Screening of Hepatitis G and Epstein-Barr Viruses Among Voluntary non Remunerated Blood Donors (VNRBD) in Burkina Faso, West Africa
Issoufou Tao1, Cyrille Bisseye1,4*, Bolni Marius Nagalo1, Mahamoudou Sanou2, Alice Kiba2, Guzin Surat1, Tegwindé Rebeca Compaoré1, Lassina Traoré1, Jean Baptiste Nikiema1, Virginio Pietra1, Jean-Didier Zongo3 and Jacques Simpore1
1 Pietro Annigoni Biomolecular Research Centre (CERBA)/ LABIOGENE, University of Ouagadougou, Burkina Faso.
2 National Blood Transfusion Centre, Ouagadougou, Burkina Faso.
3 Laboratory of genetics, University of Ouagadougou, Burkina Faso.
4 Department of Biology, University of Sciences and Techniques of Masuku (Franceville), Gabon
2 National Blood Transfusion Centre, Ouagadougou, Burkina Faso.
3 Laboratory of genetics, University of Ouagadougou, Burkina Faso.
4 Department of Biology, University of Sciences and Techniques of Masuku (Franceville), Gabon
Correspondence
to:
Dr. Cyrille Bisseye, MSc, PhD. Pietro Annigoni Biomolecular Research
Centre (CERBA)/ LABIOGENE, University of Ouagadougou, Burkina Faso. 01
BP 364 Ouagadougou 01; Ouagadougou, Burkina Faso, West Africa. E-mail: cbisseye@gmail.com
Published: September 2, 2013
Received: July 9, 2013
Accepted: August 14, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013053, DOI 10.4084/MJHID.2013.053
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
In
most sub-Saharan countries screening of blood-transmitted infections
includes mainly HIV, HBV, HCV and syphilis. Many viruses such as
Hepatitis G (HGV) and Epstein-Barr virus (EBV) which also carry a risk
of transmission by blood transfusion raise the question of the extent
of screening for these pathogens. This work aims to evaluate the
prevalence of HGV and EBV in first-time blood donors in Ouagadougou.
The prevalence of HGV and EBV in 551 blood donors was 7.4% and 5.4% respectively. HGV prevalence was significantly higher in blood donors with hepatitis B antigens and positive for HCV compared to donors negative for HCV and no hepatitis B antigens (respectively p<0.001 and p=0.004). EBV prevalence was higher among blood donors of < 20 years age group. HBV and HCV positive individuals are not eligible for blood donation.
This study shows significant results with regard to the prevalence of HGV and EBV prevalence in blood donors in Burkina Faso and emphasizes the need for a general screening.
The prevalence of HGV and EBV in 551 blood donors was 7.4% and 5.4% respectively. HGV prevalence was significantly higher in blood donors with hepatitis B antigens and positive for HCV compared to donors negative for HCV and no hepatitis B antigens (respectively p<0.001 and p=0.004). EBV prevalence was higher among blood donors of < 20 years age group. HBV and HCV positive individuals are not eligible for blood donation.
This study shows significant results with regard to the prevalence of HGV and EBV prevalence in blood donors in Burkina Faso and emphasizes the need for a general screening.
Introduction
Despite the remarkable progress in detecting transfusion-transmissible infectious pathogens, blood transfusion still remains a significant mode of transmission of infectious agents. Given the wide variety of blood-borne agents, there is an urgent need to determine their epidemiology.
Burkina Faso suffers from a shortage of blood products, with merely 3.1 units per 1,000 habitants as opposed to 10 units per 1,000 inhabitants required,[1] due to malaria, malnutrition, emergency surgery, childbirth and traffic accidents.[2-4]
The main infectious diseases detected among blood donors are human immunodeficiency virus (HIV), along with Hepatitis B and C viruses (HBV and HCV). The screening of viruses such as Hepatitis G (HGV), Epstein-Barr virus (EBV), Cytomegalovirus (CMV) and Human Lymphotropic T Cell Virus (HLTV) remains unaddressed.
In a recent report GBV-C (HGV), GBV-A and GBV-D have been classified in a new genus Pegivirus within the Family Flaviviridae.[5] The clinical role of HGV is not entirely clarified yet. HGV is not associated with any hepatic morphological modifications.[6] However, a recent study showed an association between HGV infection with aplastic anemia and extrahepatic diseases.[7]
The prevalence of HGV in the world varies from 2% in the United States of America to 12.2% in Brazil and 18% in Egypt and South Africa, respectively.[8] The virus is mainly transmitted via infectious blood and parenterally,[9,10] while sexual transmission lacks significant evidence.[8]
Another neglected virus not diagnosed among blood donors in Burkina Faso is EBV. In immunocompetent individuals EBV is in a latent phase; and infected people show no clinical symptoms. Indeed, 90% of adults are EBV asymptomatic carriers. In immunosuppressed individuals the virus is reactivated and becomes oncogenic.[11] EBV is etiologically associated with Burkitt's lymphoma (a B cell-derived tumor), nasopharyngeal carcinoma,[12] Hodgkin’s lymphoma[13] and breast cancer.[14-16] However, its mode of transmission remains controversial.
In Africa, early infections with EBV have been described in children, while in developed countries the primary infection is delayed until adulthood.[17]
In Burkina Faso, the prevalence of HGV and EBV are unknown to date. This study aims to determine the prevalence of HGV and EBV among blood donors in Burkina Faso and examine sociodemographic factors associated with both viral infections.
Materials and Methods
Blood donors. Blood samples were collected using standard procedures from voluntary non remunerated blood donors (VNRBD) at the Regional Blood Transfusion Centre of Ouagadougou (RBTC) between February and September 2012. VNRBD were all healthy subjects, selected after responding to a panel of questions comprising a medical history. Healthy individuals aged 17-65 years and over 50 kg were eligible for blood donation.
All samples were screened for hepatitis B virus surface antigen (HBsAg), antibodies to HIV, Treponema pallidum and HCV. Samples were kept at −20 °C for further analysis.
This study was approved by the CERBA/Saint Camille Ethics Committee. The consent of all study subjects was obtained before blood collection.
Serological analysis. HBsAg and antibodies to HCV and HIV were detected using a fourth generation ELISA (ARCHITECT-i1000SR-ABBOTT, Santa Clara, California, United States of America).
All reactive samples for HBsAg, HCV and HIV were re-tested using a second ELISA (Bio-Rad, Marnes la Coquette, France). A result was considered positive if both the first and second tests were positive. Antibodies to Treponema pallidum were detected using a rapid plasma reagin (RPR) test (Cypress Diagnostics, Langdorp, Belgium) and confirmed with a Treponema pallidum haemagglutination (TPHA) test (Cypress Diagnostics).
Hepatitis G virus RNA extraction and reverse transcription. Viral RNA was extracted from 140 μL of plasma using the QIAmp viral RNA extraction kit (Qiagen, Hilden, Germany) following the manufacturer's instructions and was reverse transcribed using the HGV 340/625 IC PCR kit (Reverse transcription-PCR) with electrophoretic Detection (Sacace Biotechnologies, Como, Italy).
EBV DNA extraction and amplification. Viral DNA was extracted from 200 μL of plasma using the QIAmp viral DNA extraction kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The extracted DNA was used for PCR amplification using the EBV-290 PCR kit with electrophoretic Detection (Sacace Biotechnologies, Como, Italy).
Statistical analysis. Data were analyzed using EPI-Info version 6.04 dfr (CDC, Atlanta, United States of America). A chi-square test was applied to compare proportions. Odds ratio was calculated to determine risk factors associated with HGV. P-values <0.05 were considered statistically significant.
Results
We recruited 551 blood donors of whom 84.2% were men and 15.8% were women and the majority (61.9%) was between 20 and 29 of age. Five hundred forty eight subjects were first-time blood donors (99.5%), while 3 individuals were repeat donors (0.5%). Most of the blood donors belonged to blood group O (43.9%) and 92.7% were rhesus positive. The subjects were mainly recruited from urban settings (77.1%) Table 1. The overall seroprevalence of HBV, HCV, HIV and Treponema pallidum were of 12.9%, 3.3%, 2.2% and 2.7%, respectively (Table 2).
Screening for HGV in blood donors by viral RNA showed that 41 (7.4%) were positive. The prevalence of HGV infection was of 7.3% (40/548) in first time donors. HGV was principally associated with HBV (5.6%), HCV (1.0%) and syphilis (0.4%)
No co-infection of HGV and HIV was observed among the blood donors (Table 2).
As shown in Table 3, the prevalence of HGV was not related to sex (7.5 vs 6.9%) and age group. This prevalence was similar in urban compared to rural areas; but was five and seven times higher in HBV and HCV-positive blood donors compared to HBV and HCV-negative blood donors (p<0.001; p=0.004).
EBV viral DNA was found in 5.4% (30/551) of blood donors. No significant statistical association was observed between EBV and the socio-demographic characteristics. EBV prevalence was studied in HIV and syphilis positive subjects as well as negative subjects. EBV prevalence was similar in HIV seropositive (8.3%) compared to HIV seronegative (6.1%) individuals, while it was higher among blood donors of <20 years old age group compared with 20-29 age groups (p<0.001) (Table 3).
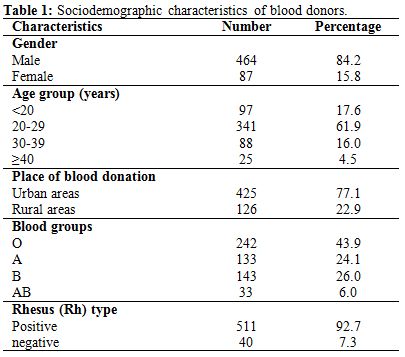
Table 1. Sociodemographic characteristics of blood donors.
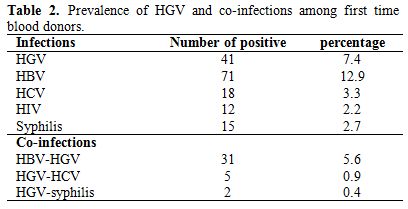
Table 2. Prevalence of HGV and co-infections among first time blood donors.
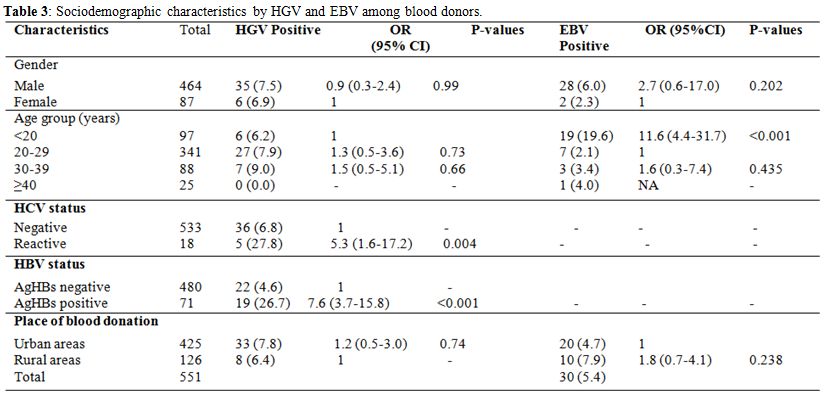
Table 3. Sociodemographic characteristics by HGV and EBV among blood donors.
Discussion
To this date, HGV prevalence is still unknown in Burkina Faso in blood donors as well as in the general population. Previous studies showed that the worldwide prevalence of HGV among blood donors is between 0.9 and 10%.[18,19]
This study documents that 7.4% of the blood donors are HGV carriers. This high prevalence of HGV is in accordance with previous studies which reported that HGV was 10 times higher in Africa than anywhere else.[8] Different HGV prevalences have been reported in Africa and elsewhere. In healthy Kuwaitian, Jordanian and Tunisian blood donors the HGV prevalence of 24.6; 9.8% and 5.3%, have been reported, respectively.[20,21] In central Africa, HGV prevalence of 10.3% and 12.6% was also found among pregnant women.[22,23]
The presence of HGV viral RNA was significantly higher in blood donors who had hepatitis B surface antigens (HBsAg) and were positive to anti-HCV compared to blood donors who were negative for HBsAg and anti-HCV. In fact, it is shown that HCV and HGV risk factors are very similar.[24] A study showed that the frequency of anti-HCV antibodies was higher among HGV positive compared with HGV negative subjects.[25] HBsAg and anti-HCV positive individuals are not eligible for blood donation. In this study, infection by HGV was not associated with age, either with the place of donation or sex. The high prevalence of HGV in blood donors who are HBsAg positive in Burkina Faso confirms that the infection is higher in patients at risk such as dialysis patients.[26-28]
We report for the first time the prevalence of EBV in blood donors in Burkina Faso, The prevalence of 5.4% found in a our study is less than the prevalence of 20% reported by Adjei et al.[13] in blood donors in Ghana. This difference could be due to the type of diagnostic used in these two studies: viral DNA screening and anti-EBV serology. A correlation between EBV and HIV-1 infection was also found in the same study. Here, we report a lack of correlation between EBV and HIV-1 infection. This could be explained by the low number of HIV-1 positive subjects studied.
EBV prevalence was higher in the age group <20 years. This is in accordance with previous studies which have shown early EBV infections among young children in Africa.[17,29] However, EBV prevalence was higher in blood donors from rural areas (9.4%) compared to those from urban areas (5.2%) but the difference observed is not statistically significant.
The high prevalence of EBV in blood donors questions the transmission of this virus through blood transfusion not only in immunodepressed patients, but also in patients who are carriers of Plasmodium falciparum. In immunocompromised subjects, the possible reactivation of the virus causes a higher risk of Burkitt lymphoma.[30] In subjects carriers of Plasmodium falciparum there is a loss of viral control when the anti-EBV immunity is altered.[31]
This study found a high prevalence of infection with blood-borne pathogen in the blood donors’ population. It is necessary to conduct an epidemiologic investigation to evaluate the residual risk in recipients. Other, the multiplex PCR could be an alternative to reduce the high cost of using molecular techniques inpoor countries such as Burkina Faso.
Conclusion
This study reports for the first time HGV and EBV prevalence in Burkina Faso among blood donors. Further studies will help to define their impact on infected humans.
Acknowledgments. We would like to thank all the participants of this study, The National blood transfusion centre staff, the Italian Episcopal Conference (C.E.I.) and Union Economique et Monétaire Ouest Africaine (UEMOA) for their support. Also Prof. Andres Reyes from Bunker Hill Community College, MA, USA for critically reading the manuscript.
Despite the remarkable progress in detecting transfusion-transmissible infectious pathogens, blood transfusion still remains a significant mode of transmission of infectious agents. Given the wide variety of blood-borne agents, there is an urgent need to determine their epidemiology.
Burkina Faso suffers from a shortage of blood products, with merely 3.1 units per 1,000 habitants as opposed to 10 units per 1,000 inhabitants required,[1] due to malaria, malnutrition, emergency surgery, childbirth and traffic accidents.[2-4]
The main infectious diseases detected among blood donors are human immunodeficiency virus (HIV), along with Hepatitis B and C viruses (HBV and HCV). The screening of viruses such as Hepatitis G (HGV), Epstein-Barr virus (EBV), Cytomegalovirus (CMV) and Human Lymphotropic T Cell Virus (HLTV) remains unaddressed.
In a recent report GBV-C (HGV), GBV-A and GBV-D have been classified in a new genus Pegivirus within the Family Flaviviridae.[5] The clinical role of HGV is not entirely clarified yet. HGV is not associated with any hepatic morphological modifications.[6] However, a recent study showed an association between HGV infection with aplastic anemia and extrahepatic diseases.[7]
The prevalence of HGV in the world varies from 2% in the United States of America to 12.2% in Brazil and 18% in Egypt and South Africa, respectively.[8] The virus is mainly transmitted via infectious blood and parenterally,[9,10] while sexual transmission lacks significant evidence.[8]
Another neglected virus not diagnosed among blood donors in Burkina Faso is EBV. In immunocompetent individuals EBV is in a latent phase; and infected people show no clinical symptoms. Indeed, 90% of adults are EBV asymptomatic carriers. In immunosuppressed individuals the virus is reactivated and becomes oncogenic.[11] EBV is etiologically associated with Burkitt's lymphoma (a B cell-derived tumor), nasopharyngeal carcinoma,[12] Hodgkin’s lymphoma[13] and breast cancer.[14-16] However, its mode of transmission remains controversial.
In Africa, early infections with EBV have been described in children, while in developed countries the primary infection is delayed until adulthood.[17]
In Burkina Faso, the prevalence of HGV and EBV are unknown to date. This study aims to determine the prevalence of HGV and EBV among blood donors in Burkina Faso and examine sociodemographic factors associated with both viral infections.
Materials and Methods
Blood donors. Blood samples were collected using standard procedures from voluntary non remunerated blood donors (VNRBD) at the Regional Blood Transfusion Centre of Ouagadougou (RBTC) between February and September 2012. VNRBD were all healthy subjects, selected after responding to a panel of questions comprising a medical history. Healthy individuals aged 17-65 years and over 50 kg were eligible for blood donation.
All samples were screened for hepatitis B virus surface antigen (HBsAg), antibodies to HIV, Treponema pallidum and HCV. Samples were kept at −20 °C for further analysis.
This study was approved by the CERBA/Saint Camille Ethics Committee. The consent of all study subjects was obtained before blood collection.
Serological analysis. HBsAg and antibodies to HCV and HIV were detected using a fourth generation ELISA (ARCHITECT-i1000SR-ABBOTT, Santa Clara, California, United States of America).
All reactive samples for HBsAg, HCV and HIV were re-tested using a second ELISA (Bio-Rad, Marnes la Coquette, France). A result was considered positive if both the first and second tests were positive. Antibodies to Treponema pallidum were detected using a rapid plasma reagin (RPR) test (Cypress Diagnostics, Langdorp, Belgium) and confirmed with a Treponema pallidum haemagglutination (TPHA) test (Cypress Diagnostics).
Hepatitis G virus RNA extraction and reverse transcription. Viral RNA was extracted from 140 μL of plasma using the QIAmp viral RNA extraction kit (Qiagen, Hilden, Germany) following the manufacturer's instructions and was reverse transcribed using the HGV 340/625 IC PCR kit (Reverse transcription-PCR) with electrophoretic Detection (Sacace Biotechnologies, Como, Italy).
EBV DNA extraction and amplification. Viral DNA was extracted from 200 μL of plasma using the QIAmp viral DNA extraction kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The extracted DNA was used for PCR amplification using the EBV-290 PCR kit with electrophoretic Detection (Sacace Biotechnologies, Como, Italy).
Statistical analysis. Data were analyzed using EPI-Info version 6.04 dfr (CDC, Atlanta, United States of America). A chi-square test was applied to compare proportions. Odds ratio was calculated to determine risk factors associated with HGV. P-values <0.05 were considered statistically significant.
Results
We recruited 551 blood donors of whom 84.2% were men and 15.8% were women and the majority (61.9%) was between 20 and 29 of age. Five hundred forty eight subjects were first-time blood donors (99.5%), while 3 individuals were repeat donors (0.5%). Most of the blood donors belonged to blood group O (43.9%) and 92.7% were rhesus positive. The subjects were mainly recruited from urban settings (77.1%) Table 1. The overall seroprevalence of HBV, HCV, HIV and Treponema pallidum were of 12.9%, 3.3%, 2.2% and 2.7%, respectively (Table 2).
Screening for HGV in blood donors by viral RNA showed that 41 (7.4%) were positive. The prevalence of HGV infection was of 7.3% (40/548) in first time donors. HGV was principally associated with HBV (5.6%), HCV (1.0%) and syphilis (0.4%)
No co-infection of HGV and HIV was observed among the blood donors (Table 2).
As shown in Table 3, the prevalence of HGV was not related to sex (7.5 vs 6.9%) and age group. This prevalence was similar in urban compared to rural areas; but was five and seven times higher in HBV and HCV-positive blood donors compared to HBV and HCV-negative blood donors (p<0.001; p=0.004).
EBV viral DNA was found in 5.4% (30/551) of blood donors. No significant statistical association was observed between EBV and the socio-demographic characteristics. EBV prevalence was studied in HIV and syphilis positive subjects as well as negative subjects. EBV prevalence was similar in HIV seropositive (8.3%) compared to HIV seronegative (6.1%) individuals, while it was higher among blood donors of <20 years old age group compared with 20-29 age groups (p<0.001) (Table 3).

Table 1. Sociodemographic characteristics of blood donors.

Table 2. Prevalence of HGV and co-infections among first time blood donors.

Table 3. Sociodemographic characteristics by HGV and EBV among blood donors.
Discussion
To this date, HGV prevalence is still unknown in Burkina Faso in blood donors as well as in the general population. Previous studies showed that the worldwide prevalence of HGV among blood donors is between 0.9 and 10%.[18,19]
This study documents that 7.4% of the blood donors are HGV carriers. This high prevalence of HGV is in accordance with previous studies which reported that HGV was 10 times higher in Africa than anywhere else.[8] Different HGV prevalences have been reported in Africa and elsewhere. In healthy Kuwaitian, Jordanian and Tunisian blood donors the HGV prevalence of 24.6; 9.8% and 5.3%, have been reported, respectively.[20,21] In central Africa, HGV prevalence of 10.3% and 12.6% was also found among pregnant women.[22,23]
The presence of HGV viral RNA was significantly higher in blood donors who had hepatitis B surface antigens (HBsAg) and were positive to anti-HCV compared to blood donors who were negative for HBsAg and anti-HCV. In fact, it is shown that HCV and HGV risk factors are very similar.[24] A study showed that the frequency of anti-HCV antibodies was higher among HGV positive compared with HGV negative subjects.[25] HBsAg and anti-HCV positive individuals are not eligible for blood donation. In this study, infection by HGV was not associated with age, either with the place of donation or sex. The high prevalence of HGV in blood donors who are HBsAg positive in Burkina Faso confirms that the infection is higher in patients at risk such as dialysis patients.[26-28]
We report for the first time the prevalence of EBV in blood donors in Burkina Faso, The prevalence of 5.4% found in a our study is less than the prevalence of 20% reported by Adjei et al.[13] in blood donors in Ghana. This difference could be due to the type of diagnostic used in these two studies: viral DNA screening and anti-EBV serology. A correlation between EBV and HIV-1 infection was also found in the same study. Here, we report a lack of correlation between EBV and HIV-1 infection. This could be explained by the low number of HIV-1 positive subjects studied.
EBV prevalence was higher in the age group <20 years. This is in accordance with previous studies which have shown early EBV infections among young children in Africa.[17,29] However, EBV prevalence was higher in blood donors from rural areas (9.4%) compared to those from urban areas (5.2%) but the difference observed is not statistically significant.
The high prevalence of EBV in blood donors questions the transmission of this virus through blood transfusion not only in immunodepressed patients, but also in patients who are carriers of Plasmodium falciparum. In immunocompromised subjects, the possible reactivation of the virus causes a higher risk of Burkitt lymphoma.[30] In subjects carriers of Plasmodium falciparum there is a loss of viral control when the anti-EBV immunity is altered.[31]
This study found a high prevalence of infection with blood-borne pathogen in the blood donors’ population. It is necessary to conduct an epidemiologic investigation to evaluate the residual risk in recipients. Other, the multiplex PCR could be an alternative to reduce the high cost of using molecular techniques inpoor countries such as Burkina Faso.
Conclusion
This study reports for the first time HGV and EBV prevalence in Burkina Faso among blood donors. Further studies will help to define their impact on infected humans.
Acknowledgments. We would like to thank all the participants of this study, The National blood transfusion centre staff, the Italian Episcopal Conference (C.E.I.) and Union Economique et Monétaire Ouest Africaine (UEMOA) for their support. Also Prof. Andres Reyes from Bunker Hill Community College, MA, USA for critically reading the manuscript.
References
- Dahourou H, Tapko JB, Kienou K, Nebie K,
Sanou M. Recruitment of blood donors in Burkina Faso: how to avoid
donations from family members? Biologicals. 2010; 38(1): 39-42.
http://dx.doi.org/10.1016/j.biologicals.2009.10.017 PMid:20144550

- Lund TC, Hume H, Allain JP, McCullough J,
Dzik W. The blood supply in Sub-Saharan Africa: Needs, challenges, and
solutions. Transfus Apher Sci. 2013 Jul 17.

- Matsuyama T, Iranami H, Fujii K, Inoue M,
Nakagawa R, Kawashima K. Risk factors for postoperative mortality and
morbidities in emergency surgeries. J Anesth. 2013 May 23.
http://dx.doi.org/10.1007/s00540-013-1639-z

- Krstic SN, Alempijevic T, Popovic N,
Jovanovic D, Mihailovic V, Sijacki A. [Blood replacement in severely
injured patients]. Acta Chir Iugosl. 2010;57(1):107-13.
http://dx.doi.org/10.2298/ACI1001110K PMid:20681210

- Stapleton JT, Foung S, Muerhoff AS, Bukh J,
Simmonds P. The GB viruses: a review and proposed classification of
GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family
Flaviviridae. J Gen Virol. 2011; 92(Pt 2): 233-46.
http://dx.doi.org/10.1099/vir.0.027490-0 PMid:21084497 PMCid:PMC3081076

- Loginov AS, Sharafanova TI, Reshetniak VI,
Il'chenko L, Shepeleva SD, Serova TI, Tkachev VD. [HGV and TTV - new
hepatitis viruses]. Ter Arkh. 2000; 72(11): 9-13. PMid:11270960

- Rauff B, Idrees M, Shah SA, Butt S, Butt
AM, Ali L, Hussain A, Irshad Ur R, Ali M. Hepatitis associated aplastic
anemia: a review. Virol J. 2011; 8(87). PMid:21352606 PMCid:PMC3052191

- Sathar M, Soni P, York D. GB virus
C/hepatitis G virus (GBV-C/HGV): still looking for a disease. Int J Exp
Pathol. 2000; 81(5): 305-22.
http://dx.doi.org/10.1046/j.1365-2613.2000.00166.x PMid:11168678
PMCid:PMC2517736

- Ohto H, Ujiie N, Sato A, Okamoto H, Mayumi
M. Mother-to-infant transmission of GB virus type C/HGV. Transfusion.
2000; 40(6): 725-30.
http://dx.doi.org/10.1046/j.1537-2995.2000.40060725.x
PMid:10864996

- Lefrere JJ, Sender A, Mercier B, Mariotti
M, Pernot F, Soulie JC, Malvoisin A, Berry M, Gabai A, Lattes F, Galiay
JC, Pawlak C, de Lachaux V, Chauveau V, Hreiche G, Larsen M, Ferec C,
Parnet-Mathieu F, Roudot-Thoraval F, Brossard Y. High rate of GB virus
type C/HGV transmission from mother to infant: possible implications
for the prevalence of infection in blood donors. Transfusion. 2000;
40(5): 602-7. http://dx.doi.org/10.1046/j.1537-2995.2000.40050602.x
PMid:10827267

- Sarmati L. HHV-8 infection in African children. Herpes. 2004; 11(2): 50-3. PMid:15955270

- Perret C. Methylation profile as a new tool
for classification of hepatocellular carcinoma. J Hepatol. 2011; 54(4):
602-3. http://dx.doi.org/10.1016/j.jhep.2010.10.015 PMid:21145852

- Adjei AA, Armah HB, Gbagbo F, Boamah I,
Adu-Gyamfi C, Asare I. Seroprevalence of HHV-8, CMV, and EBV among the
general population in Ghana, West Africa. BMC Infect Dis. 2008 8(111).

- Bonnet M, Guinebretiere JM, Kremmer E,
Grunewald V, Benhamou E, Contesso G, Joab I. Detection of Epstein-Barr
virus in invasive breast cancers. J Natl Cancer Inst. 1999; 91(16):
1376-81. http://dx.doi.org/10.1093/jnci/91.16.1376 PMid:10451442

- Magrath I, Bhatia K. Breast cancer: a new
Epstein-Barr virus-associated disease? J Natl Cancer Inst. 1999:
91(16): 1349-50. http://dx.doi.org/10.1093/jnci/91.16.1349 PMid:10451431

- Yasui Y, Potter JD, Stanford JL, Rossing
MA, Winget MD, Bronner M, Daling J. Breast cancer risk and "delayed"
primary Epstein-Barr virus infection. Cancer Epidemiol Biomarkers Prev.
2001; 10(1): 9-16. PMid:11205495

- Biggar RJ, Henle W, Fleisher G, Bocker J,
Lennette ET, Henle G. Primary Epstein-Barr virus infections in African
infants. I. Decline of maternal antibodies and time of infection. Int J
Cancer. 1978; 22(3): 239-43. http://dx.doi.org/10.1002/ijc.2910220304
PMid:212369

- Masuko K, Mitsui T, Iwano K, Yamazaki C,
Okuda K, Meguro T, Murayama N, Inoue T, Tsuda F, Okamoto H, Miyakawa Y,
Mayumi M. Infection with hepatitis GB virus C in patients on
maintenance hemodialysis. N Engl J Med. 1996; 334(23): 1485-90.
http://dx.doi.org/10.1056/NEJM199606063342301 PMid:8618602

- Bassit L, Kleter B, Ribeiro-dos-Santos G,
Maertens G, Sabino E, Chamone D, Quint W, Saez-Alquezar A. Hepatitis G
virus: prevalence and sequence analysis in blood donors of Sao Paulo,
Brazil. Vox Sang. 1998; 74(2): 83-7.
http://dx.doi.org/10.1159/000030910 PMid:9501405

- Odeh RA, Yasin S, Nasrallah G, Babi Y.
Rates of infection and phylogenetic analysis of GB virus-C among
Kuwaiti and Jordanian blood donors. Intervirology. 2010;53(6):402-7.
http://dx.doi.org/10.1159/000317290 PMid:20606462

- Mastouri M, Safer IL, Pozzetto B, Bourlet
T, Khedher M. [Prevalence of hepatitis G virus among Tunisian blood
donors]. East Mediterr Health J. 2005 Sep-Nov;11(5-6):1053-60.
PMid:16761677

- Tuveri R, Perret JL, Delaporte E,
Nguemby-Mbina C, D'Allones LR, Henzel D, et al. Prevalence and genetic
variants of hepatitis GB-C/HG and TT viruses in Gabon, equatorial
Africa. Am J Trop Med Hyg. 2000 Sep-Oct;63(3-4):192-8. PMid:11388514

- Liu HF, Muyembe-Tamfum JJ, Dahan K,
Desmyter J, Goubau P. High prevalence of GB virus C/hepatitis G virus
in Kinshasa, Democratic Republic of Congo: a phylogenetic analysis. J
Med Virol. 2000 Feb;60(2):159-65.
http://dx.doi.org/10.1002/(SICI)1096-9071(200002)60:2<159::AID-JMV9>3.0.CO;2-V

- Carman WF. Infections associated with
medical intervention: hepatitis viruses and HGV. Br Med Bull. 1998;
54(3): 731-48. http://dx.doi.org/10.1093/oxfordjournals.bmb.a011723
PMid:10326297

- Fabrizi F, Lunghi G, Bacchini G, Corti M,
Guarnori I, Raffaele L, Erba G, Pagano A, Locatelli F. Hepatitis G
virus infection in chronic dialysis patients and kidney transplant
recipients. Nephrol Dial Transplant. 1997; 12(8): 1645-51.
http://dx.doi.org/10.1093/ndt/12.8.1645 PMid:9269643

- Hammad AM, Zaghloul MH. Hepatitis G virus
infection in Egyptian children with chronic renal failure (single
centre study). Ann Clin Microbiol Antimicrob. 2009; 8(36).
PMid:20015406 PMCid:PMC2804678

- Hinrichsen H, Leimenstoll G, Stegen G,
Schrader H, Folsch UR, Schmidt WE. Prevalence of and risk factors for
hepatitis G (HGV) infection in haemodialysis patients: a multicentre
study. Nephrol Dial Transplant. 2002; 17(2): 271-5.
http://dx.doi.org/10.1093/ndt/17.2.271 PMid:11812878

- Eslamifar A, Hamkar R, Ramezani A, Ahmadi
F, Gachkar L, Jalilvand S, Adibi L, Atabak S, Khameneh A, Ghadimi R,
Aghakhani A. Hepatitis G virus exposure in dialysis patients. Int Urol
Nephrol. 2007; 39(4): 1257-63.
http://dx.doi.org/10.1007/s11255-007-9267-x PMid:17786579

- Minhas V, Brayfield BP, Crabtree KL,
Kankasa C, Mitchell CD, Wood C. Primary gamma-herpesviral infection in
Zambian children. BMC Infect Dis. 2010; 10(115). PMid:20462453
PMCid:PMC2881090

- Gasser O, Bihl F, Sanghavi S, Rinaldo C,
Rowe D, Hess C, Stablein D, Roland M, Stock P, Brander C.
Treatment-dependent loss of polyfunctional CD8+ T-cell responses in
HIV-infected kidney transplant recipients is associated with
herpesvirus reactivation. Am J Transplant. 2009; 9(4): 794-803.
http://dx.doi.org/10.1111/j.1600-6143.2008.02539.x PMid:19298451
PMCid:PMC2746278

- Whittle HC, Brown J, Marsh K, Greenwood BM,
Seidelin P, Tighe H, Wedderburn L. T-cell control of Epstein-Barr
virus-infected B cells is lost during P. falciparum malaria. Nature.
1984; 312(5993): 449-50. http://dx.doi.org/10.1038/312449a0
PMid:6095104
