Impact of Pretransplant Donor and Recipient Cytomegalovirus Serostatus on Outcome for Multiple Myeloma Patients Undergoing Reduced Intensity Conditioning Allogeneic Stem Cell Transplantation
Jean El-Cheikh1,2, Raynier Devillier1,2, Roberto Crocchiolo1,2, Sabine F�rst1,2, Boris Calmels3, Catherine Faucher1,2, Anne Marie Stoppa2, Angela Granata1,2, Luca Castagna1,2, Patrick Ladaique3, Claude Lemarie3, Reda Bouabdallah2, Christine Zandotti4, Michele Merlin5, Pierre Berger5, Christian Chabannon3 and Didier Blaise1,2.
1
Unit� de Transplantation et de Th�rapie Cellulaire (U2T), Institut
Paoli-Calmettes, Marseille, France.
2 D�partement d’Onco-H�matologie, Institut Paoli-Calmettes, Marseille, France.
3 Centre de Th�rapie Cellulaire, Institut Paoli-Calmettes, Marseille, France.
4 D�partement de Microbiologie, Institut Paoli-Calmettes, Marseille, France.
5 Service de Virologie, H�pital de la Timone, Marseille, France.
2 D�partement d’Onco-H�matologie, Institut Paoli-Calmettes, Marseille, France.
3 Centre de Th�rapie Cellulaire, Institut Paoli-Calmettes, Marseille, France.
4 D�partement de Microbiologie, Institut Paoli-Calmettes, Marseille, France.
5 Service de Virologie, H�pital de la Timone, Marseille, France.
Correspondence
to:
Jean El Cheikh, M.D. Unit� de Transplantation et de Th�rapie Cellulaire
(U2T), Institut Paoli-Calmettes, 232 Boulevard Ste Marguerite, 13009
Marseille Cedex 09, France. Tel: +33 491 223823; Fax: +33 491 223579.
E-Mail: elcheikhj@ipc.unicancer.fr
Published: April 10, 2013
Received: February 25, 2013
Accepted: March 26, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013026, DOI 10.4084/MJHID.2013.026
This article is available on PDF format at:

This is an Open
Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Scope
of the study was to investigate the impact of pre-transplant CMV
serostatus of the donor and/or recipient on the outcome of patients
undergoing allogeneic hematopoietic stem cell transplantation
(Allo-SCT) for Multiple Myeloma (MM). To our knowledge no data are
available in the literature about this issue.
We retrospectively followed 99 consecutive patients who underwent reduced-intensity conditioning (RIC) Allo-SCT for MM in our cancer center at Marseille between January 2000 and January 2012. Based upon CMV serostatus, patients were classified as low risk (donor [D]-/recipient [R]-) 17 patients (17.1%), intermediate risk (D+/R) 14 patients (14.1%), or high risk – either (D-/R+) 31 patients (31.3%) or (D+/R+), 37 patients (37.3%).
Cumulative incidence of CMV reactivation was 39% with a median time of 61 days (26–318). Three patients (3%) developed CMV disease. Two factors were associated with CMV reactivation: CMV serostatus group (low: 0% vs. intermediate: 29% vs. high: 50%; p=0.001) and the presence of grade II–IV acute GvHD (Hazard Ratio: HR=2.1 [1.1–3.9]). Thirty-six of the 39 patients (92%) with CMV reactivation did not present positive detection of CMV after a 21-day median duration preemptive treatment with ganciclovir. Cumulative incidence of day 100 grade II–IV acute GvHD, 1-year chronic GvHD and day 100 transplantation related mortality (TRM) were 37%, 36% and 9%, respectively. CMV reactivation and serostatus were not associated with increased GvHD and TRM or short survival. Only the presence of acute GvHD as a time dependent variable was significantly associated with increased TRM (p=0.005). Two-year overall and progression free survival were 56% and 34%, respectively.
Donor and recipient CMV serostatus and acute GvHD are independent factors for increased CMV reactivation in high-risk MM patients undergoing RIC Allo-SCT. However, we did not find any influence of CMV reactivation on post transplantation outcome. CMV monitoring and pre-emptive treatment strategy could in part explain these results. Novel prophylactic measures such as immunotherapy and drug prophylaxis need to be considered in this group of patients, warranting further prospective studies.
We retrospectively followed 99 consecutive patients who underwent reduced-intensity conditioning (RIC) Allo-SCT for MM in our cancer center at Marseille between January 2000 and January 2012. Based upon CMV serostatus, patients were classified as low risk (donor [D]-/recipient [R]-) 17 patients (17.1%), intermediate risk (D+/R) 14 patients (14.1%), or high risk – either (D-/R+) 31 patients (31.3%) or (D+/R+), 37 patients (37.3%).
Cumulative incidence of CMV reactivation was 39% with a median time of 61 days (26–318). Three patients (3%) developed CMV disease. Two factors were associated with CMV reactivation: CMV serostatus group (low: 0% vs. intermediate: 29% vs. high: 50%; p=0.001) and the presence of grade II–IV acute GvHD (Hazard Ratio: HR=2.1 [1.1–3.9]). Thirty-six of the 39 patients (92%) with CMV reactivation did not present positive detection of CMV after a 21-day median duration preemptive treatment with ganciclovir. Cumulative incidence of day 100 grade II–IV acute GvHD, 1-year chronic GvHD and day 100 transplantation related mortality (TRM) were 37%, 36% and 9%, respectively. CMV reactivation and serostatus were not associated with increased GvHD and TRM or short survival. Only the presence of acute GvHD as a time dependent variable was significantly associated with increased TRM (p=0.005). Two-year overall and progression free survival were 56% and 34%, respectively.
Donor and recipient CMV serostatus and acute GvHD are independent factors for increased CMV reactivation in high-risk MM patients undergoing RIC Allo-SCT. However, we did not find any influence of CMV reactivation on post transplantation outcome. CMV monitoring and pre-emptive treatment strategy could in part explain these results. Novel prophylactic measures such as immunotherapy and drug prophylaxis need to be considered in this group of patients, warranting further prospective studies.
Introduction
The introduction of allogeneic hematopoietic stem cell transplantation (Allo-SCT), and novel anti-myeloma agents such as bortezomib, thalidomide and lenalidomide has improved the outcome of patients with multiple myeloma (MM) who relapse or are refractory to standard therapies.[1,2] Allo-SCT at present is considered in the first-line therapy of MM only in young high-risk patients.[1] These advances have transformed myeloma into a chronic condition, with multiple relapses, salvage therapies and chronic immunodeficient states leading to high infection rates. Human cytomegalovirus (CMV) infection remains one of the major complications after Allo-SCT and is associated with considerable morbidity and mortality.[3,4] The incidence of CMV infection increases with the intensity and duration of immunosuppression and occurs in roughly 70% of patients after Allo-SCT, depending on both donor and recipient serostatus.[5,6] Without antiviral intervention, about 50% of patients with culture-proven CMV infection will develop CMV disease. Despite the use of antiviral prophylaxis and pre-emptive antiviral therapy, CMV diseases continue to be reported in 15 to 25% of patients.[7-9] Frequent clinical manifestations of CMV disease are interstitial pneumonitis, gastrointestinal (GI) disease and hepatitis; retinitis, encephalitis and marrow suppression have also been described. The present approach to managing CMV disorders consists of peripheral blood monitoring using either CMV pp65 antigen (antigenemia assay) or polymerase chain reaction (PCR).[10,11]
Pre-emptive therapy with either ganciclovir or foscarnet is initiated in patients detected as positive. Factors identified for CMV reactivation are CMV seropositive status of the donor, type of conditioning regimen, use of T-cell depletion, and graft-versus-host disease (GvHD).[12,13] With the introduction of newer conditioning regimens including reduced-intensity conditioning (RIC) Allo-SCT and the increased use of in vivo T-cell depleting agents such as anti-thymocyte globulin (ATG) and monoclonal antibodies such as alemtuzumab (anti-CD52), it has become essential to determine whether a specific high-risk subgroup can be identified.[14,15] These patients could benefit from more intensive or innovative prophylactic strategies as opposed to a general pre-emptive strategy. To our knowledge no data are available in the literature about this issue. We have attempted to study CMV reactivation patterns in 99 consecutive patients with MM who underwent RIC Allo-SCT in our cancer centre at Marseille, where serial CMV monitoring has been done, to ascertain the risks of CMV reactivation.
Patients and methods
This is a retrospective analysis of MM patients who underwent RIC Allo-SCT at our centre between January 2000 and January 2012. The data were collected from the transplant database and individual medical records. A total of 99 patients (median age 53 years, range 27–67) underwent RIC Allo-SCT, including 59 males and 40 females. Ninety-six patients (97%) received one or more prior autologous transplantations (Auto-SCT). Twenty-seven patients (27%) were transplanted as the first line treatment strategy whereas 72 patients (73%) had received other treatments. Those 27 patients were considered for Allo-SCT because they had poor prognostic factors like cytogenetic aberrations (Del (13q14), and/or Del 17 and/or t (4; 14)) or they didn’t have at least partial remission (PR) after Auto-SCT. The median time between Auto-SCT and Allo-SCT was 19 months (1-89). The disease status at time of transplantation was Complete Remission (CR) or VGPR in 22%, PR or stable disease (SD) in 67%, and progression or refractory disease (PD) in 11%. Patient characteristics are shown in Table 1.
Seventy-three patients (74%) had matched related donors (MRD) and 26 patients (26%) had unrelated donors (URD). Eighteen patients (18%) had an HLA-allelically matched URD, a so-called 10/10 match, and 8 patients (8%) had 9/10 HLA-matched antigens (four of them had a DQ allele mismatch, two patients had a Cw mismatch and two had A mismatch). The graft source was peripheral blood stem cells (PBSC) in 88 patients (89%), bone marrow in nine patients (9%), and cord blood in two (2%). T-cell depletion with ATG was used in 68 patients (69%) and low-grade total body irradiation (TBI) with 2 Gys was used in 30 recipients (31%) of RIC transplants, respectively. GvHD prophylaxis consisted of cyclosporine A in 56 patients (57%), CSA + mycophenolate mofetyl (MMF) in 41 patients (41%) and MMF alone in two patients (2%). Transplant characteristics are shown in Table 2.

Table 1. Patient and transplantation characteristics.
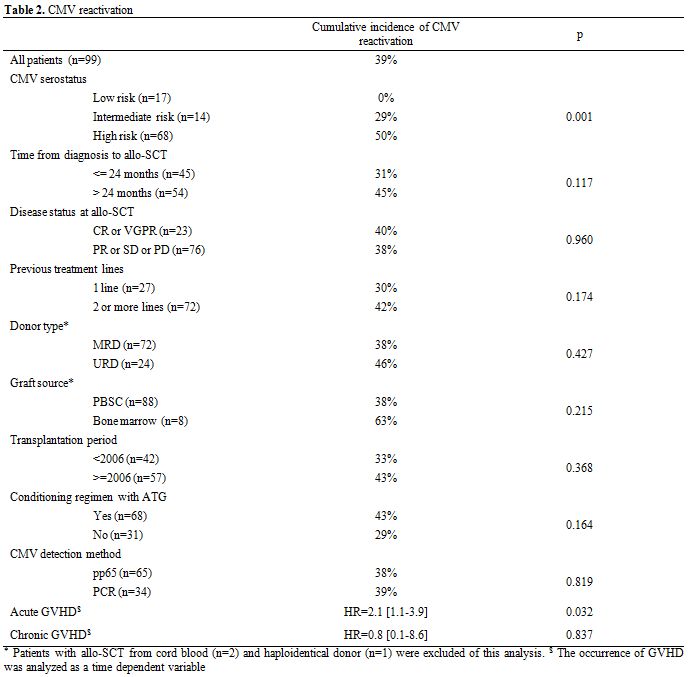
Table 2. CMV reactivation.
Based upon previous exposure to CMV, patients were classified into low risk (donor and recipient negative; D-/R-), intermediate risk (donor positive with recipient negative; D+/R-), or high risk (recipient positive with donor either negative [D-/ R+] or positive [D+/R+]). Seventeen patients (17%) were D-/R-, 14 patients (14%) were D+/R-, 31 patients (31%) were D-/R+ and 37 patients (37%) were D+/R+.
Supportive care.Our protocol in providing supportive care was the same throughout this time period. Prophylactic treatment against pneumocystis jirovecii and toxoplasmosis consisted of trimethoprim-sulfamethoxazole (10 mg/Kg/day) administered twice weekly. Patients also received daily oral amoxicillin (500 mg x 3/day) as prophylaxis against encapsulated bacteria and prophylaxis against herpes simplex virus including oral valacyclovir (500 mg x 2/day). Patients were monitored for CMV reactivation during the first 12 months after transplant, and pre-emptive therapy with ganciclovir was given if CMV reactivation occurred.
CMV monitoring and treatment. Serial weekly monitoring for CMV quantification was done using either pp65 antigen (between 2000 and 2009) or a quantitative PCR assay (COBAS R, Roche Diagnostics, Branchburg, New Jersey, USA) (from 2009 to the present). Monitoring was performed weekly initially, starting from transplantation until day 90, and then every 4–8 weeks during the next six months. If there was evidence of reactivation (pp65 >2 cells/200,000 or PCR >1000 copies/mL), treatment was started with ganciclovir (5mg/kg IV twice daily) for 2 weeks, provided two consecutive PCRs done three days apart became negative. If the PCR was still positive after 2 weeks of treatment, a maintenance therapy with ganciclovir (5mg/kg IV once /day) for another 14 days was proposed. CMV disease was diagnosed on demonstration of tissue invasion in biopsy specimens or demonstration of a positive CMV early antigen on bronchoalveolar lavage, along with clinical and radiological features consistent with CMV.
Statistics. Data are presented as medians, ranges, and 95% confidence intervals (CI). The end points analysed were overall survival (OS), progression free survival (PFS), transplant related mortality (TRM) and acute and chronic GvHD. OS was defined as the time elapsed from Allo-SCT to death, whatever the cause of death. PFS was defined as survival with no evidence of relapse or progression. Kaplan-Meier product-limit estimates were used to assess the probabilities of OS and PFS.[16] The Prentice estimate and Gray test, allowing the consideration of competing events, were used to calculate the cumulative incidences of GvHD, relapse, CMV reactivation and TRM.[17,18]
Cox regression was used to find any association between major pre-transplant variables and OS or PFS;[19] the occurrence of CMV reactivation, acute GvHD and chronic GvHD were all considered as time-dependent covariates. If two or more variables were associated with p<0.20 to each endpoint of interest, then a multivariate model was constructed. SPSS v13.0 and R 2.12.2 were used for the above cited analyses (http://www.R-project.org).
Results
CMV reactivation, disease and treatment. The cumulative incidence of CMV reactivation was 39% (n=39) with a median time of 61 [26–318] days after Allo-SCT. Twenty-three patients (59%) were under corticosteroid treatment for GvHD. There was no difference in the median time (days) of reactivation based upon its method of detection using pp65 (54 [32–162]) or quantitative PCR (65[34–162]). The incidence of CMV reactivation at 1, 3, 6 and 12 months after Allo-SCT was 30%, 35%, 37% and 39%, respectively (Figure 1). Three patients (3%) developed CMV disease (two pneumonitis and one disseminated GI and lung disease) at a median of 38 days (26–49) post Allo-SCT. There was no difference in the median time to reactivation based on donor type (MRD versus URD; 48 versus 46 days), graft source - bone marrow (45 days) versus PBSC (47 days) versus cord blood (46 days) - the year of transplant (before or after 2006), the use of ATG in the conditioning regimen, the age of the donor (more or less than 50 years) or the presence of chronic GvHD.
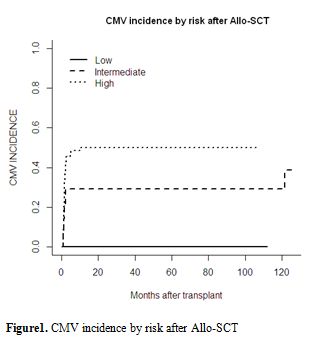
Figure 1. CMV incidence by risk after Allo-SCT.
By univariate analysis, CMV reactivation was significantly influenced by CMV serostatus risk group (high: 50% vs intermediate: 29% vs low: 0%; p=0.001; Table 2 and Figure 1). In the high-risk group characterised by a positive CMV serostatus recipient, the serostatus of the donor (positive vs. negative) did not influence CMV reactivation. The presence of acute GvHD as a time-dependent variable was associated with an increased cumulative incidence of CMV reactivation (p=0.032). Donor type, disease stage, recipient and donor ages, female-to-male graft, graft source (bone marrow versus PBSC versus cord blood), time of transplant (pre- or post-2006), the disease status, the number of lines of previous therapy and time from diagnosis to transplant, the death for other causes, the relapse of the underlying disease, or the presence of chronic GvHD, use of the new anti-myeloma drugs, use of ATG during conditioning and corticosteroid therapy did not significantly influence the incidence of CMV reactivation. Details of the results are shown in Tables 2 and 4.
By multivariate analysis, CMV reactivation was associated with CMV serostatus (HR: 3.8 [1.6–2.9], p=0.002) and the presence of acute GvHD (HR: 2.5 [1.3–4.9], p=0.006).
All 39 patients with CMV reactivation received ganciclovir. Resolution of CMV viremia occurred in 36 patients (92%) after a median of 21 days (range 7–64). Of the three patients who did not have resolution of viremia, all had extensive chronic GvHD. Two of them died of CMV disease while one patient died of disease progression. Recurrent reactivations (after resolution of the first episode with antiviral therapy) were seen in 15 patients, predominantly in patients with chronic GvHD.
Outcome after Allo-SCT. The median 2-year OS and PFS were 56% and 34%, respectively.
Cumulative incidences of day-100 grade II–IV acute GvHD, 2-year chronic GvHD and 2-year extensive chronic GvHD were 37%, 36% and 29%, respectively. The median TRM at 100 days and one year was 9% and 21%, respectively. All the 10 analyzable pathogenic microorganisms are classified in the table 3 by descending order of frequency.
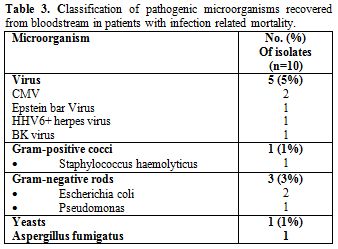
Table 3. Classification of pathogenic microorganisms recovered from bloodstream in patients with infection related mortality.
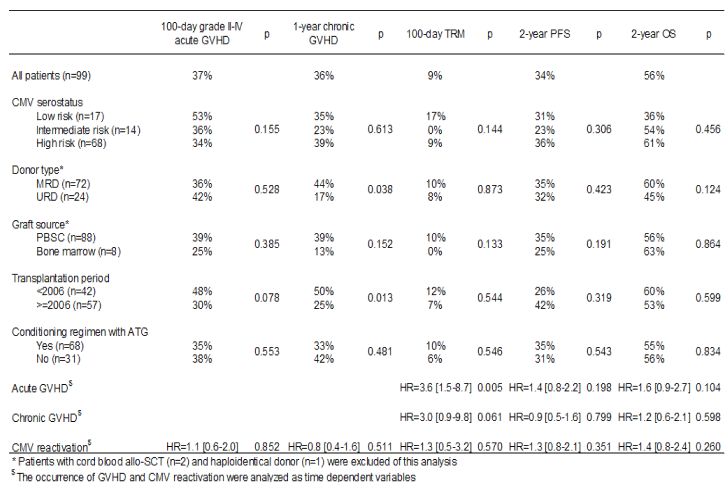
Table 4. Transplantation outcome.
No impact of pretransplant CMV serostatus on engraftment occurred in any patients. Causes of death were relatively similar between the two groups except for deaths that occurred due to CMV in the group that had reactivation. There was no increase in mortality related to bacterial or other viral infections in patients with CMV reactivation. By univariate analysis, CMV serostatus (low risk versus intermediate risk versus high risk) had no impact on the OS (Figure 2).
Other factors, although evaluated, such as donor type (MRD vs. URD), disease stage, patient and donor ages, female-to-male graft, graft source (bone marrow versus PBSC versus cord blood, time of transplant (before or after 2006), use of the new anti-myeloma drugs, use of ATG during conditioning and corticosteroid therapy did not significantly influence OS, PFS or TRM. CMV reactivation analysed as a time dependent variable did not influence OS (HR=1.4 [0.8–2.4]) (Table 4). Only the presence of acute GvHD as a time dependent variable was significantly associated with increased TRM (p=0.005) (Table 4).
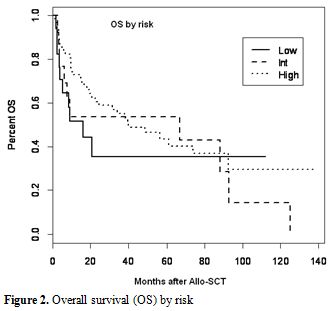
Figure 2. Overall survival (OS) by risk.
Discussion
Infections in patients with MM represent a clinical challenge. The list of potential pathogens is long and changes over the disease course.[20] The aim of this study was to discern the effects of pre-transplant CMV serostatus on the outcome of Allo-SCT in MM patients. To the best of our knowledge, this is the first and largest study reporting on the role of CMV serostatus prior to RIC Allo-SCT in MM patients.
CMV infection remains a major problem following Allo-SCT and different groups have adopted different treatment strategies to reduce the morbidity and mortality related to CMV. One strategy was to serially monitor patients in post Allo-SCT for CMV and start pre-emptive therapy with ganciclovir or foscarnet once there was evidence of reactivation.[6,8,21] This practice was established in our institution in 2000, and analysis of this pre-emptive strategy suggests that a third of our patients (39%) develop reactivation. Most importantly, the reactivations occurred in the high-risk groups (D-/R+ or D+/R+), suggesting pre-transplant recipient seropositivity is perhaps the most important risk factor for reactivation.[5,22] This study showed no impact of pre-transplant CMV serostatus on engraftment in the three risk groups. Previous studies suggested that the CMV serostatus of patients was important not only in terms of reactivation but also in terms of worsening TRM and reducing OS.[5,22] However, in our study, we did not observe worse outcomes in the high-risk group as compared with the other groups. Moreover, we did not find any correlation between CMV reactivation and poor outcome. Interestingly grade II–IV acute GvHD was associated with a higher incidence of CMV infection and there was no increase in mortality related to bacterial or other viral infections in patients with CMV reactivation. In addition to the immunodeficiency related to myeloma and its complications, the type of anti-myeloma therapy used also plays a role in the development of infection, but in this study we did not find an impact of the new anti-myeloma drugs on the outcome of patients after Allo-SCT in any of the three risk groups. These therapeutic strategies impact differently on the immune system, predisposing patients to various opportunistic infections.[20,23] A cumulative suppression of cell-mediated immunity is particular to MM, and results from the combined effect of repetitive use of high-dose corticosteroids, the chronic nature of the disease with multiple relapses requiring salvage therapies (almost always containing dexamethasone), and the addition of bortezomib, a powerful immunosuppressive agent or other immunomodulatory agents like thalidomide and lenalidomide in different treatment phases.[20,24,25] In addition, all patients treated with bortezomib-containing regimens should receive prophylaxis for HSV and VZV infection.[26,27] Recently, as reported by our group, the introduction of these novel agents was associated with an improved outcome and we did not observe an increased incidence of infectious disease in patients with high risk MM treated with RIC Allo-SCT.[28] Initial data on RIC transplants seemed to suggest the incidence of CMV infections with RIC is no different from Myeloablative transplantation (MAT) though the onset of CMV disease seemed to be later.[11,15] More recent data suggest there is a higher incidence of CMV reactivation with RIC transplants, more so with the addition of anti-T-cell antibodies to the conditioning regimen.[29-31] The use of MMF for GvHD prophylaxis was also not found to be associated with an increased risk of CMV reactivation, in contrast with limited published data available from both marrow and solid organ transplants that seem to suggest an increased risk with the use of MMF,[6,32] probably because the patients who had received MMF in our study did not receive ATG in the conditioning regimens. Similarly, the use of a URD and the type of graft source did not seem to impact on CMV reactivation. In a study comparing patients undergoing non-MAT from URD with MRD, the incidence of CMV reactivation was slightly higher with URD, though the difference was not statistically significant.[33] It is possible that the higher number of RIC Allo-SCT done using MRD (47%) rather than URD (26.7%) may have negated the effect of URD on CMV reactivation. Ljungman et al., in a very large study from the EBMT megafile, reported there was a strong influence of donor CMV serostatus on outcome in URD transplantations.[34] Patients receiving grafts from CMV-seropositive URD had improved OS and EFS and reduced TRM. However, Walker et al., in a large study involving 753 patients, showed that the graft source (cord blood versus PBSC versus bone marrow) did not independently contribute to CMV reactivation and that patient serostatus was the major determinant of reactivation.[35] The EBMT study could find no effect of donor CMV serostatus on the risk of acute GvHD.[34] But analyses of donor CMV serostatus on the risk of chronic GvHD gave different results. Recently, at our institution, we did not observe a higher incidence of infections in MM patients who received transplants from URD compared to patients with MRD.[36] Furthermore, in this study we observed that grade II–IV acute GvHD was associated with a higher incidence of CMV infection and disease. Unlike the EBMT study, our results indicate that only acute GvHD was a unique factor associated with a higher risk of CMV reactivation. This higher risk was found to be independent of the use of URD or the use of ATG in the conditioning regimens. The incidence of CMV disease was 3% and was limited to the high-risk group alone (D+/R+ and D-/R+).
In conclusion, our findings of comparable outcomes in the three CMV risk groups for MM patients prepared with RIC regimens are important because they suggest that CMV pre-transplant serostatus and reactivation has no influence on OS, PFS and TRM. However, novel prophylactic measures such as immunotherapy and drug prophylaxis need to be considered in this specific group of patients after acute GvHD, warranting further prospective studies.
Funding
We would like to thank the Association pour la Recherche sur le Cancer (ARC) (Pole ARECA) for their generous support of our research. Our group was supported by several grants from the French Ministry of Health as part of the Programme Hospitalier de Recherche Clinique (PHRC).
Transparency Declaration
No conflicts of interest to declare.
Author contributions
Jean El-Cheikh conceived and designed the study, collected and analysed data, performed statistical analyses, provided clinical care, and wrote and revised the manuscript; Raynier Devillier and Roberto Crocchiolo collected and analysed data, performed statistical analyses, provided clinical care and revised the manuscript. Sabine Furst, Catherine Faucher, Claire Oudin, Angela Granata, Luca Castagna, Anne Marie Stoppa and Reda Bouabdallah provided clinical care and commented on the manuscript.
Pierre Berger and Christine Zandotti performed microbiologic and virologic monitoring and controls and commented on the manuscript. Christian Chabannon, Patrick Ladaique, Boris Calmels and Claude Le Marie are in charge of the cell therapy facility that collected and delivered allogeneic the blood cell grafts infused into patients included in this analysis, and commented on the manuscript. Didier Blaise recruited patients, provided clinical care and commented on the manuscript.
Acknowledgments
We thank the nursing staff for providing excellent care for our patients and the physicians of the Hematology Department at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care.
The introduction of allogeneic hematopoietic stem cell transplantation (Allo-SCT), and novel anti-myeloma agents such as bortezomib, thalidomide and lenalidomide has improved the outcome of patients with multiple myeloma (MM) who relapse or are refractory to standard therapies.[1,2] Allo-SCT at present is considered in the first-line therapy of MM only in young high-risk patients.[1] These advances have transformed myeloma into a chronic condition, with multiple relapses, salvage therapies and chronic immunodeficient states leading to high infection rates. Human cytomegalovirus (CMV) infection remains one of the major complications after Allo-SCT and is associated with considerable morbidity and mortality.[3,4] The incidence of CMV infection increases with the intensity and duration of immunosuppression and occurs in roughly 70% of patients after Allo-SCT, depending on both donor and recipient serostatus.[5,6] Without antiviral intervention, about 50% of patients with culture-proven CMV infection will develop CMV disease. Despite the use of antiviral prophylaxis and pre-emptive antiviral therapy, CMV diseases continue to be reported in 15 to 25% of patients.[7-9] Frequent clinical manifestations of CMV disease are interstitial pneumonitis, gastrointestinal (GI) disease and hepatitis; retinitis, encephalitis and marrow suppression have also been described. The present approach to managing CMV disorders consists of peripheral blood monitoring using either CMV pp65 antigen (antigenemia assay) or polymerase chain reaction (PCR).[10,11]
Pre-emptive therapy with either ganciclovir or foscarnet is initiated in patients detected as positive. Factors identified for CMV reactivation are CMV seropositive status of the donor, type of conditioning regimen, use of T-cell depletion, and graft-versus-host disease (GvHD).[12,13] With the introduction of newer conditioning regimens including reduced-intensity conditioning (RIC) Allo-SCT and the increased use of in vivo T-cell depleting agents such as anti-thymocyte globulin (ATG) and monoclonal antibodies such as alemtuzumab (anti-CD52), it has become essential to determine whether a specific high-risk subgroup can be identified.[14,15] These patients could benefit from more intensive or innovative prophylactic strategies as opposed to a general pre-emptive strategy. To our knowledge no data are available in the literature about this issue. We have attempted to study CMV reactivation patterns in 99 consecutive patients with MM who underwent RIC Allo-SCT in our cancer centre at Marseille, where serial CMV monitoring has been done, to ascertain the risks of CMV reactivation.
Patients and methods
This is a retrospective analysis of MM patients who underwent RIC Allo-SCT at our centre between January 2000 and January 2012. The data were collected from the transplant database and individual medical records. A total of 99 patients (median age 53 years, range 27–67) underwent RIC Allo-SCT, including 59 males and 40 females. Ninety-six patients (97%) received one or more prior autologous transplantations (Auto-SCT). Twenty-seven patients (27%) were transplanted as the first line treatment strategy whereas 72 patients (73%) had received other treatments. Those 27 patients were considered for Allo-SCT because they had poor prognostic factors like cytogenetic aberrations (Del (13q14), and/or Del 17 and/or t (4; 14)) or they didn’t have at least partial remission (PR) after Auto-SCT. The median time between Auto-SCT and Allo-SCT was 19 months (1-89). The disease status at time of transplantation was Complete Remission (CR) or VGPR in 22%, PR or stable disease (SD) in 67%, and progression or refractory disease (PD) in 11%. Patient characteristics are shown in Table 1.
Seventy-three patients (74%) had matched related donors (MRD) and 26 patients (26%) had unrelated donors (URD). Eighteen patients (18%) had an HLA-allelically matched URD, a so-called 10/10 match, and 8 patients (8%) had 9/10 HLA-matched antigens (four of them had a DQ allele mismatch, two patients had a Cw mismatch and two had A mismatch). The graft source was peripheral blood stem cells (PBSC) in 88 patients (89%), bone marrow in nine patients (9%), and cord blood in two (2%). T-cell depletion with ATG was used in 68 patients (69%) and low-grade total body irradiation (TBI) with 2 Gys was used in 30 recipients (31%) of RIC transplants, respectively. GvHD prophylaxis consisted of cyclosporine A in 56 patients (57%), CSA + mycophenolate mofetyl (MMF) in 41 patients (41%) and MMF alone in two patients (2%). Transplant characteristics are shown in Table 2.

Table 1. Patient and transplantation characteristics.

Table 2. CMV reactivation.
Based upon previous exposure to CMV, patients were classified into low risk (donor and recipient negative; D-/R-), intermediate risk (donor positive with recipient negative; D+/R-), or high risk (recipient positive with donor either negative [D-/ R+] or positive [D+/R+]). Seventeen patients (17%) were D-/R-, 14 patients (14%) were D+/R-, 31 patients (31%) were D-/R+ and 37 patients (37%) were D+/R+.
Supportive care.Our protocol in providing supportive care was the same throughout this time period. Prophylactic treatment against pneumocystis jirovecii and toxoplasmosis consisted of trimethoprim-sulfamethoxazole (10 mg/Kg/day) administered twice weekly. Patients also received daily oral amoxicillin (500 mg x 3/day) as prophylaxis against encapsulated bacteria and prophylaxis against herpes simplex virus including oral valacyclovir (500 mg x 2/day). Patients were monitored for CMV reactivation during the first 12 months after transplant, and pre-emptive therapy with ganciclovir was given if CMV reactivation occurred.
CMV monitoring and treatment. Serial weekly monitoring for CMV quantification was done using either pp65 antigen (between 2000 and 2009) or a quantitative PCR assay (COBAS R, Roche Diagnostics, Branchburg, New Jersey, USA) (from 2009 to the present). Monitoring was performed weekly initially, starting from transplantation until day 90, and then every 4–8 weeks during the next six months. If there was evidence of reactivation (pp65 >2 cells/200,000 or PCR >1000 copies/mL), treatment was started with ganciclovir (5mg/kg IV twice daily) for 2 weeks, provided two consecutive PCRs done three days apart became negative. If the PCR was still positive after 2 weeks of treatment, a maintenance therapy with ganciclovir (5mg/kg IV once /day) for another 14 days was proposed. CMV disease was diagnosed on demonstration of tissue invasion in biopsy specimens or demonstration of a positive CMV early antigen on bronchoalveolar lavage, along with clinical and radiological features consistent with CMV.
Statistics. Data are presented as medians, ranges, and 95% confidence intervals (CI). The end points analysed were overall survival (OS), progression free survival (PFS), transplant related mortality (TRM) and acute and chronic GvHD. OS was defined as the time elapsed from Allo-SCT to death, whatever the cause of death. PFS was defined as survival with no evidence of relapse or progression. Kaplan-Meier product-limit estimates were used to assess the probabilities of OS and PFS.[16] The Prentice estimate and Gray test, allowing the consideration of competing events, were used to calculate the cumulative incidences of GvHD, relapse, CMV reactivation and TRM.[17,18]
Cox regression was used to find any association between major pre-transplant variables and OS or PFS;[19] the occurrence of CMV reactivation, acute GvHD and chronic GvHD were all considered as time-dependent covariates. If two or more variables were associated with p<0.20 to each endpoint of interest, then a multivariate model was constructed. SPSS v13.0 and R 2.12.2 were used for the above cited analyses (http://www.R-project.org).
Results
CMV reactivation, disease and treatment. The cumulative incidence of CMV reactivation was 39% (n=39) with a median time of 61 [26–318] days after Allo-SCT. Twenty-three patients (59%) were under corticosteroid treatment for GvHD. There was no difference in the median time (days) of reactivation based upon its method of detection using pp65 (54 [32–162]) or quantitative PCR (65[34–162]). The incidence of CMV reactivation at 1, 3, 6 and 12 months after Allo-SCT was 30%, 35%, 37% and 39%, respectively (Figure 1). Three patients (3%) developed CMV disease (two pneumonitis and one disseminated GI and lung disease) at a median of 38 days (26–49) post Allo-SCT. There was no difference in the median time to reactivation based on donor type (MRD versus URD; 48 versus 46 days), graft source - bone marrow (45 days) versus PBSC (47 days) versus cord blood (46 days) - the year of transplant (before or after 2006), the use of ATG in the conditioning regimen, the age of the donor (more or less than 50 years) or the presence of chronic GvHD.

Figure 1. CMV incidence by risk after Allo-SCT.
By univariate analysis, CMV reactivation was significantly influenced by CMV serostatus risk group (high: 50% vs intermediate: 29% vs low: 0%; p=0.001; Table 2 and Figure 1). In the high-risk group characterised by a positive CMV serostatus recipient, the serostatus of the donor (positive vs. negative) did not influence CMV reactivation. The presence of acute GvHD as a time-dependent variable was associated with an increased cumulative incidence of CMV reactivation (p=0.032). Donor type, disease stage, recipient and donor ages, female-to-male graft, graft source (bone marrow versus PBSC versus cord blood), time of transplant (pre- or post-2006), the disease status, the number of lines of previous therapy and time from diagnosis to transplant, the death for other causes, the relapse of the underlying disease, or the presence of chronic GvHD, use of the new anti-myeloma drugs, use of ATG during conditioning and corticosteroid therapy did not significantly influence the incidence of CMV reactivation. Details of the results are shown in Tables 2 and 4.
By multivariate analysis, CMV reactivation was associated with CMV serostatus (HR: 3.8 [1.6–2.9], p=0.002) and the presence of acute GvHD (HR: 2.5 [1.3–4.9], p=0.006).
All 39 patients with CMV reactivation received ganciclovir. Resolution of CMV viremia occurred in 36 patients (92%) after a median of 21 days (range 7–64). Of the three patients who did not have resolution of viremia, all had extensive chronic GvHD. Two of them died of CMV disease while one patient died of disease progression. Recurrent reactivations (after resolution of the first episode with antiviral therapy) were seen in 15 patients, predominantly in patients with chronic GvHD.
Outcome after Allo-SCT. The median 2-year OS and PFS were 56% and 34%, respectively.
Cumulative incidences of day-100 grade II–IV acute GvHD, 2-year chronic GvHD and 2-year extensive chronic GvHD were 37%, 36% and 29%, respectively. The median TRM at 100 days and one year was 9% and 21%, respectively. All the 10 analyzable pathogenic microorganisms are classified in the table 3 by descending order of frequency.

Table 3. Classification of pathogenic microorganisms recovered from bloodstream in patients with infection related mortality.

Table 4. Transplantation outcome.
No impact of pretransplant CMV serostatus on engraftment occurred in any patients. Causes of death were relatively similar between the two groups except for deaths that occurred due to CMV in the group that had reactivation. There was no increase in mortality related to bacterial or other viral infections in patients with CMV reactivation. By univariate analysis, CMV serostatus (low risk versus intermediate risk versus high risk) had no impact on the OS (Figure 2).
Other factors, although evaluated, such as donor type (MRD vs. URD), disease stage, patient and donor ages, female-to-male graft, graft source (bone marrow versus PBSC versus cord blood, time of transplant (before or after 2006), use of the new anti-myeloma drugs, use of ATG during conditioning and corticosteroid therapy did not significantly influence OS, PFS or TRM. CMV reactivation analysed as a time dependent variable did not influence OS (HR=1.4 [0.8–2.4]) (Table 4). Only the presence of acute GvHD as a time dependent variable was significantly associated with increased TRM (p=0.005) (Table 4).

Figure 2. Overall survival (OS) by risk.
Discussion
Infections in patients with MM represent a clinical challenge. The list of potential pathogens is long and changes over the disease course.[20] The aim of this study was to discern the effects of pre-transplant CMV serostatus on the outcome of Allo-SCT in MM patients. To the best of our knowledge, this is the first and largest study reporting on the role of CMV serostatus prior to RIC Allo-SCT in MM patients.
CMV infection remains a major problem following Allo-SCT and different groups have adopted different treatment strategies to reduce the morbidity and mortality related to CMV. One strategy was to serially monitor patients in post Allo-SCT for CMV and start pre-emptive therapy with ganciclovir or foscarnet once there was evidence of reactivation.[6,8,21] This practice was established in our institution in 2000, and analysis of this pre-emptive strategy suggests that a third of our patients (39%) develop reactivation. Most importantly, the reactivations occurred in the high-risk groups (D-/R+ or D+/R+), suggesting pre-transplant recipient seropositivity is perhaps the most important risk factor for reactivation.[5,22] This study showed no impact of pre-transplant CMV serostatus on engraftment in the three risk groups. Previous studies suggested that the CMV serostatus of patients was important not only in terms of reactivation but also in terms of worsening TRM and reducing OS.[5,22] However, in our study, we did not observe worse outcomes in the high-risk group as compared with the other groups. Moreover, we did not find any correlation between CMV reactivation and poor outcome. Interestingly grade II–IV acute GvHD was associated with a higher incidence of CMV infection and there was no increase in mortality related to bacterial or other viral infections in patients with CMV reactivation. In addition to the immunodeficiency related to myeloma and its complications, the type of anti-myeloma therapy used also plays a role in the development of infection, but in this study we did not find an impact of the new anti-myeloma drugs on the outcome of patients after Allo-SCT in any of the three risk groups. These therapeutic strategies impact differently on the immune system, predisposing patients to various opportunistic infections.[20,23] A cumulative suppression of cell-mediated immunity is particular to MM, and results from the combined effect of repetitive use of high-dose corticosteroids, the chronic nature of the disease with multiple relapses requiring salvage therapies (almost always containing dexamethasone), and the addition of bortezomib, a powerful immunosuppressive agent or other immunomodulatory agents like thalidomide and lenalidomide in different treatment phases.[20,24,25] In addition, all patients treated with bortezomib-containing regimens should receive prophylaxis for HSV and VZV infection.[26,27] Recently, as reported by our group, the introduction of these novel agents was associated with an improved outcome and we did not observe an increased incidence of infectious disease in patients with high risk MM treated with RIC Allo-SCT.[28] Initial data on RIC transplants seemed to suggest the incidence of CMV infections with RIC is no different from Myeloablative transplantation (MAT) though the onset of CMV disease seemed to be later.[11,15] More recent data suggest there is a higher incidence of CMV reactivation with RIC transplants, more so with the addition of anti-T-cell antibodies to the conditioning regimen.[29-31] The use of MMF for GvHD prophylaxis was also not found to be associated with an increased risk of CMV reactivation, in contrast with limited published data available from both marrow and solid organ transplants that seem to suggest an increased risk with the use of MMF,[6,32] probably because the patients who had received MMF in our study did not receive ATG in the conditioning regimens. Similarly, the use of a URD and the type of graft source did not seem to impact on CMV reactivation. In a study comparing patients undergoing non-MAT from URD with MRD, the incidence of CMV reactivation was slightly higher with URD, though the difference was not statistically significant.[33] It is possible that the higher number of RIC Allo-SCT done using MRD (47%) rather than URD (26.7%) may have negated the effect of URD on CMV reactivation. Ljungman et al., in a very large study from the EBMT megafile, reported there was a strong influence of donor CMV serostatus on outcome in URD transplantations.[34] Patients receiving grafts from CMV-seropositive URD had improved OS and EFS and reduced TRM. However, Walker et al., in a large study involving 753 patients, showed that the graft source (cord blood versus PBSC versus bone marrow) did not independently contribute to CMV reactivation and that patient serostatus was the major determinant of reactivation.[35] The EBMT study could find no effect of donor CMV serostatus on the risk of acute GvHD.[34] But analyses of donor CMV serostatus on the risk of chronic GvHD gave different results. Recently, at our institution, we did not observe a higher incidence of infections in MM patients who received transplants from URD compared to patients with MRD.[36] Furthermore, in this study we observed that grade II–IV acute GvHD was associated with a higher incidence of CMV infection and disease. Unlike the EBMT study, our results indicate that only acute GvHD was a unique factor associated with a higher risk of CMV reactivation. This higher risk was found to be independent of the use of URD or the use of ATG in the conditioning regimens. The incidence of CMV disease was 3% and was limited to the high-risk group alone (D+/R+ and D-/R+).
In conclusion, our findings of comparable outcomes in the three CMV risk groups for MM patients prepared with RIC regimens are important because they suggest that CMV pre-transplant serostatus and reactivation has no influence on OS, PFS and TRM. However, novel prophylactic measures such as immunotherapy and drug prophylaxis need to be considered in this specific group of patients after acute GvHD, warranting further prospective studies.
Funding
We would like to thank the Association pour la Recherche sur le Cancer (ARC) (Pole ARECA) for their generous support of our research. Our group was supported by several grants from the French Ministry of Health as part of the Programme Hospitalier de Recherche Clinique (PHRC).
Transparency Declaration
No conflicts of interest to declare.
Author contributions
Jean El-Cheikh conceived and designed the study, collected and analysed data, performed statistical analyses, provided clinical care, and wrote and revised the manuscript; Raynier Devillier and Roberto Crocchiolo collected and analysed data, performed statistical analyses, provided clinical care and revised the manuscript. Sabine Furst, Catherine Faucher, Claire Oudin, Angela Granata, Luca Castagna, Anne Marie Stoppa and Reda Bouabdallah provided clinical care and commented on the manuscript.
Pierre Berger and Christine Zandotti performed microbiologic and virologic monitoring and controls and commented on the manuscript. Christian Chabannon, Patrick Ladaique, Boris Calmels and Claude Le Marie are in charge of the cell therapy facility that collected and delivered allogeneic the blood cell grafts infused into patients included in this analysis, and commented on the manuscript. Didier Blaise recruited patients, provided clinical care and commented on the manuscript.
Acknowledgments
We thank the nursing staff for providing excellent care for our patients and the physicians of the Hematology Department at the Institut Paoli-Calmettes for their important study contributions and dedicated patient care.
References
- Crawley C,
Lalancette M, Szydlo R, Gilleece
M, Peggs K, Mackinnon S et al. Outcomes for reduced-intensity
allogeneic transplantation for multiple myeloma: an analysis of
prognostic factors from the Chronic Leukaemia Working Party of the
EBMT. Blood 2005; 105: 4532-4539. http://dx.doi.org/10.1182/blood-2004-06-2387
PMid:15731182

- Kumar SK, Rajkumar
SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma
and the impact of novel therapies. Blood 2008; 111: 2516-2520. http://dx.doi.org/10.1182/blood-2007-10-116129
PMid:17975015 PMCid:2254544

- Bacigalupo A,
Tedone E, Sanna MA, Moro F,
Van Lint MT, Grazi G et al. CMV infections following allogeneic BMT:
risk factors, early treatment and correlation with transplant related
mortality. Haematologica 1992; 77: 507-513. PMid:1337746

- Ljungman P. CMV
infections after hematopoietic stem cell transplantation. Bone Marrow
Transplant 2008; 42 Suppl 1: S70-S72. http://dx.doi.org/10.1038/bmt.2008.120
PMid:18724309

- Boeckh M, Nichols
WG. The impact of
cytomegalovirus serostatus of donor and recipient before hematopoietic
stem cell transplantation in the era of antiviral prophylaxis and
preemptive therapy. Blood 2004; 103: 2003-2008. http://dx.doi.org/10.1182/blood-2003-10-3616
PMid:14644993

- George B, Pati N,
Gilroy N, Ratnamohan M,
Huang G, Kerridge I et al. Pre-transplant cytomegalovirus (CMV)
serostatus remains the most important determinant of CMV reactivation
after allogeneic hematopoietic stem cell transplantation in the era of
surveillance and preemptive therapy. Transpl. Infect Dis 2010; 12:
322-329. http://dx.doi.org/10.1111/j.1399-3062.2010.00504.x
PMid:20487414

- Verma A, Devine S,
Morrow M, Chen YH,
Mihalov M, Peace D et al. Low incidence of CMV viremia and disease
after allogeneic peripheral blood stem cell transplantation. Role of
pretransplant ganciclovir and post-transplant acyclovir. Bone Marrow
Transplant 2003; 31: 813-816. http://dx.doi.org/10.1038/sj.bmt.1703916
PMid:12732890

- Ljungman P,
Reusser P, de la Camara R,
Einsele H, Engelhard D, Ribaud P et al. Management of CMV infections:
recommendations from the infectious diseases working party of the EBMT.
Bone Marrow Transplant 2004; 33: 1075-1081. http://dx.doi.org/10.1038/sj.bmt.1704505
PMid:15077131

- Zaia JA, Schmidt
GM, Chao NJ, Rizk NW,
Nademanee AP, Niland JC et al. Preemptive ganciclovir administration
based solely on asymptomatic pulmonary cytomegalovirus infection in
allogeneic bone marrow transplant recipients: long-term follow-up. Biol
Blood Marrow Transplant 1995; 1: 88-93. PMid:9118297

- Nitsche A, Oswald
O, Steuer N, Schetelig
J, Radonic A, Thulke S et al. Quantitative real-time PCR compared with
pp65 antigen detection for cytomegalovirus (CMV) in 1122 blood
specimens from 77 patients after allogeneic stem cell transplantation:
which test better predicts CMV disease development? Clin Chem 2003; 49:
1683-1685. http://dx.doi.org/10.1373/49.10.1683
PMid:14500600

- Schetelig J,
Oswald O, Steuer N, Radonic
A, Thulke S, Held TK et al. Cytomegalovirus infections in allogeneic
stem cell recipients after reduced-intensity or myeloablative
conditioning assessed by quantitative PCR and pp65-antigenemia. Bone
Marrow Transplant 2003; 32: 695-701. http://dx.doi.org/10.1038/sj.bmt.1704164
PMid:13130317

- Ljungman P,
Perez-Bercoff L, Jonsson J,
Avetisyan G, Sparrelid E, Aschan J et al. Risk factors for the
development of cytomegalovirus disease after allogeneic stem cell
transplantation. Haematologica 2006; 91: 78-83. PMid:16434374

- Miller W, Flynn
P, McCullough J, Balfour
HH, Jr., Goldman A, Haake R et al. Cytomegalovirus infection after bone
marrow transplantation: an association with acute graft-v-host disease.
Blood 1986; 67: 1162-1167. PMid:3006831

- Chakrabarti S,
Mackinnon S, Chopra R,
Kottaridis PD, Peggs K, O'Gorman P et al. High incidence of
cytomegalovirus infection after nonmyeloablative stem cell
transplantation: potential role of Campath-1H in delaying immune
reconstitution. Blood 2002; 99: 4357-4363. http://dx.doi.org/10.1182/blood.V99.12.4357
PMid:12036862

- Hill QA, Hill A,
Collyns TA, Pearce RM,
Cook G. Similar lymphocyte recovery and CMV reactivation profiles
between reduced intensity conditioning with alemtuzumab and
myeloablative allogeneic stem cell transplantation. Bone Marrow
Transplant 2008; 41: 749-751. http://dx.doi.org/10.1038/sj.bmt.1705974
PMid:18195685

- Kaplan EL, Meier
P. Nonparametric estimation from incomplete observations. J Am Stat
Assoc 1958; 53: 457-481. http://dx.doi.org/10.1080/01621459.1958.10501452

- Fine J. A
proportional hazards model for the subdistribution of a competing risk.
J Am Stat Assoc 1999; 94: 496-509. http://dx.doi.org/10.1080/01621459.1999.10474144

- Gooley TA,
Leisenring W, Crowley J, Storer
BE. Estimation of failure probabilities in the presence of competing
risks: new representations of old estimators. Stat. Med 1999; 18:
695-706. http://dx.doi.org/10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O

- Cox
DR. Regression models and life tables. Journal of the Royal Statistical
Society: Serie B (Methodological) 1972; 34: 187-220.

- Nucci M, Anaissie
E. Infections in
patients with multiple myeloma in the era of high-dose therapy and
novel agents. Clin Infect Dis 2009; 49: 1211-1225. http://dx.doi.org/10.1086/605664
PMid:19769539

- Ljungman P.
Molecular monitoring of viral
infections after hematopoietic stem cell transplantation. Int J Hematol
2010; 91: 596-601. http://dx.doi.org/10.1007/s12185-010-0570-4
PMid:20414752

- Nichols WG, Corey
L, Gooley T, Davis C,
Boeckh M. High risk of death due to bacterial and fungal infection
among cytomegalovirus (CMV)-seronegative recipients of stem cell
transplants from seropositive donors: evidence for indirect effects of
primary CMV infection. J Infect Dis 2002; 185: 273-282. http://dx.doi.org/10.1086/338624
PMid:11807708

- Haslett PA,
Hanekom WA, Muller G, Kaplan
G. Thalidomide and a thalidomide analogue drug costimulate
virus-specific CD8+ T cells in vitro. J Infect Dis 2003; 187: 946-955. http://dx.doi.org/10.1086/368126
PMid:12660941

- Weber DM, Chen C,
Niesvizky R, Wang M,
Belch A, Stadtmauer EA et al. Lenalidomide plus dexamethasone for
relapsed multiple myeloma in North America. N Engl J Med 2007; 357:
2133-2142. http://dx.doi.org/10.1056/NEJMoa070596
PMid:18032763

- Schutt P,
Brandhorst D, Stellberg W, Poser
M, Ebeling P, Muller S et al. Immune parameters in multiple myeloma
patients: influence of treatment and correlation with opportunistic
infections. Leuk Lymphoma 2006; 47: 1570-1582. http://dx.doi.org/10.1080/10428190500472503
PMid:23469683 PMCid:3610087

- Mitsiades CS,
Davies FE, Laubach JP,
Joshua D, San MJ, Anderson KC et al. Future directions of
next-generation novel therapies, combination approaches, and the
development of personalized medicine in myeloma. J Clin Oncol 2011; 29:
1916-1923. http://dx.doi.org/10.1200/JCO.2010.34.0760
PMid:21482978

- Laubach JP,
Mahindra A, Mitsiades CS,
Schlossman RL, Munshi NC, Ghobrial IM et al. The use of novel agents in
the treatment of relapsed and refractory multiple myeloma. Leukemia
2009; 23: 2222-2232. http://dx.doi.org/10.1038/leu.2009.179
PMid:19741729 PMCid:3133628

- El-Cheikh J,
Crocchiolo R, Boher JM, Furst
S, Stoppa AM, Faucher C et al. Allogeneic transplant for myeloma in the
era of new drugs: have the outcomes improved? Leuk Lymphoma 2012; 53:
1630-1632. http://dx.doi.org/10.3109/10428194.2012.656630
PMid:23469683 PMCid:3610087

- Chakrabarti S.
Increased CMV infection
following nonmyeloablative allogeneic stem cell transplantation: a
search for the guilty. Blood 2003; 101: 2071. http://dx.doi.org/10.1182/blood-2002-11-3598
PMid:12584145

- George B,
Kerridge I, Gilroy N, Huang G,
Hertzberg M, Gottlieb D et al. Fludarabine-based reduced intensity
conditioning transplants have a higher incidence of cytomegalovirus
reactivation compared with myeloablative transplants. Bone Marrow
Transplant 2010; 45: 849-855. http://dx.doi.org/10.1038/bmt.2009.273
PMid:19915635

- Nivison-Smith I,
Dodds AJ, Doocey R, Ganly
P, Gibson J, Ma DD et al. Allogeneic hematopoietic cell transplant for
multiple myeloma using reduced intensity conditioning therapy,
1998-2006: factors associated with improved survival outcome. Leuk
Lymphoma 2011; 52: 1727-1735. http://dx.doi.org/10.3109/10428194.2011.582201
PMid:23469683 PMCid:3610087

- Hambach L,
Stadler M, Dammann E, Ganser A,
Hertenstein B. Increased risk of complicated CMV infection with the use
of mycophenolate mofetil in allogeneic stem cell transplantation. Bone
Marrow Transplant 2002; 29: 903-906. http://dx.doi.org/10.1038/sj.bmt.1703583
PMid:12080355

- Junghanss C,
Storb R, Maris MB, Carter RA,
Sandmaier BM, Maloney DG et al. Impact of unrelated donor status on the
incidence and outcome of cytomegalovirus infections after
non-myeloablative allogeneic stem cell transplantation. Br J Haematol
2003; 123: 662-670. http://dx.doi.org/10.1046/j.1365-2141.2003.04671.x
PMid:14616970

- Ljungman P, Brand
R, Einsele H, Frassoni
F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome
of CMV-seropositive recipients after unrelated donor stem cell
transplantation: an EBMT megafile analysis. Blood 2003; 102: 4255-4260.
http://dx.doi.org/10.1182/blood-2002-10-3263
PMid:12933590

- Walker CM, van
Burik JA, De For TE,
Weisdorf DJ. Cytomegalovirus infection after allogeneic
transplantation: comparison of cord blood with peripheral blood and
marrow graft sources. Biol Blood Marrow Transplant 2007; 13: 1106-1115.
http://dx.doi.org/10.1016/j.bbmt.2007.06.006
PMid:17697973

- El-Cheikh J,
Crocchiolo R, Boher JM, Furst
S, Stoppa AM, Ladaique P et al. Comparable outcomes between Unrelated
and Related donors after Reduced Intensity Conditioning Allogeneic
hematopoietic Stem Cell Transplantation in patients with high risk
Multiple Myeloma. Eur. J Haematol 2012. http://dx.doi.org/10.1111/j.1600-0609.2012.01777.x
PMid:22385049
