Malaria in Pregnancy
Ebako Ndip Takem1 and Umberto D’Alessandro1,2
1Medical
Research Council Unit, Fajara, The Gambia
2Institute of Tropical Medicine, Antwerp, Belgium
2Institute of Tropical Medicine, Antwerp, Belgium
Correspondence
to: Prof. Umberto D’Alessandro, Institute of Tropical
Medicine, Antwerp, Belgium. E-mail: udalessandro@mrc.gm
Published: January 2, 2013
Received: September 28, 2012
Accepted: November 11, 2012
Meditter J Hematol Infect Dis 2013, 5(1): e2013010, DOI 10.4084/MJHID.2013.010
This article is available on PDF format at:

This is an Open
Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Pregnant
women have a higher risk of malaria compared to non-pregnant women.
This review provides an update on knowledge acquired since 2000 on P.
falciparum and P.vivax infections in pregnancy. Maternal risk factors
for malaria in pregnancy (MiP) include low maternal age, low parity,
and low gestational age. The main effects of MIP include maternal
anaemia, low birth weight (LBW), preterm delivery and increased infant
and maternal mortality.
P. falciparum infected erythrocytes sequester in the placenta by expressing surface antigens, mainly variant surface antigen (VAR2CSA), that bind to specific receptors, mainly chondroitin sulphate A. In stable transmission settings, the higher malaria risk in primigravidae can be explained by the non-recognition of these surface antigens by the immune system. Recently, placental sequestration has been described also for P.vivax infections. The mechanism of preterm delivery and intrauterine growth retardation is not completely understood, but fever (preterm delivery), anaemia, and high cytokines levels have been implicated.
Clinical suspicion of MiP should be confirmed by parasitological diagnosis. The sensitivity of microscopy, with placenta histology as the gold standard, is 60% and 45% for peripheral and placental falciparum infections in African women, respectively. Compared to microscopy, RDTs have a lower sensitivity though when the quality of microscopy is low RDTs may be more reliable.
Insecticide treated nets (ITN) and intermittent preventive treatment in pregnancy (IPTp) are recommended for the prevention of MiP in stable transmission settings. ITNs have been shown to reduce malaria infection and adverse pregnancy outcomes by 28-47%. Although resistance is a concern, SP has been shown to be equivalent to MQ and AQ for IPTp. For the treatment of uncomplicated malaria during the first trimester, quinine plus clindamycin for 7 days is the first line treatment and artesunate plus clindamycin for 7 days is indicated if this treatment fails; in the 2nd and 3rd trimester first line treatment is an artemisinin-based combination therapy (ACT) known to be effective in the region or artesunate and clindamycin for 7 days or quinine and clindamycin. For severe malaria, in the second and third trimester parenteral artesunate is preferred over quinine. In the first trimester, both artesunate and quinine (parenteral) may be considered as options. Nevertheless, treatment should not be delayed and should be started immediately with the most readily available drug.
P. falciparum infected erythrocytes sequester in the placenta by expressing surface antigens, mainly variant surface antigen (VAR2CSA), that bind to specific receptors, mainly chondroitin sulphate A. In stable transmission settings, the higher malaria risk in primigravidae can be explained by the non-recognition of these surface antigens by the immune system. Recently, placental sequestration has been described also for P.vivax infections. The mechanism of preterm delivery and intrauterine growth retardation is not completely understood, but fever (preterm delivery), anaemia, and high cytokines levels have been implicated.
Clinical suspicion of MiP should be confirmed by parasitological diagnosis. The sensitivity of microscopy, with placenta histology as the gold standard, is 60% and 45% for peripheral and placental falciparum infections in African women, respectively. Compared to microscopy, RDTs have a lower sensitivity though when the quality of microscopy is low RDTs may be more reliable.
Insecticide treated nets (ITN) and intermittent preventive treatment in pregnancy (IPTp) are recommended for the prevention of MiP in stable transmission settings. ITNs have been shown to reduce malaria infection and adverse pregnancy outcomes by 28-47%. Although resistance is a concern, SP has been shown to be equivalent to MQ and AQ for IPTp. For the treatment of uncomplicated malaria during the first trimester, quinine plus clindamycin for 7 days is the first line treatment and artesunate plus clindamycin for 7 days is indicated if this treatment fails; in the 2nd and 3rd trimester first line treatment is an artemisinin-based combination therapy (ACT) known to be effective in the region or artesunate and clindamycin for 7 days or quinine and clindamycin. For severe malaria, in the second and third trimester parenteral artesunate is preferred over quinine. In the first trimester, both artesunate and quinine (parenteral) may be considered as options. Nevertheless, treatment should not be delayed and should be started immediately with the most readily available drug.
Introduction
Epidemiology. Malaria in pregnancy (MiP) is a major public health problem in endemic countries. There is a wealth of evidence showing that the risk of malaria (both infection and clinical disease) is higher in pregnant than in non-pregnant women, possibly due to the immunological, hormonal changes or other factors occurring during pregnancy. Most of the available evidence is on Plasmodium falciparum and P. vivax, though for the latter, there is much less information than for P. falciparum, while little is known on P. ovale and P. malariae, the other two human malaria species. This review will focus on P.falciparum and P. vivax, with the objective of providing an update on the recently acquired knowledge (since the year 2000).
Burden. Where transmission is stable and relatively high, mainly in sub-Saharan Africa, adults have acquired immunity against malaria, including pregnant women who, despite the immune tolerance occurring during pregnancy, are able to control but not clear malaria infections. Therefore, in this high risk group, asymptomatic infections are common while clinical malaria is relatively rare. A recent review of studies, carried out in sub-Saharan Africa between 2000 and 2011, reports that malaria prevalence in pregnant women attending antenatal clinics was 29.5% (95%CI: 22.4 -36.5) in East and Southern Africa, and 35.1% (95%CI: 28.2-41.9) in West and Central Africa, while the prevalence of placenta malaria was 26.5% (95%CI: 16.7-36.4) in East and Southern Africa, and 38% (95%CI: 28.4-47.6) in West and Central Africa.[1] More recently (studies published since 2008), the reported malaria prevalence (by microscopy unless specified otherwise) was lower, reflecting the recent decrease in malaria transmission observed in several African countries[2-11] (Table 1). Most of the prevalence estimates were done by microscopy and they would probably be higher if more sensitive methods like PCR[12] or placental histology[13] were used. In addition, blood samples were collected at different times during pregnancy, increasing the difficulty of comparing different estimates.
In areas of low, unstable malaria transmission, mainly Asia-Pacific region and South America, pregnant women have a lower acquired immunity and malaria infections are more likely to evolve towards clinical disease. The number of pregnancies occurring in these areas has been estimated at 70.5 million in 2007.[14] In the Asia-Pacific region, the median proportion of women with peripheral infection has been estimated at 15.3% and that of placenta malaria at 11%.[15] For South and Central America, less data on the burden of malaria in pregnancy is available (Table 2). In Peru, the cumulative incidence of clinical malaria in pregnant women for the period January-August 2004 and 2005 was 43.1% as compared to 31.6% in non-pregnant women.[16] This study also suggested that subclinical malaria infections may occur frequently among pregnant women in this region, despite the relatively low transmission, and that passive surveillance, i.e. data collection at health facilities, may underestimate the actual burden of MiP. In Colombia, the prevalence of malaria among parturient women attending the local hospital was 13% when determined by microscopy and 32% by PCR.[17] In the same study, the prevalence of placenta malaria was 9% by microscopy and 26% by PCR, while 2% and 13% of cord blood samples were positive by microscopy and PCR, respectively.
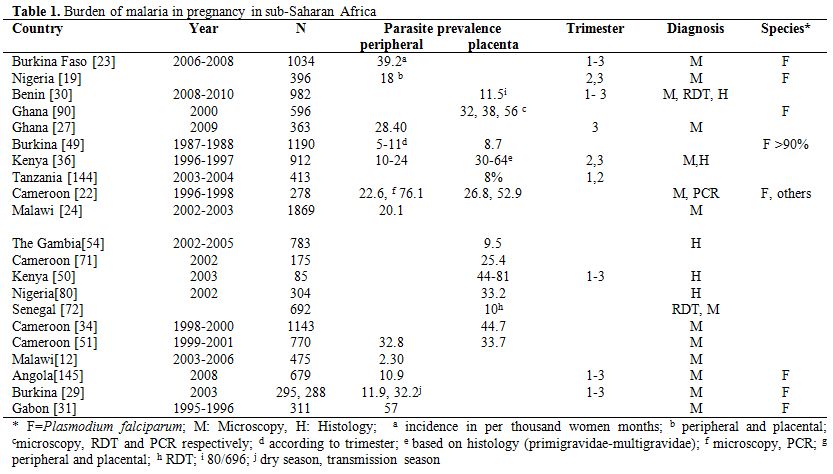
Table 1. Burden of malaria in pregnancy in sub-Saharan Africa.
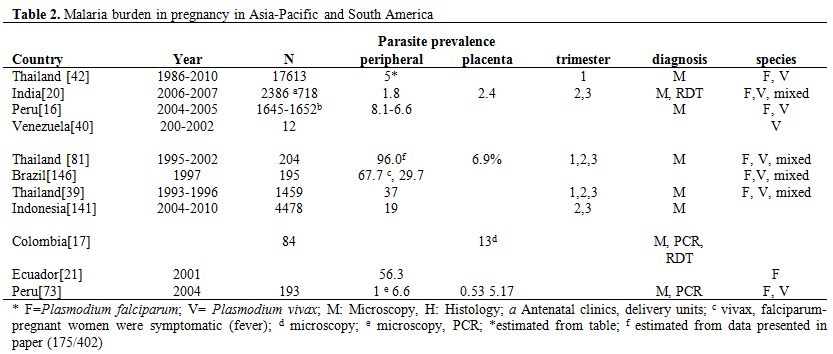
Table 2. Malaria burden in pregnancy in Asia-Pacific and South America.
Risk Factors. Maternal factors associated with the risk of malaria in pregnancy include maternal age, parity and gestational age. It is well established that younger women (primigravidae and multigravidae), particularly adolescents, are at higher risk of malaria infection than older women,[18-20] and this is independent of parity.[20-22] Parity also affects the risk of malaria as primigravidae are at higher risk than multigravidae,[18-20,23-24] though less in low transmission settings,[15] while in epidemic areas, the risk is not affected by parity.[25] Most of the available data on malaria relate to the second and third trimesters.[12,19,26-27] The peak of malaria prevalence seems to occur during the second trimester.[28] Studies on malaria burden in the first trimester of pregnancy are scarce, but it is believed that the rates are similar to that of the second trimester. However, considering the difficulty of collecting this information (pregnant women start to attend the antenatal clinic after the first trimester), and of determining the gestational age with accuracy, it is unclear whether the risk starts to increase towards the end of the first trimester. Indeed, in Burkina Faso, malaria prevalence was higher during the first as compared to the second and third trimesters.[29]
Effects of Malaria Infection. The effect of malaria infection during pregnancy will depend on the degree of acquired immunity, which in turn depends on the intensity of transmission.
Maternal effects. Where transmission is stable, such as in most of sub-Saharan Africa, most infections are asymptomatic but increase substantially the risk of anaemia.[19,26,30-31] This occurs over a background of physiological anaemia of pregnancy due to increased blood volume. Both symptomatic and asymptomatic infections can cause anaemia. Severe anaemia is more often observed in stable transmission settings,[32-34] and more in primigravidae than in multigravidae.[35-36] Malaria infections in the first or second trimester of pregnancy increase the risk of anaemia,[24,30] though one study reported an increased risk also for infections occurring in the third trimester.[30] Preventing malaria infection by intermittent preventive treatment during pregnancy (IPTp) reduces the risk of anaemia.[27,37-38]
Where malaria transmission is unstable, malaria can cause maternal anaemia,[18,35,39-40] more in primigravidae than in multigravidae and for falciparum infections more than for vivax infections.[18,35] Nevertheless, severe anaemia is less common in these settings.[39,41]
In places where malaria transmission is stable, little is known on the importance of malaria infection as a cause of miscarriage. Where malaria transmission is unstable, malaria as a cause of miscarriage seems more common, as the majority of infections evolve towards a clinical attack with fever, which may by itself determine miscarriage. Indeed, non malarial fevers also independently increase the risk of miscarriage.[18,42] Nevertheless, asymptomatic infections, i.e. slide confirmed malaria with no history of fever in the previous 48 hours and temperature <37.5˚C, was also associated with miscarriage.[7]
Maternal mortality associated to malaria is probably under-reported. Malaria was an important cause of maternal death in some studies,[43-45] while in others it was not as frequent.[46] The substantial reduction in maternal mortality observed in Thailand after the implementation of early detection and treatment of malaria suggests that malaria is an important contributor to maternal mortality.[47] When not a direct cause of death (severe malaria),[47] malaria in pregnancy is often reported as co-morbidity, e.g. with eclampsia, in conditions associated with maternal mortality.[44,48]
Perinatal effects. Malaria increases the risk of low birth weight (LBW),[19,23,30,49-51] particularly in primigravidae, and this risk seems to be higher for infections in first or second trimester,[23-24,30,49] though in one study this was true also for infections occurring late in pregnancy.[49] In high malaria transmission settings, such an effect is due to intrauterine growth retardation (IUGR) rather than pre-term delivery, as most infections are asymptomatic. A meta-analysis of 32 cross-sectional data in Africa, showed malaria prevention in pregnancy is associated with 21% (95% CI= 14-27) reduction in LBW.[52]
In unstable transmission settings, preterm deliveries, still births and neonatal deaths have been associated with malaria.18 P.vivax infections are also associated with LBW, and this effect appears to be similar in all pregnancies. In women with a single infection of P.vivax or P.falciparum detected and treated in the first trimester, no significant effect on gestation or birth weight was observed compared to women who also attended in the first trimester but who never had malaria detected in pregnancy.[42]
New born and infant effects. Fewer studies on malaria in pregnant women have evaluated infant outcomes. Congenital malaria can occur in the neonatal period and can contribute to infant morbidity and mortality.[53] Placenta malaria, especially active infection, has been linked to neonatal and infant mortality.[53] A recent study in The Gambia has showed that malaria infection during pregnancy influences infant’s growth, independently of LBW.[54] It also increases the risk of infant’s death and perinatal mortality, by causing LBW.[39,53,55] This is confirmed by the reduction neonatal mortality, up to 60%, observed after the implementation of preventive interventions targeted to pregnant women, e.g. intermittent preventive treatment.[56-57] In primi- and secundi-gravidae, malaria prevention with IPTp or insecticide-treated bed nets was significantly associated with a 18% decreased risk of neonatal mortality.[52]
Later childhood, adolescence and adulthood effects. The long term effects of malaria in pregnancy have not been studied. However, malaria causes IUGR leading to LBW, which may be related to diseases occurring during adulthood, including some cancers and the metabolic syndrome.[58]
Pathophysiology
Pregnant women are at higher risk of contracting malaria than non-pregnant women. This increased susceptibility can be explained by the immunological changes induced by pregnancy, by hormonal factors,[59] and by the higher attractiveness of pregnant women to mosquitoes.[60-61] In addition, P. falciparum -infected erythrocytes in pregnant women bind to specific receptors, i.e. chondroitin sulphate A (CSA), and sequester in the placenta.[62-63] They rarely bind to the other two commonly described receptors in non-pregnant individuals, i.e. CD36 and the intracellular adhesion molecule (ICAM-1). In pregnancy, the parasite antigens expressed on infected erythrocytes are collectively known as variant surface antigen-pregnancy associated malaria (VSAPAM). They are different from those expressed in non-pregnant individuals and in stable transmission settings are not recognised by the immune system, explaining the higher risk in primigravidae.[64] The binding of the variant surface antigen (VAR2CSA) with chondroitin sulphate A has been implicated in the pathology of falciparum malaria in pregnancy.[65-68] The VAR2CSA belongs to the family of the erythrocyte membrane protein (PfEMP1), is encoded by the var2csa gene and its expression has been described in pregnant women with falciparum malaria.[69] Levels of anti-VAR2CSA specific IgGs increase with parity, cannot be found in men and are associated with a favourable pregnancy outcome[64-66] so that the malaria risk decreases with increasing parity. Besides the antibody responses to VSAPAM, cytokine responses such as Th1, Th2, interleukins, TNF and regulators, IFN gamma,[70-72] and monocytes[73] have been observed in pregnant women with malaria. Rosetting, a phenomenon consisting of parasite-free erythrocytes surrounding parasite-infected erythrocytes and commonly observed in non-pregnant individuals, has been implicated in the pathogenesis of severe malaria[74-75] but is uncommon in pregnant women with falciparum malaria.[76]
The sequestration of P. vivax in the placenta, though until recently thought not to occur, has been described,[77-78] with the involvement of ICAM-1 and CSA as receptors.
The effects of hormonal changes on pregnancy associated malaria have been described in few studies and are subject to debate. Increased cortisol levels have been associated with increased risk of malaria in pregnant women.[79]
The increased attractiveness of pregnant women to mosquitoes may be explained by physiological and behavioural changes occurring during pregnancy. Physiological changes include increased exhaled breath and increased abdominal temperature that may render pregnant women more easily detectable by mosquitoes. Behavioural changes are represented by the fact that pregnant women urinate twice as frequently as non-pregnant women, resulting in an increased exposure to mosquito bites at night because they have to leave the protection of their bed nets.[60-61]
Malaria-associated placental changes have been described for stable[72,80] and unstable transmission settings.[73,81] They include presence of parasites, inflammatory changes and hemozoin (pigment) deposition. Placental changes have been characterised into four levels, i.e. acute (parasites present, malaria pigment absent), chronic (parasites and malaria pigment present), past infection (no parasite but pigment present) and no infection (both parasites and malaria pigment absent).[82] Recently, a 2-parameter grading system, distinguishing between inflammation and pigment deposition, has been proposed as it correlates with pregnancy outcomes, in both a stable transmission setting in Tanzania, and an unstable setting in Thailand.[73]
It is unclear what the mechanism at the basis of malaria-related preterm delivery is, though fever, anaemia, and high levels of TNF alpha or interleukin 10 have been identified as important risk factors.[18,83-84]
LBW due to IUGR is associated with maternal anaemia,[83,85] and elevated levels of cytokines.[70] Although the exact mechanism has not been elucidated, it appears to be due to chronic infections that cause reduced foetal circulation and placental insufficiency.[86] Placental endocrine changes related to falciparum infection have been suggested as another possible mechanism leading to IUGR.[87]
P.vivax is different from P. falciparum as it infects immature erythrocytes (reticulocytes), limiting the parasite densities. In addition, it can relapse during pregnancy due to the activation of liver hypnozoites. Vivax parasites do not frequently express variant surface antigens, at the basis of placenta sequestration, so that this does not occur frequently.[81] Therefore, P. vivax probably affects birth weight, and increases the risk of miscarriage and preterm birth through a systemic rather than a local effect. Nevertheless, the mechanisms at the basis of these observations are not completely understood.
Clinical Presentation
Diagnosis. The diagnosis of malaria in pregnancy is essential to prevent its deleterious effects to the mother and the foetus. Unfortunately, the clinical signs of malaria in pregnant women are usually non specific, and where transmission is stable, most infections are asymptomatic. Therefore, suspected malaria cases should be confirmed by parasitological diagnosis,[88] usually by microscopy and/or rapid diagnostic tests. Nevertheless, other methods such as PCR and placental histology can be also used, though the latter can be done only after delivery so that it cannot be used for the management of infections occurring during pregnancy.
Microscopy is one of the most widely used methods for diagnosing malaria, including during pregnancy. It has some advantages such as the possibility of determining the parasite density and species. However, its major disadvantage, besides the need of a regular power supply, is its sensitivity, which cannot go below 10-15 parasites per Ál. Therefore, a substantial proportion of infected pregnant women would not be detected because of extremely low parasite densities or of parasites sequestered in the placenta, though both conditions have deleterious effects on the mother’s and her offspring’s health.
Several studies have investigated the use of microscopy for the diagnosis of MiP in stable malaria transmission settings in Africa.[89-91] When taking placenta histology as the reference test, the sensitivity of peripheral blood microscopy for _P. falciparum infections (4 studies) was 60% (95% CI=50-69) and that of placental microscopy 45% (95% CI=34-56).[13]
In settings with unstable malaria transmission, there are few studies on the sensitivity of microscopy on peripheral blood collected during pregnancy.[13]
Rapid diagnostic tests (RDT), detecting circulating malaria antigens, can also be used. Generally, the sensitivity of RDTs for the diagnosis of malaria in pregnancy is lower than that of microscopy. However, the time needed for the diagnosis is shorter than for microscopy and the training required for their use is minimal. Although RDT can detect malaria antigens, they cannot estimate the parasite density. The sensitivity of RDT on peripheral blood using peripheral microscopy as a reference test is estimated at 81% (95% CI= 55-95), and the sensitivity of RDT on placental blood was 81% (95% CI= 62-92) using placental microscopy as the reference.[13]
PCR, which detects parasite DNA, can also be used for the diagnosis of malaria infection but is not readily available in health facilities. In stable transmission settings, the sensitivity of PCR was >80% when using microscopy as the reference.[13] PCR sensitivity has not been estimated against placental histology as reference test.
Severe malaria. Severe malaria in pregnancy is more common in unstable transmission settings because of the lower immunity pregnant women have. Generally, women in the second and third trimesters of pregnancy are at a higher risk of developing severe malaria compared to non-pregnant adults. In low transmission settings, severe malaria in pregnancy is usually associated with pulmonary oedema, hypoglycaemia and severe anaemia. Mortality in pregnant women with severe malaria and treated with either artesunate and quinine varied between 9% and 12%.[92]
Prevention and Treatment
Prevention. The most widely used interventions to prevent malaria in pregnancy are insecticide-treated bed nets (ITN), including Long-Lasting Insecticidal Nets (LLINs), and intermittent preventive treatment in pregnancy (IPTp).
While ITNs have shown a substantial reduction in malaria morbidity and mortality in children,[93-96] in pregnant women, it has been associated with a decrease in maternal parasitaemia (38%), anaemia (41%) and LBW (28%),[97] and 47% reduction in maternal anaemia.[98] In one study, there was no evidence of a reduction in anaemia and parasitaemia.[99]
IPTp is the administration of therapeutic doses of an antimalarial, currently sulfadoxine-pyrimethamine (SP), at least twice during pregnancy, in the second and third trimester, irrespective of the presence of a malaria infection. The WHO recommends its use and many sub-Saharan African countries have included it in their malaria control program. In stable transmission settings, many trials have shown that SP given as IPTp is efficacious in preventing the adverse consequences of malaria during pregnancy (Table 3).[100-104] However, SP resistance represents a major threat. A study in Benin has showed that, despite the presence of molecular markers of resistance, SP remained efficacious.[105] This has been confirmed by a review reporting that IPTp with SP is effective up to a certain level of SP resistance.[106] Nevertheless, finding an alternative to SP for IPTp is important. Adding amodiaquine to SP was efficacious but not better than SP alone.[107] Mefloquine (MQ), thanks to its long elimination half-life, could be a good alternative to SP as it would provide a long post-treatment prophylactic period. Indeed, a trial in Benin showed that for IPTp MQ was as good as SP in preventing LBW. MQ was more efficacious than SP in preventing placental malaria, clinical malaria and maternal anaemia at delivery. However, MQ was less well tolerated than SP, potentially compromising its large scale use as IPTp.[108-109]
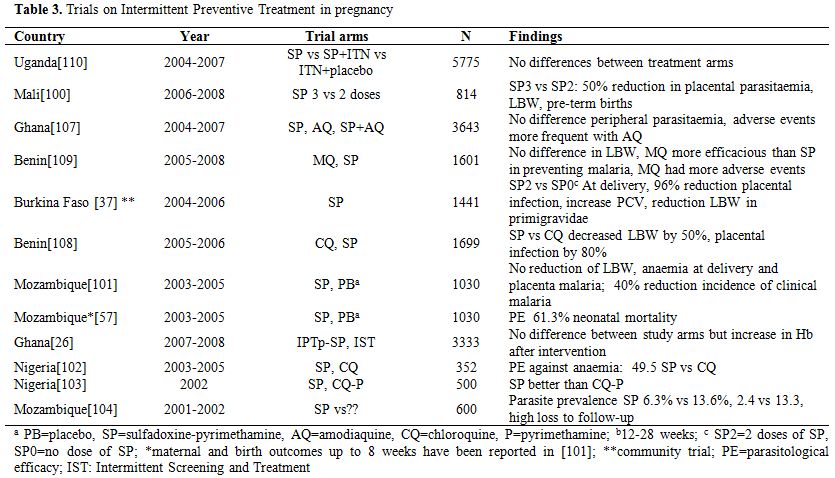
Table 3. Trials on Intermittent Preventive Treatment in pregnancy.
There is no evidence that one of the methods is better than the other[110] and the combined use appears to be better than individual use.
A different approach is systematic screening for malaria infections at regular intervals and treatment of the positive women, which may be more appropriate in settings where malaria transmission is low and the risk of infection between antenatal visits is also low. It has already be shown to have similar protective efficacy than IPTp but additional trials for a more thorough evaluation of this intervention are probably needed.[26] Due to drug resistant malaria, it has been the only form of malaria control on the Thai-Burmese border for more than 20 years, impacting significantly on maternal mortality rates.[47]
In future, vaccines specifically designed to prevent MiP may become available; VAR2CSA, in the early stages of development, seems the most promising candidate.[111-116] However, there are still several uncertainties, including the number of antigenic variants to be combined for an optimal response, the timing of the vaccine, e.g. during pregnancy or at puberty, whether only first pregnancies should be targeted, and the length of follow up for children born to vaccinated mothers.[111-112,117]
Treatment. It is recommended that pregnant women with malaria are treated after parasitological confirmation of the diagnosis, reducing the unnecessary exposure to antimalarials of both the mother and the foetus.
First trimester. Clinical trials on the safety and efficacy of antimalarials in pregnancy usually exclude women in the first trimester of pregnancy so that the evidence is based on observational studies (Table 4). Artemisinin derivatives were relatively safe (n=1937) in the first trimester of pregnancy[42,118-119] and the cumulative failure rate reported in only one study was 6.6% across all trimesters (n=461).[118] No major adverse event was observed in 377 women with known pregnancy outcome and exposed to artemisinins in the first trimester.[42,119-121] However, only 1 study[120] out of 4, was a randomised controlled trial though the treatment was given during a mass campaign and the exposure was thus inadvertent; the birth weight of newborns delivered by women exposed to artesunate during the first trimester was similar to that of the other pregnant women. According to recommendations,[88] chloroquine, quinine, clindamycin and proguanil can be considered safe in the first trimester.
In case of uncomplicated malaria in the first trimester, a combination of quinine + clindamycin for 7 days is recommended.
In case of severe malaria, parenteral antimalarials are recommended.[88] In the first trimester, the risk of hypoglycaemia is lower and the uncertainties on the safety of the artemisinins derivatives are greater. Nevertheless, considering that treatment should not be delayed and that artesunate reduces the risk of death, both artesunate and quinine (parenteral) may be considered as options. Treatment should be started immediately with the most readily available drug.[90]
Second and third trimesters. There is more experience on the use of artemisinin derivatives in the second and third trimesters of pregnancy. Evidence is available from both trials[122-127] and observational studies[128-131] involving pregnant women (Table 4). Data available indicate that ACTs are relatively safe for the foetus when taken after the first trimester of pregnancy. A recent review of treatment studies carried out in pregnant women from 1998-2009, reported a parasitological failure >5% in 3 out of 11 trials.[132] In the second trimester, ACTs that are known to be effective in the area, or 7 days artesunate+ clindamycin, or 7 days quinine+ clindamycin are recommended for uncomplicated malaria.[88] In case of severe malaria, parenteral artesunate is preferable because it saves the life of the mother. Several studies have shown that the kinetics of artemisinins derivatives, most specifically of the active metabolite dihydroartemisinin, is modified during pregnancy.[133-134]
Amodiaquine (AQ) has been shown to be efficacious in pregnant women with falciparum malaria in Ghana and Tanzania.[122,125] Day 28 parasitological failure rates were 3% for AQ monotherapy,[122] 0-1% for the combination AQ+SP,[122,125] and 4.5% for the combination AS+AQ.[125] It was relatively safe and well tolerated and associated with some minor side effects (nausea, weakness, dizziness). Blood dyscrasias were not a problem associated with its use. A pharmacokinetics study on AQ for treatment of P.vivax in pregnancy conducted in Thailand indicates the doses are similar to that of non-pregnant adults.[131,135]
There are fewer reports on the efficacy and safety of mefloquine (MQ) for MiP. High cure rates have been reported in Thailand, for the combination of MQ+AS (cure rate of 98.2% at day 63).[126] One study reported minor side effects.[109] However, there are concerns about still births and neuropsychiatric disorders. There are currently some ongoing clinical studies which will provide useful data on the safety, efficacy and pharmacokinetics of MQ in pregnant women (Table 5). The combination AS+ MQ is being evaluated in studies in Africa and Asia (NCT00852423, NCT00701961, NCT01054248, CTRI/2009/091/001055TEMP, NCT01054248).
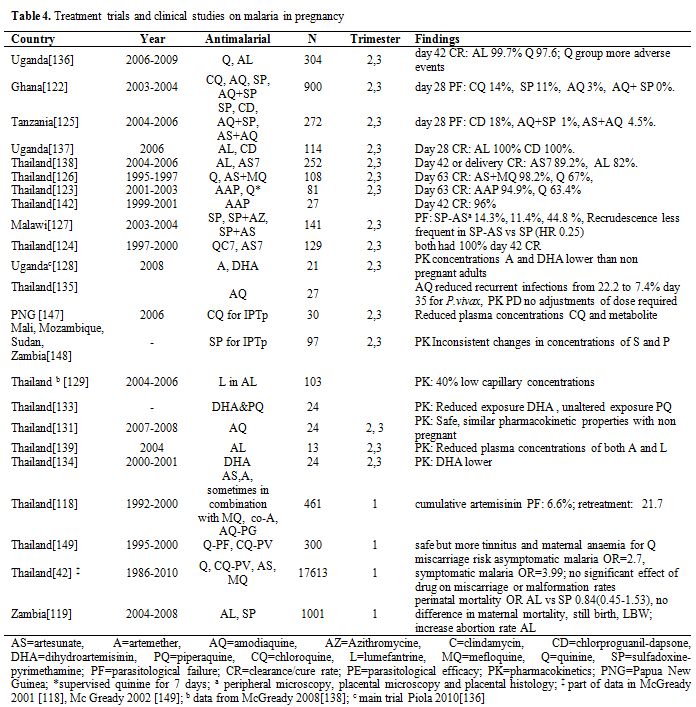
Table 4. Treatment trials and clinical studies on malaria in pregnancy.
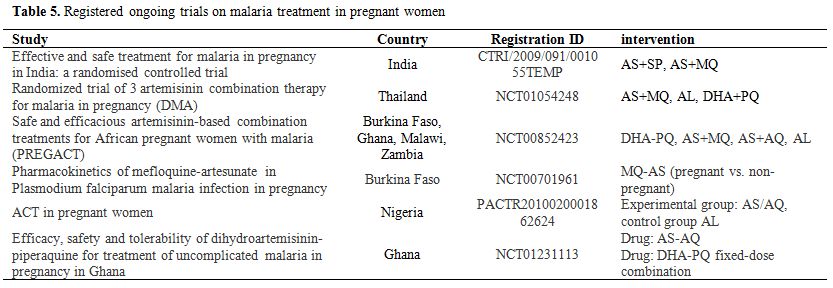
Table 5. Registered ongoing trials on malaria treatment in pregnant women.
In Uganda, in an area of relatively high transmission and hence with pregnant women having some acquired immunity, artemether-Lumefantrine (AL) was efficacious, with cure rates >95%.[136-137] However, in Thailand the cure rate at day 42 was only 82%,[138] possibly due to the low day 7 lumefantrine concentrations. AL was safe and well tolerated.[136-138] As for other antimalarial treatments, pharmacokinetics may be altered during pregnancy, with plasma concentrations lower than expected.[129,139] AL is currently being evaluated in Thailand and in four sites in sub-Saharan Africa (NCT01054248, NCT00852423).
Dihydroartemisinin piperaquine (DHAPQ) was highly effective in women with multiple recrudescent infections on the Thai-Burmese border.[140] DHAPQ is used in the Western Pacific for malaria in pregnant women.[141] DHA-PQ is currently being evaluated in 3 studies in Africa and Asia (NCT00852423, NCT01054248, NCT01231113). Cure rates and PK are reassuring.
In Thailand, atovaquone-proguanil in combination with artesunate (AAP) was associated with high cure rates (>95%) and was relatively safe,[123,142] though the sample size was small. In Thailand, plasma concentrations of AAP were lower in pregnant than in non pregnant women.[143]
Conclusions
This review shows that although the deleterious effects of MiP to both the mother and the child are well documented, the mechanisms involved are still relatively unknown, particularly where transmission is low and unstable. The diagnosis of MiP is challenging, as peripheral microscopy will miss a large proportion of infected women with parasites sequestered in the placenta. MiP can be prevented by currently available control methods, i.e. ITNs and IPTp, but the challenge is attaining a high coverage, particularly for women with the highest risk such as adolescent primigravidae. It is still unclear what would be the alternative to SP for the IPTp.
The burden of P. vivax MiP, which is substantial in the Asia-Pacific region and in South America has been relatively neglected. It is generally believed that vivax infections are milder than falciparum ones, but this is based on few studies. There is also the need of having more sensitive diagnostic methods for vivax infections, as it would help improving early diagnosis and appropriate management. Finally, information of the safety and efficacy of antimalarials during pregnancy is growing, though this is true mainly for the second and third trimester. For the first trimester, treatment options are still extremely limited and evidence is mainly based on pharmacovigilance data on accidental exposures.
Epidemiology. Malaria in pregnancy (MiP) is a major public health problem in endemic countries. There is a wealth of evidence showing that the risk of malaria (both infection and clinical disease) is higher in pregnant than in non-pregnant women, possibly due to the immunological, hormonal changes or other factors occurring during pregnancy. Most of the available evidence is on Plasmodium falciparum and P. vivax, though for the latter, there is much less information than for P. falciparum, while little is known on P. ovale and P. malariae, the other two human malaria species. This review will focus on P.falciparum and P. vivax, with the objective of providing an update on the recently acquired knowledge (since the year 2000).
Burden. Where transmission is stable and relatively high, mainly in sub-Saharan Africa, adults have acquired immunity against malaria, including pregnant women who, despite the immune tolerance occurring during pregnancy, are able to control but not clear malaria infections. Therefore, in this high risk group, asymptomatic infections are common while clinical malaria is relatively rare. A recent review of studies, carried out in sub-Saharan Africa between 2000 and 2011, reports that malaria prevalence in pregnant women attending antenatal clinics was 29.5% (95%CI: 22.4 -36.5) in East and Southern Africa, and 35.1% (95%CI: 28.2-41.9) in West and Central Africa, while the prevalence of placenta malaria was 26.5% (95%CI: 16.7-36.4) in East and Southern Africa, and 38% (95%CI: 28.4-47.6) in West and Central Africa.[1] More recently (studies published since 2008), the reported malaria prevalence (by microscopy unless specified otherwise) was lower, reflecting the recent decrease in malaria transmission observed in several African countries[2-11] (Table 1). Most of the prevalence estimates were done by microscopy and they would probably be higher if more sensitive methods like PCR[12] or placental histology[13] were used. In addition, blood samples were collected at different times during pregnancy, increasing the difficulty of comparing different estimates.
In areas of low, unstable malaria transmission, mainly Asia-Pacific region and South America, pregnant women have a lower acquired immunity and malaria infections are more likely to evolve towards clinical disease. The number of pregnancies occurring in these areas has been estimated at 70.5 million in 2007.[14] In the Asia-Pacific region, the median proportion of women with peripheral infection has been estimated at 15.3% and that of placenta malaria at 11%.[15] For South and Central America, less data on the burden of malaria in pregnancy is available (Table 2). In Peru, the cumulative incidence of clinical malaria in pregnant women for the period January-August 2004 and 2005 was 43.1% as compared to 31.6% in non-pregnant women.[16] This study also suggested that subclinical malaria infections may occur frequently among pregnant women in this region, despite the relatively low transmission, and that passive surveillance, i.e. data collection at health facilities, may underestimate the actual burden of MiP. In Colombia, the prevalence of malaria among parturient women attending the local hospital was 13% when determined by microscopy and 32% by PCR.[17] In the same study, the prevalence of placenta malaria was 9% by microscopy and 26% by PCR, while 2% and 13% of cord blood samples were positive by microscopy and PCR, respectively.

Table 1. Burden of malaria in pregnancy in sub-Saharan Africa.

Table 2. Malaria burden in pregnancy in Asia-Pacific and South America.
Risk Factors. Maternal factors associated with the risk of malaria in pregnancy include maternal age, parity and gestational age. It is well established that younger women (primigravidae and multigravidae), particularly adolescents, are at higher risk of malaria infection than older women,[18-20] and this is independent of parity.[20-22] Parity also affects the risk of malaria as primigravidae are at higher risk than multigravidae,[18-20,23-24] though less in low transmission settings,[15] while in epidemic areas, the risk is not affected by parity.[25] Most of the available data on malaria relate to the second and third trimesters.[12,19,26-27] The peak of malaria prevalence seems to occur during the second trimester.[28] Studies on malaria burden in the first trimester of pregnancy are scarce, but it is believed that the rates are similar to that of the second trimester. However, considering the difficulty of collecting this information (pregnant women start to attend the antenatal clinic after the first trimester), and of determining the gestational age with accuracy, it is unclear whether the risk starts to increase towards the end of the first trimester. Indeed, in Burkina Faso, malaria prevalence was higher during the first as compared to the second and third trimesters.[29]
Effects of Malaria Infection. The effect of malaria infection during pregnancy will depend on the degree of acquired immunity, which in turn depends on the intensity of transmission.
Maternal effects. Where transmission is stable, such as in most of sub-Saharan Africa, most infections are asymptomatic but increase substantially the risk of anaemia.[19,26,30-31] This occurs over a background of physiological anaemia of pregnancy due to increased blood volume. Both symptomatic and asymptomatic infections can cause anaemia. Severe anaemia is more often observed in stable transmission settings,[32-34] and more in primigravidae than in multigravidae.[35-36] Malaria infections in the first or second trimester of pregnancy increase the risk of anaemia,[24,30] though one study reported an increased risk also for infections occurring in the third trimester.[30] Preventing malaria infection by intermittent preventive treatment during pregnancy (IPTp) reduces the risk of anaemia.[27,37-38]
Where malaria transmission is unstable, malaria can cause maternal anaemia,[18,35,39-40] more in primigravidae than in multigravidae and for falciparum infections more than for vivax infections.[18,35] Nevertheless, severe anaemia is less common in these settings.[39,41]
In places where malaria transmission is stable, little is known on the importance of malaria infection as a cause of miscarriage. Where malaria transmission is unstable, malaria as a cause of miscarriage seems more common, as the majority of infections evolve towards a clinical attack with fever, which may by itself determine miscarriage. Indeed, non malarial fevers also independently increase the risk of miscarriage.[18,42] Nevertheless, asymptomatic infections, i.e. slide confirmed malaria with no history of fever in the previous 48 hours and temperature <37.5˚C, was also associated with miscarriage.[7]
Maternal mortality associated to malaria is probably under-reported. Malaria was an important cause of maternal death in some studies,[43-45] while in others it was not as frequent.[46] The substantial reduction in maternal mortality observed in Thailand after the implementation of early detection and treatment of malaria suggests that malaria is an important contributor to maternal mortality.[47] When not a direct cause of death (severe malaria),[47] malaria in pregnancy is often reported as co-morbidity, e.g. with eclampsia, in conditions associated with maternal mortality.[44,48]
Perinatal effects. Malaria increases the risk of low birth weight (LBW),[19,23,30,49-51] particularly in primigravidae, and this risk seems to be higher for infections in first or second trimester,[23-24,30,49] though in one study this was true also for infections occurring late in pregnancy.[49] In high malaria transmission settings, such an effect is due to intrauterine growth retardation (IUGR) rather than pre-term delivery, as most infections are asymptomatic. A meta-analysis of 32 cross-sectional data in Africa, showed malaria prevention in pregnancy is associated with 21% (95% CI= 14-27) reduction in LBW.[52]
In unstable transmission settings, preterm deliveries, still births and neonatal deaths have been associated with malaria.18 P.vivax infections are also associated with LBW, and this effect appears to be similar in all pregnancies. In women with a single infection of P.vivax or P.falciparum detected and treated in the first trimester, no significant effect on gestation or birth weight was observed compared to women who also attended in the first trimester but who never had malaria detected in pregnancy.[42]
New born and infant effects. Fewer studies on malaria in pregnant women have evaluated infant outcomes. Congenital malaria can occur in the neonatal period and can contribute to infant morbidity and mortality.[53] Placenta malaria, especially active infection, has been linked to neonatal and infant mortality.[53] A recent study in The Gambia has showed that malaria infection during pregnancy influences infant’s growth, independently of LBW.[54] It also increases the risk of infant’s death and perinatal mortality, by causing LBW.[39,53,55] This is confirmed by the reduction neonatal mortality, up to 60%, observed after the implementation of preventive interventions targeted to pregnant women, e.g. intermittent preventive treatment.[56-57] In primi- and secundi-gravidae, malaria prevention with IPTp or insecticide-treated bed nets was significantly associated with a 18% decreased risk of neonatal mortality.[52]
Later childhood, adolescence and adulthood effects. The long term effects of malaria in pregnancy have not been studied. However, malaria causes IUGR leading to LBW, which may be related to diseases occurring during adulthood, including some cancers and the metabolic syndrome.[58]
Pathophysiology
Pregnant women are at higher risk of contracting malaria than non-pregnant women. This increased susceptibility can be explained by the immunological changes induced by pregnancy, by hormonal factors,[59] and by the higher attractiveness of pregnant women to mosquitoes.[60-61] In addition, P. falciparum -infected erythrocytes in pregnant women bind to specific receptors, i.e. chondroitin sulphate A (CSA), and sequester in the placenta.[62-63] They rarely bind to the other two commonly described receptors in non-pregnant individuals, i.e. CD36 and the intracellular adhesion molecule (ICAM-1). In pregnancy, the parasite antigens expressed on infected erythrocytes are collectively known as variant surface antigen-pregnancy associated malaria (VSAPAM). They are different from those expressed in non-pregnant individuals and in stable transmission settings are not recognised by the immune system, explaining the higher risk in primigravidae.[64] The binding of the variant surface antigen (VAR2CSA) with chondroitin sulphate A has been implicated in the pathology of falciparum malaria in pregnancy.[65-68] The VAR2CSA belongs to the family of the erythrocyte membrane protein (PfEMP1), is encoded by the var2csa gene and its expression has been described in pregnant women with falciparum malaria.[69] Levels of anti-VAR2CSA specific IgGs increase with parity, cannot be found in men and are associated with a favourable pregnancy outcome[64-66] so that the malaria risk decreases with increasing parity. Besides the antibody responses to VSAPAM, cytokine responses such as Th1, Th2, interleukins, TNF and regulators, IFN gamma,[70-72] and monocytes[73] have been observed in pregnant women with malaria. Rosetting, a phenomenon consisting of parasite-free erythrocytes surrounding parasite-infected erythrocytes and commonly observed in non-pregnant individuals, has been implicated in the pathogenesis of severe malaria[74-75] but is uncommon in pregnant women with falciparum malaria.[76]
The sequestration of P. vivax in the placenta, though until recently thought not to occur, has been described,[77-78] with the involvement of ICAM-1 and CSA as receptors.
The effects of hormonal changes on pregnancy associated malaria have been described in few studies and are subject to debate. Increased cortisol levels have been associated with increased risk of malaria in pregnant women.[79]
The increased attractiveness of pregnant women to mosquitoes may be explained by physiological and behavioural changes occurring during pregnancy. Physiological changes include increased exhaled breath and increased abdominal temperature that may render pregnant women more easily detectable by mosquitoes. Behavioural changes are represented by the fact that pregnant women urinate twice as frequently as non-pregnant women, resulting in an increased exposure to mosquito bites at night because they have to leave the protection of their bed nets.[60-61]
Malaria-associated placental changes have been described for stable[72,80] and unstable transmission settings.[73,81] They include presence of parasites, inflammatory changes and hemozoin (pigment) deposition. Placental changes have been characterised into four levels, i.e. acute (parasites present, malaria pigment absent), chronic (parasites and malaria pigment present), past infection (no parasite but pigment present) and no infection (both parasites and malaria pigment absent).[82] Recently, a 2-parameter grading system, distinguishing between inflammation and pigment deposition, has been proposed as it correlates with pregnancy outcomes, in both a stable transmission setting in Tanzania, and an unstable setting in Thailand.[73]
It is unclear what the mechanism at the basis of malaria-related preterm delivery is, though fever, anaemia, and high levels of TNF alpha or interleukin 10 have been identified as important risk factors.[18,83-84]
LBW due to IUGR is associated with maternal anaemia,[83,85] and elevated levels of cytokines.[70] Although the exact mechanism has not been elucidated, it appears to be due to chronic infections that cause reduced foetal circulation and placental insufficiency.[86] Placental endocrine changes related to falciparum infection have been suggested as another possible mechanism leading to IUGR.[87]
P.vivax is different from P. falciparum as it infects immature erythrocytes (reticulocytes), limiting the parasite densities. In addition, it can relapse during pregnancy due to the activation of liver hypnozoites. Vivax parasites do not frequently express variant surface antigens, at the basis of placenta sequestration, so that this does not occur frequently.[81] Therefore, P. vivax probably affects birth weight, and increases the risk of miscarriage and preterm birth through a systemic rather than a local effect. Nevertheless, the mechanisms at the basis of these observations are not completely understood.
Clinical Presentation
Diagnosis. The diagnosis of malaria in pregnancy is essential to prevent its deleterious effects to the mother and the foetus. Unfortunately, the clinical signs of malaria in pregnant women are usually non specific, and where transmission is stable, most infections are asymptomatic. Therefore, suspected malaria cases should be confirmed by parasitological diagnosis,[88] usually by microscopy and/or rapid diagnostic tests. Nevertheless, other methods such as PCR and placental histology can be also used, though the latter can be done only after delivery so that it cannot be used for the management of infections occurring during pregnancy.
Microscopy is one of the most widely used methods for diagnosing malaria, including during pregnancy. It has some advantages such as the possibility of determining the parasite density and species. However, its major disadvantage, besides the need of a regular power supply, is its sensitivity, which cannot go below 10-15 parasites per Ál. Therefore, a substantial proportion of infected pregnant women would not be detected because of extremely low parasite densities or of parasites sequestered in the placenta, though both conditions have deleterious effects on the mother’s and her offspring’s health.
Several studies have investigated the use of microscopy for the diagnosis of MiP in stable malaria transmission settings in Africa.[89-91] When taking placenta histology as the reference test, the sensitivity of peripheral blood microscopy for _P. falciparum infections (4 studies) was 60% (95% CI=50-69) and that of placental microscopy 45% (95% CI=34-56).[13]
In settings with unstable malaria transmission, there are few studies on the sensitivity of microscopy on peripheral blood collected during pregnancy.[13]
Rapid diagnostic tests (RDT), detecting circulating malaria antigens, can also be used. Generally, the sensitivity of RDTs for the diagnosis of malaria in pregnancy is lower than that of microscopy. However, the time needed for the diagnosis is shorter than for microscopy and the training required for their use is minimal. Although RDT can detect malaria antigens, they cannot estimate the parasite density. The sensitivity of RDT on peripheral blood using peripheral microscopy as a reference test is estimated at 81% (95% CI= 55-95), and the sensitivity of RDT on placental blood was 81% (95% CI= 62-92) using placental microscopy as the reference.[13]
PCR, which detects parasite DNA, can also be used for the diagnosis of malaria infection but is not readily available in health facilities. In stable transmission settings, the sensitivity of PCR was >80% when using microscopy as the reference.[13] PCR sensitivity has not been estimated against placental histology as reference test.
Severe malaria. Severe malaria in pregnancy is more common in unstable transmission settings because of the lower immunity pregnant women have. Generally, women in the second and third trimesters of pregnancy are at a higher risk of developing severe malaria compared to non-pregnant adults. In low transmission settings, severe malaria in pregnancy is usually associated with pulmonary oedema, hypoglycaemia and severe anaemia. Mortality in pregnant women with severe malaria and treated with either artesunate and quinine varied between 9% and 12%.[92]
Prevention and Treatment
Prevention. The most widely used interventions to prevent malaria in pregnancy are insecticide-treated bed nets (ITN), including Long-Lasting Insecticidal Nets (LLINs), and intermittent preventive treatment in pregnancy (IPTp).
While ITNs have shown a substantial reduction in malaria morbidity and mortality in children,[93-96] in pregnant women, it has been associated with a decrease in maternal parasitaemia (38%), anaemia (41%) and LBW (28%),[97] and 47% reduction in maternal anaemia.[98] In one study, there was no evidence of a reduction in anaemia and parasitaemia.[99]
IPTp is the administration of therapeutic doses of an antimalarial, currently sulfadoxine-pyrimethamine (SP), at least twice during pregnancy, in the second and third trimester, irrespective of the presence of a malaria infection. The WHO recommends its use and many sub-Saharan African countries have included it in their malaria control program. In stable transmission settings, many trials have shown that SP given as IPTp is efficacious in preventing the adverse consequences of malaria during pregnancy (Table 3).[100-104] However, SP resistance represents a major threat. A study in Benin has showed that, despite the presence of molecular markers of resistance, SP remained efficacious.[105] This has been confirmed by a review reporting that IPTp with SP is effective up to a certain level of SP resistance.[106] Nevertheless, finding an alternative to SP for IPTp is important. Adding amodiaquine to SP was efficacious but not better than SP alone.[107] Mefloquine (MQ), thanks to its long elimination half-life, could be a good alternative to SP as it would provide a long post-treatment prophylactic period. Indeed, a trial in Benin showed that for IPTp MQ was as good as SP in preventing LBW. MQ was more efficacious than SP in preventing placental malaria, clinical malaria and maternal anaemia at delivery. However, MQ was less well tolerated than SP, potentially compromising its large scale use as IPTp.[108-109]

Table 3. Trials on Intermittent Preventive Treatment in pregnancy.
There is no evidence that one of the methods is better than the other[110] and the combined use appears to be better than individual use.
A different approach is systematic screening for malaria infections at regular intervals and treatment of the positive women, which may be more appropriate in settings where malaria transmission is low and the risk of infection between antenatal visits is also low. It has already be shown to have similar protective efficacy than IPTp but additional trials for a more thorough evaluation of this intervention are probably needed.[26] Due to drug resistant malaria, it has been the only form of malaria control on the Thai-Burmese border for more than 20 years, impacting significantly on maternal mortality rates.[47]
In future, vaccines specifically designed to prevent MiP may become available; VAR2CSA, in the early stages of development, seems the most promising candidate.[111-116] However, there are still several uncertainties, including the number of antigenic variants to be combined for an optimal response, the timing of the vaccine, e.g. during pregnancy or at puberty, whether only first pregnancies should be targeted, and the length of follow up for children born to vaccinated mothers.[111-112,117]
Treatment. It is recommended that pregnant women with malaria are treated after parasitological confirmation of the diagnosis, reducing the unnecessary exposure to antimalarials of both the mother and the foetus.
First trimester. Clinical trials on the safety and efficacy of antimalarials in pregnancy usually exclude women in the first trimester of pregnancy so that the evidence is based on observational studies (Table 4). Artemisinin derivatives were relatively safe (n=1937) in the first trimester of pregnancy[42,118-119] and the cumulative failure rate reported in only one study was 6.6% across all trimesters (n=461).[118] No major adverse event was observed in 377 women with known pregnancy outcome and exposed to artemisinins in the first trimester.[42,119-121] However, only 1 study[120] out of 4, was a randomised controlled trial though the treatment was given during a mass campaign and the exposure was thus inadvertent; the birth weight of newborns delivered by women exposed to artesunate during the first trimester was similar to that of the other pregnant women. According to recommendations,[88] chloroquine, quinine, clindamycin and proguanil can be considered safe in the first trimester.
In case of uncomplicated malaria in the first trimester, a combination of quinine + clindamycin for 7 days is recommended.
In case of severe malaria, parenteral antimalarials are recommended.[88] In the first trimester, the risk of hypoglycaemia is lower and the uncertainties on the safety of the artemisinins derivatives are greater. Nevertheless, considering that treatment should not be delayed and that artesunate reduces the risk of death, both artesunate and quinine (parenteral) may be considered as options. Treatment should be started immediately with the most readily available drug.[90]
Second and third trimesters. There is more experience on the use of artemisinin derivatives in the second and third trimesters of pregnancy. Evidence is available from both trials[122-127] and observational studies[128-131] involving pregnant women (Table 4). Data available indicate that ACTs are relatively safe for the foetus when taken after the first trimester of pregnancy. A recent review of treatment studies carried out in pregnant women from 1998-2009, reported a parasitological failure >5% in 3 out of 11 trials.[132] In the second trimester, ACTs that are known to be effective in the area, or 7 days artesunate+ clindamycin, or 7 days quinine+ clindamycin are recommended for uncomplicated malaria.[88] In case of severe malaria, parenteral artesunate is preferable because it saves the life of the mother. Several studies have shown that the kinetics of artemisinins derivatives, most specifically of the active metabolite dihydroartemisinin, is modified during pregnancy.[133-134]
Amodiaquine (AQ) has been shown to be efficacious in pregnant women with falciparum malaria in Ghana and Tanzania.[122,125] Day 28 parasitological failure rates were 3% for AQ monotherapy,[122] 0-1% for the combination AQ+SP,[122,125] and 4.5% for the combination AS+AQ.[125] It was relatively safe and well tolerated and associated with some minor side effects (nausea, weakness, dizziness). Blood dyscrasias were not a problem associated with its use. A pharmacokinetics study on AQ for treatment of P.vivax in pregnancy conducted in Thailand indicates the doses are similar to that of non-pregnant adults.[131,135]
There are fewer reports on the efficacy and safety of mefloquine (MQ) for MiP. High cure rates have been reported in Thailand, for the combination of MQ+AS (cure rate of 98.2% at day 63).[126] One study reported minor side effects.[109] However, there are concerns about still births and neuropsychiatric disorders. There are currently some ongoing clinical studies which will provide useful data on the safety, efficacy and pharmacokinetics of MQ in pregnant women (Table 5). The combination AS+ MQ is being evaluated in studies in Africa and Asia (NCT00852423, NCT00701961, NCT01054248, CTRI/2009/091/001055TEMP, NCT01054248).

Table 4. Treatment trials and clinical studies on malaria in pregnancy.

Table 5. Registered ongoing trials on malaria treatment in pregnant women.
In Uganda, in an area of relatively high transmission and hence with pregnant women having some acquired immunity, artemether-Lumefantrine (AL) was efficacious, with cure rates >95%.[136-137] However, in Thailand the cure rate at day 42 was only 82%,[138] possibly due to the low day 7 lumefantrine concentrations. AL was safe and well tolerated.[136-138] As for other antimalarial treatments, pharmacokinetics may be altered during pregnancy, with plasma concentrations lower than expected.[129,139] AL is currently being evaluated in Thailand and in four sites in sub-Saharan Africa (NCT01054248, NCT00852423).
Dihydroartemisinin piperaquine (DHAPQ) was highly effective in women with multiple recrudescent infections on the Thai-Burmese border.[140] DHAPQ is used in the Western Pacific for malaria in pregnant women.[141] DHA-PQ is currently being evaluated in 3 studies in Africa and Asia (NCT00852423, NCT01054248, NCT01231113). Cure rates and PK are reassuring.
In Thailand, atovaquone-proguanil in combination with artesunate (AAP) was associated with high cure rates (>95%) and was relatively safe,[123,142] though the sample size was small. In Thailand, plasma concentrations of AAP were lower in pregnant than in non pregnant women.[143]
Conclusions
This review shows that although the deleterious effects of MiP to both the mother and the child are well documented, the mechanisms involved are still relatively unknown, particularly where transmission is low and unstable. The diagnosis of MiP is challenging, as peripheral microscopy will miss a large proportion of infected women with parasites sequestered in the placenta. MiP can be prevented by currently available control methods, i.e. ITNs and IPTp, but the challenge is attaining a high coverage, particularly for women with the highest risk such as adolescent primigravidae. It is still unclear what would be the alternative to SP for the IPTp.
The burden of P. vivax MiP, which is substantial in the Asia-Pacific region and in South America has been relatively neglected. It is generally believed that vivax infections are milder than falciparum ones, but this is based on few studies. There is also the need of having more sensitive diagnostic methods for vivax infections, as it would help improving early diagnosis and appropriate management. Finally, information of the safety and efficacy of antimalarials during pregnancy is growing, though this is true mainly for the second and third trimester. For the first trimester, treatment options are still extremely limited and evidence is mainly based on pharmacovigilance data on accidental exposures.
References
- Chico RM, Mayaud
P, Ariti C, Mabey D,
Ronsmans C, and Chandramohan D, Prevalence of malaria and sexually
transmitted and reproductive tract infections in pregnancy in
sub-Saharan Africa: a systematic review. JAMA. 2012; 307(19): 2079-86. http://dx.doi.org/10.1001/jama.2012.3428
PMid:22665107

- Teklehaimanot HD,
Teklehaimanot A,
Kiszewski A, Rampao HS, and Sachs JD, Malaria in Sao Tome and principe:
on the brink of elimination after three years of effective antimalarial
measures. Am J Trop Med Hyg. 2009; 80(1): 133-40.
PMid:19141851

- Lee PW, Liu CT,
Rampao HS, do Rosario VE,
and Shaio MF, Pre-elimination of malaria on the island of Principe.
Malar J. 2010; 9: 26. http://dx.doi.org/10.1186/1475-2875-9-26
PMid:20089158 PMCid:2823607

- Beiersmann C,
Bountogo M, Tiendrebeogo J,
De Allegri M, Louis VR, Coulibaly B, Ye M, and Mueller O, Falciparum
malaria in young children of rural Burkina Faso: comparison of survey
data in 1999 with 2009. Malar J. 2011; 10: 296. http://dx.doi.org/10.1186/1475-2875-10-296
PMid:21989335 PMCid:3200185

- Bouyou-Akotet MK,
Mawili-Mboumba DP, Kendjo
E, Mabika-Mamfoumbi M, Ngoungou EB, Dzeing-Ella A, Pemba-Mihindou M,
Ibinga E, Efame-Eya E, Planche T, Kremsner PG, and Kombila M, Evidence
of decline of malaria in the general hospital of Libreville, Gabon from
2000 to 2008. Malar J. 2009; 8: 300. http://dx.doi.org/10.1186/1475-2875-8-300
PMid:20017905 PMCid:2806380

- Otten M, Aregawi
M, Were W, Karema C, Medin
A, Bekele W, Jima D, Gausi K, Komatsu R, Korenromp E, Low-Beer D, and
Grabowsky M, Initial evidence of reduction of malaria cases and deaths
in Rwanda and Ethiopia due to rapid scale-up of malaria prevention and
treatment. Malar J. 2009; 8: 14. http://dx.doi.org/10.1186/1475-2875-8-14
PMid:19144183 PMCid:2653503

- Graves PM, Osgood
DE, Thomson MC, Sereke K,
Araia A, Zerom M, Ceccato P, Bell M, Del Corral J, Ghebreselassie S,
Brantly EP, and Ghebremeskel T, Effectiveness of malaria control during
changing climate conditions in Eritrea, 1998-2003. Trop Med Int Health.
2008; 13(2): 218-28. http://dx.doi.org/10.1111/j.1365-3156.2007.01993.x
PMid:21176056 PMCid:3499407

- Bhattarai A, Ali
AS, Kachur SP, Martensson
A, Abbas AK, Khatib R, Al-Mafazy AW, Ramsan M, Rotllant G, Gerstenmaier
JF, Molteni F, Abdulla S, Montgomery SM, Kaneko A, and Bjorkman A,
Impact of artemisinin-based combination therapy and insecticide-treated
nets on malaria burden in Zanzibar. PLoS Med. 2007; 4(11): e309. http://dx.doi.org/10.1371/journal.pmed.0040309
PMid:17988171 PMCid:2062481

- O'Meara WP, Bejon
P, Mwangi TW, Okiro EA,
Peshu N, Snow RW, Newton CR, and Marsh K, Effect of a fall in malaria
transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;
372(9649): 1555-62. http://dx.doi.org/10.1016/S0140-6736(08)61655-4

- Ceesay SJ,
Casals-Pascual C, Erskine J,
Anya SE, Duah NO, Fulford AJ, Sesay SS, Abubakar I, Dunyo S, Sey O,
Palmer A, Fofana M, Corrah T, Bojang KA, Whittle HC, Greenwood BM, and
Conway DJ, Changes in malaria indices between 1999 and 2007 in The
Gambia: a retrospective analysis. Lancet. 2008; 372(9649): 1545-54. http://dx.doi.org/10.1016/S0140-6736(08)61654-2

- Ceesay SJ,
Casals-Pascual C, Nwakanma DC,
Walther M, Gomez-Escobar N, Fulford AJC, Takem EN, Nogaro S, Bojang KA,
Corrah T, Jaye MC, Taal MA, Sonko AAJ, and Conway DJ, Continued Decline
of Malaria in The Gambia with Implications for Elimination. PLoS ONE.
2010; 5(8): e12242. http://dx.doi.org/10.1371/journal.pone.0012242
PMid:20805878 PMCid:2923605

- Rantala AM,
Taylor SM, Trottman PA,
Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, and Meshnick SR,
Comparison of real-time PCR and microscopy for malaria parasite
detection in Malawian pregnant women. Malar J. 2010; 9: 269. http://dx.doi.org/10.1186/1475-2875-9-269
PMid:20925928 PMCid:2984567

- Kattenberg JH,
Ochodo EA, Boer KR,
Schallig HD, Mens PF, and Leeflang MM, Systematic review and
meta-analysis: rapid diagnostic tests versus placental histology,
microscopy and PCR for malaria in pregnant women. Malar J. 2011; 10:
321. http://dx.doi.org/10.1186/1475-2875-10-321
PMid:22035448 PMCid:3228868

- Dellicour S,
Tatem AJ, Guerra CA, Snow RW,
and ter Kuile FO, Quantifying the number of pregnancies at risk of
malaria in 2007: a demographic study. PLoS Med. 2010; 7(1): e1000221. http://dx.doi.org/10.1371/journal.pmed.1000221
PMid:20126256 PMCid:2811150

- Rijken MJ,
McGready R, Boel ME,
Poespoprodjo R, Singh N, Syafruddin D, Rogerson S, and Nosten F,
Malaria in pregnancy in the Asia-Pacific region. Lancet Infect Dis.
2012; 12(1): 75-88. http://dx.doi.org/10.1016/S1473-3099(11)70315-2

- Parekh FK,
Hernandez JN, Krogstad DJ,
Casapia WM, and Branch OH, Prevalence and risk of Plasmodium falciparum
and P. vivax malaria among pregnant women living in the hypoendemic
communities of the Peruvian Amazon. Am J Trop Med Hyg. 2007; 77(3):
451-7. PMid:17827359

- Campos IM, Uribe
ML, Cuesta C,
Franco-Gallego A, Carmona-Fonseca J, and Maestre A, Diagnosis of
gestational, congenital, and placental malaria in Colombia: comparison
of the efficacy of microscopy, nested polymerase chain reaction, and
histopathology. Am J Trop Med Hyg. 2011; 84(6): 929-35. http://dx.doi.org/10.4269/ajtmh.2011.10-0507
PMid:21633030 PMCid:3110370

- Poespoprodjo JR,
Fobia W, Kenangalem E,
Lampah DA, Warikar N, Seal A, McGready R, Sugiarto P, Tjitra E, Anstey
NM, and Price RN, Adverse pregnancy outcomes in an area where
multidrug-resistant plasmodium vivax and Plasmodium falciparum
infections are endemic. Clin Infect Dis. 2008; 46(9): 1374-81. http://dx.doi.org/10.1086/586743
PMid:18419439 PMCid:2875100

- Ayoola OO,
Whatmore A, Balogun WO, Jarrett
OO, Cruickshank JK, and Clayton PE, Maternal malaria status and
metabolic profiles in pregnancy and in cord blood: relationships with
birth size in Nigerian infants. Malar J. 2012; 11: 75. http://dx.doi.org/10.1186/1475-2875-11-75
PMid:22429464 PMCid:3325162

- Hamer DH, Singh
MP, Wylie BJ, Yeboah-Antwi
K, Tuchman J, Desai M, Udhayakumar V, Gupta P, Brooks MI, Shukla MM,
Awasthy K, Sabin L, MacLeod WB, Dash AP, and Singh N, Burden of malaria
in pregnancy in Jharkhand State, India. Malar J. 2009; 8: 210. http://dx.doi.org/10.1186/1475-2875-8-210
PMid:19728882 PMCid:2744702

- Espinoza E,
Hidalgo L, and Chedraui P, The
effect of malarial infection on maternal-fetal outcome in Ecuador. J
Matern Fetal Neonatal Med. 2005; 18(2): 101-5. http://dx.doi.org/10.1080/147670500231989
PMid:16203594

- Walker-Abbey A,
Djokam RR, Eno A, Leke RF,
Titanji VP, Fogako J, Sama G, Thuita LH, Beardslee E, Snounou G, Zhou
A, and Taylor DW, Malaria in pregnant Cameroonian women: the effect of
age and gravidity on submicroscopic and mixed-species infections and
multiple parasite genotypes. Am J Trop Med Hyg. 2005; 72(3): 229-35.
PMid:15772312

- Valea I, Tinto H,
Drabo MK, Huybregts L,
Sorgho H, Ouedraogo JB, Guiguemde RT, van Geertruyden JP, Kolsteren P,
and D'Alessandro U, An analysis of timing and frequency of malaria
infection during pregnancy in relation to the risk of low birth weight,
anaemia and perinatal mortality in Burkina Faso. Malar J. 2012; 11: 71.
http://dx.doi.org/10.1186/1475-2875-11-71
PMid:22433778 PMCid:3338396

- Kalilani L,
Mofolo I, Chaponda M, Rogerson
SJ, and Meshnick SR, The effect of timing and frequency of Plasmodium
falciparum infection during pregnancy on the risk of low birth weight
and maternal anemia. Trans R Soc Trop Med Hyg. 2010; 104(6): 416-22. http://dx.doi.org/10.1016/j.trstmh.2010.01.013
PMid:20207387

- Newman RD,
Hailemariam A, Jimma D, Degifie
A, Kebede D, Rietveld AE, Nahlen BL, Barnwell JW, Steketee RW, and
Parise ME, Burden of malaria during pregnancy in areas of stable and
unstable transmission in Ethiopia during a nonepidemic year. J Infect
Dis. 2003; 187(11): 1765-72. http://dx.doi.org/10.1086/374878
PMid:12751034

- Tagbor H, Bruce
J, Agbo M, Greenwood B,
and Chandramohan D, Intermittent screening and treatment versus
intermittent preventive treatment of malaria in pregnancy: a randomized
controlled non-inferiority trial. PLoS ONE. 2010; 5(12): e14425. http://dx.doi.org/10.1371/journal.pone.0014425
PMid:21203389 PMCid:3010999

- Wilson NO, Ceesay
FK, Obed SA, Adjei AA,
Gyasi RK, Rodney P, Ndjakani Y, Anderson WA, Lucchi NW, and Stiles JK,
Intermittent preventive treatment with sulfadoxine-pyrimethamine
against malaria and anemia in pregnant women. Am J Trop Med Hyg. 2011;
85(1): 12-21. http://dx.doi.org/10.4269/ajtmh.2011.10-0512
PMid:21734118 PMCid:3122337

- Agbor-Enoh ST,
Achur RN, Valiyaveettil M,
Leke R, Taylor DW, and Gowda DC, Chondroitin sulfate proteoglycan
expression and binding of Plasmodium falciparum-infected erythrocytes
in the human placenta during pregnancy. Infect Immun. 2003; 71(5):
2455-61. http://dx.doi.org/10.1128/IAI.71.5.2455-2461.2003
PMid:12704116 PMCid:153269

- Coulibaly SO,
Gies S, and D'Alessandro U,
Malaria burden among pregnant women living in the rural district of
Boromo, Burkina Faso. Am J Trop Med Hyg. 2007; 77(6 Suppl): 56-60.
PMid:18165475

- Huynh BT, Fievet
N, Gbaguidi G, Dechavanne
S, Borgella S, Guezo-Mevo B, Massougbodji A, Ndam NT, Deloron P, and
Cot M, Influence of the timing of malaria infection during pregnancy on
birth weight and on maternal anemia in Benin. Am J Trop Med Hyg. 2011;
85(2): 214-20. http://dx.doi.org/10.4269/ajtmh.2011.11-0103
PMid:21813837 PMCid:3144815

- Bouyou-Akotet MK,
Ionete-Collard DE,
Mabika-Manfoumbi M, Kendjo E, Matsiegui PB, Mavoungou E, and Kombila M,
Prevalence of Plasmodium falciparum infection in pregnant women in
Gabon. Malar J. 2003; 2: 18. http://dx.doi.org/10.1186/1475-2875-2-18
PMid:12919637 PMCid:183856

- van Eijk AM,
Ayisi JG, Slutsker L, Ter
Kuile FO, Rosen DH, Otieno JA, Shi YP, Kager PA, Steketee RW, and
Nahlen BL, Effect of haematinic supplementation and malaria prevention
on maternal anaemia and malaria in western Kenya. Trop Med Int Health.
2007; 12(3): 342-52. http://dx.doi.org/10.1111/j.1365-3156.2006.01787.x
PMid:21176056 PMCid:3499407

- Tarimo SD,
Appraisal on the prevalence of
malaria and anaemia in pregnancy and factors influencing uptake of
intermittent preventive therapy with sulfadoxine-pyrimethamine in
Kibaha district, Tanzania. East Afr J Public Health. 2007; 4(2): 80-3.
PMid:18085136

- Achidi EA, Kuoh
AJ, Minang JT, Ngum B,
Achimbom BM, Motaze SC, Ahmadou MJ, and Troye-Blomberg M, Malaria
infection in pregnancy and its effects on haemoglobin levels in women
from a malaria endemic area of Fako Division, South West Province,
Cameroon. J Obstet Gynaecol. 2005; 25(3): 235-40. http://dx.doi.org/10.1080/01443610500060628
PMid:23130816 PMCid:3498373

- Guyatt HL and
Snow RW, The epidemiology
and burden of Plasmodium falciparum-related anemia among pregnant women
in sub-Saharan Africa. Am J Trop Med Hyg. 2001; 64(1-2 Suppl):
36-44.

- Shulman CE,
Marshall T, Dorman EK, Bulmer
JN, Cutts F, Peshu N, and Marsh K, Malaria in pregnancy: adverse
effects on haemoglobin levels and birthweight in primigravidae and
multigravidae. Trop Med Int Health. 2001; 6(10): 770-8. http://dx.doi.org/10.1046/j.1365-3156.2001.00786.x
PMid:11679125

- Gies S, Coulibaly
SO, Ouattara FT, and
D'Alessandro U, Individual efficacy of intermittent preventive
treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae
in rural Burkina Faso: impact on parasitaemia, anaemia and birth
weight. Trop Med Int Health. 2009; 14(2): 174-82. http://dx.doi.org/10.1111/j.1365-3156.2008.02215.x
PMid:21176056 PMCid:3499407

- Kayentao K, Kodio
M, Newman RD, Maiga H,
Doumtabe D, Ongoiba A, Coulibaly D, Keita AS, Maiga B, Mungai M, Parise
ME, and Doumbo O, Comparison of intermittent preventive treatment with
chemoprophylaxis for the prevention of malaria during pregnancy in
Mali. J Infect Dis. 2005; 191(1): 109-16. http://dx.doi.org/10.1086/426400
PMid:15593011

- Luxemburger C,
McGready R, Kham A, Morison
L, Cho T, Chongsuphajaisiddhi T, White NJ, and Nosten F, Effects of
malaria during pregnancy on infant mortality in an area of low malaria
transmission. Am J Epidemiol. 2001; 154(5): 459-65. http://dx.doi.org/10.1093/aje/154.5.459
PMid:11532788

- Rodriguez-Morales
AJ, Sanchez E, Vargas M,
Piccolo C, Colina R, Arria M, and Franco-Paredes C, Pregnancy outcomes
associated with Plasmodium vivax malaria in northeastern Venezuela. Am
J Trop Med Hyg. 2006; 74(5): 755-7. PMid:16687675

- O'Donnell A,
Raiko A, Clegg JB, Weatherall
DJ, and Allen SJ, Southeast Asian ovalocytosis and pregnancy in a
malaria-endemic region of Papua New Guinea. Am J Trop Med Hyg. 2007;
76(4): 631-3. PMid:17426161

- McGready R, Lee
SJ, Wiladphaingern J,
Ashley EA, Rijken MJ, Boel M, Simpson JA, Paw MK, Pimanpanarak M, Mu O,
Singhasivanon P, White NJ, and Nosten FH, Adverse effects of falciparum
and vivax malaria and the safety of antimalarial treatment in early
pregnancy: a population-based study. Lancet Infect Dis. 2012; 12(5):
388-96. http://dx.doi.org/10.1016/S1473-3099(11)70339-5

- Romagosa C, Ordi
J, Saute F, Quinto L,
Machungo F, Ismail MR, Carrilho C, Osman N, Alonso PL, and Menendez C,
Seasonal variations in maternal mortality in Maputo, Mozambique: the
role of malaria. Trop Med Int Health. 2007; 12(1): 62-7.
PMid:17207149

- Anya SE, Seasonal
variation in the risk
and causes of maternal death in the Gambia: malaria appears to be an
important factor. Am J Trop Med Hyg. 2004; 70(5): 510-3.
PMid:15155982

- Ali AA, Okud A,
Khojali A, and Adam I,
High incidence of obstetric complications in Kassala Hospital, Eastern
Sudan. J Obstet Gynaecol. 2012; 32(2): 148-9. http://dx.doi.org/10.3109/01443615.2011.637140
PMid:23130816 PMCid:3498373

- Somigliana E,
Sabino A, Schrettenbrunner
C, Nkurunziza R, Okello E, and Manenti F, A comprehensive and
integrated project to improve reproductive health at Oyam district,
northern Uganda: insights from maternal death review at the district
hospital. Arch Gynecol Obstet. 2011; 283(3): 645-9. http://dx.doi.org/10.1007/s00404-010-1780-y
PMid:21113718

- McGready R, Boel
M, Rijken MJ, Ashley EA,
Cho T, Moo O, Paw MK, Pimanpanarak M, Hkirijareon L, Carrara VI, Lwin
KM, Phyo AP, Turner C, Chu CS, van Vugt M, Price RN, Luxemburger C, ter
Kuile FO, Tan SO, Proux S, Singhasivanon P, White NJ, and Nosten FH,
Effect of early detection and treatment on malaria related maternal
mortality on the north-western border of Thailand 1986-2010. PLoS ONE.
2012; 7(7): e40244. http://dx.doi.org/10.1371/journal.pone.0040244
PMid:22815732 PMCid:3399834

- Adam I, Elhassan
EM, Mohmmed AA, Salih MM,
and Elbashir MI, Malaria and pre-eclampsia in an area with unstable
malaria transmission in Central Sudan. Malar J. 2011; 10: 258. http://dx.doi.org/10.1186/1475-2875-10-258
PMid:21899731 PMCid:3224261

- Cottrell G, Mary
JY, Barro D, and Cot M,
The importance of the period of malarial infection during pregnancy on
birth weight in tropical Africa. Am J Trop Med Hyg. 2007; 76(5):
849-54. PMid:17488903

- Kassam SN,
Nesbitt S, Hunt LP, Oster N,
Soothill P, and Sergi C, Pregnancy outcomes in women with or without
placental malaria infection. Int J Gynaecol Obstet. 2006; 93(3):
225-32. http://dx.doi.org/10.1016/j.ijgo.2006.02.021
PMid:20695826 PMCid:3465272

- Akum AE, Kuoh AJ,
Minang JT, Achimbom BM,
Ahmadou MJ, and Troye-Blomberg M, The effect of maternal, umbilical
cord and placental malaria parasitaemia on the birthweight of newborns
from South-western Cameroon. Acta Paediatr. 2005; 94(7): 917-23. http://dx.doi.org/10.1080/08035250510028605
PMid:16188815

- Eisele TP, Larsen
DA, Anglewicz PA,
Keating J, Yukich J, Bennett A, Hutchinson P, and Steketee RW, Malaria
prevention in pregnancy, birthweight, and neonatal mortality: a
meta-analysis of 32 national cross-sectional datasets in Africa. Lancet
Infect Dis. 2012. http://dx.doi.org/10.1016/S1473-3099(12)70222-0

- Bardaji A,
Sigauque B, Sanz S, Maixenchs
M, Ordi J, Aponte JJ, Mabunda S, Alonso PL, and Menendez C, Impact of
malaria at the end of pregnancy on infant mortality and morbidity. J
Infect Dis. 2011; 203(5): 691-9. http://dx.doi.org/10.1093/infdis/jiq049
PMid:21199881 PMCid:3071276

- Walther B, Miles
DJ, Crozier S, Waight P,
Palmero MS, Ojuola O, Touray E, van der Sande M, Whittle H,
Rowland-Jones S, and Flanagan KL, Placental malaria is associated with
reduced early life weight development of affected children independent
of low birth weight. Malar J. 2010; 9: 16. http://dx.doi.org/10.1186/1475-2875-9-16
PMid:20074331 PMCid:2841609

- Diamond-Smith N,
Singh N, Gupta RK, Dash
A, Thimasarn K, Campbell OM, and Chandramohan D, Estimating the burden
of malaria in pregnancy: a case study from rural Madhya Pradesh, India.
Malar J. 2009; 8: 24. http://dx.doi.org/10.1186/1475-2875-8-24
PMid:19210797 PMCid:2647551

- Titaley CR,
Dibley MJ, Roberts CL, and
Agho K, Combined iron/folic acid supplements and malaria prophylaxis
reduce neonatal mortality in 19 sub-Saharan African countries. Am J
Clin Nutr. 2010; 92(1): 235-43. http://dx.doi.org/10.3945/ajcn.2009.29093
PMid:20504976

- Menendez C,
Bardaji A, Sigauque B, Sanz S,
Aponte JJ, Mabunda S, and Alonso PL, Malaria prevention with IPTp
during pregnancy reduces neonatal mortality. PLoS ONE. 2010; 5(2):
e9438.

- Christensen DL,
Kapur A, and Bygbjerg IC,
Physiological adaption to maternal malaria and other adverse exposure:
low birth weight, functional capacity, and possible metabolic disease
in adult life. Int J Gynaecol Obstet. 2011; 115 Suppl 1: S16-9.
PMid:22099434

- Rogerson SJ,
Hviid L, Duffy PE, Leke RF,
and Taylor DW, Malaria in pregnancy: pathogenesis and immunity. Lancet
Infect Dis. 2007; 7(2): 105-17. http://dx.doi.org/10.1016/S1473-3099(07)70022-1

- Lindsay S, Ansell
J, Selman C, Cox V,
Hamilton K, and Walraven G, Effect of pregnancy on exposure to malaria
mosquitoes. Lancet. 2000; 355(9219): 1972. http://dx.doi.org/10.1016/S0140-6736(00)02334-5

- Ansell J,
Hamilton KA, Pinder M, Walraven
GE, and Lindsay SW, Short-range attractiveness of pregnant women to
Anopheles gambiae mosquitoes. Trans R Soc Trop Med Hyg. 2002; 96(2):
113-6. http://dx.doi.org/10.1016/S0035-9203(02)90271-3

- Cserti CM and
Dzik WH, The ABO blood group system and Plasmodium falciparum malaria.
Blood. 2007; 110(7): 2250-8. http://dx.doi.org/10.1182/blood-2007-03-077602
PMid:17502454

- Fairhurst RM and
Wellems TE, Modulation of
malaria virulence by determinants of Plasmodium falciparum erythrocyte
membrane protein-1 display. Curr Opin Hematol. 2006; 13(3): 124-30. http://dx.doi.org/10.1097/01.moh.0000219655.73162.42
PMid:16567953

- Staalsoe T,
Shulman CE, Bulmer JN,
Kawuondo K, Marsh K, and Hviid L, Variant surface antigen-specific IgG
and protection against clinical consequences of pregnancy-associated
Plasmodium falciparum malaria. Lancet. 2004; 363(9405): 283-9. http://dx.doi.org/10.1016/S0140-6736(03)15386-X

- Salanti A,
Dahlback M, Turner L, Nielsen
MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K,
Hviid L, and Theander TG, Evidence for the involvement of VAR2CSA in
pregnancy-associated malaria. J Exp Med. 2004; 200(9): 1197-203. http://dx.doi.org/10.1084/jem.20041579
PMid:15520249 PMCid:2211857

- Tuikue Ndam NG,
Salanti A, Bertin G,
Dahlback M, Fievet N, Turner L, Gaye A, Theander T, and Deloron P, High
level of var2csa transcription by Plasmodium falciparum isolated from
the placenta. J Infect Dis. 2005; 192(2): 331-5. http://dx.doi.org/10.1086/430933
PMid:15962229

- Ofori MF,
Staalsoe T, Bam V, Lundquist M,
David KP, Browne EN, Akanmori BD, and Hviid L, Expression of variant
surface antigens by Plasmodium falciparum parasites in the peripheral
blood of clinically immune pregnant women indicates ongoing placental
infection. Infect Immun. 2003; 71(3): 1584-6. http://dx.doi.org/10.1128/IAI.71.3.1584-1586.2003
PMid:12595482 PMCid:148875

- Duffy PE and
Fried M, Plasmodium falciparum adhesion in the placenta. Curr Opin
Microbiol. 2003; 6(4): 371-6. http://dx.doi.org/10.1016/S1369-5274(03)00090-0

- Sander AF,
Salanti A, Lavstsen T, Nielsen
MA, Theander TG, Leke RG, Lo YY, Bobbili N, Arnot DE, and Taylor DW,
Positive selection of Plasmodium falciparum parasites with multiple
var2csa-type PfEMP1 genes during the course of infection in pregnant
women. J Infect Dis. 2011; 203(11): 1679-85. http://dx.doi.org/10.1093/infdis/jir168
PMid:21592998 PMCid:3096795

- Thevenon AD, Zhou
JA, Megnekou R, Ako S,
Leke RG, and Taylor DW, Elevated levels of soluble TNF receptors 1 and
2 correlate with Plasmodium falciparum parasitemia in pregnant women:
potential markers for malaria-associated inflammation. J Immunol. 2010;
185(11): 7115-22. http://dx.doi.org/10.4049/jimmunol.1002293
PMid:20980627 PMCid:2988086

- Achidi EA,
Apinjoh TO, and Titanji VP,
Malaria parasitemia and systemic cytokine bias in pregnancy. Int J
Gynaecol Obstet. 2007; 97(1): 15-20. http://dx.doi.org/10.1016/j.ijgo.2006.12.015
PMid:20695826 PMCid:3465272

- Sarr D, Aldebert
D, Marrama L, Frealle E,
Gaye A, Brahim HO, Niang M, Dangou JM, Mercereau-Puijalon O, Lehesran
JY, and Jambou R, Chronic infection during placental malaria is
associated with up-regulation of cycloxygenase-2. Malar J. 2010; 9: 45.
http://dx.doi.org/10.1186/1475-2875-9-45
PMid:20144201 PMCid:2831904

- Parekh FK,
Davison BB, Gamboa D, Hernandez
J, and Branch OH, Placental histopathologic changes associated with
subclinical malaria infection and its impact on the fetal environment.
Am J Trop Med Hyg. 2010; 83(5): 973-80. http://dx.doi.org/10.4269/ajtmh.2010.09-0445
PMid:21036823 PMCid:2963955

- Cockburn IA,
Mackinnon MJ, O'Donnell A,
Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC, and Rowe JA, A
human complement receptor 1 polymorphism that reduces Plasmodium
falciparum rosetting confers protection against severe malaria. Proc
Natl Acad Sci U S A. 2004; 101(1): 272-7. http://dx.doi.org/10.1073/pnas.0305306101
PMid:14694201 PMCid:314175

- Horata N,
Kalambaheti T, Craig A, and
Khusmith S, Sequence variation of PfEMP1-DBLalpha in association with
rosette formation in Plasmodium falciparum isolates causing severe and
uncomplicated malaria. Malar J. 2009; 8: 184. http://dx.doi.org/10.1186/1475-2875-8-184
PMid:19650937 PMCid:3224928

- Rogerson SJ,
Beeson JG, Mhango CG,
Dzinjalamala FK, and Molyneux ME, Plasmodium falciparum rosette
formation is uncommon in isolates from pregnant women. Infect Immun.
2000; 68(1): 391-3. http://dx.doi.org/10.1128/IAI.68.1.391-393.2000
PMid:10603414 PMCid:97147

- Chotivanich K,
Udomsangpetch R, Suwanarusk
R, Pukrittayakamee S, Wilairatana P, Beeson JG, Day NP, and White NJ,
Plasmodium vivax adherence to placental glycosaminoglycans. PLoS ONE.
2012; 7(4): e34509. http://dx.doi.org/10.1371/journal.pone.0034509
PMid:22529919 PMCid:3328474

- Carvalho BO,
Lopes SC, Nogueira PA,
Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM,
Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA,
Araujo MO, Russell B, Suwanarusk R, Snounou G, Renia L, and Costa FT,
On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect
Dis. 2010; 202(4): 638-47. http://dx.doi.org/10.1086/654815
PMid:20617923

- Bouyou-Akotet MK,
Adegnika AA, Agnandji
ST, Ngou-Milama E, Kombila M, Kremsner PG, and Mavoungou E, Cortisol
and susceptibility to malaria during pregnancy. Microbes Infect. 2005;
7(11-12): 1217-23. http://dx.doi.org/10.1016/j.micinf.2005.04.008
PMid:16002311

- Adebami OJ, Owa
JA, Oyedeji GA, Oyelami
OA, and Omoniyi-Esan GO, Associations between placental and cord blood
malaria infection and fetal malnutrition in an area of malaria
holoendemicity. Am J Trop Med Hyg. 2007; 77(2): 209-13.
PMid:17690388

- McGready R,
Davison BB, Stepniewska K, Cho
T, Shee H, Brockman A, Udomsangpetch R, Looareesuwan S, White NJ,
Meshnick SR, and Nosten F, The effects of Plasmodium falciparum and P.
vivax infections on placental histopathology in an area of low malaria
transmission. Am J Trop Med Hyg. 2004; 70(4): 398-407.
PMid:15100454

- Ismail MR, Ordi
J, Menendez C, Ventura PJ,
Aponte JJ, Kahigwa E, Hirt R, Cardesa A, and Alonso PL, Placental
pathology in malaria: a histological, immunohistochemical, and
quantitative study. Hum Pathol. 2000; 31(1): 85-93. http://dx.doi.org/10.1016/S0046-8177(00)80203-8

- Tako EA, Zhou A,
Lohoue J, Leke R, Taylor
DW, and Leke RF, Risk factors for placental malaria and its effect on
pregnancy outcome in Yaounde, Cameroon. Am J Trop Med Hyg. 2005; 72(3):
236-42. PMid:15772313

- Suguitan AL, Jr.,
Cadigan TJ, Nguyen TA,
Zhou A, Leke RJ, Metenou S, Thuita L, Megnekou R, Fogako J, Leke RG,
and Taylor DW, Malaria-associated cytokine changes in the placenta of
women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg.
2003; 69(6): 574-81. PMid:14740871

- Adam I, Elhassan
EM, Haggaz AE, Ali AA,
and Adam GK, A perspective of the epidemiology of malaria and anaemia
and their impact on maternal and perinatal outcomes in Sudan. J Infect
Dev Ctries. 2011; 5(2): 83-7. http://dx.doi.org/10.3855/jidc.1282

- Conroy AL,
McDonald CR, Silver KL, Liles
WC, and Kain KC, Complement activation: a critical mediator of adverse
fetal outcomes in placental malaria? Trends Parasitol. 2011; 27(7):
294-9. http://dx.doi.org/10.1016/j.pt.2011.02.005
PMid:21493146

- Umbers AJ, Boeuf
P, Clapham C, Stanisic
DI, Baiwog F, Mueller I, Siba P, King CL, Beeson JG, Glazier J, and
Rogerson SJ, Placental malaria-associated inflammation disturbs the
insulin-like growth factor axis of fetal growth regulation. J Infect
Dis. 2011; 203(4): 561-9. http://dx.doi.org/10.1093/infdis/jiq080
PMid:21216864 PMCid:3071224

- WHO, Guidelines
for the treatment of malaria, Second edition. 2010: Geneva,
Switzerland. p. 1-32.
- Mayor A, Moro L,
Aguilar R, Bardaj A,
Cister P, Serra-Casas E, Sigaque B, Alonso PL, Ordi J, and Menndez C,
How Hidden Can Malaria Be in Pregnant Women? Diagnosis by Microscopy,
Placental Histology, Polymerase Chain Reaction and Detection of
Histidine-Rich Protein 2 in Plasma. Clinical Infectious Diseases. 2012;
54(11): 1561-1568. http://dx.doi.org/10.1093/cid/cis236
PMid:22447794

- Mockenhaupt FP,
Ulmen U, von Gaertner C,
Bedu-Addo G, and Bienzle U, Diagnosis of placental malaria. J Clin
Microbiol. 2002; 40(1): 306-8. http://dx.doi.org/10.1128/JCM.40.1.306-308.2002
PMid:11773140 PMCid:120131

- VanderJagt TA,
Ikeh EI, Ujah IO, Belmonte
J, Glew RH, and VanderJagt DJ, Comparison of the OptiMAL rapid test and
microscopy for detection of malaria in pregnant women in Nigeria. Trop
Med Int Health. 2005; 10(1): 39-41. http://dx.doi.org/10.1111/j.1365-3156.2004.01349.x
PMid:15655012

- Dondorp A, Nosten
F, Stepniewska K, Day N,
and White N, Artesunate versus quinine for treatment of severe
falciparum malaria: a randomised trial. Lancet. 2005; 366(9487):
717-25. http://dx.doi.org/10.1016/S0140-6736(05)67176-0

- Leenstra T,
Phillips-Howard PA, Kariuki
SK, Hawley WA, Alaii JA, Rosen DH, Oloo AJ, Nahlen BL, Kager PA, and
ter Kuile FO, Permethrin-treated bed nets in the prevention of malaria
and anemia in adolescent schoolgirls in western Kenya. Am J Trop Med
Hyg. 2003; 68(4 Suppl): 86-93. PMid:12749490

- ter Kuile FO,
Terlouw DJ, Kariuki SK,
Phillips-Howard PA, Mirel LB, Hawley WA, Friedman JF, Shi YP, Kolczak
MS, Lal AA, Vulule JM, and Nahlen BL, Impact of permethrin-treated bed
nets on malaria, anemia, and growth in infants in an area of intense
perennial malaria transmission in western Kenya. Am J Trop Med Hyg.
2003; 68(4 Suppl): 68-77. PMid:12749488

- Jayasooriya S,
Hislop A, Peng Y,
Croom-Carter D, Jankey Y, Bell A, Dong T, Rowland-Jones S, Rickinson A,
Walther M, and Whittle H, Revisiting the effect of acute P. falciparum
malaria on Epstein-Barr virus: host balance in the setting of reduced
malaria endemicity. PLoS ONE. 2012; 7(2): e31142. http://dx.doi.org/10.1371/journal.pone.0031142
PMid:22347443 PMCid:3275582

- Phillips-Howard
PA, Nahlen BL, Kolczak MS,
Hightower AW, ter Kuile FO, Alaii JA, Gimnig JE, Arudo J, Vulule JM,
Odhacha A, Kachur SP, Schoute E, Rosen DH, Sexton JD, Oloo AJ, and
Hawley WA, Efficacy of permethrin-treated bed nets in the prevention of
mortality in young children in an area of high perennial malaria
transmission in western Kenya. Am J Trop Med Hyg. 2003; 68(4 Suppl):
23-9. PMid:12749482

- ter Kuile FO,
Terlouw DJ, Phillips-Howard
PA, Hawley WA, Friedman JF, Kariuki SK, Shi YP, Kolczak MS, Lal AA,
Vulule JM, and Nahlen BL, Reduction of malaria during pregnancy by
permethrin-treated bed nets in an area of intense perennial malaria
transmission in western Kenya. Am J Trop Med Hyg. 2003; 68(4 Suppl):
50-60. PMid:12749486

- Njagi JK,
Magnussen P, Estambale B, Ouma
J, and Mugo B, Prevention of anaemia in pregnancy using
insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly
malarious area of Kenya: a randomized controlled trial. Trans R Soc
Trop Med Hyg. 2003; 97(3): 277-82. http://dx.doi.org/10.1016/S0035-9203(03)90141-6

- Browne EN, Maude
GH, and Binka FN, The
impact of insecticide-treated bednets on malaria and anaemia in
pregnancy in Kassena-Nankana district, Ghana: a randomized controlled
trial. Trop Med Int Health. 2001; 6(9): 667-76. http://dx.doi.org/10.1046/j.1365-3156.2001.00759.x
PMid:11555433

- Diakite OS,
Kayentao K, Traore BT, Djimde
A, Traore B, Diallo M, Ongoiba A, Doumtabe D, Doumbo S, Traore MS, Dara
A, Guindo O, Karim DM, Coulibaly S, Bougoudogo F, Ter Kuile FO, Danis
M, and Doumbo OK, Superiority of 3 over 2 doses of intermittent
preventive treatment with sulfadoxine-pyrimethamine for the prevention
of malaria during pregnancy in mali: a randomized controlled trial.
Clin Infect Dis. 2011; 53(3): 215-23. http://dx.doi.org/10.1093/cid/cir374
PMid:21765069

- Menendez C,
Bardaji A, Sigauque B,
Romagosa C, Sanz S, Serra-Casas E, Macete E, Berenguera A, David C,
Dobano C, Naniche D, Mayor A, Ordi J, Mandomando I, Aponte JJ, Mabunda
S, and Alonso PL, A randomized placebo-controlled trial of intermittent
preventive treatment in pregnant women in the context of insecticide
treated nets delivered through the antenatal clinic. PLoS ONE. 2008;
3(4): e1934

- Asa OO, Onayade
AA, Fatusi AO, Ijadunola
KT, and Abiona TC, Efficacy of intermittent preventive treatment of
malaria with sulphadoxine-pyrimethamine in preventing anaemia in
pregnancy among Nigerian women. Matern Child Health J. 2008; 12(6):
692-8. http://dx.doi.org/10.1007/s10995-008-0319-3
PMid:18274885

- Tukur IU,
Thacher TD, Sagay AS, and
Madaki JK, A comparison of sulfadoxine-pyrimethamine with chloroquine
and pyrimethamine for prevention of malaria in pregnant Nigerian women.
Am J Trop Med Hyg. 2007; 76(6): 1019-23. PMid:17556604

- Challis K, Osman
NB, Cotiro M, Nordahl G,
Dgedge M, and Bergstrom S, Impact of a double dose of
sulphadoxine-pyrimethamine to reduce prevalence of pregnancy malaria in
southern Mozambique. Trop Med Int Health. 2004; 9(10): 1066-73. http://dx.doi.org/10.1111/j.1365-3156.2004.01307.x
PMid:15482398

- Bertin G, Briand
V, Bonaventure D,
Carrieu A, Massougbodji A, Cot M, and Deloron P, Molecular markers of
resistance to sulphadoxine-pyrimethamine during intermittent preventive
treatment of pregnant women in Benin. Malar J. 2011; 10: 196. http://dx.doi.org/10.1186/1475-2875-10-196
PMid:21767415 PMCid:3199903

- ter Kuile FO,
van Eijk AM, and Filler SJ,
Effect of sulfadoxine-pyrimethamine resistance on the efficacy of
intermittent preventive therapy for malaria control during pregnancy: a
systematic review. JAMA. 2007; 297(23): 2603-16. http://dx.doi.org/10.1001/jama.297.23.2603
PMid:17579229

- Clerk CA, Bruce
J, Affipunguh PK, Mensah
N, Hodgson A, Greenwood B, and Chandramohan D, A randomized, controlled
trial of intermittent preventive treatment with
sulfadoxine-pyrimethamine, amodiaquine, or the combination in pregnant
women in Ghana. J Infect Dis. 2008; 198(8): 1202-11. http://dx.doi.org/10.1086/591944
PMid:18752443

- Briand V,
Denoeud L, Massougbodji A, and
Cot M, Efficacy of intermittent preventive treatment versus chloroquine
prophylaxis to prevent malaria during pregnancy in Benin. J Infect Dis.
2008; 198(4): 594-601. http://dx.doi.org/10.1086/590114
PMid:18598190

- Briand V,
Bottero J, Noel H, Masse V,
Cordel H, Guerra J, Kossou H, Fayomi B, Ayemonna P, Fievet N,
Massougbodji A, and Cot M, Intermittent treatment for the prevention of
malaria during pregnancy in Benin: a randomized, open-label equivalence
trial comparing sulfadoxine-pyrimethamine with mefloquine. J Infect
Dis. 2009; 200(6): 991-1001. http://dx.doi.org/10.1086/605474
PMid:19656069

- Ndyomugyenyi R,
Clarke SE, Hutchison CL,
Hansen KS, and Magnussen P, Efficacy of malaria prevention during
pregnancy in an area of low and unstable transmission: an
individually-randomised placebo-controlled trial using intermittent
preventive treatment and insecticide-treated nets in the Kabale
Highlands, southwestern Uganda. Trans R Soc Trop Med Hyg. 2011;
105(11): 607-16. http://dx.doi.org/10.1016/j.trstmh.2011.07.012
PMid:21962292

- Hviid L, The
role of Plasmodium
falciparum variant surface antigens in protective immunity and vaccine
development. Hum Vaccin. 2010; 6(1): 84-9. http://dx.doi.org/10.4161/hv.6.1.9602
PMid:19823032

- Menendez C,
Alonso P, and Universitat de
B, Guidelines and considerations for testing malaria vaccines in
pregnant women. Hum Vaccin. 2010; 6(1): 21-6. PMid:19946207

- Crompton PD,
Pierce SK, and Miller LH,
Advances and challenges in malaria vaccine development. J Clin Invest.
2010; 120(12): 4168-78. http://dx.doi.org/10.1172/JCI44423
PMid:21123952 PMCid:2994342

- Duffy PE and
Fried M, Antibodies that
inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are
associated with increased birth weight and the gestational age of
newborns. Infect Immun. 2003; 71(11): 6620-3. http://dx.doi.org/10.1128/IAI.71.11.6620-6623.2003
PMid:14573685 PMCid:219546

- Magistrado PA,
Minja D, Doritchamou J,
Ndam NT, John D, Schmiegelow C, Massougbodji A, Dahlback M, Ditlev SB,
Pinto VV, Resende M, Lusingu J, Theander TG, Salanti A, and Nielsen MA,
High efficacy of anti DBL4varepsilon-VAR2CSA antibodies in inhibition
of CSA-binding Plasmodium falciparum-infected erythrocytes from
pregnant women. Vaccine. 2011; 29(3): 437-43. http://dx.doi.org/10.1016/j.vaccine.2010.10.080
PMid:21075162

- Duffy PE and
Fried M, Pregnancy malaria: cryptic disease, apparent solution. Mem
Inst Oswaldo Cruz. 2011; 106 Suppl 1: 64-9. http://dx.doi.org/10.1590/S0074-02762011000900008

- Hviid L, The
case for PfEMP1-based
vaccines to protect pregnant women against Plasmodium falciparum
malaria. Expert Rev Vaccines. 2011; 10(10): 1405-14. http://dx.doi.org/10.1586/erv.11.113
PMid:21988306

- McGready R, Cho
T, Keo NK, Thwai KL,
Villegas L, Looareesuwan S, White NJ, and Nosten F, Artemisinin
antimalarials in pregnancy: a prospective treatment study of 539
episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis.
2001; 33(12): 2009-16. http://dx.doi.org/10.1086/324349
PMid:11712093

- Manyando C,
Mkandawire R, Puma L, Sinkala
M, Mpabalwani E, Njunju E, Gomes M, Ribeiro I, Walter V, Virtanen M,
Schlienger R, Cousin M, Chipimo M, and Sullivan FM, Safety of
artemether-lumefantrine in pregnant women with malaria: results of a
prospective cohort study in Zambia. Malar J. 2010; 9: 249. http://dx.doi.org/10.1186/1475-2875-9-249
PMid:20809964 PMCid:2944339

- Deen JL, von
Seidlein L, Pinder M,
Walraven GE, and Greenwood BM, The safety of the combination artesunate
and pyrimethamine-sulfadoxine given during pregnancy. Trans R Soc Trop
Med Hyg. 2001; 95(4): 424-8. http://dx.doi.org/10.1016/S0035-9203(01)90204-4

- Adam I, Elhassan
EM, Omer EM, Abdulla MA,
Mahgoub HM, and Adam GK, Safety of artemisinins during early pregnancy,
assessed in 62 Sudanese women. Ann Trop Med Parasitol. 2009; 103(3):
205-10. http://dx.doi.org/10.1179/136485909X398285
PMid:19341535

- Tagbor H, Bruce
J, Browne E, Randal A,
Greenwood B, and Chandramohan D, Efficacy, safety, and tolerability of
amodiaquine plus sulphadoxine-pyrimethamine used alone or in
combination for malaria treatment in pregnancy: a randomised trial.
Lancet. 2006; 368(9544): 1349-56. http://dx.doi.org/10.1016/S0140-6736(06)69559-7

- McGready R,
Ashley EA, Moo E, Cho T,
Barends M, Hutagalung R, Looareesuwan S, White NJ, and Nosten F, A
randomized comparison of artesunate-atovaquone-proguanil versus quinine
in treatment for uncomplicated falciparum malaria during pregnancy. J
Infect Dis. 2005; 192(5): 846-53. http://dx.doi.org/10.1086/432551
PMid:16088834

- McGready R, Cho
T, Samuel, Villegas L,
Brockman A, van Vugt M, Looareesuwan S, White NJ, and Nosten F,
Randomized comparison of quinine-clindamycin versus artesunate in the
treatment of falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg.
2001; 95(6): 651-6. http://dx.doi.org/10.1016/S0035-9203(01)90106-3

- Mutabingwa TK,
Muze K, Ord R, Briceno M,
Greenwood BM, Drakeley C, and Whitty CJ, Randomized trial of
artesunate+amodiaquine, sulfadoxine-pyrimethamine+amodiaquine,
chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania.
PLoS ONE. 2009; 4(4): e5138. http://dx.doi.org/10.1371/journal.pone.0005138
PMid:19352498 PMCid:2662423

- McGready R,
Brockman A, Cho T, Cho D, van
Vugt M, Luxemburger C, Chongsuphajaisiddhi T, White NJ, and Nosten F,
Randomized comparison of mefloquine-artesunate versus quinine in the
treatment of multidrug-resistant falciparum malaria in pregnancy. Trans
R Soc Trop Med Hyg. 2000; 94(6): 689-93. http://dx.doi.org/10.1016/S0035-9203(00)90235-9

- Kalilani L,
Mofolo I, Chaponda M,
Rogerson SJ, Alker AP, Kwiek JJ, and Meshnick SR, A randomized
controlled pilot trial of azithromycin or artesunate added to
sulfadoxine-pyrimethamine as treatment for malaria in pregnant women.
PLoS ONE. 2007; 2(11): e1166. http://dx.doi.org/10.1371/journal.pone.0001166
PMid:18000538 PMCid:2048661

- Tarning J,
Kloprogge F, Piola P, Dhorda
M, Muwanga S, Turyakira E, Nuengchamnong N, Nosten F, Day N, White N,
Guerin P, and Lindegardh N, Population pharmacokinetics of Artemether
and dihydroartemisinin in pregnant women with uncomplicated Plasmodium
falciparum malaria in Uganda. Malaria Journal. 2012; 11(1): 293. http://dx.doi.org/10.1186/1475-2875-11-293
PMid:22913677 PMCid:3502166

- Tarning J,
McGready R, Lindegardh N,
Ashley EA, Pimanpanarak M, Kamanikom B, Annerberg A, Day NP,
Stepniewska K, Singhasivanon P, White NJ, and Nosten F, Population
pharmacokinetics of lumefantrine in pregnant women treated with
artemether-lumefantrine for uncomplicated Plasmodium falciparum
malaria. Antimicrob Agents Chemother. 2009; 53(9): 3837-46. http://dx.doi.org/10.1128/AAC.00195-09
PMid:19564366 PMCid:2737887

- Schlagenhauf P,
Blumentals WA, Suter P,
Regep L, Vital-Durand G, Schaerer MT, Boutros MS, Rhein H-G, and
Adamcova M, Pregnancy and Fetal Outcomes After Exposure to Mefloquine
in the Pre- and Periconception Period and During Pregnancy. Clinical
Infectious Diseases. 2012. http://dx.doi.org/10.1093/cid/cis215
PMid:22495078 PMCid:3348951

- Rijken MJ,
McGready R, Jullien V, Tarning
J, Lindegardh N, Phyo AP, Win AK, Hsi P, Cammas M, Singhasivanon P,
White NJ, and Nosten F, Pharmacokinetics of amodiaquine and
desethylamodiaquine in pregnant and postpartum women with Plasmodium
vivax malaria. Antimicrob Agents Chemother. 2011; 55(9): 4338-42. http://dx.doi.org/10.1128/AAC.00154-11
PMid:21709098 PMCid:3165320

- McGready R,
White NJ, and Nosten F,
Parasitological efficacy of antimalarials in the treatment and
prevention of falciparum malaria in pregnancy 1998 to 2009: a
systematic review. BJOG. 2011; 118(2): 123-35. http://dx.doi.org/10.1111/j.1471-0528.2010.02810.x

- Tarning J,
Rijken MJ, McGready R, Phyo
AP, Hanpithakpong W, Day NP, White NJ, Nosten F, and Lindegardh N,
Population pharmacokinetics of dihydroartemisinin and piperaquine in
pregnant and nonpregnant women with uncomplicated malaria. Antimicrob
Agents Chemother. 2012; 56(4): 1997-2007. http://dx.doi.org/10.1128/AAC.05756-11
PMid:22252822 PMCid:3318332

- McGready R,
Stepniewska K, Ward SA, Cho
T, Gilveray G, Looareesuwan S, White NJ, and Nosten F, Pharmacokinetics
of dihydroartemisinin following oral artesunate treatment of pregnant
women with acute uncomplicated falciparum malaria. Eur J Clin
Pharmacol. 2006; 62(5): 367-71.
http://dx.doi.org/10.1007/s00228-006-0118-y
PMid:16552504

- Tarning J,
Chotsiri P, Jullien V, Rijken
MJ, Bergstrand M, Cammas M, McGready R, Singhasivanon P, Day NP, White
NJ, Nosten F, and Lindegardh N, Population pharmacokinetic and
pharmacodynamic modeling of amodiaquine and desethylamodiaquine in
women with Plasmodium vivax malaria during and after pregnancy.
Antimicrob Agents Chemother. 2012. http://dx.doi.org/10.1128/AAC.01242-12
PMid:22926572 PMCid:3486620

- Piola P,
Nabasumba C, Turyakira E, Dhorda
M, Lindegardh N, Nyehangane D, Snounou G, Ashley EA, McGready R, Nosten
F, and Guerin PJ, Efficacy and safety of artemether-lumefantrine
compared with quinine in pregnant women with uncomplicated Plasmodium
falciparum malaria: an open-label, randomised, non-inferiority trial.
Lancet Infect Dis. 2010; 10(11): 762-9. http://dx.doi.org/10.1016/S1473-3099(10)70202-4

- Kaye DK,
Nshemerirwe R, Mutyaba TS, and
Ndeezi G, A randomized clinical trial comparing safety, clinical and
parasitological response to artemether-lumefantrine and
chlorproguanil-dapsone in treatment of uncomplicated malaria in
pregnancy in Mulago hospital, Uganda. J Infect Dev Ctries. 2008; 2(2):
135-9. http://dx.doi.org/10.3855/T2.2.135
PMid:19738339

- McGready R, Tan
SO, Ashley EA,
Pimanpanarak M, Viladpai-Nguen J, Phaiphun L, Wustefeld K, Barends M,
Laochan N, Keereecharoen L, Lindegardh N, Singhasivanon P, White NJ,
and Nosten F, A randomised controlled trial of artemether-lumefantrine
versus artesunate for uncomplicated plasmodium falciparum treatment in
pregnancy. PLoS Med. 2008; 5(12): e253. http://dx.doi.org/10.1371/journal.pmed.0050253
PMid:19265453 PMCid:2605900

- McGready R,
Stepniewska K, Lindegardh N,
Ashley EA, La Y, Singhasivanon P, White NJ, and Nosten F, The
pharmacokinetics of artemether and lumefantrine in pregnant women with
uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006; 62(12):
1021-31. http://dx.doi.org/10.1007/s00228-006-0199-7
PMid:17053895

- Rijken MJ,
McGready R, Phyo AP,
Lindegardh N, Tarning J, Laochan N, Than HH, Mu O, Win AK,
Singhasivanon P, White N, and Nosten F, Pharmacokinetics of
dihydroartemisinin and piperaquine in pregnant and nonpregnant women
with uncomplicated falciparum malaria. Antimicrob Agents Chemother.
2011; 55(12): 5500-6. http://dx.doi.org/10.1128/AAC.05067-11
PMid:21947392 PMCid:3232755

- Poespoprodjo JR,
Fobia W, Kenangalem E,
Hasanuddin A, Sugiarto P, Tjitra E, Anstey NM, and Price RN, Highly
effective therapy for maternal malaria associated with a lower risk of
vertical transmission. J Infect Dis. 2011; 204(10): 1613-9. http://dx.doi.org/10.1093/infdis/jir558
PMid:21908728 PMCid:3192188

- McGready R, Keo
NK, Villegas L, White NJ,
Looareesuwan S, and Nosten F, Artesunate-atovaquone-proguanil rescue
treatment of multidrug-resistant Plasmodium falciparum malaria in
pregnancy: a preliminary report. Trans R Soc Trop Med Hyg. 2003; 97(5):
592-4. http://dx.doi.org/10.1016/S0035-9203(03)80040-8

- McGready R,
Stepniewska K, Edstein MD,
Cho T, Gilveray G, Looareesuwan S, White NJ, and Nosten F, The
pharmacokinetics of atovaquone and proguanil in pregnant women with
acute falciparum malaria. Eur J Clin Pharmacol. 2003; 59(7): 545-52. http://dx.doi.org/10.1007/s00228-003-0652-9
PMid:12955371

- Kabanywanyi AM,
Macarthur JR, Stolk WA,
Habbema JD, Mshinda H, Bloland PB, Abdulla S, and Kachur SP, Malaria in
pregnant women in an area with sustained high coverage of
insecticide-treated bed nets. Malar J. 2008; 7: 133. http://dx.doi.org/10.1186/1475-2875-7-133
PMid:18644118 PMCid:2500040

- Campos PA,
Valente B, Campos RB,
Goncalves L, Rosario VE, Varandas L, and Silveira H, Plasmodium
falciparum infection in pregnant women attending antenatal care in
Luanda, Angola. Rev Soc Bras Med Trop. 2012; 45(3): 369-74. http://dx.doi.org/10.1590/S0037-86822012000300017
PMid:22760138

- Martinez-Espinosa
FE, Daniel-Ribeiro CT,
and Alecrim WD, Malaria during pregnancy in a reference centre from the
Brazilian Amazon: unexpected increase in the frequency of Plasmodium
falciparum infections. Mem Inst Oswaldo Cruz. 2004; 99(1): 19-21. http://dx.doi.org/10.1590/S0074-02762004000100003

- Karunajeewa HA,
Salman S, Mueller I,
Baiwog F, Gomorrai S, Law I, Page-Sharp M, Rogerson S, Siba P, Ilett
KF, and Davis TM, Pharmacokinetics of chloroquine and
monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother.
2010; 54(3): 1186-92. http://dx.doi.org/10.1128/AAC.01269-09
PMid:20086162 PMCid:2825967

- Nyunt MM, Adam
I, Kayentao K, van Dijk J,
Thuma P, Mauff K, Little F, Cassam Y, Guirou E, Traore B, Doumbo O,
Sullivan D, Smith P, and Barnes KI, Pharmacokinetics of sulfadoxine and
pyrimethamine in intermittent preventive treatment of malaria in
pregnancy. Clin Pharmacol Ther. 2010; 87(2): 226-34. http://dx.doi.org/10.1038/clpt.2009.177
PMid:19776738

- McGready R,
Thwai KL, Cho T, Samuel,
Looareesuwan S, White NJ, and Nosten F, The effects of quinine and
chloroquine antimalarial treatments in the first trimester of
pregnancy. Trans R Soc Trop Med Hyg. 2002; 96(2): 180-4. http://dx.doi.org/10.1016/S0035-9203(02)90297-X
