was confirmed after ASCT. The combination of VD with cyclophosphamide (VCD) was able to induce a VGPR or better in over 60% of the patients,[25] similar results were reported using VD+ doxorubicin (PAD).[26] Lenalidomide was studied in a randomized trial in combination to high (RD) vs low (Rd) doses dexamethasone,[27] after 4 courses patients were allowed to undergo ASCT or to proceed with the same therapy; even though response rate was significantly higher in the RD group, survival was the same due to the higher toxicity experienced by the RD group.
A further improvement in the results obtained with novel drugs±steroids±chemotherapy was achieved combining two novel drugs with dexamethasone (Table 2). The combination bortezomib-thalidomide and dexamethasone (VTD) was randomly compared to thalidomide-dexamethasone (TD) as induction therapy prior to ASCT, yielding a significant advantage in terms of response, both CR and VGPR.[28] These data were confirmed by a recent study of the Pethema group.[29] A bortezomib+thalidomide-containing regimen was also used in Total Therapy 3 trial,[30] in the context of a polychemotherapy program involving induction, ASCT, consolidation and maintenance; as compared to Total Therapy 2, in which only TD was used,[21] a significant prolongation of EFS was observed. These results so far indicate that induction therapy in preparation to ASCT should include bortezomib+dexamethasone+an immunomodulating agent, either thalidomide or lenalidomide, that is presently being explored in phase II trials.[31]

Table 1. Thalidomide-containing induction regimens.

Table 2. Major drug combinations used as induction therapy.
Controversial Issues
Consolidation, Maintenance or Both? The administration of some kind of treatment upon completion of major therapy in order to improve/maintain its efficacy represents the standard of care in several lymphoproliferative neoplasms such as acute lymphoblastic leukemia, low grade lymphoma or mantle cell lymphoma, and for this reason it has been considered an attractive option also for MM. Several groups have addressed the issue of post transplantation treatment, and interesting results have been reported; at present, however, no data can definitely support a treatment over another, and no drug has been formally approved for the therapy of MM at this disease stage.
Consolidation therapy is defined as a short course of treatment administered after ASCT aiming at further reduce tumor load (Table 3). A study from the nordic group[32] has evaluated the efficacy of a short course of Bortezomib, and an increased percentage of CRs was observed. Two different studies analyzed the effects of a short course of Bortezomib-thalidomide-dexamethasone (VTD) administered as consolidation after ASCT, both trials showed that a molecular response can be achieved in up to 60% of the patients.[33-35] Maintenance therapy is defined as long-term treatment aiming at preventing disease recurrence or progression. Alpha interferon has been widely tested after ASCT and despite two reports showing an improved survival, side effects greatly overcome the possible advantage, so that this approach has been definitely abandoned.[36] A limited efficacy was also reported with long term use of steroids.[37] Thalidomide has been studied in six trials[21-22,38-41] (Table 4), in 3 of which the drug was used also in induction phase. Although all the trials showed an advantage in terms of EFS or PFS; an OS advantage for patients treated with thalidomide was observed only in 2 trials. A major concern regarding the use of this drug as maintenance therapy is the high percentage of patients dropping out due to long term side effects, specifically peripheral neuropathy.[38-41] Furthermore, the likelihood of selecting MM clones resistant to thalidomide and responsible for short post-relapse survival should probably be taken into consideration[21-22,41] as well as the limited efficacy of the drugs in case of poor-risk cytogenetic.[41] Due to its favorable toxicity profile, and specifically to the lack of long-term neurological toxicity, lenalidomide has been tested as maintenance therapy in two randomized studies,[42-43] both of them showed a significant advance in TTP, while OS was significantly improved only in one study.[43] Side effects were mainly hematological, a higher percentage of second primary malignancies were observed in Lenalidomide-treated patients,[42,43] however this data need further observation as it is clear that survival benefit outgrows the risk of death from second malignancies.[44] A recent report analyzed the role of bortezomib maintenance after ASCT;[26] patients showed a significant advantage in terms of PFS and OS, even though the potential of neurological toxicity should be taken into consideration.
Despite these interesting results, however, data are not mature to recommend a specific strategy, and the issue of consolidation and/or maintenance treatment remains still debated.

Table 3. Regimens used as consolidation therapy.

Table 4. Maintenance regimens.
Upfront or Salvage ASCT? Early studies on ASCT in MM were performed in patients with relapsed/refractory disease but, due to the poor result that were obtained,[45] the procedure is now preferentially employed in newly diagnosed patients.[46] Furthermore, a timely-dependant application of ASCT seems to be crucial in determining an optimal response.[47] A randomized study from the French group,[48] however, demonstrated a comparable outcome in terms of survival in patients undergoing early vs deferred ASCT (64.4 vs 64 months OS). These data were obtained when only chemotherapeutic agents were available; it is now evident that new drugs, when applied during induction, are able to determine a deeper response than that obtained with conventional chemotherapy combinations. Several groups have thus designed studies aimed at evaluating efficacy of long term treatment with new drugs as compared to ASCT,[49,50] applying transplant only upon relapse. Results that have been published so far failed to show a difference in patients survival even though early ASCT is related to a shorter duration of treatment and drug exposure. A recent retrospective study has shown that, in patients treated with thalidomide or lenalidomide followed by early stem cell mobilization,[51] comparable results were achieved after early vs late ASCT. Data from further studies are awaited
Is ASCT Feasible in Elderly Patients? Patients aged > 65 years are not considered good candidates to ASCT as their survival is significantly shorter than that observed in younger patients (50% vs 68% at 5 years,[52]). Several reports, however, have identified a “grey zone” represented by patients aged 65-70 in good clinical conditions, that could potentially take advantage from this procedure. In particular, a randomized study conducted in these patients has demonstrated that intermediate dose melphalan (10mg/sqm) with PBSC support results into a significantly prolonged event-free and overall survival as compared to melphalan-prednisone.[53] On the other hand, a later study conducted in older patients (65-75 years) failed to show an advantage of intermediate dose melphalan as compared to MP, and both regimens were inferior to the combination melphalan-prednisone and thalidomide.[54] At present, however, no data can unequivocably establish whether an ASCT program including new drugs can be useful in older patients as it happens in younger ones. At present only one phase II study has been reported, aimed at evaluating the toxicity and the efficacy of bortezomib and lenalidomide included in pre-transplant induction and post transplant consolidation and maintenance in patients aged 65-75 years.[55] The percentage of patients obtaining a CR increased progressively through the various phases of the treatment program (13% after induction, 43% after transplant and 73% during consolidation/maintenance) and hematological and non-hematological toxicities were acceptable. These data indicate that, in selected elderly patients, an ASCT program including new drugs can be safely performed, thus representing a possible therapeutic option.
Is ASCT the Best Treatment for High-Risk Patients? In recent years, many attempts have been made in order to identify patients at high risk of relapse and poor survival, and several parameters have been taken into consideration. The simplest and cheapest one is the International Staging System (ISS) prognostic model,[56] designed by the IMWG, based on beta-2 microglobulin and albumin level; a significantly different survival (62 months, 44 months and 29 months) was shown in stage 1, 2 or 3 patients, respectively. The major pitfall of this risk stratification is that it does not take into account cytogenetic alterations, that are now considered the main parameter affecting patients prognosis. No agreement does still exist on which, among fluorescence-in situ hybridization (FISH), comparative genomic hybridization (CGH) and gene expression profile (GEP) is the best method to use in order to detect chromosomal abnormalities. However, patients showing t (4;14), t (14;16) deletion 17q[57] or 1q abnormalities[57,58] carry a worse prognosis and should be treated differently from patients with no chromosomal abnormality.[59] Very few data however, are presently available concerning the efficacy of different therapeutic regimens in poor risk patients. A bortezomib -containing induction therapy seems to be able to overcome the adverse prognosis carried by t(4;14).[28] This is not the case for thalidomide,[60] especially in maintenance trials[37] while conflicting results were reported regarding lenalidomide-dexamethasone induction.[61] On the other hand, patients with 17q deletion seem not to benefit from Bortezomib followed by ASCT.[62] Dose dense regimens, upfront myeloablative allogeneic stem cell transplantation or novel agents are presently proposed for high risk patients, in the context of clinical trials, aiming at finding a proper therapeutic approach.
Autologous, Allogeneic or Tandem Autologous-Allogeneic SCT? Myeloablative allogeneic bone marrow transplantation (allo-BMT) or, later on, allo-SCT for the treatment of MM was introduced in the early 80s by several Institutions.[63] This procedure allowed to demonstrate that high dose chemo/radiotherapy coupled with the graft versus myeloma (GVM) effect could overcome drug resistance and induce long-lasting complete remission; transplant-related mortality (TRM), however, remained a major issue for many years, with most of the trials reporting mortality rates ranging from 30 to 50%.[64-65] On the other hand, allo-SCT can result into a more frequent molecular CR and decreased probability of relapse as compared to ASCT;[66] therefore it is likely that this procedure is probably the only therapeutic approach which has the potential ability to eradicate the myeloma clone. A decrease in TRM could be achieved using of non-myeloablative preparative regimens (RIC-allo-SCT), aimed at reducing conditioning-related toxicity while sparing GVM effect. A great variety of preparative regimens have been used, either including low dose (2Gy) total body irradiation with fludarabine or intermediate-dose melphalan plus fludarabine; a favorable outcome is more frequently observed in non-heavily pretreated patients and in chemosensitive disease.[67] A tandem strategy of high-dose melphalan and ASCT followed by RIC-allo-SCT has been propsed by several groups, in order to further decrease tumor burden prior to induce GVM effect. A direct comparison of double ASCT versus tandem ASCT followed by RIC-allo-SCT led to controversial results; with the autologous+allogeneic strategy resulting superior according to Bruno and Bjorkstrand[68-69] and inferior according to Moreau and Krishnan.[70-71] A recently published meta analysis concluded that ASCT followed by RIC-allo-SCT is associated with a higher percentage of CR, but TRM is also higher, thus leading to lack of improvement of PFS and OS.[72]
Concluding Remarks
In the last few years the outcome of MM patients has significantly improved with the introduction of novel drugs in the clinical practice. The inclusion of thalidomide, lenalidomide or bortezomib in various combinations, in the different phases of an ASCT program, increases the percentage of patients achieving a CR, thus potentially leading to patients cure. Data are not mature, so far, to establish whether a combination of new drugs, administered for a prolonged period of time, could render ASCT unnecessary. At present, in many US Institutions, both physicians and patients are in favor of a delayed ASCT policy, in order to avoid complications related to the period of myelosuppression related to the procedure. It cannot be taken for granted, however, that patients quality of life is worse in case of a short time myelosuppression as in ASCT, rather than in case ofa prolonged therapy with any of the new drugs that are presently available and whose side effects are well known. At present, at least in Europe, ASCT is still considered the standard of care for young patients with newly diagnosed MM, and the issue is how the results can be further improved. A number of new drugs are presently being tested in MM, at various disease phases. Among them carfilzomib, an irreversible proteasome inhibitor, that after having proven effective in relapsed/refractory disease, has been tested in combination with lenalidomide in newly diagnosed MM patients[73] inducing up to 40% stringently defined CR. Pomalidomide, a thalidomide derivative, has demonstrated to be effective even in lenalidomide or bortazomib-refractory patients.[74] These drugs will be probably included into induction therapy prior to ASCT in order to further improve disease eradication.
References
- Yemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277-300 http://dx.doi.org/10.3322/caac.20073 PMid:20610543

- Bergsagel L, De Sprague CC, Austin C,
Griffith KM. Evaluation of new chemotherapeutic agents in the treatment
of multiple myeloma. IV. L-phenilalanine mustard (NSC-8806). Cancer
Chemotherapy Report 1962; 21:87-99 PMid:13867794

- Gregory WM, Richards MA, Malpas JS.
Combination chemotherapy versus melphalan and prednisolone in the
treatment of multiple myeloma: an overview of published trials. J Clin
Oncol 1992;10:334-342 PMid:1531068

- Ben-Efraim S, Bocian RC, Mokyr MB, Dray S.
Increase in the effectiveness of melphalan therapy with progression of
MOPC-315 plasmacytoma tumor growth. Cancer Immunol Immunother
1983;15:101-107 http://dx.doi.org/10.1007/BF00199699

- Selby P, McElwain TJ, Nandi AC et al.
Multiple myeloma treated with high-dose intravenous melphalan. Br J
Haematol 1987;66:55-62 PMid:3593657

- Barlogie B, Hall R, Zander A, Dicke K,
Alexanian R. High-dose melphalan with autologos bone marrow
transplantation for multiple myeloma. Blood 1986;67:1298-1301
PMid:3516252

- Alexanian R, Dimpoulos M, Hester J,
Delasalle K, Champlin R. Early myeloablative therapy for multiple
myeloma. Blood 1994:84:4278-4282 PMid:7994043

- Attal M, Harousseau JL, Stoppa AM et al.A
prospective randomized trial of autologous bone marrow transplantation
and chemotherapy in multiple myeloma.N Engl J Med 1996;335:91-97 http://dx.doi.org/10.1056/NEJM199607113350204 PMid:8649495

- Child JA, Morgan GJ, Davies FE et al.
High-dose chemotherapy with hematopoietic stem-cell rescue for multiple
myeloma. N Engl J Med 2003;348:1875-1883 http://dx.doi.org/10.1056/NEJMoa022340 PMid:12736280

- Attal M, Harousseau JL, Facon T et al.
Single versus double autologous stem cell transplantation for multiple
myeloma. N Engl J Med 2003;349:2495-2502 http://dx.doi.org/10.1056/NEJMoa032290 PMid:14695409

- Cavo M, Tosi P. Zamagni E et al.
Prospective, randomized study of single compared with double autologous
stem cell transplantation for multiple myeloma: Bologna '96 clinical
study. J Clin Oncol 2007;25:2434-2441 PMid:17485707

- Kumar SK, Dingli D, Lacy MQ et al.
Autologous stem cell transplantation in patients of 70 years and older
with multiple myeloma: results of a matched-pair analysis. Am J Hematol
2008;83:614-617 http://dx.doi.org/10.1002/ajh.21191 PMid:18429054

- Martinelli G, Terragna C, Zamagni E et al.
Molecular remission after allogeneic or autologous transplantation of
hematopoietic stem cells for multiple myeloma. J Clin Oncol
2000;18:2273-2281 PMid:10829048

- Paiva B, Vidriales MB, Cerver J et al.
Multiparameter flow cytometric remission is the most relevant
prognostic factor for multiple myeloma patients who undergo autologous
stem cell transplantation. Blood 2008;112:4017-4023 PMid:18669875
PMCid:2581991

- Durie BG, Harousseau JL, San Miguel J et
al. International uniform response criteria for multiple myeloma.
Leukemia 2006;20:1467-1473 http://dx.doi.org/10.1038/sj.leu.2404284 PMid:16855634

- Chanan-Khan A, Giralt S. Importance of
achieving a complete response inmultiple myeloma and the impact of
novel agents. J Clin Oncol 2010; 28:2612-2624 http://dx.doi.org/10.1200/JCO.2009.25.4250 PMid:20385994

- Barlogie B, Anaisie E, Haessler J et al.
Complete remission sustained 3 years from treatment initiation is a
powerful surrogate for extended survival in multiple myeloma. Cancer
2008;113:355-359 http://dx.doi.org/10.1002/cncr.23546 PMid:18470907

- Cavo M, Rajkumar SV, Palumbo A et al.
International myeloma working group consensus approach to the treatment
of multiple myeloma patients who are candidates for autologous stem
cell transplantation. Blood 2011;117:6063-6073 http://dx.doi.org/10.1182/blood-2011-02-297325 PMid:21447828 PMCid:3293742

- Cavo M, Zamagni E, Tosi P et al.
Superiority of thalidomide and deamethasone over
vincristine-doxorubicin-dexamethasone (VAD) as primary therapy in
preparation for autologous transplantation for multiple myeloma. Blood
2005;106:35-39 http://dx.doi.org/10.1182/blood-2005-02-0522 PMid:15761019

- Rajkumar SV, Blood E, Vesole D Fonseca R,
Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone
compared with dexamethasone alone in newly diagnosed multiple myeloma:a
clinical trial coordinated by the Eastern Cooperative Oncology Group. J
Clin Oncol 2006;24:431-436 http://dx.doi.org/10.1200/JCO.2005.03.0221 PMid:16365178

- Barlogie B, Tricot G, Anaissie E et al.
Thalidomide and hematopoietic stem cell transplantation for multiple
myeloma. N Engl J Med 006:354:1021-1030

- Lokhorst HM, van der Holt B, Zweegman S et
al. A randomized phase III study on the effect of thalidomide combined
with adriamicyn, dexamethasone and high-dose melphalan, followed by
thalidomide maintenance in patients with multiple myeloma. Blood
2010;11:1113-1120 http://dx.doi.org/10.1182/blood-2009-05-222539 PMid:19880501

- Harousseau JL, Attal M, Leleu X et al.
Bortezomib pus dexamethasone as induction treatment prior to autologous
stem cell transplantation in patients with newly diagnosed multiple
myeloma:results of a IFM phase II study.Haematologica
2006;91:1498-1505 PMid:17043025

- Harousseau JL, Attal M, Avet-Loiseau H et
al. Bortezomib plus dexamethasone is superior to vincristin plus
doxorubicin plus dexamethasone as induction treatment prior to
autologous stem cell transplantation in newly diagnosed multiple
myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol
2010;28:4621-4629 http://dx.doi.org/10.1200/JCO.2009.27.9158 PMid:20823406

- Reeder CB, Reece DE, Kukreti V et al.
Cyclophosphamide, bortezomib and dexamethasone for newly diagnosed
multiple myeloma:high response rates in a phase II clinical trial.
Leukemia 2009;23:1337-1341 http://dx.doi.org/10.1038/leu.2009.26 PMid:19225538 PMCid:2711213

- Sonneveld P, Schmidt-Wolf IG, van der Holt
B et al. Bortezomib induction and maintenance treatment in patients
with newly diagnosed multiple myeloma:results of the randomized phase
III HOVON-65/GMMG-HD4 trial. J Clin Oncol 2012;30:2946-2955 http://dx.doi.org/10.1200/JCO.2011.39.6820 PMid:22802322

- Rajkumar SV, Jacobus S, Callander NS et
al. Lenalidomide plus high dose dexamethasone versus lenalidomide plus
low dose dexamethasone as initial therapy for newly diagnosed multiple
myeloma: an open label randomized controlled trial. Lancet Oncol
2010;11:29-37 http://dx.doi.org/10.1016/S1470-2045(09)70284-0

- Cavo M, Tacchetti P, Patriarca F et al.
Bortezomib thalidomide and dexamehasone compared with thalidomide and
dexamethasone as induction before and consolidation therapy afyter
double autologous stem cell transplantation in newly diagnosed multiple
myeloma:result froma rndomized phase III study. Lancet 2010;
379:2075-2085 http://dx.doi.org/10.1016/S0140-6736(10)61424-9

- Rosinol L, Oriol A, Teruel AL et al.
Superiority of bortezomib, thalidomide and dexamethasone (VTD) as
induction pretransplantation therapy in multiple myeloma: a randomized
Pethema/GEM study. Bloog 2012;120:1589-1596 http://dx.doi.org/10.1182/blood-2012-02-408922 PMid:22791289

- Pineda-Roman M, Zangari M, Haessler J et
al. Sustained complete remissions in multiple myeloma linked to
bortezomib in total therapy 3: comparisons with total therapy 2. Br J
Haematol 2008;140:625-634 http://dx.doi.org/10.1111/j.1365-2141.2007.06921.x PMid:18302711

- Richardson P, Weller E, Lonial S et al.
Lenalidomide, bortezomib and dexamethasone combination therapy in
patients with newly diagnosed multiple myeloma. Blood 2010;116:679-686 http://dx.doi.org/10.1182/blood-2010-02-268862 PMid:20385792 PMCid:3324254

- Mellqvist UH, Westin J, Gimsing P et al.
Improved response rate with bortezomib consolidation after high dose
melphalan: first results of a Nordic Myeloma Study Group randomized
phase III trial. Blood 2009;114:530

- Ladetto M, Pagliano G, Ferrero S et al.
Major tumor shrinking and persistent molecular remissions after
consolidation with bortezomib, thalidomide, and dexamethasone in
patients with autografted myeloma. J Clin Oncol, 2010; 28:2077-2084

- Cavo M, Pantani L, Petrucci MT ety al.
Bortezomib-thalidomide-dexamethasone is superior to
thalidomide-dexamethasone as consolidation therapy after autologous
hematopoietic stem cell transplantation in patients with newly
diagnosed multiple myeloma. Blood 2012;120:9-19 http://dx.doi.org/10.1182/blood-2012-02-408898 PMid:22498745

- Terragna C, Zamagni E, Petrucci MT et al.
Molecular remission after bortezomib-thalidomide-dexamethasone compared
with thalidomide-dexamethasone as consolidation therapy after double
autologous transplantation for multipkle myeloma:results of a
qualitative and quantitative analysis. Blood 2010;116:861 PMid:20705764

- Fritz E, Ludwig H. Interferon-alpha
treatment in multiple myeloma:meta-analysis of 30 randomized trials
among 3948 patients. Ann Oncol 2000;11:1427-1436 http://dx.doi.org/10.1023/A:1026548226770 PMid:11142483

- Berenson JR, Crowley JJ, Grogan TM et al.
Maintenance therapy with alternate-day prednisone improves survival in
multiple myeloma patients. Blood 2002;99:3163-3168 http://dx.doi.org/10.1182/blood.V99.9.3163 PMid:11964279

- Attal M. Harousseau JL, Leyvraz S et al.
Maintenance therapy with thalidomide improves survival in patients with
multiple myeloma. Blood 2006;108:289-3294 PMid:16873668

- Spencer A, Prince HM, Roberts AW et al.
Consolidation therapy with low-dose thalidomide and prednisolone
prolongs the survival of multiple myeloma patients undergoing a single
autologous stem cell transplantation procedure. J Clin Oncol
2009;27:1788-1793 http://dx.doi.org/10.1200/JCO.2008.18.8573 PMid:19273705

- Stewart KA, Trudel S, Bahlis NJ et al. A
randomized phase III trial of thalidomide and prednisone as maintenance
therapy following autologous stem cell transplantation (ASCT) in
patients with multiple myeloma (MM): the NCIC CTG MY10 trial. Blood
2010;116:39

- Morgan GJ, Gregory WM, Davies FE et al.
The role of maintenance thalidomide therapy in multiple myeloma: MRC
myeloma IX results and meta-analysis. Blood 2012;119:7-15 http://dx.doi.org/10.1182/blood-2011-06-357038 PMid:22021371

- Attal M, Lauwers-Cances V, Marit G et al.
Lenalidomide maintenance after stem cell transplantation for multiple
myeloma. N Engl J Med 2012;366:1782-1791 http://dx.doi.org/10.1056/NEJMoa1114138 PMid:22571202

- McCarthy PL, Owzar K, Hofmeister CC et al:
Lenalidomide after stem cell transplantation for multiple myeloma. N
Engl J Med 2012;366:1770-1781 http://dx.doi.org/10.1056/NEJMoa1114083 PMid:22571201

- Palumbo A, Freeman J, Weiss L, Fenaux P.
The clinical safety of lenalidomide in multiple myeloma and
myelodysplastic syndromes. Expert Opin Drug Saf 2012, 11:107-120 http://dx.doi.org/10.1517/14740338.2011.619975 PMid:22066855

- Vesole DH, Crowley JJ, Catchatourian R et
al. High-dose melphalan with autotransplantation for refractory
multiple myeloma: results of a Southwest Oncology Group phase II trial.
J Clin Oncol 1999;17:2173-2179 PMid:10561273

- Barosi G, Boccadoro M, Cavo M et al.
Management of multile myeloma and related disorders:guidelines from the
Italian Society of Hematology, (SIE), the Italian Society of
Experimental Hematology (SIES) and Italian Group for Bone Marrow
Transplantation (GITMO) Haematologica 2004;89:717-741 PMid:15194540

- Barlogie B, Jagannath S, Desikan KR et al.
Total therapy with tandem transplants for newly diagnosed multiple
myeloma. Blood 1999;93:55-65 PMid:9864146

- Fermand JP, Ravaud P, Chevret S et al.
High-dose therapy and autologous peripheral blood stem cell
transplantation in multiple myeloma: up-front or rescue treatment?
Results of a multicenter sequential randomized clinical trial. Blood
1998;92:3131-3136 PMid:9787148

- Rajkumar SV, Jacobus S, Callander NS et
al. Lenalidomide plus high-dose dexamethasone as initial therapy for
newly diagnosed multiple myeloma: an open label randomised controlled
trial. Lancet Oncol 2010; 11:29-37 http://dx.doi.org/10.1016/S1470-2045(09)70284-0

- Palumbo A, Rajkumar SV. Multiple myeloma:
chemotherapy or transplantation in the era of new drugs. Eur J Haematol
2010;84:379-390 http://dx.doi.org/10.1111/j.1600-0609.2010.01431.x PMid:20345446

- Kumar SK, Lacy MQ, Dispenzieri A et al.
Early versus delayed autologous stem cell transplantation after
immunomodulatory agent-based induction therapy in patients with newly
diagnosed multiple myeloma. Cancer 2012;118;1585-1592 http://dx.doi.org/10.1002/cncr.26422 PMid:22009602

- Barlogie B, Tricot G, Anaissie E et al:
Thalidomide and hematopoietic cell transplantation for multiple
myeloma. N Engl J Med 2006; 354:1021-1030 http://dx.doi.org/10.1056/NEJMoa053583 PMid:16525139

- Palumbo A, Bringhen S, Petrucci MT et al:
Intermediate -dose melphalan improves survival of myeloma patients aged
50 to 70: results of a randomized controlled trial PMid:15265788

- Facon T, Mary JY, Hulin C et al: Melphalan
and prednisone plus thalidomide versus melphalan and prednisone alone
or reduced intensity autologous stem cell transplantation in elederly
patienys with multiple myeloma (IFM 99-06): a randomised trial. Lancet
2007; 370:1209-1218 http://dx.doi.org/10.1016/S0140-6736(07)61537-2

- Palumbo A, Gay F, Falco P et al:
Bortezomib as induction before autologous transplantation, followed by
lenalidomide as consolidation-maintenance in untreated myeloma
patients. J Clin Oncol 2010;28:800-807 http://dx.doi.org/10.1200/JCO.2009.22.7561 PMid:20048187

- Greipp PR, San Miguel J, Durie BG et al. International staging system for multiple myeloma. J Clin Oncol 2005;20:3412-3420 http://dx.doi.org/10.1200/JCO.2005.04.242 PMid:15809451

- Avet Loiseau H, Attal M, Campion L et al.
Long-term analysis of the IFM99 trials for myeloma: cytogenetic
abnormalities [t(4;14), del (17p), 1q gains] play a major role in
defining long-term survival. J Clin Oncol 2012;30:1949-1952 http://dx.doi.org/10.1200/JCO.2011.36.5726 PMid:22547600

- Sawyer JR, Tian E, Thomas E et al.
Evidence for a novel mechanism for gene amplification in multiple
myeloma: 1q12 pericentromeric heterochromatin mediates
breakage-fusion-bridge cycles of a 1q12 approximately 23 amplicon. Br J
Haematol 2009;147:484-494 http://dx.doi.org/10.1111/j.1365-2141.2009.07869.x PMid:19744130

- Stewart AK, Bergsagel PL, Greipp PR et al.
A practical guide to defining high-risk myeloma for clinical trials,
patients counseling and choice of therapy. Mayo Clin Proc
2007:21:529-534

- Zamagni E, Testoni N, Terragna C et al.
Prognostic impact of cytogenetic abnormalities on outcomes of newly
diagnosed multiple myeloma patients treated with
thalidomide-dexamethasone incorporated into double autologous stem cell
transplantation: an analysis of 593 patients. Blood 2010;116:3562

- Kapoor P, Kumar S, Fonseca R et al. Impact
of risk stratification on outcome among patients with multiple myeloma
receiving initial therapy with lenalidomide and dexamethasone. Blood
2009;114:518-521 http://dx.doi.org/10.1182/blood-2009-01-202010 PMid:19324902 PMCid:2713462

- Avet Loiseau H, Leleu X, Roussel MM et al.
Bortezomib plus dexamethasone induction improves outcome of patients
with t(4;14) but not outcome of patients with del (17p). J Clin Oncol
2010;28:4630-4634 http://dx.doi.org/10.1200/JCO.2010.28.3945 PMid:20644101

- Bensinger WI., Buckner CD., Anasetti C. et
al.: Allogeneic marrow transplantation for multiple myeloma: an
analysis of risk factors on outcome. Blood 1996; 88:2787-2793
PMid:8839877

- Gahrton G, Tura S, Ljungman P et al.
Allogeneic bone marrow transplantation in multiple myeloma. European
Group for Bone Marrow Transplantation. N Engl J Med 1991;325:1267-1273 http://dx.doi.org/10.1056/NEJM199110313251802 PMid:1922221

- Gahrton G, Svensson H, Cavo M et al.
Progress in allogeneic bone marrow and peripferal blood stem cell
transplantation in multiple myeloma: a comparison between transplants
performed 1983-93 and 1994-8 at European Group for Bone Marrow
Transplantation Centres. Br J Haematol 2001; 113:209-216

- Martinelli G., Terragna C., Zamagni E. et
al. Molecular remission after allogeneic or autologous transplantation
of hematopoietic stem cell for multiple myeloma. J Clin Oncol 2000;
18:2273-2281 PMid:10829048

- Crawley C, Lalancette M, Szydlo R et al.
Outcomes for reduced-intensity allogeneic transplantation for multiple
myeloma: an analysis of prognostic factors from the Chronic Leukaemia
Working Party of the EBMT. Blood. 2005 Jun 1;105(11):4532-4539 http://dx.doi.org/10.1182/blood-2004-06-2387 PMid:15731182

- Bruno B, Rotta M, Patriarca F et al. A
comparison of allografting with autografting for newly diagnosed
myeloma. N Engl J Med 2007;356:1110-1120 http://dx.doi.org/10.1056/NEJMoa065464 PMid:17360989

- Bjorkstrand B, Iacobelli S, Hegenbart U et
al. Tandem autologous/reduced intensity conditioning allogeneic stem
cell transplantation versus autologous transplantation in myeloma:
long-term follow-up. J Clin Oncol 2011;29:3016-3022 http://dx.doi.org/10.1200/JCO.2010.32.7312 PMid:21730266

- Moreau P, Garban F, Attal M et al.
Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing
nonmyeloablative allotransplantation with autologous transplantation in
high-risk de novo multiple myeloma. Blood 2008;112:3914-3915 http://dx.doi.org/10.1182/blood-2008-07-168823 PMid:18948589

- Krishnan A, Pasquinui MC, Logan B et al.
Autologous haematopoietic stem cell transplantation followed by
allogeneic or autologous haematopoietic stem cell transplantation in
patients with multiple myeloma (BMT CTN 0102): a phase 3 biological
assignment trial. Lancet Oncol 2011;12:1195-1203 http://dx.doi.org/10.1016/S1470-2045(11)70243-1

- Armeson KE, Hill EG, Costa LJ. Tandem
autologous vs autologous plus reduced intensity allogeneic
transplantation in the upfront management of multiple myeloma:
meta-analysis of trials with biological assignment PMid:22964593

- Jakubowiak AJ, Dytfeld D, Griffith KA et
al: A phase study of carfilzomib in combination with lenalidomide
and low-dose dexamethasone as frontline treatment for multiple myeloma.
Blood 2012 http://dx.doi.org/10.1182/blood-2012-04-422683 PMid:22665938

- Lacy MQ, Allred JB, Gertz MA et al:
Pomalidomide plus low-dose dexamethasone in myeloma refractory to both
bortezomib and lenalidomide: comparison of two dosing strategies in
dual refractory disease. Blood 2011;118:2970-2975 http://dx.doi.org/10.1182/blood-2011-04-348896 PMid:21690557 PMCid:3291492
