Ara-C, Idarubicine and Gentuzumab Ozogamicin (AIM) as Salvage Treatment in Advanced Acute Myeloid Leukemia Patients
Saveria Capria, Silvia Maria Trisolini, Clara Minotti, Caterina Stefanizzi, Luisa Cardarelli, Martina Canichella, Claudio Cartoni, Daniela Diverio, Maria Stefania De Propris, Marco Mancini, Alessandra Micozzi, Robin Foà and Giovanna Meloni
Hematology, Department of Cellular Biotechnologies and Hematology, “Sapienza” University of Rome, Rome, Italy
Correspondence
to:
Saveria Capria. Hematology, Department of Cellular Biotechnologies and
Hematology, “Sapienza” University of Rome, Via Benevento 6 00161Rome,
Italy. Tel:+39-06-49974741, Fax:+39-06-44241984. E-mail: capria@bce.uniroma1.it
Published: November 6, 2012
Received: August 25, 2012
Accepted: October 4, 2012
Meditter J Hematol Infect Dis 2012, 4(1): e2012072, DOI 10.4084/MJHID.2012.072
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Long-term
survival of relapsed/refractory acute myeloid leukemia (AML) remains a
major problem, particularly in patients not eligible for
transplantation.
We hereby evaluated the feasibility and efficacy of adding Gemtuzumab Ozogamicin to salvage chemotherapy (Ara-C, Idarubicine, Peg-Filgrastim) in relapsed/refractory AML. The main endpoints were: the rate of complete remissions (CR) and the proportion of patients capable of undergoing a stem cell transplant.
Fourty-two patients were enrolled. The overall CR rate was 76% and no induction deaths were reported. In 56% of patients, a transplant procedure could be performed. The treatment schedule proved feasible and well tolerated, providing a high CR rate and a useful bridge to transplant.
We hereby evaluated the feasibility and efficacy of adding Gemtuzumab Ozogamicin to salvage chemotherapy (Ara-C, Idarubicine, Peg-Filgrastim) in relapsed/refractory AML. The main endpoints were: the rate of complete remissions (CR) and the proportion of patients capable of undergoing a stem cell transplant.
Fourty-two patients were enrolled. The overall CR rate was 76% and no induction deaths were reported. In 56% of patients, a transplant procedure could be performed. The treatment schedule proved feasible and well tolerated, providing a high CR rate and a useful bridge to transplant.
Introduction
Patients with recurrent or refractory acute myeloid leukemia (AML) have a poor prognostic likelihood. Salvage regimens generally include high-dose Ara-C, but whenever a second CR is attained, the median duration of a second relapse-free interval (RFI) is considerably shorter than the first one, highlighting the need to develop novel therapies.[1-2] In patients who are able to undergo an allogeneic hematopoietic stem cell transplant (HSCT), long-term survival rates range from 25% to 40%.[4] However, only a minority of patients can benefit from a HSCT because of co-morbidities and treatment-related toxicities. Thus, the goal of novel therapies should be to achieve complete remissions (CR) with the lowest possible toxicities, in order to provide a bridge to transplant.[3]
Gentuzumab Ozogamicin (GO) is a conjugate of an anti-CD33 humanized monoclonal antibody and the anthracycline calicheamicin with an attractive mechanism of action, since the CD33 antigen/antibody complex is rapidly internalized in the leukemic cell prior to drug delivery.[4] Pivotal studies of (GO) as single agent at 9 mg/m2 in relapsed AML have shown an overall response rate of 28% and encouraging results have been obtained in phase II trials in which it has been employed in combination with other cytotoxic agents.[5-8]
The EORTC/GIMEMA leukemia group tested in a phase II study (AML-15) the feasibility and safety of the sequential combination of GO and conventional chemotherapy in patients older than 60 years of age with untreated AML, confirming the feasibility and activity of such a treatment strategy in this group of patients.[9]
Moreover, several phase II trials have utilized the combination of Mylotarg with chemotherapy in AML patients with advanced disease, reporting a response rate between 12% and 42%, and a high treatment-related mortality.[10-12] The main dose limiting toxicity of the compound is liver toxicity and the incidence of veno-occlusive disease; thus, when combined with chemotherapy, it is currently used at lower doses.[13]
In this retrospective analysis we evaluated the efficacy and safety of the administration of GO 3 mg/m2 after intensive chemotherapy consisting of intermediate doses of Ara-C and Idarubicine, and followed by Peg-filgrastim in adult patients with refractory/relapsed AML The main endpoints of the study were: the rate of second CR, the survival rate and the possibility of patients achieving a CR to undergo a transplant procedure.
Patients and Methods
In this study we retrospectively analyzed the outcome of 42 adult patients with advanced phase AML consecutively treated in our Institute between 2005 and 2010. The main inclusion criteria were: diagnosis of AML with the exclusion of acute promyelocytic leukemia patients; first or subsequent relapse or refractory disease; adequate cardiac function (LVEF ≥50%); positivity of blast cells to the CD33 antigen.
The median age was 47 years (range 19-63). Twenty patients were females and 22 males. Thirteen patients had refractory disease, 24 patients were in 1st relapse (median 1st CR duration 11 months, range 1-95) and 5 patients had more advanced disease. One patient in 1st relapse had received an allograft in 1st CR and suffered the relapse 95 months after the 1st CR. Nine patients (22%) had high-risk cytogenetic features and 10 (25%) were FLT3 ITD+. Eleven of the 42 patients had received a stem cell transplant in 1st CR; more in particular, 8 patients had undergone an autologous transplant and 3 an allogeneic stem cell transplant.
The biological and clinical risk factors of the patients are detailed in Table 1.
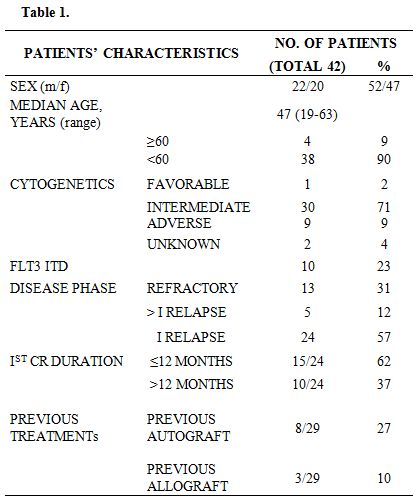
Table 1
Salvage Regimen and Supportive Care. The chemotherapy regimen (AIM) consisted of intermediate dose of Ara-C (1 g/m2/day by four-hour intravenous infusion on days 1-5), Idarubicine (8 mg/m2/day by one hour intravenous infusion on days 1, 3 and 5), GO (3 mg/m2/day by two hours intravenous infusion on day 6) and Peg-filgrastim 6 mg fixed dose by subcutaneous injection on day 8.
Before GO administration, all patients received steroids (40 mg of methylprednisolone), antihistamine and acetaminophen 1g orally to prevent or ameliorate the infusion-related symptoms. Prophylactic oral quinolones were given when the neutrophil count fell below 0.5 x 109/l. Acyclovir i.v. (250 mg/8h) was started on day +9 to prevent Herpes virus infection. Intravenous broad spectrum antibiotics were given for fever during aplasia. In case of persistence of fever over 72 hours after the beginning of antibiotic therapy, a work-up was performed to investigate the presence of a fungal infection (cranial, paranasal and thorax CT scan, evaluation of galactomannan serum levels and plugs). For patients who had experienced a fungal infection during previous treatments, antifungal therapy was given at the end of the chemotherapy regimen as secondary prophylactic treatment. All blood products were irradiated prior to infusion to prevent graft-versus-host reactions. Low-dose heparin for veno-occlusive disease (VOD) prophylaxis was not scheduled in our patients.
Response Criteria. A CR was defined as a cellular bone marrow with less than 5% of blasts, a normalization of peripheral blood counts and a disappearance of all clinical features related to leukemia persisting for more than one month. A patient was defined as refractory if either progression of disease during induction or a persistent bone marrow blastosis on day +28 from the start of chemotherapy were recorded. Relapse was defined as a reappearance of leukemic blasts in the peripheral blood or ≥5% blasts in the bone marrow in two consecutive bone marrow aspirates performed at a one week interval.[14]
Statistical Analysis. Data were analyzed as of June 2012. Overall survival (OS) was calculated from the start of chemotherapy to the date of death from any cause or of the last follow-up. Disease-free-survival (DFS) was calculated from the date of CR to the date of relapse or death and to the last follow up for patients in CR. OS and DFS probabilities were estimated using the Kaplan-Meier method as calculated according to the SPSS software.
Results
The overall CR rate was 76% (32/42 patients). The median time to remission was 27 days (range 16-94). Two patients achieved a CR after two induction cycles. Ten patients (24%) proved refractory, but only 5/10 belonged to a high-risk category (4 FLT3 ITD+, 1 secondary leukemia). No induction-related deaths were reported. The results are shown in Table 2.
The median OS and disease-free survival (DFS) times are 8 months (range 5-50) and 7 months (range 1-50), respectively. The two-year OS and DFS rates are 29% and 25%, respectively (Fig.1, Fig.2).The cumulative rates of relapse are 41% and 45% at 1 and 2 years, respectively. Among the 32 responding patients 25 have died. The cause of death was AML in 12 patients, infection in 1 patient and transplant-related toxicity in 12 patients. None of the transplant-related deaths was due to the occurrence of VOD.
By log rank analysis, consolidation treatment before transplant had no significant impact on survival (p: 0.5).
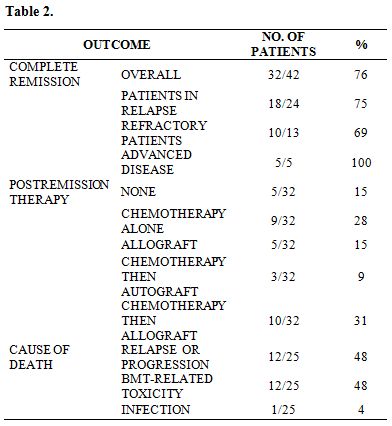
Table 2
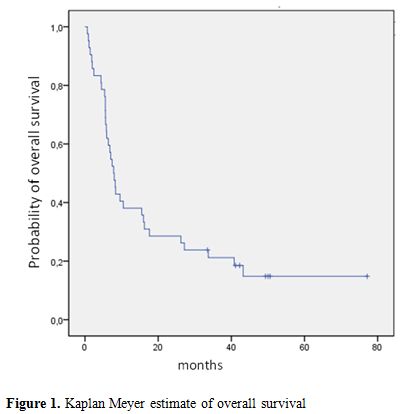
Figure 1. Kaplan Meyer estimate of overall survival
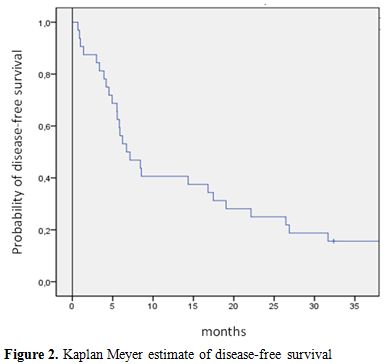
Figure 2. Kaplan Meyer estimate of disease-free survival
Post-remission Therapy. Patients achieving a CR were submitted, as soon as possible, to a transplant procedure. In the case of a delay in donor availability, they received a consolidation course with the same chemotherapy schedule. Among the 32 patients who achieved a CR, 5 did not receive further chemotherapy after the AIM regimen because of early relapse (n=1), poor performance status (n=1) and planned donor lymphocyte infusion (DLI) (n=3), because of a previous allograft. Twenty-two patients received 1 or 2 consolidation cycles.
In 3 patients, chemotherapy was followed by an autograft. Fifteen patients could proceed to an allogeneic HSCT, directly after AIM induction (n=5) or after consolidation chemotherapy (n=10). Allogeneic stem cell transplant consisted of a standard matched related HSCT in 4 patients, a matched unrelated allogeneic HSCT in 7 patients, a haploidentical HSCT in 4.
Toxicity. The median time required to attain an absolute neutrophil count in excess of 0.5 x 109/L was 19 days (range 16-50). A sustained platelet count exceeding 50 x 109/L was reached after a median of 27 days (range 17-108). The median duration of neutropenia was 14 days (range 5-55). Extra-hematological toxicity was mild: 7 patients (17%) suffered a grade III-IV mucositis according to the WHO classification. No patient presented alopecia. Thirty patients had fever; in 20 of them (48%), a bacteremia was documented.
Out of 42 patients, 15 (36%) had experienced fungal infections during previous treatments and therefore received successful systemic prophylactic antifungal therapy during aplasia. Among the remaining patients, 2 developed a fungal infection: pneumonia in 1 case and a spleen abscess in the other.
Neither VOD nor other sign of hepatic toxicity occurred.
Discussion
Although the possibility of achieving a CR in patients with AML has substantially improved over the last decade, relapsed/refractory AML has a very poor prognosis with standard chemotherapy; thus, the possibility of undergoing a transplant procedure has to be considered the goal for any re-induction therapy.[15]
Recently, the development of targeted strategies with compounds more specifically directed against leukemic cells, like the FLT3 inhibitors and the farnesyl-transferase inhibitors, have opened new possibilities for the management of relapsed and refractory AML. GO is the only compound for which there are a number of phase II/III studies in the literature.[9-13] In the phase III MRC AML15 study, a single dose of GO at 3 mg/m2 during the first and third course of chemotherapy was associated with a significant survival benefit in the subset of patients with favourable cytogenetics. Recently the ALFA 0701 study showed that the addiction of fractionated doses of GO to standard chemotherapy (3/3/3 regimen) improves the survival outcome in patients aged 50-70 years with the novo AML. The authors conclude that the fractionated regimen allows the delivery of a high cumulative dose of GO without excessive toxicity.[16,17] In our study, using a new salvage schedule combining chemotherapy with GO and Peg-filgrastim (AIM), we report a high overall CR rate (76%) in the high-risk group of relapsed/refractory AML patients, most of whom had already received a stem cell transplant in 1st CR, had unfavorable molecular and cytogenetic markers, and a 1st CR duration shorter than one year. Despite the relatively limited patient sample size, the remission rate recorded is comparable to that obtained in newly diagnosed AML patients.
The therapeutic regimen was well tolerated. No induction deaths were observed and the hematological toxicity proved comparable to that of other salvage schedules previously reported. The low fungal infection rate reported is related to a high prevalence of antifungal prophylaxis (36%) because of previous fungal infections. No patient developed a VOD or other hepatotoxic symptoms; this occurred both in patients who had received a transplant procedure in 1st CR and in those treated with azole antifungal agents, which can be considered as other potential factors contributing to liver toxicity. Gut toxicity was nearly absent and no patient presented alopecia. Possible explanations for this extremely mild morbidity may be the choice of utilizing an intermediate dose of Ara-C, which makes it feasible also in heavily pretreated patients, and the use of a reduced dose of GO at 3 mg/m2 based on its documented low toxicity profile.
The protocol design has several advantages: first of all, it combines two of the most efficient agents in AML, such as intermediate doses of Ara-C and Idarubicin, with the anti-CD33 antibody; secondly, GO is administered at the end of chemotherapy, after the debulking phase. Finally, the addition of Peg-filgrastim at the end of chemotherapy might enhance GO-induced apoptosis and inhibit P-gp expression in AML cells.[18,19]
Chevallier and colleagues reported their experience on 62 patients with primary resistant or relapsed AML treated with Mitoxantrone, intermediate-dose Ara-C and Gentuzumab Ozogamicin, documenting a 2-year overall survival of 41%.[20] The main difference between the present study and the French one is that in the latter the proportion of patients achieving a response was 63%, and only 12/39 patients (30%) proceeded to an allogeneic transplant, which was in the majority of cases a reduced intensity conditioning transplant.
In recent years, the development of reduced intensity conditioning regimens has enabled older and more comorbid patients to undergo an allogeneic HSCT. These approaches rely more heavily on the potent graft-versus leukemia (GVL) effects for tumor eradication/control than on chemotherapy doses and are associated with survival rates between 28% and 54% also because of the low risk of morbidity and mortality.[21]
In our series, the median OS from relapse is 8 months with the 2-year projected OS and DFS of 29% and 25%, respectively. The relapse rate is similar to that reported in the literature, but 48% of deaths were related to transplant procedures either performed directly after treatment or after a subsequent relapse.
Unfortunately, due to the results reported by the SWOG Group in the phase III S0106 study,[22] documenting an increased number of deaths during induction therapy in the GO group, GO has recently been withdrawn from the market in the US and Europe, and this has prevented us to increase the patient population. Nevertheless our results suggest that the AIM regimen is safe and feasible, capable of providing a high response rate in relapsed/refractory AML patients and of enabling a high proportion of patients to proceed to a transplant procedure. In this category of heavily pretreated patients, a non-myeloablative allogeneic transplant may contribute to improve the long-term survival probability.
Patients with recurrent or refractory acute myeloid leukemia (AML) have a poor prognostic likelihood. Salvage regimens generally include high-dose Ara-C, but whenever a second CR is attained, the median duration of a second relapse-free interval (RFI) is considerably shorter than the first one, highlighting the need to develop novel therapies.[1-2] In patients who are able to undergo an allogeneic hematopoietic stem cell transplant (HSCT), long-term survival rates range from 25% to 40%.[4] However, only a minority of patients can benefit from a HSCT because of co-morbidities and treatment-related toxicities. Thus, the goal of novel therapies should be to achieve complete remissions (CR) with the lowest possible toxicities, in order to provide a bridge to transplant.[3]
Gentuzumab Ozogamicin (GO) is a conjugate of an anti-CD33 humanized monoclonal antibody and the anthracycline calicheamicin with an attractive mechanism of action, since the CD33 antigen/antibody complex is rapidly internalized in the leukemic cell prior to drug delivery.[4] Pivotal studies of (GO) as single agent at 9 mg/m2 in relapsed AML have shown an overall response rate of 28% and encouraging results have been obtained in phase II trials in which it has been employed in combination with other cytotoxic agents.[5-8]
The EORTC/GIMEMA leukemia group tested in a phase II study (AML-15) the feasibility and safety of the sequential combination of GO and conventional chemotherapy in patients older than 60 years of age with untreated AML, confirming the feasibility and activity of such a treatment strategy in this group of patients.[9]
Moreover, several phase II trials have utilized the combination of Mylotarg with chemotherapy in AML patients with advanced disease, reporting a response rate between 12% and 42%, and a high treatment-related mortality.[10-12] The main dose limiting toxicity of the compound is liver toxicity and the incidence of veno-occlusive disease; thus, when combined with chemotherapy, it is currently used at lower doses.[13]
In this retrospective analysis we evaluated the efficacy and safety of the administration of GO 3 mg/m2 after intensive chemotherapy consisting of intermediate doses of Ara-C and Idarubicine, and followed by Peg-filgrastim in adult patients with refractory/relapsed AML The main endpoints of the study were: the rate of second CR, the survival rate and the possibility of patients achieving a CR to undergo a transplant procedure.
Patients and Methods
In this study we retrospectively analyzed the outcome of 42 adult patients with advanced phase AML consecutively treated in our Institute between 2005 and 2010. The main inclusion criteria were: diagnosis of AML with the exclusion of acute promyelocytic leukemia patients; first or subsequent relapse or refractory disease; adequate cardiac function (LVEF ≥50%); positivity of blast cells to the CD33 antigen.
The median age was 47 years (range 19-63). Twenty patients were females and 22 males. Thirteen patients had refractory disease, 24 patients were in 1st relapse (median 1st CR duration 11 months, range 1-95) and 5 patients had more advanced disease. One patient in 1st relapse had received an allograft in 1st CR and suffered the relapse 95 months after the 1st CR. Nine patients (22%) had high-risk cytogenetic features and 10 (25%) were FLT3 ITD+. Eleven of the 42 patients had received a stem cell transplant in 1st CR; more in particular, 8 patients had undergone an autologous transplant and 3 an allogeneic stem cell transplant.
The biological and clinical risk factors of the patients are detailed in Table 1.

Table 1
Salvage Regimen and Supportive Care. The chemotherapy regimen (AIM) consisted of intermediate dose of Ara-C (1 g/m2/day by four-hour intravenous infusion on days 1-5), Idarubicine (8 mg/m2/day by one hour intravenous infusion on days 1, 3 and 5), GO (3 mg/m2/day by two hours intravenous infusion on day 6) and Peg-filgrastim 6 mg fixed dose by subcutaneous injection on day 8.
Before GO administration, all patients received steroids (40 mg of methylprednisolone), antihistamine and acetaminophen 1g orally to prevent or ameliorate the infusion-related symptoms. Prophylactic oral quinolones were given when the neutrophil count fell below 0.5 x 109/l. Acyclovir i.v. (250 mg/8h) was started on day +9 to prevent Herpes virus infection. Intravenous broad spectrum antibiotics were given for fever during aplasia. In case of persistence of fever over 72 hours after the beginning of antibiotic therapy, a work-up was performed to investigate the presence of a fungal infection (cranial, paranasal and thorax CT scan, evaluation of galactomannan serum levels and plugs). For patients who had experienced a fungal infection during previous treatments, antifungal therapy was given at the end of the chemotherapy regimen as secondary prophylactic treatment. All blood products were irradiated prior to infusion to prevent graft-versus-host reactions. Low-dose heparin for veno-occlusive disease (VOD) prophylaxis was not scheduled in our patients.
Response Criteria. A CR was defined as a cellular bone marrow with less than 5% of blasts, a normalization of peripheral blood counts and a disappearance of all clinical features related to leukemia persisting for more than one month. A patient was defined as refractory if either progression of disease during induction or a persistent bone marrow blastosis on day +28 from the start of chemotherapy were recorded. Relapse was defined as a reappearance of leukemic blasts in the peripheral blood or ≥5% blasts in the bone marrow in two consecutive bone marrow aspirates performed at a one week interval.[14]
Statistical Analysis. Data were analyzed as of June 2012. Overall survival (OS) was calculated from the start of chemotherapy to the date of death from any cause or of the last follow-up. Disease-free-survival (DFS) was calculated from the date of CR to the date of relapse or death and to the last follow up for patients in CR. OS and DFS probabilities were estimated using the Kaplan-Meier method as calculated according to the SPSS software.
Results
The overall CR rate was 76% (32/42 patients). The median time to remission was 27 days (range 16-94). Two patients achieved a CR after two induction cycles. Ten patients (24%) proved refractory, but only 5/10 belonged to a high-risk category (4 FLT3 ITD+, 1 secondary leukemia). No induction-related deaths were reported. The results are shown in Table 2.
The median OS and disease-free survival (DFS) times are 8 months (range 5-50) and 7 months (range 1-50), respectively. The two-year OS and DFS rates are 29% and 25%, respectively (Fig.1, Fig.2).The cumulative rates of relapse are 41% and 45% at 1 and 2 years, respectively. Among the 32 responding patients 25 have died. The cause of death was AML in 12 patients, infection in 1 patient and transplant-related toxicity in 12 patients. None of the transplant-related deaths was due to the occurrence of VOD.
By log rank analysis, consolidation treatment before transplant had no significant impact on survival (p: 0.5).

Table 2

Figure 1. Kaplan Meyer estimate of overall survival

Figure 2. Kaplan Meyer estimate of disease-free survival
Post-remission Therapy. Patients achieving a CR were submitted, as soon as possible, to a transplant procedure. In the case of a delay in donor availability, they received a consolidation course with the same chemotherapy schedule. Among the 32 patients who achieved a CR, 5 did not receive further chemotherapy after the AIM regimen because of early relapse (n=1), poor performance status (n=1) and planned donor lymphocyte infusion (DLI) (n=3), because of a previous allograft. Twenty-two patients received 1 or 2 consolidation cycles.
In 3 patients, chemotherapy was followed by an autograft. Fifteen patients could proceed to an allogeneic HSCT, directly after AIM induction (n=5) or after consolidation chemotherapy (n=10). Allogeneic stem cell transplant consisted of a standard matched related HSCT in 4 patients, a matched unrelated allogeneic HSCT in 7 patients, a haploidentical HSCT in 4.
Toxicity. The median time required to attain an absolute neutrophil count in excess of 0.5 x 109/L was 19 days (range 16-50). A sustained platelet count exceeding 50 x 109/L was reached after a median of 27 days (range 17-108). The median duration of neutropenia was 14 days (range 5-55). Extra-hematological toxicity was mild: 7 patients (17%) suffered a grade III-IV mucositis according to the WHO classification. No patient presented alopecia. Thirty patients had fever; in 20 of them (48%), a bacteremia was documented.
Out of 42 patients, 15 (36%) had experienced fungal infections during previous treatments and therefore received successful systemic prophylactic antifungal therapy during aplasia. Among the remaining patients, 2 developed a fungal infection: pneumonia in 1 case and a spleen abscess in the other.
Neither VOD nor other sign of hepatic toxicity occurred.
Discussion
Although the possibility of achieving a CR in patients with AML has substantially improved over the last decade, relapsed/refractory AML has a very poor prognosis with standard chemotherapy; thus, the possibility of undergoing a transplant procedure has to be considered the goal for any re-induction therapy.[15]
Recently, the development of targeted strategies with compounds more specifically directed against leukemic cells, like the FLT3 inhibitors and the farnesyl-transferase inhibitors, have opened new possibilities for the management of relapsed and refractory AML. GO is the only compound for which there are a number of phase II/III studies in the literature.[9-13] In the phase III MRC AML15 study, a single dose of GO at 3 mg/m2 during the first and third course of chemotherapy was associated with a significant survival benefit in the subset of patients with favourable cytogenetics. Recently the ALFA 0701 study showed that the addiction of fractionated doses of GO to standard chemotherapy (3/3/3 regimen) improves the survival outcome in patients aged 50-70 years with the novo AML. The authors conclude that the fractionated regimen allows the delivery of a high cumulative dose of GO without excessive toxicity.[16,17] In our study, using a new salvage schedule combining chemotherapy with GO and Peg-filgrastim (AIM), we report a high overall CR rate (76%) in the high-risk group of relapsed/refractory AML patients, most of whom had already received a stem cell transplant in 1st CR, had unfavorable molecular and cytogenetic markers, and a 1st CR duration shorter than one year. Despite the relatively limited patient sample size, the remission rate recorded is comparable to that obtained in newly diagnosed AML patients.
The therapeutic regimen was well tolerated. No induction deaths were observed and the hematological toxicity proved comparable to that of other salvage schedules previously reported. The low fungal infection rate reported is related to a high prevalence of antifungal prophylaxis (36%) because of previous fungal infections. No patient developed a VOD or other hepatotoxic symptoms; this occurred both in patients who had received a transplant procedure in 1st CR and in those treated with azole antifungal agents, which can be considered as other potential factors contributing to liver toxicity. Gut toxicity was nearly absent and no patient presented alopecia. Possible explanations for this extremely mild morbidity may be the choice of utilizing an intermediate dose of Ara-C, which makes it feasible also in heavily pretreated patients, and the use of a reduced dose of GO at 3 mg/m2 based on its documented low toxicity profile.
The protocol design has several advantages: first of all, it combines two of the most efficient agents in AML, such as intermediate doses of Ara-C and Idarubicin, with the anti-CD33 antibody; secondly, GO is administered at the end of chemotherapy, after the debulking phase. Finally, the addition of Peg-filgrastim at the end of chemotherapy might enhance GO-induced apoptosis and inhibit P-gp expression in AML cells.[18,19]
Chevallier and colleagues reported their experience on 62 patients with primary resistant or relapsed AML treated with Mitoxantrone, intermediate-dose Ara-C and Gentuzumab Ozogamicin, documenting a 2-year overall survival of 41%.[20] The main difference between the present study and the French one is that in the latter the proportion of patients achieving a response was 63%, and only 12/39 patients (30%) proceeded to an allogeneic transplant, which was in the majority of cases a reduced intensity conditioning transplant.
In recent years, the development of reduced intensity conditioning regimens has enabled older and more comorbid patients to undergo an allogeneic HSCT. These approaches rely more heavily on the potent graft-versus leukemia (GVL) effects for tumor eradication/control than on chemotherapy doses and are associated with survival rates between 28% and 54% also because of the low risk of morbidity and mortality.[21]
In our series, the median OS from relapse is 8 months with the 2-year projected OS and DFS of 29% and 25%, respectively. The relapse rate is similar to that reported in the literature, but 48% of deaths were related to transplant procedures either performed directly after treatment or after a subsequent relapse.
Unfortunately, due to the results reported by the SWOG Group in the phase III S0106 study,[22] documenting an increased number of deaths during induction therapy in the GO group, GO has recently been withdrawn from the market in the US and Europe, and this has prevented us to increase the patient population. Nevertheless our results suggest that the AIM regimen is safe and feasible, capable of providing a high response rate in relapsed/refractory AML patients and of enabling a high proportion of patients to proceed to a transplant procedure. In this category of heavily pretreated patients, a non-myeloablative allogeneic transplant may contribute to improve the long-term survival probability.
References
- Lee S, Tallman MS, Oken MM, Cassileth PA,
Bennett JM, Wiernik PH, Rowe JM. Duration of second complete remission
compared with first complete remission in patients with acute myeloid
leukemia. Leukemia 2000; 14: 1345-1348. http://dx.doi.org/10.1038/sj.leu.2401853 PMid:10942227

- Giles F, O'Brien S, Cortes J, Verstovsek S,
Bueso-Ramos C, Shan J, Pierce S, Garcia-Manero G, Keating M, Kantarjian
H. Outcome of patients with acute myelogenous leukemia after second
salvage therapy. Cancer. 2005 Aug 1; 104: 547-54. http://dx.doi.org/10.1002/cncr.21187 PMid:15973664

- Horwitz ME. Reduced intensity versus
myeloablative allogeneic stem cell transplantation for the treatment of
acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid
leukemia. Curr Opin Oncol. 2011;23:197-202 http://dx.doi.org/10.1097/CCO.0b013e328342b82a PMid:21252669

- Morris KL, Adams JA, Liu JA. Effect of
gemtuzumab ozogamicin on acute myeloid leukaemia blast cells in vitro,
as a single agent and combined with other cytotoxic agents. British
Journal of Haematology 2006; 135, 509-512 http://dx.doi.org/10.1111/j.1365-2141.2006.06326.x PMid:17054677

- Larson RA, Sievers EL, Stadtmauer EA,
Löwenberg B, Estey EH, Dombret H, Theobald M, Voliotis D, Bennett JM,
Richie M, Leopold LH, Berger MS, Sherman ML, Loken MR, van Dongen JJ,
Bernstein ID, Appelbaum FR. Final report of the efficacy and safety of
gemtuzumab ozogamicin (Mylotarg) in patients with CD33-Positive acute
myeloid leukemia in first recurrence. Cancer 2005; 104:1442-52. http://dx.doi.org/10.1002/cncr.21326 PMid:16116598

- Taksin AL, Legrand O, Raffoux E, de Revel
T, Thomas X, Contentin N, Bouabdallah R, Pautas C, Turlure P, Reman O,
Gardin C, Varet B, de Botton S, Pousset F, Farhat H, Chevret S, Dombret
H, Castaigne S. High efficacy and safety profile of fractionated doses
of Mylotarg as induction therapy in patients with relapsed acute
myeloblastic leukemia: a prospective study of the Alfa group. Leukemia
2007; 21: 66-71. http://dx.doi.org/10.1038/sj.leu.2404434 PMid:17051246

- Alvarado Y, Tsimberidou A, Kantarjian H,
Cortes J, Garcia-Manero G, Faderl S, Thomas D, Estey E, Giles FJ. Pilot
study of Mylotarg, idarubicin and cytarabine combination regimen in
patients with primary resistant or relapsed acute myeloid leukemia.
Cancer Chemother Pharmacol 2002; 51:87-90. http://dx.doi.org/10.1007/s00280-002-0546-z PMid:12497211

- Kell WJ, Burnett AK, Chopra R, Yin JA,
Clark RE, Rohatiner A, Culligan D, Hunter A, Prentice AG, Milligan DW.
A feasibility study of simultaneous administration of gemtuzumab
ozogamicin with intensive chemotherapy in induction and consolidation
in younger patients with acute myeloid leukemia. Blood.
2003;102:4277-83. http://dx.doi.org/10.1182/blood-2003-05-1620 PMid:12933575

- Amadori S, Suciu S, Stasi R, Willemze R,
Mandelli F, Selleslag D, Denzlinger C, Muus P, Stauder R, Berneman Z,
Pruijt J, Nobile F, Cassibba V, Marie JP, Beeldens F, Baila L, Vignetti
M, de Witte T. Gemtuzumab ozogamicin (Mylotarg) as single-agent
treatment for frail patients 61 years of age and older with acute
myeloid leukemia: final results of AML-15B, a phase 2 study of the
European Organisation for Research and Treatment of Cancer and Gruppo
Italiano Malattie Ematologiche dell'Adulto Leukemia Groups. Leukemia.
2005;19:1768-73. http://dx.doi.org/10.1038/sj.leu.2403901 PMid:16079891

- Apostolidou E, Cortes J, Tsimberidou A,
Estey E, Kantarjian H, Giles FJ. Pilot study of gemtuzumab ozogamicin,
liposomal daunorubicin, cytarabine and cyclosporine regimen in patients
with refractory acute myelogenous leukemia. Leuk Res. 2003; 27: 887-91 http://dx.doi.org/10.1016/S0145-2126(03)00021-3

- Chevallier P, Roland V, Mahé B,
Juge-Morineau N, Dubruille V, Guillaume T, Vigouroux S, Moreau P,
Milpied N, Garand R, Avet-Loiseau H, Harousseau JL. Administration of
mylotarg 4 days after beginning of a chemotherapy including
intermediate-dose aracytin and mitoxantrone (MIDAM regimen) produces a
high rate of complete hematologic remission in patients with CD33+
primary resistant or relapsed acute myeloid leukemia. Leuk Res. 2005;
29:1003-7. http://dx.doi.org/10.1016/j.leukres.2005.02.005 PMid:16038726

- Walter RB, Gooley TA, van der Velden VH,
Loken MR, van Dongen JJ, Flowers DA, Bernstein ID, Appelbaum FR. CD33
expression and P-glycoprotein-mediated drug efflux inversely correlate
and predict clinical outcome in patients with acute myeloid leukemia
treated with gemtuzumab ozogamicin monotherapy. Blood. 2007; 109:
4168-70. http://dx.doi.org/10.1182/blood-2006-09-047399 PMid:17227830 PMCid:1885511

- Malfuson JV, Konopacki J, Thepenier C,
Eddou H, Foissaud V, de Revel T.. Fractionated doses of Gentuzumab
ozogamicin combined with 3+7 induction chemotherapy as salvage
treatment for young patients with acute myeloid leukemia in first
relapse. Ann Hematol epub 21 July 2012

- Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA,
Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B,
Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the
International Working Group for Diagnosis, Standardization of Response
Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic
Trials in Acute Myeloid Leukemia.; International Working Group for
Diagnosis, Standardization of Response Criteria, Treatment Outcomes,
and Reporting Standards for Therapeutic Trials in Acute Myeloid
Leukemia. J Clin Oncol. 2003; 21: 4642-9. http://dx.doi.org/10.1200/JCO.2003.04.036 PMid:14673054

- Giles F, Verstovsek S, Garcia-Manero G,
Thomas D, Ravandi F, Wierda W, Ferrajoli A, Kornblau S, Jabbour E, Shan
J, O'brien S, Albitar M, Kantarjian H. Validation of the European
Prognostic Index for younger adult patiens with acute myeloid leukemia
in first relapse. British Journal of Hematology 2006; 134: 58-60. http://dx.doi.org/10.1111/j.1365-2141.2006.06106.x PMid:16803568

- Burnett AK, Hills RK, Milligan D, Kjeldsen
L, Kell J, Russell NH, Yin JA, Hunter A, Goldstone AH, Wheatley K.
Identification of patients with acute myeloblastic leukemia who benefit
from the addition of gentuzumab ozogamicin: results of the MRC AML 15
trial. J clin Oncol 2011; 29:369-77. http://dx.doi.org/10.1200/JCO.2010.31.4310 PMid:21172891

- Castaigne S, Pautas C, Terré C, Raffoux E,
Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, de
Revel T, Gastaud L, de Gunzburg N, Contentin N, Henry E, Marolleau JP,
Aljijakli A, Rousselot P, Fenaux P, Preudhomme C, Chevret S, Dombret H;
Acute Leukemia. French Association. Effect of gentuzumab ozogamicin on
survival of adult patients with de-novo acute myeloid leukaemia
(ALFA-0701): a randomized, open-label, phase 3 study. Lancet.
2012;379:1508-16 http://dx.doi.org/10.1016/S0140-6736(12)60485-1

- Rutella S, Bonanno G, Procoli A, Mariotti
A, Lucia MB, Contemi AM, Cauda R, Fianchi L, Scambia G, Pagano L, Leone
G. Granulocyte colony-stimulating factor enhances the in vitro
cytotoxicity of gemtuzumab ozogamicin against acute myeloid leukemia
cell lines and primary blast cells. Exp Hematol. 2006;341: 54-65. http://dx.doi.org/10.1016/j.exphem.2005.10.003 PMid:16413391

- Piccirillo N, De Matteis S, De Vita S,
Laurenti L, Chiusolo P, Sorà F, Reddiconto G, d'Onofrio G, Leone G,
Sica S. Kinetics of peg-filgrastim after high-dose chemotherapy and
autologous peripheral blood stem cell transplantation. Bone Marrow
Transplant. 2007;406: 579-83. http://dx.doi.org/10.1038/sj.bmt.1705772 PMid:17637690

- Chevallier P, Delaunay J, Turlure P,
Pigneux A, Hunault M, Garand R, Guillaume T, Avet-Loiseau H, Dmytruk N,
Girault S, Milpied N, Ifrah N, Mohty M, Harousseau JL. Long-term
disease-free survival after gemtuzumab, intermediate-dose cytarabine,
and mitoxantrone in patients with CD33(+) primary resistant or relapsed
acute myeloid leukemia. J Clin Oncol. 2008; 26: 5192-7. http://dx.doi.org/10.1200/JCO.2007.15.9764 PMid:18854573

- Gyurkocza B, Storb R, Storer BE, Chauncey
TR, Lange T, Shizuru JA, Langston AA, Pulsipher MA, Bredeson CN,
Maziarz RT, Bruno B, Petersen FB, Maris MB, Agura E, Yeager A, Bethge
W, Sahebi F, Appelbaum FR, Maloney DG, Sandmaier BM. Nonmyeloablative
allogeneic hematopoietic cell transplantation in patients with acute
myeloid leukemia. J Clin Oncol. 2010; 28: 2859-67. http://dx.doi.org/10.1200/JCO.2009.27.1460 PMid:20439626 PMCid:2903320

- Petersdorf S, Kopecky K, Stuart RK, Larson
RA, Nevill TJ, Stenke J, Slovak ML, Tallman MS, Willman CL, Erba H, and
Appelbaum FR. Preliminary Results of Southwest Oncology Group Study
S0106: An International Intergroup Phase 3 Randomized Trial Comparing
the Addition of Gemtuzumab Ozogamicin to Standard Induction Therapy
Versus Standard Induction Therapy Followed by a Second Randomization to
Post-Consolidation Gemtuzumab Ozogamicin Versus No Additional Therapy
for Previously Untreated Acute Myeloid Leukemia. Blood (ASH Annual
Meeting Abstracts), Nov 2009; 114: 790