Assessment of Diagnostic Techniques of Urinary Tuberculosis
Khaled Ghaleb1, Magdy Afifi2 and Mohamad El-Gohary3
1 Department of applied medical science,Faculty of Applied Medical Science, king Khalid University, Saudia Arabia, Bisha
2 Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Assuit 71524, Egypt
3 Department of Internal Medicine, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
2 Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Assuit 71524, Egypt
3 Department of Internal Medicine, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
Correspondence
to:
Dr. Khaled Ghaleb, Department of applied medical science,Faculty of
Applied Medical Science, king Khalid University, Saudia Arabia,Bisha.
Tel: +201119338055 Egypt, +966595388496 Saudia Arabia. E-mail: kh_ghaleb4@hotmail.com
Published: June 3, 2013
Received: January 26, 2013
Accepted: April 26, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013034, DOI 10.4084/MJHID.2013.034
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Early
diagnosis of active tuberculosis remains an elusive challenge. In
addition, one third of the world's population is latently infected with
Mycobacterium tuberculosis (Mtb) and up to 10% of infected individuals
develop tuberculosis (TB) in their lifetime. In this investigation, the
incidence of urinary tuberculosis among renal patients was studied.
Three hundreds urine samples were processed for detection of Mtb by
Ziehl-Neelsen (ZN) smear examination, Lowenstein Jensen (LJ) medium,
radiometric BACTEC460 system as well as polymerase chain reaction (PCR)
followed by DNA Enzyme Immunoassay (DEIA) test. Out of 300 urine
samples, 2 were positive by both ZN smears and LJ medium with incidence
rate of 0.66 %, 3 positive samples by BACTEC460 culture system with
incidence of 1%. PCR assay gave more positive results than smear and
culture examination (i.e. 8 positive samples with incidence rate of
2.6%). The specificities were 25% for both ZN smears and LJ medium,
37.5% for BACTEC460 culture system, and 100% for PCR test, while
sensitivities of all assays were 100%. Thus PCR is a rapid and
sensitive method for the early diagnosis of urinary tuberculosis.
Introduction
Tuberculosis kills over 1.7 million people worldwide every year and nearly 40% of patients with active tuberculosis remain undiagnosed because of the poor sensitivity of the current, century old diagnostic method.[1] The situation is further exacerbated with the increasing incidence of drug resistant TB.[2] Early diagnosis of TB remains an elusive challenge, especially in individuals with disseminated TB and HIV co-infection.[3]
Early diagnosis plays a vital role in control of TB. Diagnosis of mycobacterial infections, however, remains an enigma. Although acid fast bacilli (AFB) microscopy and LJ culture remains the cornerstone of the diagnosis of TB, these traditional bacteriological methods are either slow or their sensitivity is quite low, especially with clinical samples that contain small number of organisms.[4] This can affect treatment by either delaying it or causing inappropriate empiric therapy for TB to subjects without mycobacterial infections or with atypical mycobacteria.[5]
Urogenital tuberculosis (UGTB) is among the most common manifestations of extrapulmonary tuberculosis (EPTB) worldwide.[6,7] Because of its insidious evolution and late onset of symptoms, the diagnosis and treatment are notoriously delayed, resulting in significant morbidity (e.g. end-stage renal failure, shrunken bladder, and testicular destruction).[8-10] UGTB is mainly caused by members of the Mtb complex. Nevertheless, several mycobacteria species other than tuberculosis are also associated with urogenital infections.[11,12] Many efforts have been made to develop faster diagnostic assays for UGTB.[13,14] However, the optimal treatment cannot be initiated on the basis of rapid tests alone, because they fail to assess mycobacterial viability or provide affordable phenotypic drug susceptibility testing. Similarly, fast diagnostic methods, such as matrix-assisted laser desorption ionization-time of flight-mass spectrometry or genotype, which are useful in tuberculosis lung infections, have the same drawbacks.[15-17] Therefore, urine or tissue culture remains essential in the diagnosis of UGTB and its treatment.
UGTB is the second most common extrapulmonary presentation of tuberculosis, affecting 8–15% of the patients with pulmonary tuberculosis.[8] From the lungs, the kidneys are affected through hematogenic dissemination, with subsequent inv olvement of the ureters and bladder through descending infection of the collecting system. The genital organs are involved through hematogenic (prostate and epydidimus) or retrograde canalicular dissemination.[8,18] UGTB affects all age ranges, but predominates in men in their fourth or fifth decades. The diagnosis is often delayed, especially in developing countries, due to the evolution insidious with few and unspecific symptoms, along with a lack of awareness of physicians.[19,20]
Several studies have been done to detect Mtb s in urine and other clinical samples by, conventional ZN (Ziehl-Neelson) stained acid fast bacilli (AFB) microscopy and culture by LJ and radiometric BACTEC system[21,22] as well as by amplifying different DNA sequences of Mtb by PCR test.[23] In addition, the pathogen-specific biomarkers can be applied for the rapid and effective diagnosis of TB. It is likely that detection of a combination of biomarkers offers greater reliability of TB diagnosis, rather than detection of any single pathogen biomarker.[3]
The aim of this study was to compare different techniques for the diagnosis of renal tuberculosis, including Ziehle-Neelsen(ZN) smear, Lowenstein Jensen(LJ) medium, Bactec 460 radiometric culture system as well as PCR followed by DIA hybridization.
Materials and Methods
Three hundred urine samples were collected from the outpatient department of the Azhar University's hospital of the School of Medicine in Assiut, Egypt from January until June 2009. The samples were collected in the morning from the admitted patients from urology department in the hospital. The patients were divided into three groups (I, II, III), each group comprised 100 patients. Group 1 (control group), com¬prised apparently healthy patients, Group II, comprised a patient with chronic renal failure, and Group III, comprised patients with suspected infection (Table 1).
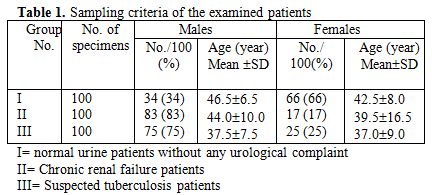
Table 1. Sampling criteria of the examined patients
Processing of specimens. Urine specimens were treated with NALC-NaOH method for the decontamination.[24] Ziehl-Neelsen (ZN) staining method was done as described previously.[25] Lowenstein-Jensen medium (Becton- Dinkinson Microbiology Systems, Cockeysille, MD, USA) was prepared.[26] The processed specimens were inoculated into a Bactec 12 B vial.[24] The BACTEC460 instrument (Becton Dickinson Diagnostic Instrument System, Sparks, MD, USA) was achieved, which was significantly superior to the conventional method.[22]
Polymerase Chain Reaction (PCR). DNA extraction. The urine specimens were treated as previously mentioned for the decontamination and liquefaction to obtain the pellet for DNA extraction.[24] The bacterial pellet was resuspended in 180 ul buffer ATL (supplied in QIAamp Tissue Kit, QIAGEN GmbH, Hilden, Germany). The extraction of TB DNA was performed according to QIAGEN kit protocol. The eluted DNA can be stored at-20°C until use in PCR.
Primers and PCR assay. The PCR assay was performed with the primers pair p1(5’CCTGCGAGCGTAGCGTAGGCGG-‘3) p2(5’-CTCGTCCAGTCCAGCGCCGCTTCGG-‘3) located within IS6110 sequence repeated 10-12 times in the chromosome of M. Tuberculosis, these primers gives a 123 bp product and synthesized by Sorin BIomedica Diagnostics, Saluggia, VC, Italy which gives. Ten µl of the extract were subjected to PCR in a final volume of 100µl in 0.2 ml microcentrifuge tubes containing 1X PCR buffer (10 mM Tris-HCl, pH 8.0,2.5 mM MgCl2, 50 mM KCl, gelatin 0.01%w/v), 0.2 mM (each) of the four deoxyribonucleoside triphosphates (dATP, dTTP, dGTP and dCTP), 2.5 U of taq polymerase were all supplied from STRATA-GENE reagent kit (Alameda, California, USA) and 50 pM each primer. The PCR conditions for IS6110 DNA amplification were an initial denaturation step of 94°C for 5 min, followed by 35 cycles of 94°C for 2 min, 68°C for 2 min, 72° for 2 min. and a final extension step at 72°C for 10 min. The PCR procedure was accomplished with a thermocycler TC 9600 (Perkin-Elmer Cetus). Each experiment included positive and negative control tubes. The products of amplification were then analyzed by agarose gel electrophoresis followed by hybridization with DNA Enzyme Immunoassay (DEIA test).
DNA Enzyme Immunoassay (DEIA test). One hundred µl hybridization buffer were dispensed into each well, and 200µl controls and denatured samples into their respective wells. The strip was sealed with card board sealer, incubated at 45°C for 30 min and then washed. The anti-DS-DNA solution was prepared at the end of the first incubation and 100µl diluted Anti-DS. The DNA were dispensed into each well except for the blank, the strip was sealed and incubated for 1h. at room temperature, then washed. The enzyme tracer solution was prepared at the end of the incubation. Hundred µl diluted enzyme tracer solution were dispensed into all the wells except for the blank, the strip was sealed and incubated for 1 hr at room temperature. The chromogen-substrate was prepared at the end of third incubation. At the end of incubation, substrate strip was washed. Hundred µl chromogen – substrate were dispensed into each well, incubated for 30min at room temperature, away from light. Two hundred µl of blocking reagent (GENE Kit DEIA, Sorin Biomedica Diagnostics, Saluggia,VC, Italy) were then dispensed into all the wells. The strip was read using ELISA reader photometer with 450-630 nm filter. The instrument was rested with blank, before reading the wells at 450 nm. Absorbance values at least 0.170 greater than the mean negative control value indicated that hybridization had taken place.
Statistical analysis
The incidences of all techniques were performed with the Statistical Package for Social Science (SPSS version 12.0) using the Z test and P value.
Results
Characteristics of the studied patients are summarized in Table 1; 300 urine samples were provided into three groups (I, II, III) as previously mentioned.
The most common presenting symptoms in groups II and III patients with urinary TB were sterile pyuria (17% and 22 %), haematuria (2% and 4%) in group I and II, respectively. In addition, Dysuria (3%) and persistent dysuria (25%) are reported only in group II (Table 2).
Out of 300 urine specimens, 2 samples were detected by both ZN smear and LJ medium giving an incidence rate of 0.66%, 3 samples were also found to be contaminated in BACTEC culture giving an incidence rate of 1%. Accordingly, PCR assay and non-isotopic hybridization probe test gave more positive results than smear and culture examinations (i.e. 8 samples were detected and gave an incidence rate of 2.6%). The results are given in Table 3.
The results show that ZN smear examination has a sensitivity of 25% and a specificity of 100%. For LJ media culture, sensitivity was 25% and specificity was 100%. BACTEC culture showed a sensitivity of 37.5% and a specificity of 100%. In comparison, PCR was found to have a much higher sensitivity of 100% and a specificity of 100% (Table 4).
The specificity, sensitivity and speed of PCR test in diagnosis of M. tuberculosis infection shown in this study should encourage the use of this technique in diagnosis of urinary TB. Acid-fast bacilli (AFB) smear were performed on all specimens. The reading of semiquantitative results had indicated the present number of bacteria (Table 5).
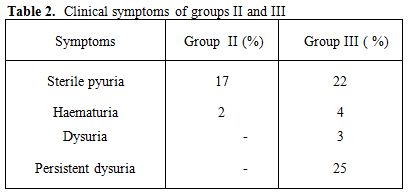
Table 2. Clinical symptoms of groups II and III
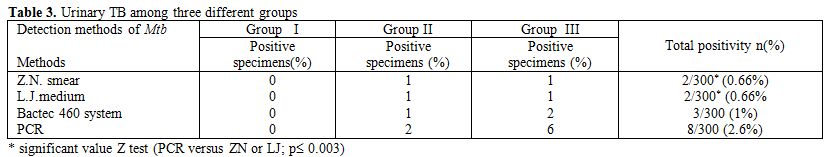
Table 3. Urinary TB among three different groups
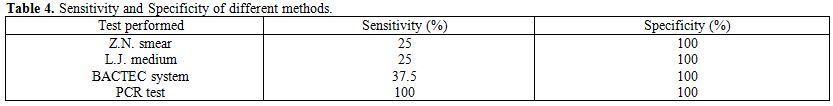
Table 4. Sensitivity and Specificity of different methods
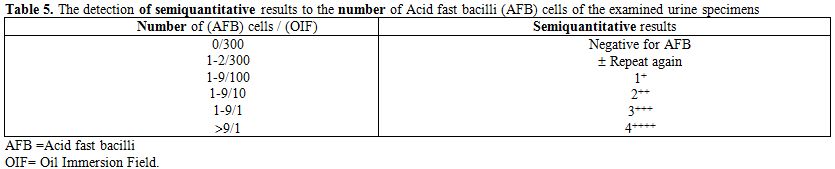
Table 5. The detection of semiquantitative results to the number of Acid fast bacilli (AFB) cells of the examined urine specimens
In this study, two positive specimens for M. tuberculosis were detected by both ZN smear and LJ medium out of 300 specimens, one positive specimen was found in group II and one positive specimen was found in group III (Table 3). The control specimens (group I) were completely negative by LJ medium.
Three positive specimens of M. tuberculosis were detected by Bactec culture system out of 300 urine specimens (1%). These positive samples were 1 in group II and 2 in group III. The control specimens (group I) were completely negative by Bactec460 culture (Table 3), while the sensitivity of BACTEC 460 system was 37.5% and the specificity was 100% (Table 4).
The detection of M. tuberculosis DNA in urine specimens by PCR and non-isotopic hybridization was elevated for laboratory diagnosis of urinary tract M. tuberculosis infection. After amplifying DNA by PCR, the samples were detected by agarose gel electrophoresis to see the specific band for M. tuberculosis. Positive specimens were seen in agarose gel containing 123 bp band located within IS6110 sequence repeated 10-12 times in the chromosome of M. tuberculosis. The 100 bp ladder was used as depicted in Figure 1.
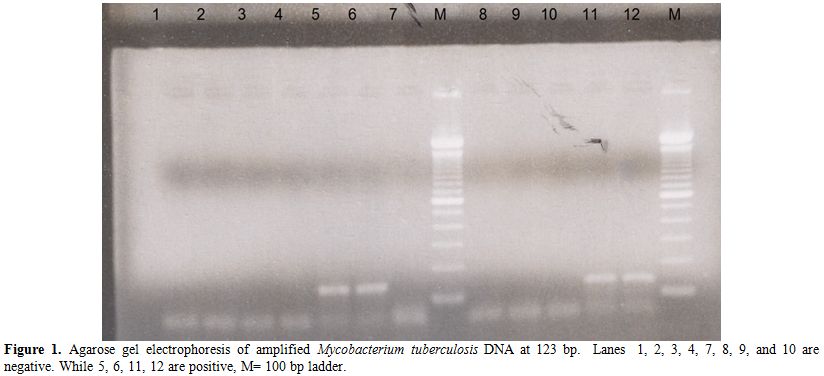
Figure 1. Agarose gel electrophoresis of amplified Mycobacterium tuberculosis DNA at 123 bp. Lanes 1, 2, 3, 4, 7, 8, 9, and 10 are negative. While 5, 6, 11, 12 are positive, M= 100 bp ladder.
Negative specimens were shown by absence of 123 bp band. The results of gel electrophoresis of amplified product were 8 positive specimens for M. tuberculosis. Six of which had a clear band of 123 bp and other two had a weak or faint 123 bp band, these two specimens considered positive after confirmation using hybridization by non-isotopic hybridization to the specific probe. Hybridization to the specific probe was performed to all samples on the amplified products. Results of non-isotopic hybridization probe was performed and read at 450 nm, mean OD of the DEIA negative control was 0.05 A450 and positive control was 3.0 A450. Therefore, the OD cut-off value calculated in this study was 0.170 A450 and the samples were classified as negative at OD450< 0.170 or positive at OD450>=0.170. The eight positive specimens had OD in the range of 0.750- 1.5 A450. The positive control used in PCR reaction gave OD value >2.0 and 0.450 A450.
To conclude, molecular diagnosis of tuberculosis by PCR has a great potential than ZN smear examination, LJ medium, and BACTEC radiometric culture, to improve the clinicians' ability to diagnose urinary tuberculosis. This will ensure early treatment to patients and prevent further transmission of disease.
Discussion
Early identification of tuberculosis in the clinical setting is of great importance in order for specific therapy to be swiftly initiated because TB is the leading cause of death worldwide attributable to a single infectious disease agent. UGTB is the second most common form of extrapulmonary tuberculosis in countries with a severe epidemic and the third most common form in regions with a low incidence of tuberculosis.[27]
Because of the insidious evolution and late onset of TB symptoms, the diagnosis and treatment are notoriously delayed, resulting in significant morbidity (e.g. end-stage renal failure, shrunken bladder, and testicular destruction).[8-10]
In our context, the most common presenting symptoms in patients in this study with UTB are sterile pyuria (17% and 22%), haematuria (2% and 4%) in group I and II, respectively. In addition, dysuria (3%) and persistent dysuria (25%) are reported only in group II.
Therefore, the diagnosis of UTB is often delayed not only because of its insidious evolution accompanied by few nonspecific symptoms, but also because of the long time required to confirm the diagnosis by classical conventional methods of cultivation,[28] the only utilized in most developing countries.
Sterile pyuria (81%), pyuria (48%) and hematuria (18%) have been reported in patients with renal tuberculosis.[13] Moreover, Narayan[29] found that the most common presenting symptoms in patients with UTB are irritative voiding symptoms and hematuria. Histopathology is characteristic but there could be problems to get a representative specimen, and non-specific features can sometimes complicate the diagnosis. Nevertheless, antigen-based serological tests have the potential of providing inexpensive and robust tools for diagnosis of TB under the conditions encountered commonly in developing countries such as Egypt.
However, immunological diagnosis is often inconclusive because antibodies and the delayed type hypersensitivity response persist for a long time after the subsidence of sub-clinical or clinical disease. Although a number of TB specific seroantigens have been identified in recent years, these vary in their sensitivities and specificities. Therefore, newer and reliable diagnostic methods are needed for early and precise detection of TB and efforts are ongoing in various laboratories to improve the performance of TB diagnostic tests either through improvements in conventional tools or by using newer diagnostic modalities that are based on nucleic acid amplification or antigen/antibody detection, etc. The salient achievements made towards improving the diagnosis of TB are summarized in this study.
In our investigation, the incidence of urinary tuberculosis among renal patients was studied. Three hundreds urine samples were processed for detection of Mtb by ZN smear examination, LJ medium culture, radiometric BACTEC460 culture as well as PCR and non-isotopic hybridization probe test.
The results show that ZN smear examination has a sensitivity of 25% and a specificity of 100%. To make such detection of UGTB faster, one could use media such as Middlebrook (solid or liquid) or Lowenstein-Jensen in the microcalorimetric vials, such as was proposed in other studies.[30-33] Those media could then be inoculated with urine samples previously concentrated by centrifugation and decontaminated using the N-acetyl-L-cysteine-sodium hydroxide procedure to inactivate contaminants and avoid mixed bacterial growth.
The sensitivities of BACTEC culture method were found to be much higher compared to LJ i.e 37.5% and a specificity of 100% as well as the mean detection time for Mtb was 24.03 days by LJ medium culture, 12.89 days by BACTEC culture as reported.[14,34,35]
Another noteworthy aspect of our study was in the diagnosis by PCR. Its results revealed 8 positive samples out of 300 specimens with incidence of 2.6%. Moreover, in comparison with the previous techniques, PCR was found to have the highest sensitivity of 100% and a specificity of 100%. This specificity, sensitivity and speed of PCR test followed by non-isotopic hybridization probe test in diagnosis of M. tuberculosis infection in this study, should encourage the use of this method in diagnosis of TB compared the performance of ZN smear, LJ media, and BACTAC 460 culture system in urine samples for diagnosis of TB as reported by others[21,23,34] as well as Immunoassay test.[3,36-38] However, immunological diagnosis is often inconclusive because antibodies and the delayed type hypersensitivity response persist for a long time after the subsidence of sub-clinical or clinical disease. Although a number of TB specific seroantigens have been identified in recent years, these vary in their sensitivities and specificities.
With the use of PCR test and non-isotopic hybridization probe test, we are able to detect M. tuberculosis in 4 times than both ZN smear and LJ culture and 2.66 times than BACTAC460 culture system.
PCR test detected M. tuberculosis in less than one day, compared to average 24.03 days required for detection by conventional (LJ) and 12.89 days by radiometric BACTEC technique, as supported.[39] Similar measurements using bactec culture and polymerase chain reaction methods have been performed for diagnosis of tuberculosis and found that a significant difference was seen in the sensitivities of 74.4% for PCR test, 33.79% for ZN smear examination, 48.9% for LJ culture and 55.8% for BACTEC culture (P<0.05). However, there was no significant difference (P>0.05) as far as specificity of different tests was concerned.[21]
PCR test was also shown to be reasonably sensitive in diagnosis of TB.[13,38,40-44]
Taken together, the present demonstration emphasized that among various diagnostic methods used for urinary tuberculosis, PCR, as a recent method, offers a promising new approach for rapid, safe, specific, and reproducible determination of M. tuberculosis infection.
Tuberculosis kills over 1.7 million people worldwide every year and nearly 40% of patients with active tuberculosis remain undiagnosed because of the poor sensitivity of the current, century old diagnostic method.[1] The situation is further exacerbated with the increasing incidence of drug resistant TB.[2] Early diagnosis of TB remains an elusive challenge, especially in individuals with disseminated TB and HIV co-infection.[3]
Early diagnosis plays a vital role in control of TB. Diagnosis of mycobacterial infections, however, remains an enigma. Although acid fast bacilli (AFB) microscopy and LJ culture remains the cornerstone of the diagnosis of TB, these traditional bacteriological methods are either slow or their sensitivity is quite low, especially with clinical samples that contain small number of organisms.[4] This can affect treatment by either delaying it or causing inappropriate empiric therapy for TB to subjects without mycobacterial infections or with atypical mycobacteria.[5]
Urogenital tuberculosis (UGTB) is among the most common manifestations of extrapulmonary tuberculosis (EPTB) worldwide.[6,7] Because of its insidious evolution and late onset of symptoms, the diagnosis and treatment are notoriously delayed, resulting in significant morbidity (e.g. end-stage renal failure, shrunken bladder, and testicular destruction).[8-10] UGTB is mainly caused by members of the Mtb complex. Nevertheless, several mycobacteria species other than tuberculosis are also associated with urogenital infections.[11,12] Many efforts have been made to develop faster diagnostic assays for UGTB.[13,14] However, the optimal treatment cannot be initiated on the basis of rapid tests alone, because they fail to assess mycobacterial viability or provide affordable phenotypic drug susceptibility testing. Similarly, fast diagnostic methods, such as matrix-assisted laser desorption ionization-time of flight-mass spectrometry or genotype, which are useful in tuberculosis lung infections, have the same drawbacks.[15-17] Therefore, urine or tissue culture remains essential in the diagnosis of UGTB and its treatment.
UGTB is the second most common extrapulmonary presentation of tuberculosis, affecting 8–15% of the patients with pulmonary tuberculosis.[8] From the lungs, the kidneys are affected through hematogenic dissemination, with subsequent inv olvement of the ureters and bladder through descending infection of the collecting system. The genital organs are involved through hematogenic (prostate and epydidimus) or retrograde canalicular dissemination.[8,18] UGTB affects all age ranges, but predominates in men in their fourth or fifth decades. The diagnosis is often delayed, especially in developing countries, due to the evolution insidious with few and unspecific symptoms, along with a lack of awareness of physicians.[19,20]
Several studies have been done to detect Mtb s in urine and other clinical samples by, conventional ZN (Ziehl-Neelson) stained acid fast bacilli (AFB) microscopy and culture by LJ and radiometric BACTEC system[21,22] as well as by amplifying different DNA sequences of Mtb by PCR test.[23] In addition, the pathogen-specific biomarkers can be applied for the rapid and effective diagnosis of TB. It is likely that detection of a combination of biomarkers offers greater reliability of TB diagnosis, rather than detection of any single pathogen biomarker.[3]
The aim of this study was to compare different techniques for the diagnosis of renal tuberculosis, including Ziehle-Neelsen(ZN) smear, Lowenstein Jensen(LJ) medium, Bactec 460 radiometric culture system as well as PCR followed by DIA hybridization.
Materials and Methods
Three hundred urine samples were collected from the outpatient department of the Azhar University's hospital of the School of Medicine in Assiut, Egypt from January until June 2009. The samples were collected in the morning from the admitted patients from urology department in the hospital. The patients were divided into three groups (I, II, III), each group comprised 100 patients. Group 1 (control group), com¬prised apparently healthy patients, Group II, comprised a patient with chronic renal failure, and Group III, comprised patients with suspected infection (Table 1).

Table 1. Sampling criteria of the examined patients
Processing of specimens. Urine specimens were treated with NALC-NaOH method for the decontamination.[24] Ziehl-Neelsen (ZN) staining method was done as described previously.[25] Lowenstein-Jensen medium (Becton- Dinkinson Microbiology Systems, Cockeysille, MD, USA) was prepared.[26] The processed specimens were inoculated into a Bactec 12 B vial.[24] The BACTEC460 instrument (Becton Dickinson Diagnostic Instrument System, Sparks, MD, USA) was achieved, which was significantly superior to the conventional method.[22]
Polymerase Chain Reaction (PCR). DNA extraction. The urine specimens were treated as previously mentioned for the decontamination and liquefaction to obtain the pellet for DNA extraction.[24] The bacterial pellet was resuspended in 180 ul buffer ATL (supplied in QIAamp Tissue Kit, QIAGEN GmbH, Hilden, Germany). The extraction of TB DNA was performed according to QIAGEN kit protocol. The eluted DNA can be stored at-20°C until use in PCR.
Primers and PCR assay. The PCR assay was performed with the primers pair p1(5’CCTGCGAGCGTAGCGTAGGCGG-‘3) p2(5’-CTCGTCCAGTCCAGCGCCGCTTCGG-‘3) located within IS6110 sequence repeated 10-12 times in the chromosome of M. Tuberculosis, these primers gives a 123 bp product and synthesized by Sorin BIomedica Diagnostics, Saluggia, VC, Italy which gives. Ten µl of the extract were subjected to PCR in a final volume of 100µl in 0.2 ml microcentrifuge tubes containing 1X PCR buffer (10 mM Tris-HCl, pH 8.0,2.5 mM MgCl2, 50 mM KCl, gelatin 0.01%w/v), 0.2 mM (each) of the four deoxyribonucleoside triphosphates (dATP, dTTP, dGTP and dCTP), 2.5 U of taq polymerase were all supplied from STRATA-GENE reagent kit (Alameda, California, USA) and 50 pM each primer. The PCR conditions for IS6110 DNA amplification were an initial denaturation step of 94°C for 5 min, followed by 35 cycles of 94°C for 2 min, 68°C for 2 min, 72° for 2 min. and a final extension step at 72°C for 10 min. The PCR procedure was accomplished with a thermocycler TC 9600 (Perkin-Elmer Cetus). Each experiment included positive and negative control tubes. The products of amplification were then analyzed by agarose gel electrophoresis followed by hybridization with DNA Enzyme Immunoassay (DEIA test).
DNA Enzyme Immunoassay (DEIA test). One hundred µl hybridization buffer were dispensed into each well, and 200µl controls and denatured samples into their respective wells. The strip was sealed with card board sealer, incubated at 45°C for 30 min and then washed. The anti-DS-DNA solution was prepared at the end of the first incubation and 100µl diluted Anti-DS. The DNA were dispensed into each well except for the blank, the strip was sealed and incubated for 1h. at room temperature, then washed. The enzyme tracer solution was prepared at the end of the incubation. Hundred µl diluted enzyme tracer solution were dispensed into all the wells except for the blank, the strip was sealed and incubated for 1 hr at room temperature. The chromogen-substrate was prepared at the end of third incubation. At the end of incubation, substrate strip was washed. Hundred µl chromogen – substrate were dispensed into each well, incubated for 30min at room temperature, away from light. Two hundred µl of blocking reagent (GENE Kit DEIA, Sorin Biomedica Diagnostics, Saluggia,VC, Italy) were then dispensed into all the wells. The strip was read using ELISA reader photometer with 450-630 nm filter. The instrument was rested with blank, before reading the wells at 450 nm. Absorbance values at least 0.170 greater than the mean negative control value indicated that hybridization had taken place.
Statistical analysis
The incidences of all techniques were performed with the Statistical Package for Social Science (SPSS version 12.0) using the Z test and P value.
Results
Characteristics of the studied patients are summarized in Table 1; 300 urine samples were provided into three groups (I, II, III) as previously mentioned.
The most common presenting symptoms in groups II and III patients with urinary TB were sterile pyuria (17% and 22 %), haematuria (2% and 4%) in group I and II, respectively. In addition, Dysuria (3%) and persistent dysuria (25%) are reported only in group II (Table 2).
Out of 300 urine specimens, 2 samples were detected by both ZN smear and LJ medium giving an incidence rate of 0.66%, 3 samples were also found to be contaminated in BACTEC culture giving an incidence rate of 1%. Accordingly, PCR assay and non-isotopic hybridization probe test gave more positive results than smear and culture examinations (i.e. 8 samples were detected and gave an incidence rate of 2.6%). The results are given in Table 3.
The results show that ZN smear examination has a sensitivity of 25% and a specificity of 100%. For LJ media culture, sensitivity was 25% and specificity was 100%. BACTEC culture showed a sensitivity of 37.5% and a specificity of 100%. In comparison, PCR was found to have a much higher sensitivity of 100% and a specificity of 100% (Table 4).
The specificity, sensitivity and speed of PCR test in diagnosis of M. tuberculosis infection shown in this study should encourage the use of this technique in diagnosis of urinary TB. Acid-fast bacilli (AFB) smear were performed on all specimens. The reading of semiquantitative results had indicated the present number of bacteria (Table 5).

Table 2. Clinical symptoms of groups II and III

Table 3. Urinary TB among three different groups

Table 4. Sensitivity and Specificity of different methods

Table 5. The detection of semiquantitative results to the number of Acid fast bacilli (AFB) cells of the examined urine specimens
In this study, two positive specimens for M. tuberculosis were detected by both ZN smear and LJ medium out of 300 specimens, one positive specimen was found in group II and one positive specimen was found in group III (Table 3). The control specimens (group I) were completely negative by LJ medium.
Three positive specimens of M. tuberculosis were detected by Bactec culture system out of 300 urine specimens (1%). These positive samples were 1 in group II and 2 in group III. The control specimens (group I) were completely negative by Bactec460 culture (Table 3), while the sensitivity of BACTEC 460 system was 37.5% and the specificity was 100% (Table 4).
The detection of M. tuberculosis DNA in urine specimens by PCR and non-isotopic hybridization was elevated for laboratory diagnosis of urinary tract M. tuberculosis infection. After amplifying DNA by PCR, the samples were detected by agarose gel electrophoresis to see the specific band for M. tuberculosis. Positive specimens were seen in agarose gel containing 123 bp band located within IS6110 sequence repeated 10-12 times in the chromosome of M. tuberculosis. The 100 bp ladder was used as depicted in Figure 1.

Figure 1. Agarose gel electrophoresis of amplified Mycobacterium tuberculosis DNA at 123 bp. Lanes 1, 2, 3, 4, 7, 8, 9, and 10 are negative. While 5, 6, 11, 12 are positive, M= 100 bp ladder.
Negative specimens were shown by absence of 123 bp band. The results of gel electrophoresis of amplified product were 8 positive specimens for M. tuberculosis. Six of which had a clear band of 123 bp and other two had a weak or faint 123 bp band, these two specimens considered positive after confirmation using hybridization by non-isotopic hybridization to the specific probe. Hybridization to the specific probe was performed to all samples on the amplified products. Results of non-isotopic hybridization probe was performed and read at 450 nm, mean OD of the DEIA negative control was 0.05 A450 and positive control was 3.0 A450. Therefore, the OD cut-off value calculated in this study was 0.170 A450 and the samples were classified as negative at OD450< 0.170 or positive at OD450>=0.170. The eight positive specimens had OD in the range of 0.750- 1.5 A450. The positive control used in PCR reaction gave OD value >2.0 and 0.450 A450.
To conclude, molecular diagnosis of tuberculosis by PCR has a great potential than ZN smear examination, LJ medium, and BACTEC radiometric culture, to improve the clinicians' ability to diagnose urinary tuberculosis. This will ensure early treatment to patients and prevent further transmission of disease.
Discussion
Early identification of tuberculosis in the clinical setting is of great importance in order for specific therapy to be swiftly initiated because TB is the leading cause of death worldwide attributable to a single infectious disease agent. UGTB is the second most common form of extrapulmonary tuberculosis in countries with a severe epidemic and the third most common form in regions with a low incidence of tuberculosis.[27]
Because of the insidious evolution and late onset of TB symptoms, the diagnosis and treatment are notoriously delayed, resulting in significant morbidity (e.g. end-stage renal failure, shrunken bladder, and testicular destruction).[8-10]
In our context, the most common presenting symptoms in patients in this study with UTB are sterile pyuria (17% and 22%), haematuria (2% and 4%) in group I and II, respectively. In addition, dysuria (3%) and persistent dysuria (25%) are reported only in group II.
Therefore, the diagnosis of UTB is often delayed not only because of its insidious evolution accompanied by few nonspecific symptoms, but also because of the long time required to confirm the diagnosis by classical conventional methods of cultivation,[28] the only utilized in most developing countries.
Sterile pyuria (81%), pyuria (48%) and hematuria (18%) have been reported in patients with renal tuberculosis.[13] Moreover, Narayan[29] found that the most common presenting symptoms in patients with UTB are irritative voiding symptoms and hematuria. Histopathology is characteristic but there could be problems to get a representative specimen, and non-specific features can sometimes complicate the diagnosis. Nevertheless, antigen-based serological tests have the potential of providing inexpensive and robust tools for diagnosis of TB under the conditions encountered commonly in developing countries such as Egypt.
However, immunological diagnosis is often inconclusive because antibodies and the delayed type hypersensitivity response persist for a long time after the subsidence of sub-clinical or clinical disease. Although a number of TB specific seroantigens have been identified in recent years, these vary in their sensitivities and specificities. Therefore, newer and reliable diagnostic methods are needed for early and precise detection of TB and efforts are ongoing in various laboratories to improve the performance of TB diagnostic tests either through improvements in conventional tools or by using newer diagnostic modalities that are based on nucleic acid amplification or antigen/antibody detection, etc. The salient achievements made towards improving the diagnosis of TB are summarized in this study.
In our investigation, the incidence of urinary tuberculosis among renal patients was studied. Three hundreds urine samples were processed for detection of Mtb by ZN smear examination, LJ medium culture, radiometric BACTEC460 culture as well as PCR and non-isotopic hybridization probe test.
The results show that ZN smear examination has a sensitivity of 25% and a specificity of 100%. To make such detection of UGTB faster, one could use media such as Middlebrook (solid or liquid) or Lowenstein-Jensen in the microcalorimetric vials, such as was proposed in other studies.[30-33] Those media could then be inoculated with urine samples previously concentrated by centrifugation and decontaminated using the N-acetyl-L-cysteine-sodium hydroxide procedure to inactivate contaminants and avoid mixed bacterial growth.
The sensitivities of BACTEC culture method were found to be much higher compared to LJ i.e 37.5% and a specificity of 100% as well as the mean detection time for Mtb was 24.03 days by LJ medium culture, 12.89 days by BACTEC culture as reported.[14,34,35]
Another noteworthy aspect of our study was in the diagnosis by PCR. Its results revealed 8 positive samples out of 300 specimens with incidence of 2.6%. Moreover, in comparison with the previous techniques, PCR was found to have the highest sensitivity of 100% and a specificity of 100%. This specificity, sensitivity and speed of PCR test followed by non-isotopic hybridization probe test in diagnosis of M. tuberculosis infection in this study, should encourage the use of this method in diagnosis of TB compared the performance of ZN smear, LJ media, and BACTAC 460 culture system in urine samples for diagnosis of TB as reported by others[21,23,34] as well as Immunoassay test.[3,36-38] However, immunological diagnosis is often inconclusive because antibodies and the delayed type hypersensitivity response persist for a long time after the subsidence of sub-clinical or clinical disease. Although a number of TB specific seroantigens have been identified in recent years, these vary in their sensitivities and specificities.
With the use of PCR test and non-isotopic hybridization probe test, we are able to detect M. tuberculosis in 4 times than both ZN smear and LJ culture and 2.66 times than BACTAC460 culture system.
PCR test detected M. tuberculosis in less than one day, compared to average 24.03 days required for detection by conventional (LJ) and 12.89 days by radiometric BACTEC technique, as supported.[39] Similar measurements using bactec culture and polymerase chain reaction methods have been performed for diagnosis of tuberculosis and found that a significant difference was seen in the sensitivities of 74.4% for PCR test, 33.79% for ZN smear examination, 48.9% for LJ culture and 55.8% for BACTEC culture (P<0.05). However, there was no significant difference (P>0.05) as far as specificity of different tests was concerned.[21]
PCR test was also shown to be reasonably sensitive in diagnosis of TB.[13,38,40-44]
Taken together, the present demonstration emphasized that among various diagnostic methods used for urinary tuberculosis, PCR, as a recent method, offers a promising new approach for rapid, safe, specific, and reproducible determination of M. tuberculosis infection.
References
- Green C, Huggett JF, Talbot E, Mwaba P,
Reither K, Zumla AI (2009). Rapid diagnosis of tuberculosis through the
detection of mycobacterial DNA in urine by nucleic acid amplification
methods. Lancet Infect Dis. 9: 505-11 http://dx.doi.org/10.1016/S1473-3099(09)70149-5

- Dietrich G, Viret JF, Hesh J (2003).
Mycobacterium bovis BCG based vaccine against tuberculosis: novel
developments. Vaccine 21:667-670. http://dx.doi.org/10.1016/S0264-410X(02)00577-7

- Mukundan H, Kumar S, Price DN, Ray SM, Lee
Y, Min S, Eum S, Kubicek-Sutherland J, Resnick JM, Grace WK, Anderson
AS, Hwang SH, Cho SN, Via LE, Barry C, Sakamuri R, Swanson BI (2012).
Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich
immunoassay format using a waveguide-based optical biosensor.
Tuberculosis 92: 407-41. http://dx.doi.org/10.1016/j.tube.2012.05.009 PMid:22710249

- Jonas V, Alden MJ, Curry JI, Kamisango K,
Knott CA, Lankford R, Wolfe JM, Moore DF (1993). Detection and
identification of Mycobacterium tuberculosis directly from sputum
sediments by amplification of rRNA. J Clin Microbio 31:2410-241.
PMid:8408564 PMCid:265770

- Noel AB, Lecossier D, Nassif X, Birgite
Gicquel, Frebault VL, Hance AJ (1989). Rapid diagnosis of tuberculosis
by amplification of Mycobacterial DNA in clinical samples. Lancet
2(8671):1069-1071.

- Figueiredo AA, Lucon AM, Junior RF (2008). Epidemiology of urogenital tuberculosis worldwide. Int J Urol 15:827-832. http://dx.doi.org/10.1111/j.1442-2042.2008.02099.x PMid:18637157

- Jacob JT, Nguyen TM, Ray SM (2008). Male genital tuberculosis. Lancet Infect Dis. 8:335-342. http://dx.doi.org/10.1016/S1473-3099(08)70101-4

- Wise GJ, Marella VK.(2003) Genitourinary manifestations of tuberculosis. Urol Clin N Am 30(1):111–21. http://dx.doi.org/10.1016/S0094-0143(02)00123-4

- Wise GJ, Shteynshlyuger A (2008). An update on lower urinary tract tuberculosis. Curr Urol Rep.9:305-313. http://dx.doi.org/10.1007/s11934-008-0053-9 PMid:18765130

- Zarrabi AD, Heyns CF (2009). Clinical
features of confirmed versus suspected urogenital tuberculosis in
region with extremely high prevalence of pulmonary tuberculosis.
Urology 74:41-45. http://dx.doi.org/10.1016/j.urology.2008.12.083 PMid:19428090

- Hepper NG, Karlson AG, Leary FJ (1971).
Genitourinary infection due to Mycobacterium kansasii. Mayo Clin Proc.
46:387-390. PMid:5088980

- Brooker WJ, Aufderheide AC (1980). Genitourinary tract infections due to atypical mycobacteria. J Urol 124:242-244.

- Van VollenHoven P, Heyns CF, de Beer PM
(1996). Polymerase chain reaction in the diagnosis of urinary tract
tuberculosis. Urol Res. 24: 107-111. http://dx.doi.org/10.1007/BF00431088 PMid:8740980

- Hillemann D, Richter E, Rüsch-Gerdes S
(2006). Use of the BACTEC mycobacteria growth indicator tube 960
automated system for recovery of mycobacteria from 9,558 extrapulmonary
specimens, including urine samples. J Clin Microbiol 44:4014-4017. http://dx.doi.org/10.1128/JCM.00829-06 PMid:17005737 PMCid:1698359

- Boehme CC, Nabeta P, Hillemann D (2010).
Rapid molecular detection of tuberculosis and rifampin resistance. N
Engl J Med 363:1005-1015.

- Lotz A, Ferroni A, Beretti JL (2010).
Rapid identification of mycobacterial whole cells in solid and liquid
culture media by matrixassisted laser desorption ionization-time of
flight mass spectrometry. J Clin Microbiol 48:4481-4486

- Saleeb PG, Drake SK, Murray PR (2011).
Identification of mycobacteria in solid-culture media by
matrix-assisted laser desorption ionization-time of flight mass
spectrometry. J Clin Microbiol. 49:1790-1794. http://dx.doi.org/10.1128/JCM.02135-10 PMid:21411597 PMCid:3122647

- Leite OHM (2001). Tuberculosis. Prob Gen Surg 18(4):69–78. http://dx.doi.org/10.1097/00013452-200112000-00012

- Ferrie BG, Rundle JSH (1985).
Genito-urinary tuberculosis in Glasgow 1970 to 1979: a review of 230
patients. Scott Med J 30:30–4. PMid:3983618

- Schubert GE, Haltaufderheide T, Golz R
(1992). Frequency of urogenital tuberculosis in an unselected autopsy
series from 1928 to 1949 and 1976 to 1989. Eur Urol 21:216–23.
PMid:1499628

- Negi SS, Khan SF, Gupta S, Pasha ST, Khare
S, Lal S (2005). Comparison of the conventional diagnostic modalities,
bactec culture and polymerase chain reaction test for diagnosis of
tuberculosis. Indian J Med Microbiol 23:29-33. http://dx.doi.org/10.4103/0255-0857.13869 PMid:15928418

- Lakshmi V, Patil MA, Subhadha K, Himabindu
V (2006). Isolation of mycobacteria by Bactec 460 TB system from
clinical specimens. Indian J Med Microbiol 24:124-6. http://dx.doi.org/10.4103/0255-0857.25197 PMid:16687864

- Montenegro SH, Gilman RH, Sheen P, Cama R,
Cavides L, Hopper T, Chambers R, Oberhelman RA (2003). Improved
detection of M.tuberculosis in Peruvian children by use of heminested
IS6110 PCR assay. Clin Infec Dis 36:16-23. http://dx.doi.org/10.1086/344900 PMid:12491196

- Baron EJ, Finagold SM. (Eds.)(1994) Mycobacteria, chapter 13. In: Bailey and Scott's Diagnostic Microbiology, 9th Ed. (The CV Mosby Company, St Luis) 590-633
- International Union Against Tuberculosis
(1978). Technical guide for sputum examination for tuberculosis by
direct microscopy. Bull Int Union Tuberc Lung Dis 53 (Suppl 2): 4–16

- Takahashi H, Foster V (1983). Detection and recovery of mycobacteria by a radiometric procedure. J Clin Microb 117:380-381

- Kulchavenya E, Kim CS, Bulanova O, Zhukova
I (2012). Male genital tuberculosis: epidemiology and diagnostic. World
J Urol 30(1):15-21. http://dx.doi.org/10.1007/s00345-011-0695-y PMid:21604018

- Bonkat G, Bachmann A, Solokhina A, Widmer
AF, Frei R, Gasser TC, Braissant O (2012). Growth of Mycobacteria in
Urine Determined by Isothermal Microcalorimetry: Implications for
Urogenital Tuberculosis and Other Mycobacterial Infections. Urology 80:
1163.e9 –1163.e12

- Narayan AS (1982). Overview of renal tuberculosis. Urology 19: 231-236. http://dx.doi.org/10.1016/0090-4295(82)90490-3

- Selvakumar N, Rahman F, Garg R,
Rajasekaran S, Mohan NS, Thyagarajan K (2002). Evaluation of the phenol
ammonium sulfate sedimentation smear microscopy method for diagnosis of
pulmonary tuberculosis. J Clin Microbiol 40:3017-20. http://dx.doi.org/10.1128/JCM.40.8.3017-3020.2002 PMid:12149368 PMCid:120642

- Braissant O, Wirz D, Göpfert B (2010).
"The heat is on": rapid microcalorimetric detection of mycobacteria in
culture. Tuberculosis (Edinb) 90:57-59. http://dx.doi.org/10.1016/j.tube.2009.11.001 PMid:19969505

- Rodríguez D, Daniels AU, Urrusti JL
(2011). Evaluation of a low-cost calorimetric approach for rapid
detection of tuberculosis and other mycobacteria in culture. J Appl
Microbiol 111:1016-1024. http://dx.doi.org/10.1111/j.1365-2672.2011.05117.x PMid:21797951

- Howell M, Wirz D, Daniels AU (2012).
Application of a microcalorimetric method for determining drug
susceptibility in mycobacterium species. J Clin Microbiol 50:16-20. http://dx.doi.org/10.1128/JCM.05556-11 PMid:22090404 PMCid:3256699

- Wang SX, Tay L (1999). Evaluation of three
nucleic acid amplification methods for direct detection of
Mycobacterium tuberculosis complex in respiratory specimens. J Clin
Microbiol 37: 1932–1934. PMid:10325349 PMCid:84988

- Dinnes J, Deeks J, Kunst H (2007). A
systematic review of rapid diagnostic tests for the detection of
tuberculosis infection. Health Technol Assess 11:1-196. PMid:17266837

- Ruhwald M, Bjerregaard-Andersen M, Rabna
P, Kofoed K, Eugen-Olsen J, Ravn P (2007). CXCL10/IP-10 release is
induced by incubation of whole blood from tuberculosis patients with
ESAT-6, CFP10 and TB7.7. Microbes Infect 9:806-812. http://dx.doi.org/10.1016/j.micinf.2007.02.021 PMid:17533146

- Hong SC, Lee J, Shin H, Kim C, Park JY,
Koh K, Kim H, Chang CL, Lee J (2011). Clinical immunosensing of
tuberculosis CFP-10 in patient urine by surface plasmon resonance
spectroscopy. Sensors and Actuators B 160:1434–1438. http://dx.doi.org/10.1016/j.snb.2011.10.006

- Bibovaa I, Linhartovaa I, Staneka O,
Rusnakovac V, Kubistac M, Suchaneke M, Vasakovaf M, Seboa P (2012).
Detection of immune cell response to M. tuberculosis–specific antigens
by quantitative polymerase chain reaction. Diagnostic Microbiology and
Infectious Disease 72:68–78. http://dx.doi.org/10.1016/j.diagmicrobio.2011.09.024 PMid:22085772

- Pfyffer GE, Welscher HM, Kissling P,
Cieslak C, Casal MJ, Gutierrez J, Gerdes SR (1997). Comparison of the
Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid
culture for recovery of Acid fast bacilli. J Clin Microbiol 35:364-368.
PMid:9003597 PMCid:229581

- Cousins DV, Wilton SD, Francis BR (1992).
Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J
Clin Microbiol 30: 255-258. PMid:1734065 PMCid:265037

- Kolk AH, Schuitema ARJ, Kuijper S (1992).
Detection of Mycobacterium tuberculosis in clinical samples by using
polymerase chain reaction and a non-radioactive detection system. J
Clin Microbiol 30: 2567-2575. PMid:1400955 PMCid:270480

- Missirliu A, Gasman D, Vogt B (1996).
Genito-urinary tuberculosis: Rapid diagnosis using the polymerase chain
reaction. Eur Urol 30: 523-524. PMid:8977081

- Gillespie SH, McHugh TD (1997). Monitoring
the therapy of pulmonary tuberculosis by nested polymerase chain
reaction. Journal of Infection 35: 324-325. http://dx.doi.org/10.1016/S0163-4453(97)93878-0

- Zümrütdal A, Yıldız I, Ozelsancak R,
Canpolat T (2011). Chronic renal failure: unexpected late sequela of
pulmonary tuberculosis after 30 years. Mikrobiyol Bul 45:366-70.
PMid:21644081
