Von Willebrand Factor Abnormalities Studied in the Mouse Model: What We Learned about VWF Functions
Caterina Casari1,2, Peter J. Lenting1,2, Olivier D. Christophe1,2 and Cécile V. Denis1,2
1 INSERM U770, Le Kremlin-Bicętre, F-94276, France
2 Univ Paris-Sud, UMR_S770, Le Kremlin-Bicętre, F-94276, France
2 Univ Paris-Sud, UMR_S770, Le Kremlin-Bicętre, F-94276, France
Correspondence
to:
Cécile V. Denis, INSERM U770, 80 rue du Général Leclerc, 94276 Le Kremlin-Bicętre Cedex, France. Tel: 33-1-49-59-56-05. E-mail: cecile.denis@inserm.fr
Published: July 10, 2013
Received: April 17, 2013
Accepted: June 26, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013047, DOI 10.4084/MJHID.2013.047
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Up
until recently, von Willebrand Factor (VWF) structure-function
relationships have only been studied through in vitro approaches. A
powerful technique known as hydrodynamic gene transfer, which allows
transient expression of a transgene by mouse hepatocytes, has led to an
important shift in VWF research. Indeed this approach has now enabled
us to transiently express a number of VWF mutants in VWF-deficient mice
in order to test the relative importance of specific residues in
different aspects of VWF biology and functions in an in vivo setting.
As a result, mice reproducing various types of von Willebrand disease
have been generated, models that will be useful to test new therapies.
This approach also allowed a more precise identification of the
importance of VWF interaction with subendothelial collagens and with
platelets receptors in hemostasis and thrombosis. The recent advances
gathered from these studies as well as the pros and cons of the
technique will be reviewed here.
Although
von Willebrand disease (VWD) was first recognized as early as 1926 by
Erik von Willebrand,[1] it is only in the 1970’s that the protein
responsible for this disease, i.e.
von Willebrand factor was formally recognized as an independent entity
not to be confused with coagulation factor VIII (FVIII). Other articles
in this special issue have extensively developed all aspects related to
VWF functions in hemostasis/ thrombosis as well as beyond these fields
and also the pathological aspects with regard to VWD. Some of these
progresses were made possible thanks to the availability of animal
models of VWD. Indeed, VWD has been described as naturally occurring in
a number of different species such as pigs, dogs, cats, and mice.[2,3]
In addition, the progress made in the last 20 years in genetic
manipulation has allowed the engineering of additional models of VWD
especially murine models. A knock-out mouse deficient in VWF was
generated and was shown to adequately model severe human VWD.[4] This
model has proven invaluable to study specific aspects of VWF functions
and has uncovered many unsuspected leads, regenerating the research
interest for this protein.[5,6] However, after a few years, the
limitations of the model became apparent. Indeed, it does not allow a
more subtle analysis of the relative importance of the different VWF
domains. When considering the large size of VWF and its capacity to
interact with various ligands, the necessity to evaluate independently
these interactions appeared increasingly crucial. In addition, the
motivation to evaluate in vivo
the effect of mutations detected in VWD patients, also fueled interest
in gaining access to additional mouse models reproducing such defects.
Using the homologous recombination technique to engineer genetically
modified mice expressing mutated VWF (knock-in mice) would be the ideal
approach but it is time-consuming, expensive and technically
challenging. An alternative consists in using the hydrodynamic
injection technique, which allows transient expression of a transgene
by mouse hepatocytes. This approach has now been implemented with
success in several laboratories and in this article, we will review the
new information that was gathered from its use.
Hydrodynamic injection: application to VWF expression. Hydrodynamic injection is akin to an in vivo transfection method (Figure 1). It consists in a rapid injection of a large volume of plasmid DNA without any other viral or non-viral vector in the lateral tail vein of mice.[7] The volume and speed of injection have been shown to strongly influence the efficiency of gene delivery to hepatocytes. In order to be fully optimal, injection should be completed within 5 seconds with a volume of injection corresponding to 10% of the body weight of the mouse (i.e. 2 ml for a 20 g mouse).[8] After undergoing a successful injection, the mice go through an apathetic phase (shock) that is transient. In C57BL/6 mice, recovery occurs after approximately 20-30 minutes and the survival rate is 100%. The following mechanism has been proposed to explain hepatocytes transfection: by exceeding cardiac capacity, the large DNA volume accumulates in the lower vena cava. This leads to a sharp increase in venous blood pressure and a backflow of the solution towards organs connected to the vena cava. The liver has the most extensive structure and absorbs the majority of the volume injected (>90%). The elevated pressure generated by hydrodynamic injection opens sinusoid fenestrae allowing the solution to reach hepatocytes. On average, 10-40% of hepatocytes are transfected. It is rather amazing that despite the “traumatic” aspect of the technique, liver biological parameters are only slightly modified and on a temporary basis. Indeed, only levels of serum alanine aminotransferase and of aspartate aminotransferase are elevated after hydrodynamic gene delivery (4-20 fold according to different studies) but they return to baseline three days later. Histological studies also showed that less than 5% of the liver has suffered from hepatic damage. Other parameters such as alkaline phosphatase, total bilirubin, albumin, total proteins and Na+, K+ and Cl- ions are not modified.[7]
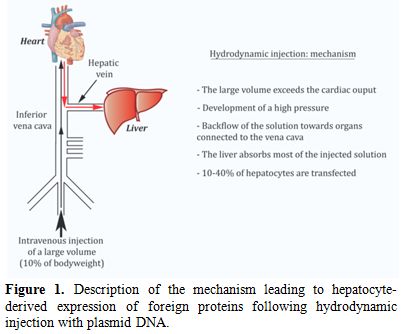
Figure 1. Description of the mechanism leading to hepatocyte-derived expression of foreign proteins following hydrodynamic injection with plasmid DNA.
This technique has now been used successfully to express functionally active VWF in VWF-deficient mice.[9] Several potential hurdles had to be overcome before such a result could be obtained. First, there was a species-barrier since human VWF interacts poorly with its platelet receptor glycoprotein (GP) Ibα, in mice. As a consequence, only murine VWF (mVWF) cDNA can be used. mVWF cDNA was therefore subcloned in different vectors, either with a CMV promoter[10] or with a liver-specific promoter (murine albumin or α1-antitrypsin).[11,12] In all cases, high pressure injection of 50-100 µg of mVWF cDNA resulted in high levels of expression of VWF antigen in plasma of VWF-deficient mice (Figure 2). Vectors with liver-specific promoters allowed relatively long-term expression (a few weeks compared to a couple of days for vectors with a CMV promoter). A temporary decrease in platelet counts could also be observed but was not apparent anymore after 72 hours (Figure 2). Immunostaining showed that, as expected, VWF is expressed by hepatocytes and not by endothelial cells.[13] This hepatocyte-expression of VWF represented the second hurdle since it was not clear whether this cell type had the necessary machinery to multimerize VWF, a critical criteria for VWF biological activity. Fortunately, we were able to show that this hepatocyte-derived VWF was indeed multimerized (Figure 2) and that it also had the capacity to bind endogenous FVIII, leading to restoration of plasma FVIII levels, which are normally strongly reduced in VWF-deficient mice. Finally the ultimate proof of the functional quality of liver-expressed mVWF was demonstrated by its capacity to correct bleeding time in VWF-deficient mice.[10] In addition, thrombus formation after ferric-chloride induced injury was also restored in the knockout mice expressing plasma mVWF.[11,12]
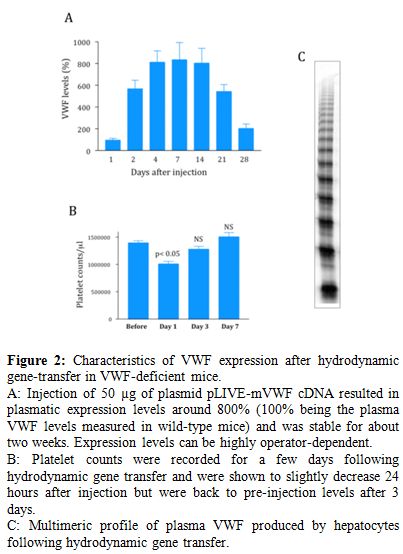
Figure 2. Characteristics of VWF expression after hydrodynamic gene-transfer in VWF-deficient mice.
A: Injection of 50 µg of plasmid pLIVE-mVWF cDNA resulted in plasmatic expression levels around 800% (100% being the plasma VWF levels measured in wild-type mice) and was stable for about two weeks. Expression levels can be highly operator-dependent.
B: Platelet counts were recorded for a few days following hydrodynamic gene transfer and were shown to slightly decrease 24 hours after injection but were back to pre-injection levels after 3 days.
C: Multimeric profile of plasma VWF produced by hepatocytes following hydrodynamic gene transfer.
These results show that plasma VWF is sufficient to support efficient hemostasis and thrombosis in the mouse, indicating that liver-targeted gene therapy can be considered as a viable treatment alternative for severe VWD. This model also allows the direct comparison of hemostatic capacities of wild-type versus mutant VWF.
Analysis of VWF variants carrying mutations in binding domains to different ligands. In order to play its role in hemostasis, VWF binds to a number of different ligands, among which two platelet receptors, platelet GPIbα and GPIIbIIIa as well as subendothelial collagens.[14] Thanks to many in vitro biochemical studies, the residues on human VWF involved in its binding to these ligands have been precisely identified.[15-17] Considering that these residues were perfectly conserved in mVWF, we hypothesized that the same sequences were also involved in mVWF binding to its murine ligands on platelets and in the vessel wall. We therefore mutated these residues in order to abolish binding to the different receptors.[10] To abolish mVWF binding to murine GPIbα, we mutated the lysine residue in position 1362 to an alanine. Binding of mVWF to mGPIIbIIIa was suppressed by mutating the aspartic acid residue in position 2509 to glycine (RGD sequence converted to RGG sequence). Finally to abolish binding of mVWF to collagens, three residues were mutated simultaneously: aspartic acid 1742, serine 1783 and histidine 1786 were all converted to alanine. The lack of interaction of these different VWF variants to their respective ligands was confirmed in vitro.[10] Hydrodynamic gene transfer of these three mutants showed that 1) comparable plasma mVWF expression levels were obtained, suggesting that the mutations do not alter VWF biosynthesis and 2) plasma VWF was correctly multimerized for all mutants, suggesting that the mutations do not interfere with the multimerization process.
Effect of mutations on bleeding time. Patients with VWD type 2A or 2M already made it clear that a form of VWF that could not interact with platelet GPIbα lead to bleeding symptoms.[18] Injection of the mVWF cDNA carrying the K1362A mutation in VWF-deficient mice confirmed this observation.[10] In contrast to mice injected with wild-type mVWF cDNA, these mice could not control their bleeding time in the tail clip assay and kept bleeding indefinitely, similar to untreated mice. In contrast to the expected bleeding phenotype predicted for the VWF mutant unable to bind GPIbα, no real indication of what to anticipate for lack of binding to collagens or to GPIIbIIIa could be deducted from VWD patients. Indeed, no patient with a mutation in the GPIIbIIIa binding domain has been reported so far and only very few patients with mutations in the collagen-binding region were described at the beginning of this study. Interestingly, VWF-deficient mice injected with either the collagen binding mutant or the RGG mVWF cDNAs were able to control their bleeding as well as wild-type mVWF-treated mice, indicating that these interactions are not absolutely required for the physiological process of hemostasis in the mouse model.[10]
Effect of mutations on thrombosis. Since a defect or lack thereof, in functional activity in the bleeding model is not necessarily representative of the complex role played by VWF in hemostasis/thrombosis, we also extended our examination of these mutations in an in vivo thrombosis model initiated by ferric-chloride exposure of mesenteric vessels.[12] We first showed that injection of wild-type mVWF cDNA in VWF-deficient mice was able to restore thrombus formation and vessel occlusion with a kinetic very similar to the one obtained with wild-type mice. Mice expressing the GPIbα-binding mutant did not form occlusive thrombi, similar to untreated mice. This result, added to the incapacity of this mutant to correct bleeding time, confirms the critical importance of the VWF-GPIbα interaction in maintaining efficient hemostasis/ thrombosis. More surprisingly, mice expressing the collagen binding mutant showed altered thrombosis with significantly delayed vessel occlusion (30 min versus 16 min in wild-type VWF-expressing mice).[12] However, despite this delay, the mice were able to form stable thrombi well anchored to the vessel wall. In contrast, mice expressing the GPIIb/IIIa binding mutant were more severely affected. Less than 40% of the mice expressing this mutant were able to reach occlusive thrombosis. Mice formed thrombi that were less compact and which were not efficient to stop blood flow. Portions of these thrombi constantly embolized, preventing further growth of the thrombus. This result emphasized the critical importance of the VWF-GPIIbIIIa interaction in platelet-platelet firm adhesion at high shear rates.
Altogether, results from the bleeding time as well as the thrombosis model experiments suggested that VWF interactions with fibrillar collagens and with GPIIbIIIa are more important in the pathological thrombotic process than in the physiological process of hemostasis.
Effect of mutations on stroke. The same three VWF mutants were also tested in an ischemic stroke model in mice in order to establish which VWF interactions were important in this model.[19] Stroke was induced by transient middle cerebral artery occlusion. Previous reports had already described a protection against stroke in mice deficient in VWF, while replenishing the VWF plasma compartment by hydrodynamic gene transfer with wild-type mVWF cDNA totally restored the susceptibility of the mice to cerebral ischemia.[11] Analysis of VWF-deficient mice hydrodynamically injected with the mVWF cDNAs mutated in the binding sites to fibrillar collagens, GPIbα or GPIIbIIIa revealed that interaction with GPIbα or collagens were involved in stroke development while interaction with GPIIbIIIa was not playing any role. Indeed the brain infarct volumes were significantly smaller in mice expressing the collagen mutant or the GPIbα mutant compared to mice expressing wild-type or GPIIbIIIa mutant VWF. Along the same line, the functional outcome as measured by neurological Bederson score or the grip test score were significantly higher in untreated VWF-/- mice or VWF-/- mice expressing the collagen or GPIbα mutants compared to wild-type or GPIIbIIIa-treated mice.[19] Interestingly this result not only contributes to our understanding of the molecular mechanisms involved in ischemic brain injury but by showing that VWF-GPIIbIIIa interaction is not involved in this process it also sheds some light on our comprehension of the lack of effectiveness of GPIIbIIIa blockade in experimental and clinical stroke.[20]
Application for anti-thombotic treatment. The observation that VWF interactions with fibrillar collagen or with platelet GPIIbIIIa may be more important for thrombosis than for hemostasis makes these axes interesting for new antithrombotic therapies.[12] Monoclonal antibodies directed against human VWF and specifically targeting these interactions have been described in the litterature.[21-23] However, so far the absence of accessible animal models has made it difficult to test the in vivo efficacy of such tools targeting human VWF. To bypass this species barrier, we have developed a human-murine VWF chimera where the human VWF A1 domain, i.e. the domain containing the GPIbα binding region, is replaced by its murine counterpart.[24] This chimeric molecule proved to be functional after in vivo expression (through hydrodynamic injection) in VWF-deficient mice since it supported occlusive thrombosis after ferric-chloride-induced vascular injury. Being predominantly human, the chimera still contains the epitopes recognized by monoclonal antibodies targeting the VWF-collagen of VWF-GPIIbIIIa interactions. Hydrodynamic gene transfer was therefore performed to express this chimeric molecule in VWF-deficient mice and the inhibition of thrombus formation by monoclonal antibodies was measured in the mice. Inhibition of the VWF-collagen or VWF-GPIIbIIIa interactions proved efficient to reduce thrombosis in arterioles. In particular, one of the antibodies inhibiting VWF-collagen interaction was the most powerful, preventing vessel occlusion in 7 mice out of 8 tested.[24] Hydrodynamic gene transfer of this human-murine VWF chimera thus proved to be an efficient manner to test various agents targeting human VWF in an in vivo system.
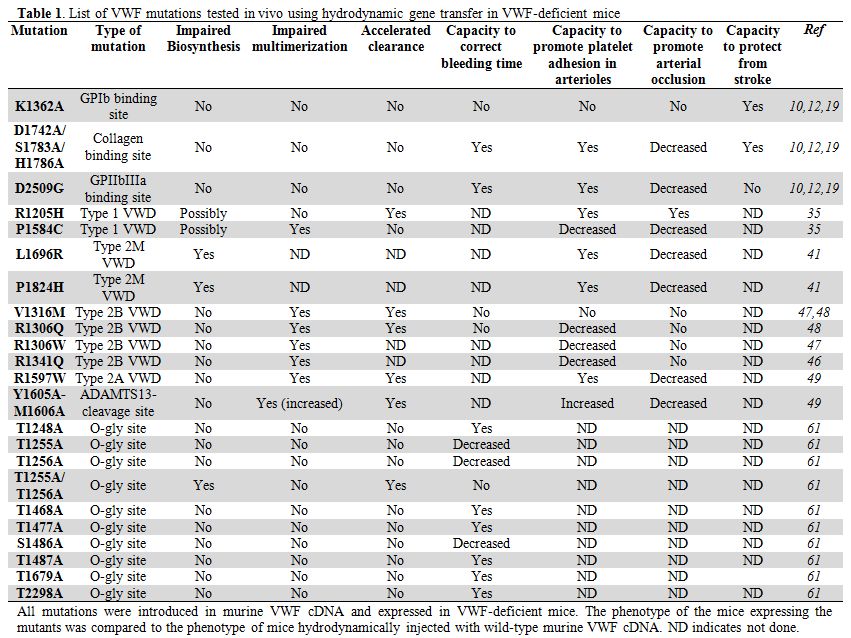
Table 1. List of VWF mutations tested in vivo using hydrodynamic gene transfer in VWF-deficient mice.
Analysis of mice reproducing different subtypes of human VWD. VWD is characterized by quantitative and/or qualitative abnormalities in VWF with heterogeneous clinical manifestations.[18,25] Our understanding of this disease has recently improved based on some large multicenter studies in Europe, Canada and the US[26,27] but also on the use of murine models of VWD. Indeed, the hydrodynamic gene transfer has now allowed detailed in vivo genotype-phenotype analysis for a number of VWD types.
Type 1 VWD. Although type 1 VWD, encompassing mild to moderate reduction in VWF levels, is by far the most common VWD type, its molecular pathogenesis is also the least well understood. Large multicenter studies in Europe, Canada and United States have recently contributed to shed light on the complex mechanisms at the basis of type 1 VWD.[26-28] These may involve defective RNA or protein synthesis, intracellular protein degradation, impaired secretion, rapid plasma clearance, all of which can result from either a mutation in the VWF gene or the presence of modifier genes interfering with VWF biology.[3,29,30] What has become increasingly clear from these studies, is the absolute need for multiple approaches to identify these complex and sometimes multiple mechanisms. Cellular studies are extremely useful to identify aspects related to synthesis and secretion, especially now that the possibility to grow blood outgrowth endothelial cells (BOECs) directly from the patient’s blood is available.[31-34] However, when it comes to plasma clearance or detection of a gene modifier, a more complex organism is needed. No transgenic type 1 VWD murine model has been generated so far. However, two common type 1 VWD mutations have been expressed in VWF-deficient mice by hydrodynamic gene transfer: the p.R1205H Vicenza mutation and the p.Y1584C mutation.[35] Results confirmed the aptitude of this in vivo murine expression system to fully recapitulate a complex clinical picture already observed in VWD patients. Indeed, both in the homozygous and heterozygous states, mice expressing either of these two mutants displayed lower VWF antigen levels compared to wild-type VWF expressing mice. Mice expressing the p.R1205H Vicenza mutant had an increased VWF propeptide/VWF antigen ratio, a feature indicating accelerated clearance of the protein. This confirms results found in patients as well as results obtained in a murine model with injection of the recombinant protein.[36,37] Mice expressing the p.Y1584C mutant displayed increased ADAMTS13 proteolysis, also supporting studies performed with patients plasma.[38,39] This model also allowed the authors to study the procoagulant function of the mutant proteins in in vivo thrombosis experiments and to do so at a fixed VWF antigen plasma concentration. This approach has the advantage to evaluate the inherent capacity of the mutant protein to promote thrombus formation, independently of the quantitative anomaly. Results showed that the p.R1205H VWF exerts a normal hemostatic function, suggesting that the associated defect in patients is purely of a quantitative nature while the p.Y1584C VWF displays a lower capacity to support thrombus formation, resembling a mild type 2A VWD phenotype, probably due to the increased ADAMTS13 cleavage.[35]
Type 2M VWD: collagen-binding mutants. So far, all the VWF mutations that have been tested using the hydrodynamic injection procedure were introduced in the murine VWF sequence. The mouse VWF amino acid sequence has 91% homology to that of human VWF,[40] allowing replacement of the same residue in the murine system as the one affected in the patients. As mentioned earlier, only murine VWF is hemostatically active in the mouse, preventing use of human VWF. However, since we had generated an active human-murine VWF chimera (hVWFmA1) in order to test the anti-thrombotic effect of monoclonal antibodies inhibiting specific VWF functions,[24] we also used the same construct to introduce VWF mutations present in the A3 domain and detected in patients with VWD.[41] Mutations in the VWF A3 domain have not been described as frequently as mutations in the platelet-binding domain (the A1 domain) but they are now being increasingly recognized.[42-44] These mutations usually lead to deficient VWF-collagen interaction and mild/moderate bleeding symptoms. Two such mutations detected through the French National Reference Center for VWD were introduced in the hVWFmA1 cDNA and injected to VWF-deficient mice using hydrodynamic gene transfer: p.L1696R and p.P1824H. In contrast to above-mentioned studies using type 2B and type 1 mutants where the in vivo studies were performed in a second step following extensive in vitro analysis, the mutants were here studied in parallel in vitro and in vivo in order to get a complete picture of the functional defects associated with the mutations. This dual approach proved extremely useful to identify the dominant-negative effect of the p.L1696R mutation on VWF expression levels in patients and in mice expressing the mutation in a heterozygous manner, compared to cellular expression systems. This result raised the possibility that the mutant protein was cleared more rapidly than wild-type VWF in vivo, a feature that cannot be detected in vitro. Mice expressing the second A3 domain mutant, p.P1824H-VWF also displayed low expression VWF levels, but the probable underlying cause, intracellular retention was this time detected both in the in vitro and the in vivo expression systems.[41] For both mutants, the thrombotic response in arterioles of mice was strongly decreased, but the low expression levels did not allow concluding as whether this effect should be attributed to the quantitative defect or to the qualitative defect.
Type 2B VWD. Type 2B VWD is characterized by gain-of-function mutations in VWF A1 domain, resulting not only in an increased affinity for GPIbα but also in an increased susceptibility to ADAMTS13.[25,45] VWF carrying common type 2B mutations, p.V1316M, p.R1306Q, p.R1306W or p.R1341Q.[25,45,46] were expressed in VWF-deficient mice by hydrodynamic gene transfer to generate type 2B VWD murine models. A clear mutation-dependent modulation of disease severity was described, with variable thrombocytopenia and loss of high molecular weight VWF multimers, presence of circulating platelet aggregates and of giant platelets and defective hemostasis and thrombosis.[47,48] Strikingly, these features parallel remarkably well with the phenotype of the patients carrying similar mutations.[46] It therefore appears that these generated transient mouse models are sensitive enough to recapitulate the myriad of defects associated with human type 2B VWD and to detect subtle differences in phenotype. Such models have been instrumental in establishing that the loss of high molecular weight multimers in vivo is caused by the enhanced susceptibility to ADAMTS13 proteolysis rather than preferential adsorption of the high multimers onto platelets.[48] These type 2B VWD mouse models will be very useful in the future, not only to understand additional factors involved in this VWD type such as the mechanisms underlying thrombocytopenia but they also represent very useful tools to test new potential treatments.
Study of ADAMTS13-cleavage mutants. Cleavage of VWF by ADAMTS13 is an important step in the regulation of VWF activity and any perturbation in the delicate balance between too little or too much cleavage leads to pathologic consequences: hemorrhagic when cleavage is excessive as is the case for VWD type 2A or thrombotic consequences when cleavage is insufficient or lacking as is the case in thrombotic thrombocytopenic purpura (TTP). In order to evaluate the in vivo consequences of interfering with VWF sequences involved in ADAMTS13 cleavage, Pruss and colleagues used hydrodynamic gene transfer to generate transient murine models expressing VWF carrying the p.R1597W mutation, the most common type 2A VWD mutation or VWF carrying mutations at the ADAMTS13 cleavage site p.Y1605A/M1606A.[49] Analysis of the VWF multimeric profile of these mice confirmed a loss of high molecular weight multimers in p.R1597-VWF expressing mice and in contrast, a profile with higher molecular weight bands in p.Y1605A/M1606A-VWF expressing mice. This effect was even further amplified when full-length ADAMTS13 cDNA was co-injected with VWF cDNA in order to compensate for the presence of the truncated ADAMTS13 form that is naturally expressed in the C57Bl/6 mouse strain[50] and that is not fully active in vivo.[51,52] Interestingly, mice expressing the cleavage-resistant mutant, even at high concentrations, did not display any sign of TTP, similar to mice deficient in ADAMTS13.[53,54] This result confirms that additional triggering events are necessary to induce TTP, at least in the murine model. The most striking observations were obtained in the in vivo thrombosis model induced by ferric-chloride injury of the cremaster muscle microcirculation. Indeed, initial platelet adhesion was not decreased in mice expressing p.R1597-VWF compared to mice expressing wild-type VWF, despite the absence of high molecular weight multimers in the former, suggesting that these most active forms are not absolutely required for this initial platelet adhesion step.[49] However, time to occlusion was severely prolonged in the p.R1597-VWF expressing mice. Such results are reminiscent of results observed with VWF-deficient mice where platelet adhesion and thrombus formation are still observed but vessel occlusion is a rare event due to the absolute requirement for VWF[55] and most likely fully multimerized VWF in order to close the last portion of the vessel where blood circulates under conditions of very high shear stress. Perhaps even more surprising was the observation that initial platelet adhesion was increased in mice expressing the p.Y1605A/M1606A-VWF mutant while vessel occlusion was lacking, a very puzzling result that remains largely unexplained so far.[49]
Study of VWF O-glycome through hydrodynamic gene transfer. VWF is a heavily glycosylated proteins with 12 N-linked and 10 O-linked glycosylation sites per mature monomer,[56] accounting for approximately 20% of the molecular weight of the protein. The role of N-glycans in VWF biology and function has been extensively studied[57-60] in contrast to the role of O-glycans. Assessing the role of such glycans using hydrodynamic gene transfer is clearly a challenge since VWF is produced in hepatocytes and not by its physiologic production sites, endothelial cells and megakaryocytes. However, heterologous cell lines such as HEK293 have been commonly used to study VWF glycosylation and brought valuable information despite the fact that they can be similarly questioned in terms of relevance. In our team, we chose the option to investigate the role of VWF O-glycans by mutating individual sites or clusters of such glycans in the context of the murine system. First, we compared hepatocyte-derived VWF to normal plasma VWF for its ability to bind to a number of lectins and found that a similar extent of terminal galactose and sialic acids were exposed on endothelial and hepatic-derived VWF.[61] Similarly, we showed that VWF produced by hepatocytes is not fundamentally different from endothelial-derived VWF in terms of peanut agglutinin recognition, a lectin recognizing T-antigen, the major O-linked glycan present in VWF.[62,63] We therefore felt confident that we could use hydrodynamic gene transfer to study the functional role of VWF O-glycans. Mice carrying mutations in one or several potential VWF O-glycosylation sites were thus generated and studied in terms of VWF expression levels, multimerization, functionality and clearance. Our results showed that O-glycosylations are not absolutely required for VWF biosynthesis and multimerization. However, two residues T1255 and T1256 appeared to be of particular importance since they are involved in the maintenance of VWF plasma levels as well as in the capacity of VWF to support hemostasis. A third residue, S1486, was also shown to play an important role in VWF function.[61]
Conclusion. In the last few years, a number of articles using hydrodynamic gene transfer to study VWF variants have been published by different teams, allowing us to acquire a good perception on the advantages and of course on the drawbacks of this very powerful approach. The benefits are rather obvious, allowing evaluation of VWF mutations in a complete organism more simply than by using in vitro transfections, making it a potential first line approach to screen such mutations. This has led to very interesting results, uncovering the relative importance of some mutations in the thrombotic process versus the hemostastic process. Furthermore, the model proved sensitive enough to detect subtle differences in phenotype, improving our comprehension of some patient’s bleeding symptoms. It is rather paradoxical that such developments were obtained while VWF is at first glance clearly not an ideal candidate target for hydrodynamic gene transfer. Indeed a number of important challenges could have hampered research progress and they did so for a while. First, VWF needs to be multimerized to be active, an additional burden for the hepatocytes. Second, human VWF is not functional in the mouse, leading to the necessity to introduce mutations in the murine cDNA, or in a chimeric human-murine VWF construct. Third, with hydrodynamic injection only the VWF plasma compartment is replenished, leaving endothelial cells, the subendothelium and platelets, VWF-free. This aspect is particularly relevant when testing the ability of mutant-VWF to correct bleeding time in mice. In our hands, we have noticed that an expression level of around 300% of VWF needs to be reached in order to obtain bleeding time correction, probably as a result of the absence of VWF in compartments besides plasma and also possibly to a slight decrease in high molecular weight multimer bands. Despite all these potential issues, hydrodynamic gene transfer appears like an extremely valuable approach to investigate a complex phenotype associated with VWF modifications, ranging from biosynthesis to function to clearance of the variant molecule.
Acknowledgements. Financial support from Agence Nationale de la Recherche (ANR-11-BSV1-010-01) and from Fondation pour la Recherche Médicale (FRM-SPF20101220866), is gratefully acknowledged.
Hydrodynamic injection: application to VWF expression. Hydrodynamic injection is akin to an in vivo transfection method (Figure 1). It consists in a rapid injection of a large volume of plasmid DNA without any other viral or non-viral vector in the lateral tail vein of mice.[7] The volume and speed of injection have been shown to strongly influence the efficiency of gene delivery to hepatocytes. In order to be fully optimal, injection should be completed within 5 seconds with a volume of injection corresponding to 10% of the body weight of the mouse (i.e. 2 ml for a 20 g mouse).[8] After undergoing a successful injection, the mice go through an apathetic phase (shock) that is transient. In C57BL/6 mice, recovery occurs after approximately 20-30 minutes and the survival rate is 100%. The following mechanism has been proposed to explain hepatocytes transfection: by exceeding cardiac capacity, the large DNA volume accumulates in the lower vena cava. This leads to a sharp increase in venous blood pressure and a backflow of the solution towards organs connected to the vena cava. The liver has the most extensive structure and absorbs the majority of the volume injected (>90%). The elevated pressure generated by hydrodynamic injection opens sinusoid fenestrae allowing the solution to reach hepatocytes. On average, 10-40% of hepatocytes are transfected. It is rather amazing that despite the “traumatic” aspect of the technique, liver biological parameters are only slightly modified and on a temporary basis. Indeed, only levels of serum alanine aminotransferase and of aspartate aminotransferase are elevated after hydrodynamic gene delivery (4-20 fold according to different studies) but they return to baseline three days later. Histological studies also showed that less than 5% of the liver has suffered from hepatic damage. Other parameters such as alkaline phosphatase, total bilirubin, albumin, total proteins and Na+, K+ and Cl- ions are not modified.[7]

Figure 1. Description of the mechanism leading to hepatocyte-derived expression of foreign proteins following hydrodynamic injection with plasmid DNA.
This technique has now been used successfully to express functionally active VWF in VWF-deficient mice.[9] Several potential hurdles had to be overcome before such a result could be obtained. First, there was a species-barrier since human VWF interacts poorly with its platelet receptor glycoprotein (GP) Ibα, in mice. As a consequence, only murine VWF (mVWF) cDNA can be used. mVWF cDNA was therefore subcloned in different vectors, either with a CMV promoter[10] or with a liver-specific promoter (murine albumin or α1-antitrypsin).[11,12] In all cases, high pressure injection of 50-100 µg of mVWF cDNA resulted in high levels of expression of VWF antigen in plasma of VWF-deficient mice (Figure 2). Vectors with liver-specific promoters allowed relatively long-term expression (a few weeks compared to a couple of days for vectors with a CMV promoter). A temporary decrease in platelet counts could also be observed but was not apparent anymore after 72 hours (Figure 2). Immunostaining showed that, as expected, VWF is expressed by hepatocytes and not by endothelial cells.[13] This hepatocyte-expression of VWF represented the second hurdle since it was not clear whether this cell type had the necessary machinery to multimerize VWF, a critical criteria for VWF biological activity. Fortunately, we were able to show that this hepatocyte-derived VWF was indeed multimerized (Figure 2) and that it also had the capacity to bind endogenous FVIII, leading to restoration of plasma FVIII levels, which are normally strongly reduced in VWF-deficient mice. Finally the ultimate proof of the functional quality of liver-expressed mVWF was demonstrated by its capacity to correct bleeding time in VWF-deficient mice.[10] In addition, thrombus formation after ferric-chloride induced injury was also restored in the knockout mice expressing plasma mVWF.[11,12]

Figure 2. Characteristics of VWF expression after hydrodynamic gene-transfer in VWF-deficient mice.
A: Injection of 50 µg of plasmid pLIVE-mVWF cDNA resulted in plasmatic expression levels around 800% (100% being the plasma VWF levels measured in wild-type mice) and was stable for about two weeks. Expression levels can be highly operator-dependent.
B: Platelet counts were recorded for a few days following hydrodynamic gene transfer and were shown to slightly decrease 24 hours after injection but were back to pre-injection levels after 3 days.
C: Multimeric profile of plasma VWF produced by hepatocytes following hydrodynamic gene transfer.
These results show that plasma VWF is sufficient to support efficient hemostasis and thrombosis in the mouse, indicating that liver-targeted gene therapy can be considered as a viable treatment alternative for severe VWD. This model also allows the direct comparison of hemostatic capacities of wild-type versus mutant VWF.
Analysis of VWF variants carrying mutations in binding domains to different ligands. In order to play its role in hemostasis, VWF binds to a number of different ligands, among which two platelet receptors, platelet GPIbα and GPIIbIIIa as well as subendothelial collagens.[14] Thanks to many in vitro biochemical studies, the residues on human VWF involved in its binding to these ligands have been precisely identified.[15-17] Considering that these residues were perfectly conserved in mVWF, we hypothesized that the same sequences were also involved in mVWF binding to its murine ligands on platelets and in the vessel wall. We therefore mutated these residues in order to abolish binding to the different receptors.[10] To abolish mVWF binding to murine GPIbα, we mutated the lysine residue in position 1362 to an alanine. Binding of mVWF to mGPIIbIIIa was suppressed by mutating the aspartic acid residue in position 2509 to glycine (RGD sequence converted to RGG sequence). Finally to abolish binding of mVWF to collagens, three residues were mutated simultaneously: aspartic acid 1742, serine 1783 and histidine 1786 were all converted to alanine. The lack of interaction of these different VWF variants to their respective ligands was confirmed in vitro.[10] Hydrodynamic gene transfer of these three mutants showed that 1) comparable plasma mVWF expression levels were obtained, suggesting that the mutations do not alter VWF biosynthesis and 2) plasma VWF was correctly multimerized for all mutants, suggesting that the mutations do not interfere with the multimerization process.
Effect of mutations on bleeding time. Patients with VWD type 2A or 2M already made it clear that a form of VWF that could not interact with platelet GPIbα lead to bleeding symptoms.[18] Injection of the mVWF cDNA carrying the K1362A mutation in VWF-deficient mice confirmed this observation.[10] In contrast to mice injected with wild-type mVWF cDNA, these mice could not control their bleeding time in the tail clip assay and kept bleeding indefinitely, similar to untreated mice. In contrast to the expected bleeding phenotype predicted for the VWF mutant unable to bind GPIbα, no real indication of what to anticipate for lack of binding to collagens or to GPIIbIIIa could be deducted from VWD patients. Indeed, no patient with a mutation in the GPIIbIIIa binding domain has been reported so far and only very few patients with mutations in the collagen-binding region were described at the beginning of this study. Interestingly, VWF-deficient mice injected with either the collagen binding mutant or the RGG mVWF cDNAs were able to control their bleeding as well as wild-type mVWF-treated mice, indicating that these interactions are not absolutely required for the physiological process of hemostasis in the mouse model.[10]
Effect of mutations on thrombosis. Since a defect or lack thereof, in functional activity in the bleeding model is not necessarily representative of the complex role played by VWF in hemostasis/thrombosis, we also extended our examination of these mutations in an in vivo thrombosis model initiated by ferric-chloride exposure of mesenteric vessels.[12] We first showed that injection of wild-type mVWF cDNA in VWF-deficient mice was able to restore thrombus formation and vessel occlusion with a kinetic very similar to the one obtained with wild-type mice. Mice expressing the GPIbα-binding mutant did not form occlusive thrombi, similar to untreated mice. This result, added to the incapacity of this mutant to correct bleeding time, confirms the critical importance of the VWF-GPIbα interaction in maintaining efficient hemostasis/ thrombosis. More surprisingly, mice expressing the collagen binding mutant showed altered thrombosis with significantly delayed vessel occlusion (30 min versus 16 min in wild-type VWF-expressing mice).[12] However, despite this delay, the mice were able to form stable thrombi well anchored to the vessel wall. In contrast, mice expressing the GPIIb/IIIa binding mutant were more severely affected. Less than 40% of the mice expressing this mutant were able to reach occlusive thrombosis. Mice formed thrombi that were less compact and which were not efficient to stop blood flow. Portions of these thrombi constantly embolized, preventing further growth of the thrombus. This result emphasized the critical importance of the VWF-GPIIbIIIa interaction in platelet-platelet firm adhesion at high shear rates.
Altogether, results from the bleeding time as well as the thrombosis model experiments suggested that VWF interactions with fibrillar collagens and with GPIIbIIIa are more important in the pathological thrombotic process than in the physiological process of hemostasis.
Effect of mutations on stroke. The same three VWF mutants were also tested in an ischemic stroke model in mice in order to establish which VWF interactions were important in this model.[19] Stroke was induced by transient middle cerebral artery occlusion. Previous reports had already described a protection against stroke in mice deficient in VWF, while replenishing the VWF plasma compartment by hydrodynamic gene transfer with wild-type mVWF cDNA totally restored the susceptibility of the mice to cerebral ischemia.[11] Analysis of VWF-deficient mice hydrodynamically injected with the mVWF cDNAs mutated in the binding sites to fibrillar collagens, GPIbα or GPIIbIIIa revealed that interaction with GPIbα or collagens were involved in stroke development while interaction with GPIIbIIIa was not playing any role. Indeed the brain infarct volumes were significantly smaller in mice expressing the collagen mutant or the GPIbα mutant compared to mice expressing wild-type or GPIIbIIIa mutant VWF. Along the same line, the functional outcome as measured by neurological Bederson score or the grip test score were significantly higher in untreated VWF-/- mice or VWF-/- mice expressing the collagen or GPIbα mutants compared to wild-type or GPIIbIIIa-treated mice.[19] Interestingly this result not only contributes to our understanding of the molecular mechanisms involved in ischemic brain injury but by showing that VWF-GPIIbIIIa interaction is not involved in this process it also sheds some light on our comprehension of the lack of effectiveness of GPIIbIIIa blockade in experimental and clinical stroke.[20]
Application for anti-thombotic treatment. The observation that VWF interactions with fibrillar collagen or with platelet GPIIbIIIa may be more important for thrombosis than for hemostasis makes these axes interesting for new antithrombotic therapies.[12] Monoclonal antibodies directed against human VWF and specifically targeting these interactions have been described in the litterature.[21-23] However, so far the absence of accessible animal models has made it difficult to test the in vivo efficacy of such tools targeting human VWF. To bypass this species barrier, we have developed a human-murine VWF chimera where the human VWF A1 domain, i.e. the domain containing the GPIbα binding region, is replaced by its murine counterpart.[24] This chimeric molecule proved to be functional after in vivo expression (through hydrodynamic injection) in VWF-deficient mice since it supported occlusive thrombosis after ferric-chloride-induced vascular injury. Being predominantly human, the chimera still contains the epitopes recognized by monoclonal antibodies targeting the VWF-collagen of VWF-GPIIbIIIa interactions. Hydrodynamic gene transfer was therefore performed to express this chimeric molecule in VWF-deficient mice and the inhibition of thrombus formation by monoclonal antibodies was measured in the mice. Inhibition of the VWF-collagen or VWF-GPIIbIIIa interactions proved efficient to reduce thrombosis in arterioles. In particular, one of the antibodies inhibiting VWF-collagen interaction was the most powerful, preventing vessel occlusion in 7 mice out of 8 tested.[24] Hydrodynamic gene transfer of this human-murine VWF chimera thus proved to be an efficient manner to test various agents targeting human VWF in an in vivo system.

Table 1. List of VWF mutations tested in vivo using hydrodynamic gene transfer in VWF-deficient mice.
Analysis of mice reproducing different subtypes of human VWD. VWD is characterized by quantitative and/or qualitative abnormalities in VWF with heterogeneous clinical manifestations.[18,25] Our understanding of this disease has recently improved based on some large multicenter studies in Europe, Canada and the US[26,27] but also on the use of murine models of VWD. Indeed, the hydrodynamic gene transfer has now allowed detailed in vivo genotype-phenotype analysis for a number of VWD types.
Type 1 VWD. Although type 1 VWD, encompassing mild to moderate reduction in VWF levels, is by far the most common VWD type, its molecular pathogenesis is also the least well understood. Large multicenter studies in Europe, Canada and United States have recently contributed to shed light on the complex mechanisms at the basis of type 1 VWD.[26-28] These may involve defective RNA or protein synthesis, intracellular protein degradation, impaired secretion, rapid plasma clearance, all of which can result from either a mutation in the VWF gene or the presence of modifier genes interfering with VWF biology.[3,29,30] What has become increasingly clear from these studies, is the absolute need for multiple approaches to identify these complex and sometimes multiple mechanisms. Cellular studies are extremely useful to identify aspects related to synthesis and secretion, especially now that the possibility to grow blood outgrowth endothelial cells (BOECs) directly from the patient’s blood is available.[31-34] However, when it comes to plasma clearance or detection of a gene modifier, a more complex organism is needed. No transgenic type 1 VWD murine model has been generated so far. However, two common type 1 VWD mutations have been expressed in VWF-deficient mice by hydrodynamic gene transfer: the p.R1205H Vicenza mutation and the p.Y1584C mutation.[35] Results confirmed the aptitude of this in vivo murine expression system to fully recapitulate a complex clinical picture already observed in VWD patients. Indeed, both in the homozygous and heterozygous states, mice expressing either of these two mutants displayed lower VWF antigen levels compared to wild-type VWF expressing mice. Mice expressing the p.R1205H Vicenza mutant had an increased VWF propeptide/VWF antigen ratio, a feature indicating accelerated clearance of the protein. This confirms results found in patients as well as results obtained in a murine model with injection of the recombinant protein.[36,37] Mice expressing the p.Y1584C mutant displayed increased ADAMTS13 proteolysis, also supporting studies performed with patients plasma.[38,39] This model also allowed the authors to study the procoagulant function of the mutant proteins in in vivo thrombosis experiments and to do so at a fixed VWF antigen plasma concentration. This approach has the advantage to evaluate the inherent capacity of the mutant protein to promote thrombus formation, independently of the quantitative anomaly. Results showed that the p.R1205H VWF exerts a normal hemostatic function, suggesting that the associated defect in patients is purely of a quantitative nature while the p.Y1584C VWF displays a lower capacity to support thrombus formation, resembling a mild type 2A VWD phenotype, probably due to the increased ADAMTS13 cleavage.[35]
Type 2M VWD: collagen-binding mutants. So far, all the VWF mutations that have been tested using the hydrodynamic injection procedure were introduced in the murine VWF sequence. The mouse VWF amino acid sequence has 91% homology to that of human VWF,[40] allowing replacement of the same residue in the murine system as the one affected in the patients. As mentioned earlier, only murine VWF is hemostatically active in the mouse, preventing use of human VWF. However, since we had generated an active human-murine VWF chimera (hVWFmA1) in order to test the anti-thrombotic effect of monoclonal antibodies inhibiting specific VWF functions,[24] we also used the same construct to introduce VWF mutations present in the A3 domain and detected in patients with VWD.[41] Mutations in the VWF A3 domain have not been described as frequently as mutations in the platelet-binding domain (the A1 domain) but they are now being increasingly recognized.[42-44] These mutations usually lead to deficient VWF-collagen interaction and mild/moderate bleeding symptoms. Two such mutations detected through the French National Reference Center for VWD were introduced in the hVWFmA1 cDNA and injected to VWF-deficient mice using hydrodynamic gene transfer: p.L1696R and p.P1824H. In contrast to above-mentioned studies using type 2B and type 1 mutants where the in vivo studies were performed in a second step following extensive in vitro analysis, the mutants were here studied in parallel in vitro and in vivo in order to get a complete picture of the functional defects associated with the mutations. This dual approach proved extremely useful to identify the dominant-negative effect of the p.L1696R mutation on VWF expression levels in patients and in mice expressing the mutation in a heterozygous manner, compared to cellular expression systems. This result raised the possibility that the mutant protein was cleared more rapidly than wild-type VWF in vivo, a feature that cannot be detected in vitro. Mice expressing the second A3 domain mutant, p.P1824H-VWF also displayed low expression VWF levels, but the probable underlying cause, intracellular retention was this time detected both in the in vitro and the in vivo expression systems.[41] For both mutants, the thrombotic response in arterioles of mice was strongly decreased, but the low expression levels did not allow concluding as whether this effect should be attributed to the quantitative defect or to the qualitative defect.
Type 2B VWD. Type 2B VWD is characterized by gain-of-function mutations in VWF A1 domain, resulting not only in an increased affinity for GPIbα but also in an increased susceptibility to ADAMTS13.[25,45] VWF carrying common type 2B mutations, p.V1316M, p.R1306Q, p.R1306W or p.R1341Q.[25,45,46] were expressed in VWF-deficient mice by hydrodynamic gene transfer to generate type 2B VWD murine models. A clear mutation-dependent modulation of disease severity was described, with variable thrombocytopenia and loss of high molecular weight VWF multimers, presence of circulating platelet aggregates and of giant platelets and defective hemostasis and thrombosis.[47,48] Strikingly, these features parallel remarkably well with the phenotype of the patients carrying similar mutations.[46] It therefore appears that these generated transient mouse models are sensitive enough to recapitulate the myriad of defects associated with human type 2B VWD and to detect subtle differences in phenotype. Such models have been instrumental in establishing that the loss of high molecular weight multimers in vivo is caused by the enhanced susceptibility to ADAMTS13 proteolysis rather than preferential adsorption of the high multimers onto platelets.[48] These type 2B VWD mouse models will be very useful in the future, not only to understand additional factors involved in this VWD type such as the mechanisms underlying thrombocytopenia but they also represent very useful tools to test new potential treatments.
Study of ADAMTS13-cleavage mutants. Cleavage of VWF by ADAMTS13 is an important step in the regulation of VWF activity and any perturbation in the delicate balance between too little or too much cleavage leads to pathologic consequences: hemorrhagic when cleavage is excessive as is the case for VWD type 2A or thrombotic consequences when cleavage is insufficient or lacking as is the case in thrombotic thrombocytopenic purpura (TTP). In order to evaluate the in vivo consequences of interfering with VWF sequences involved in ADAMTS13 cleavage, Pruss and colleagues used hydrodynamic gene transfer to generate transient murine models expressing VWF carrying the p.R1597W mutation, the most common type 2A VWD mutation or VWF carrying mutations at the ADAMTS13 cleavage site p.Y1605A/M1606A.[49] Analysis of the VWF multimeric profile of these mice confirmed a loss of high molecular weight multimers in p.R1597-VWF expressing mice and in contrast, a profile with higher molecular weight bands in p.Y1605A/M1606A-VWF expressing mice. This effect was even further amplified when full-length ADAMTS13 cDNA was co-injected with VWF cDNA in order to compensate for the presence of the truncated ADAMTS13 form that is naturally expressed in the C57Bl/6 mouse strain[50] and that is not fully active in vivo.[51,52] Interestingly, mice expressing the cleavage-resistant mutant, even at high concentrations, did not display any sign of TTP, similar to mice deficient in ADAMTS13.[53,54] This result confirms that additional triggering events are necessary to induce TTP, at least in the murine model. The most striking observations were obtained in the in vivo thrombosis model induced by ferric-chloride injury of the cremaster muscle microcirculation. Indeed, initial platelet adhesion was not decreased in mice expressing p.R1597-VWF compared to mice expressing wild-type VWF, despite the absence of high molecular weight multimers in the former, suggesting that these most active forms are not absolutely required for this initial platelet adhesion step.[49] However, time to occlusion was severely prolonged in the p.R1597-VWF expressing mice. Such results are reminiscent of results observed with VWF-deficient mice where platelet adhesion and thrombus formation are still observed but vessel occlusion is a rare event due to the absolute requirement for VWF[55] and most likely fully multimerized VWF in order to close the last portion of the vessel where blood circulates under conditions of very high shear stress. Perhaps even more surprising was the observation that initial platelet adhesion was increased in mice expressing the p.Y1605A/M1606A-VWF mutant while vessel occlusion was lacking, a very puzzling result that remains largely unexplained so far.[49]
Study of VWF O-glycome through hydrodynamic gene transfer. VWF is a heavily glycosylated proteins with 12 N-linked and 10 O-linked glycosylation sites per mature monomer,[56] accounting for approximately 20% of the molecular weight of the protein. The role of N-glycans in VWF biology and function has been extensively studied[57-60] in contrast to the role of O-glycans. Assessing the role of such glycans using hydrodynamic gene transfer is clearly a challenge since VWF is produced in hepatocytes and not by its physiologic production sites, endothelial cells and megakaryocytes. However, heterologous cell lines such as HEK293 have been commonly used to study VWF glycosylation and brought valuable information despite the fact that they can be similarly questioned in terms of relevance. In our team, we chose the option to investigate the role of VWF O-glycans by mutating individual sites or clusters of such glycans in the context of the murine system. First, we compared hepatocyte-derived VWF to normal plasma VWF for its ability to bind to a number of lectins and found that a similar extent of terminal galactose and sialic acids were exposed on endothelial and hepatic-derived VWF.[61] Similarly, we showed that VWF produced by hepatocytes is not fundamentally different from endothelial-derived VWF in terms of peanut agglutinin recognition, a lectin recognizing T-antigen, the major O-linked glycan present in VWF.[62,63] We therefore felt confident that we could use hydrodynamic gene transfer to study the functional role of VWF O-glycans. Mice carrying mutations in one or several potential VWF O-glycosylation sites were thus generated and studied in terms of VWF expression levels, multimerization, functionality and clearance. Our results showed that O-glycosylations are not absolutely required for VWF biosynthesis and multimerization. However, two residues T1255 and T1256 appeared to be of particular importance since they are involved in the maintenance of VWF plasma levels as well as in the capacity of VWF to support hemostasis. A third residue, S1486, was also shown to play an important role in VWF function.[61]
Conclusion. In the last few years, a number of articles using hydrodynamic gene transfer to study VWF variants have been published by different teams, allowing us to acquire a good perception on the advantages and of course on the drawbacks of this very powerful approach. The benefits are rather obvious, allowing evaluation of VWF mutations in a complete organism more simply than by using in vitro transfections, making it a potential first line approach to screen such mutations. This has led to very interesting results, uncovering the relative importance of some mutations in the thrombotic process versus the hemostastic process. Furthermore, the model proved sensitive enough to detect subtle differences in phenotype, improving our comprehension of some patient’s bleeding symptoms. It is rather paradoxical that such developments were obtained while VWF is at first glance clearly not an ideal candidate target for hydrodynamic gene transfer. Indeed a number of important challenges could have hampered research progress and they did so for a while. First, VWF needs to be multimerized to be active, an additional burden for the hepatocytes. Second, human VWF is not functional in the mouse, leading to the necessity to introduce mutations in the murine cDNA, or in a chimeric human-murine VWF construct. Third, with hydrodynamic injection only the VWF plasma compartment is replenished, leaving endothelial cells, the subendothelium and platelets, VWF-free. This aspect is particularly relevant when testing the ability of mutant-VWF to correct bleeding time in mice. In our hands, we have noticed that an expression level of around 300% of VWF needs to be reached in order to obtain bleeding time correction, probably as a result of the absence of VWF in compartments besides plasma and also possibly to a slight decrease in high molecular weight multimer bands. Despite all these potential issues, hydrodynamic gene transfer appears like an extremely valuable approach to investigate a complex phenotype associated with VWF modifications, ranging from biosynthesis to function to clearance of the variant molecule.
Acknowledgements. Financial support from Agence Nationale de la Recherche (ANR-11-BSV1-010-01) and from Fondation pour la Recherche Médicale (FRM-SPF20101220866), is gratefully acknowledged.
References
- von Willebrand EA. Hereditär pseudohämofili. Fin Läkäresallsk Handl. 1926;67:7-12.

- Denis CV, Wagner DD. Insights from von Willebrand disease animal models. Cell Mol Life Sci. 1999;56:977-90. http://dx.doi.org/10.1007/s000180050487

- Pendu R, Christophe OD, Denis CV. Mouse models of von Willebrand disease. J Thromb Haemost. 2009;7 Suppl 1:61-4. http://dx.doi.org/10.1111/j.1538-7836.2009.03411.x PMid:19630770

- Denis C, Methia N, Frenette PS, Rayburn H,
Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von
Willebrand disease: defects in hemostasis and thrombosis. Proc Natl
Acad Sci USA. 1998;95:9524-9. http://dx.doi.org/10.1073/pnas.95.16.9524 PMid:9689113 PMCid:PMC21371

- Denis CV, Lenting PJ. von Willebrand factor: at the crossroads of bleeding and thrombosis. Int J Hematol. 2012;95:353-61. http://dx.doi.org/10.1007/s12185-012-1041-x PMid:22477538 PMCid:PMC3677142

- Lenting PJ, Casari C, Christophe OD, Denis
CV. von Willebrand factor: the old, the new and the unknown. J Thromb
Haemost. 2012;10:2428-37. http://dx.doi.org/10.1111/jth.12008 PMid:23020315

- Liu F, Song Y, Liu D. Hydrodynamics-based
transfection in animals by systemic administration of plasmid DNA. Gene
Ther. 1999;6:1258-66. http://dx.doi.org/10.1038/sj.gt.3300947 PMid:10455434

- Feng DM, He CX, Miao CY, Lu B, Wu WJ, Ding
YF, Xue JL. Conditions affecting hydrodynamics-based gene delivery into
mouse liver in vivo. Clin Exp Pharmacol Physiol. 2004;31:850-5. http://dx.doi.org/10.1111/j.1440-1681.2004.04125.x PMid:15659048

- Lenting PJ, de Groot PG, De Meyer SF,
Vanhoorelbeke K, Pruss C, Lillicrap D, Marx I, Denis CV. Correction of
the bleeding time in von Willebrand factor (VWF)-deficient mice using
murine VWF. Blood. 2007;109:2267-8. http://dx.doi.org/10.1182/blood-2006-10-054718 PMid:17312004

- Marx I, Lenting PJ, Adler T, Pendu R,
Christophe OD, Denis CV. Correction of bleeding symptoms in von
Willebrand factor-deficient mice by liver-expressed von Willebrand
factor mutants. Arterioscler Thromb Vasc Biol. 2008;28:419-24. http://dx.doi.org/10.1161/ATVBAHA.107.159442 PMid:18187670

- De Meyer SF, Vandeputte N, Pareyn I,
Petrus I, Lenting PJ, Chuah MK, VandenDriessche T, Deckmyn H,
Vanhoorelbeke K. Restoration of plasma von Willebrand factor deficiency
is sufficient to correct thrombus formation after gene therapy for
severe von Willebrand disease. Arterioscler Thromb Vasc Biol.
2008;28:1621-6. http://dx.doi.org/10.1161/ATVBAHA.108.168369 PMid:18556568

- Marx I, Christophe OD, Lenting PJ, Rupin
A, Vallez MO, Verbeuren TJ, Denis CV. Altered thrombus formation in von
Willebrand factor-deficient mice expressing von Willebrand factor
variants with defective binding to collagen or GPIIbIIIa. Blood.
2008;112:603-9. http://dx.doi.org/10.1182/blood-2008-02-142943 PMid:18487513

- Denis CV, Marx I, Badirou I, Pendu R,
Christophe O. Mouse models to study von Willebrand factor
structure-function relationships in vivo. Hamostaseologie.
2009;29:17-8, 20. PMid:19151840

- Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120 Suppl 1:S5-9. http://dx.doi.org/10.1016/j.thromres.2007.03.011 PMid:17493665 PMCid:PMC2702526

- Beacham DA, Wise RJ, Turci SM, Handin RI.
Selective inactivation of the Arg-Gly-Asp-Ser (RGDS) binding site in
von Willebrand factor by site-directed mutagenesis. J Biol Chem.
1992;267:3409-15. PMid:1737795

- Matsushita T, Meyer D, Sadler JE.
Localization of von willebrand factor-binding sites for platelet
glycoprotein Ib and botrocetin by charged-to-alanine scanning
mutagenesis. J Biol Chem. 2000;275:11044-9. http://dx.doi.org/10.1074/jbc.275.15.11044 PMid:10753907

- Romijn RA, Westein E, Bouma B, Schiphorst
ME, Sixma JJ, Lenting PJ, Huizinga EG. Mapping the collagen-binding
site in the von Willebrand factor-A3 domain. J Biol Chem.
2003;278:15035-9. http://dx.doi.org/10.1074/jbc.M208977200 PMid:12582178

- Sadler JE, Budde U, Eikenboom JC, Favaloro
EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM,
Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F,
Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB.
Update on the pathophysiology and classification of von Willebrand
disease: a report of the Subcommittee on von Willebrand Factor. J
Thromb Haemost. 2006;4:2103-14.
http://dx.doi.org/10.1111/j.1538-7836.2006.02146.x PMid:16889557
- De Meyer SF, Schwarz T, Deckmyn H, Denis
CV, Nieswandt B, Stoll G, Vanhoorelbeke K, Kleinschnitz C. Binding of
von Willebrand factor to collagen and glycoprotein Ibalpha, but not to
glycoprotein IIb/IIIa, contributes to ischemic stroke in mice--brief
report. Arterioscler Thromb Vasc Biol. 2010;30:1949-51. http://dx.doi.org/10.1161/ATVBAHA.110.208918 PMid:20616311

- De Meyer SF, Stoll G, Wagner DD,
Kleinschnitz C. von Willebrand Factor: An Emerging Target in Stroke
Therapy. Stroke. 2012;43:599-606. http://dx.doi.org/10.1161/STROKEAHA.111.628867 PMid:22180250

- Baruch D, Denis C, Marteaux C, Schoevaert
D, Coulombel L, Meyer D. Role of von Willebrand factor associated to
extracellular matrices in platelet adhesion. Blood. 1991;77:519-27.
PMid:1991166

- Fressinaud E, Baruch D, Girma JP,
Sakariassen KS, Baumgartner HR, Meyer D. von Willebrand factor-mediated
platelet adhesion to collagen involves platelet membrane glycoprotein
IIb-IIIa as well as glycoprotein Ib. J Lab Clin Med. 1988;112:58-67.
PMid:2455762

- Girma JP, Kalafatis M, Pietu G, Lavergne
JM, Chopek MW, Edgington TS, Meyer D. Mapping of distinct von
Willebrand factor domains interacting with platelet GPIb and GPIIb/IIIa
and with collagen using monoclonal antibodies. Blood. 1986;67:1356-66.
PMid:3008890

- Navarrete AM, Casari C, Legendre P, Marx
I, Hu JR, Lenting PJ, Christophe OD, Denis CV. A murine model to
characterize the antithrombotic effect of molecules targeting human von
Willebrand factor. Blood. 2012;120:2723-32. http://dx.doi.org/10.1182/blood-2012-03-420042 PMid:22915646

- Schneppenheim R, Budde U. von Willebrand
factor: the complex molecular genetics of a multidomain and
multifunctional protein. J Thromb Haemost. 2011;9 Suppl 1:209-15. http://dx.doi.org/10.1111/j.1538-7836.2011.04324.x PMid:21781257

- James PD, Goodeve AC. von Willebrand disease. Genet Med. 2011;13:365-76. http://dx.doi.org/10.1097/GIM.0b013e3182035931 PMid:21289515

- James PD, Lillicrap D. von Willebrand
disease: clinical and laboratory lessons learned from the large von
Willebrand disease studies. Am J Hematol. 2012;87 Suppl 1:S4-11. http://dx.doi.org/10.1002/ajh.23142 PMid:22389132

- Goodeve AC. The genetic basis of von Willebrand disease. Blood Rev. 2010;24:123-34.
http://dx.doi.org/10.1016/j.blre.2010.03.003 PMid:20409624
- Shavit JA, Manichaikul A, Lemmerhirt HL,
Broman KW, Ginsburg D. Modifiers of von Willebrand factor identified by
natural variation in inbred strains of mice. Blood. 2009;114:5368-74. http://dx.doi.org/10.1182/blood-2009-07-233213 PMid:19789385 PMCid:PMC2796139

- Westrick RJ, Ginsburg D. Modifier genes for disorders of thrombosis and hemostasis. J Thromb Haemost. 2009;7 Suppl 1:132-5. http://dx.doi.org/10.1111/j.1538-7836.2009.03362.x PMid:19630785

- Berber E, James PD, Hough C, Lillicrap D.
An assessment of the pathogenic significance of the R924Q von
Willebrand factor substitution. J Thromb Haemost. 2009;7:1672-9. http://dx.doi.org/10.1111/j.1538-7836.2009.03551.x PMid:19624459

- Martin-Ramirez J, Hofman M, van den
Biggelaar M, Hebbel RP, Voorberg J. Establishment of outgrowth
endothelial cells from peripheral blood. Nat Protoc. 2012;7:1709-15. http://dx.doi.org/10.1038/nprot.2012.093 PMid:22918388

- Starke RD, Paschalaki KE, Dyer CE,
Harrison-Lavoie KJ, Cutler JA, McKinnon TA, Millar CM, Cutler DF,
Laffan MA, Randi AM. Cellular and molecular basis of von Willebrand
disease: studies on blood outgrowth endothelial cells. Blood.
2013;121:2773-84. http://dx.doi.org/10.1182/blood-2012-06-435727 PMid:23355534 PMCid:PMC3617637

- Wang JW, Bouwens EA, Pintao MC, Voorberg
J, Safdar H, Valentijn KM, de Boer HC, Mertens K, Reitsma PH, Eikenboom
JC. Analysis of storage and secretion of von Willebrand factor in blood
outgrowth endothelial cells derived from von Willebrand disease
patients. Blood. 2013;121:2762-72. http://dx.doi.org/10.1182/blood-2012-06-434373 PMid:23426949

- Pruss CM, Golder M, Bryant A, Hegadorn CA,
Burnett E, Laverty K, Sponagle K, Dhala A, Notley C, Haberichter S,
Lillicrap D. Pathologic mechanisms of type 1 VWD mutations R1205H and
Y1584C through in vitro and in vivo mouse models. Blood.
2011;117:4358-66.

http://dx.doi.org/10.1182/blood-2010-08-303727 PMid:21346256 PMCid:PMC3087484
- Casonato A, Pontara E, Sartorello F,
Cattini MG, Sartori MT, Padrini R, Girolami A. Reduced von Willebrand
factor survival in type Vicenza von Willebrand disease. Blood.
2002;99:180-4. http://dx.doi.org/10.1182/blood.V99.1.180 PMid:11756169

- Lenting PJ, Westein E, Terraube V, Ribba
AS, Huizinga EG, Meyer D, de Groot PG, Denis CV. An experimental model
to study the in vivo survival of von Willebrand factor. Basic aspects
and application to the R1205H mutation. J Biol Chem. 2004;279:12102-9. http://dx.doi.org/10.1074/jbc.M310436200 PMid:14613933

- Bowen DJ, Collins PW. An amino acid
polymorphism in von Willebrand factor correlates with increased
susceptibility to proteolysis by ADAMTS13. Blood. 2004;103:941-7.
http://dx.doi.org/10.1182/blood-2003-05-1505 PMid:14525793

- Keeney S, Grundy P, Collins PW, Bowen DJ.
C1584 in von Willebrand factor is necessary for enhanced proteolysis by
ADAMTS13 in vitro. Haemophilia. 2007;13:405-8. http://dx.doi.org/10.1111/j.1365-2516.2007.01470.x PMid:17610557

- Chitta MS, Duhe RJ, Kermode JC. Cloning of
the cDNA for murine von Willebrand factor and identification of
orthologous genes reveals the extent of conservation among diverse
species. Platelets. 2007;18:182-98. http://dx.doi.org/10.1080/09537100600938816 PMid:17497430

- Legendre P, Navarrete AM, Rayes J, Casari
C, Boisseau P, Ternisien C, Caron C, Fressinaud E, Goudemand J,
Veyradier A, Denis CV, Lenting PJ, Christophe OD. Mutations in the A3
domain of Von Willebrand factor inducing combined qualitative and
quantitative defects in the protein. Blood. 2013;121:2135-43. http://dx.doi.org/10.1182/blood-2012-09-456038 PMid:23335371

- Flood VH, Lederman CA, Wren JS,
Christopherson PA, Friedman KD, Hoffmann RG, Montgomery RR. Absent
collagen binding in a VWF A3 domain mutant: utility of the VWF:CB in
diagnosis of VWD. J Thromb Haemost. 2010;8:1431-3. http://dx.doi.org/10.1111/j.1538-7836.2010.03869.x PMid:20345715

- Ribba AS, Loisel I, Lavergne JM,
Juhan-Vague I, Obert B, Cherel G, Meyer D, Girma JP. Ser968Thr mutation
within the A3 domain of von Willebrand factor (VWF) in two related
patients leads to a defective binding of VWF to collagen. Thromb
Haemost. 2001;86:848-54. PMid:11583318

- Riddell AF, Gomez K, Millar CM, Mellars G,
Gill S, Brown SA, Sutherland M, Laffan MA, McKinnon TA.
Characterization of W1745C and S1783A: 2 novel mutations causing
defective collagen binding in the A3 domain of von Willebrand factor.
Blood. 2009;114:3489-96. http://dx.doi.org/10.1182/blood-2008-10-184317 PMid:19687512

- Rayes J, Hommais A, Legendre P, Tout H,
Veyradier A, Obert B, Ribba AS, Girma JP. Effect of von Willebrand
disease type 2B and type 2M mutations on the susceptibility of von
Willebrand factor to ADAMTS-13. J Thromb Haemost. 2007;5:321-8.
http://dx.doi.org/10.1111/j.1538-7836.2007.02296.x PMid:17087728
- Federici AB, Mannucci PM, Castaman G,
Baronciani L, Bucciarelli P, Canciani MT, Pecci A, Lenting PJ, De Groot
PG. Clinical and molecular predictors of thrombocytopenia and risk of
bleeding in patients with von Willebrand disease type 2B: a cohort
study of 67 patients. Blood. 2009;113:526-34. http://dx.doi.org/10.1182/blood-2008-04-152280 PMid:18805962

- Golder M, Pruss CM, Hegadorn C, Mewburn J,
Laverty K, Sponagle K, Lillicrap D. Mutation-specific hemostatic
variability in mice expressing common type 2B von Willebrand disease
substitutions. Blood. 2010;115:4862-9. http://dx.doi.org/10.1182/blood-2009-11-253120 PMid:20371742

- Rayes J, Hollestelle MJ, Legendre P, Marx
I, de Groot PG, Christophe OD, Lenting PJ, Denis CV. Mutation and
ADAMTS13-dependent modulation of disease severity in a mouse model for
von Willebrand disease type 2B. Blood. 2010;115:4870-7. http://dx.doi.org/10.1182/blood-2009-11-254193 PMid:20200350

- Pruss CM, Golder M, Bryant A, Hegadorn C,
Haberichter S, Lillicrap D. Use of a mouse model to elucidate the
phenotypic effects of the von Willebrand factor cleavage mutants,
Y1605A/M1606A and R1597W. J Thromb Haemost. 2012;10:940-50.
http://dx.doi.org/10.1111/j.1538-7836.2012.04675.x PMid:22372972
- Banno F, Kaminaka K, Soejima K, Kokame K,
Miyata T. Identification of strain-specific variants of mouse Adamts13
gene encoding von Willebrand factor-cleaving protease. J Biol Chem.
2004;279:30896-903. http://dx.doi.org/10.1074/jbc.M314184200 PMid:15136581

- Banno F, Chauhan AK, Kokame K, Yang J,
Miyata S, Wagner DD, Miyata T. The distal carboxyl-terminal domains of
ADAMTS13 are required for regulation of in vivo thrombus formation.
Blood. 2009;113:5323-9. http://dx.doi.org/10.1182/blood-2008-07-169359 PMid:19109562 PMCid:PMC2686194

- Zhang P, Pan W, Rux AH, Sachais BS, Zheng
XL. The cooperative activity between the carboxyl-terminal TSP1 repeats
and the CUB domains of ADAMTS13 is crucial for recognition of von
Willebrand factor under flow. Blood. 2007;110:1887-94. http://dx.doi.org/10.1182/blood-2007-04-083329 PMid:17540842 PMCid:PMC1976376

- Banno F, Kokame K, Okuda T, Honda S,
Miyata S, Kato H, Tomiyama Y, Miyata T. Complete deficiency in ADAMTS13
is prothrombotic, but it alone is not sufficient to cause thrombotic
thrombocytopenic purpura. Blood. 2006;107:3161-6. http://dx.doi.org/10.1182/blood-2005-07-2765 PMid:16368888

- Motto DG, Chauhan AK, Zhu G, Homeister J,
Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD, Ginsburg D. Shigatoxin
triggers thrombotic thrombocytopenic purpura in genetically susceptible
ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752-61. http://dx.doi.org/10.1172/JCI26007 PMid:16200209 PMCid:PMC1240119

- Ni H, Denis CV, Subbarao S, Degen JL, Sato
TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in
arterioles of mice lacking both von Willebrand factor and fibrinogen. J
Clin Invest. 2000;106:385-92. http://dx.doi.org/10.1172/JCI9896 PMid:10930441 PMCid:PMC314330

- Titani K, Kumar S, Takio K, Ericsson LH,
Wade RD, Ashida K, Walsh KA, Chopek MW, Sadler JE, Fujikawa K. Amino
acid sequence of human von Willebrand factor. Biochemistry.
1986;25:3171-84. http://dx.doi.org/10.1021/bi00359a015 PMid:3524673

- Davies JA, Collins PW, Hathaway LS, Bowen
DJ. von Willebrand factor: evidence for variable clearance in vivo
according to Y/C1584 phenotype and ABO blood group. J Thromb Haemost.
2008;6:97-103. http://dx.doi.org/10.1111/j.1538-7836.2007.02809.x PMid:17949477

- McKinnon TA, Chion AC, Millington AJ, Lane
DA, Laffan MA. N-linked glycosylation of VWF modulates its interaction
with ADAMTS13. Blood. 2008;111:3042-9. http://dx.doi.org/10.1182/blood-2007-06-095042 PMid:17975018

- McKinnon TA, Goode EC, Birdsey GM, Nowak
AA, Chan AC, Lane DA, Laffan MA. Specific N-linked glycosylation sites
modulate synthesis and secretion of von Willebrand factor. Blood.
2010;116:640-8. http://dx.doi.org/10.1182/blood-2010-02-267450 PMid:20418283

- Wagner DD, Mayadas T, Marder VJ. Initial
glycosylation and acidic pH in the Golgi apparatus are required for
multimerization of von Willebrand factor. J Cell Biol. 1986;102:1320-4.
http://dx.doi.org/10.1083/jcb.102.4.1320 PMid:3082891

- Badirou I, Kurdi M, Legendre P, Rayes J,
Bryckaert M, Casari C, Lenting PJ, Christophe OD, Denis CV. In vivo
analysis of the role of O-glycosylations of von Willebrand factor. PLoS
One. 2012;7:e37508. http://dx.doi.org/10.1371/journal.pone.0037508 PMid:22616016 PMCid:PMC3355127

- Canis K, McKinnon TA, Nowak A, Panico M,
Morris HR, Laffan M, Dell A. The plasma von Willebrand factor O-glycome
comprises a surprising variety of structures including ABH antigens and
disialosyl motifs. J Thromb Haemost. 2010;8:137-45. http://dx.doi.org/10.1111/j.1538-7836.2009.03665.x PMid:19874459

- Samor B, Michalski JC, Mazurier C,
Goudemand M, De Waard P, Vliegenthart JF, Strecker G, Montreuil J.
Primary structure of the major O-glycosidically linked carbohydrate
unit of human von Willebrand factor. Glycoconj J. 1989;6:263-70. http://dx.doi.org/10.1007/BF01047846 PMid:2535488
