Monocyte Adhesion Molecules Expression in Patients with Chronic Hepatitis C Liver Disease
Nora E.I. El-Bassiouni1, Ola M. Mahmoud1*, Eman G El Ahwani2, Raafat A. Ibrahim3 and Azza E.I. El Bassiouny2
1 Department of Haematology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
2 Department of Immunology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
3 Department of Hepato- Gastroenterology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
2 Department of Immunology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
3 Department of Hepato- Gastroenterology, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
Correspondence
to:
Ola M. Mahmoud. Departments of Haematology, Theodor Bilharz Research
Institute, Imbaba, Giza, Egypt. Tel: 00202/24723116, Fax: 00202/
35408125. E-mail: mahmoudola5@yahoo.com
Published: September 2, 2013
Received: April 21, 2013
Accepted: August 2, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013054, DOI 10.4084/MJHID.2013.054
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Background:
Chronic viral hepatitis is histologically characterized by
predominantly periportal infiltration of mononuclear cells, including
lymphocytes and monocytes/macrophages. Intralobular infiltration of
these inflammatory cells is an ominous sign of deterioration and a
criterion for disease activity.
Objective: To assess the monocyte inflammatory milieu, monocytes adhesion molecules, their endothelial receptors, cytokines and chemokines in patients with HCV induced chronic liver disease, in an attempt to clarify the role of blood monocytes in induction of inflammation and fibrogenesis in chronic hepatitis C liver disease.
Subjects and Methods: The current study included 60 patients with chronic liver disease categorized into 2groups: Patients chronic hepatitis C (CHC) and patients with liver cirrhosis (LC), 15 patients each; 15 healthy subjects were included as normal controls. Immunophenotype characterization was carried out by flowcytometric analysis for identification of CD11a, CD11b and CD49d monocyte surface antigen expression in different groups studied. The circulating levels of the soluble adhesion molecules (sE-selectin, sICAM-1 and sVCAM-1), cytokines (TNF-α and IL-1) and chemokines (MCP-1) were also assessed by immunoassays.
Results: Data demonstrated a significant increase (p<0.01) in the surface expression of CD11a on peripheral blood monocytes and in the circulating levels sE-selectins, sICAM-1, sVCAM-1 and TNF-α in both groups of patients compared to healthy subjects. Data also revealed a significant increase (p<0.01) in the surface expression of each of CD11b and CD49d on peripheral blood monocytes and in the circulating levels sICAM-1, sVCAM-1 and TNF-α in patients with LC compared to those with CHC. Moreover, data demonstrated that the increase in surface antigen expression of each CD11a (p<0.01), CD11b (p<0.05) and CD49d (p<0.01) on circulating peripheral blood monocytes is positively correlated with the increase in the circulating levels of each of sICAM-1 and sVCAM-1 in the both groups of patients.
Conclusions: These findings suggest that the modulation of monocyte-subset recruitment into the liver via adhesion molecules or cytokines/cytokine receptors may represent promising approaches for therapeutic interventions in human liver fibrosis. Measurement of serum soluble adhesion molecules may be useful for monitoring progression of liver inflammation and fibrosis during CHC.
Objective: To assess the monocyte inflammatory milieu, monocytes adhesion molecules, their endothelial receptors, cytokines and chemokines in patients with HCV induced chronic liver disease, in an attempt to clarify the role of blood monocytes in induction of inflammation and fibrogenesis in chronic hepatitis C liver disease.
Subjects and Methods: The current study included 60 patients with chronic liver disease categorized into 2groups: Patients chronic hepatitis C (CHC) and patients with liver cirrhosis (LC), 15 patients each; 15 healthy subjects were included as normal controls. Immunophenotype characterization was carried out by flowcytometric analysis for identification of CD11a, CD11b and CD49d monocyte surface antigen expression in different groups studied. The circulating levels of the soluble adhesion molecules (sE-selectin, sICAM-1 and sVCAM-1), cytokines (TNF-α and IL-1) and chemokines (MCP-1) were also assessed by immunoassays.
Results: Data demonstrated a significant increase (p<0.01) in the surface expression of CD11a on peripheral blood monocytes and in the circulating levels sE-selectins, sICAM-1, sVCAM-1 and TNF-α in both groups of patients compared to healthy subjects. Data also revealed a significant increase (p<0.01) in the surface expression of each of CD11b and CD49d on peripheral blood monocytes and in the circulating levels sICAM-1, sVCAM-1 and TNF-α in patients with LC compared to those with CHC. Moreover, data demonstrated that the increase in surface antigen expression of each CD11a (p<0.01), CD11b (p<0.05) and CD49d (p<0.01) on circulating peripheral blood monocytes is positively correlated with the increase in the circulating levels of each of sICAM-1 and sVCAM-1 in the both groups of patients.
Conclusions: These findings suggest that the modulation of monocyte-subset recruitment into the liver via adhesion molecules or cytokines/cytokine receptors may represent promising approaches for therapeutic interventions in human liver fibrosis. Measurement of serum soluble adhesion molecules may be useful for monitoring progression of liver inflammation and fibrosis during CHC.
Introduction
Hepatitis C virus (HCV) is considered the most common causes of chronic hepatitis in Egypt. Liver fibrosis is the scarring process that represents the liver’s response to injury. Over time this process can result in cirrhosis of the liver, which once have developed the serious complications of liver disease may occur including portal hypertension, liver failure and liver cancer. Cirrhosis and liver cancer are now among the top ten causes of death worldwide, and in many developed countries liver disease is now one of the top five causes of death in middle-age.[1]
Liver damage in chronic hepatitis C (CHC) is commonly attributed to immune-mediated mechanisms.[2-3] Hepatic fibrosis is characterized by abnormal excessive accumulation of extracellular matrix accompanied by exaggerated cytokine release. Recruitment and trans-differentiation of peripheral blood cells, in particular, monocytes into injured liver[4] may play a role in this respect. Chronic viral hepatitis is histologically characterized by predominantly periportal infiltration of mononuclear cells, including lymphocytes and monocytes/macrophages. Intralobular infiltration of these inflammatory cells is an ominous sign of deterioration and a criterion for disease activity.[5]
Monocytes circulate as nonadherent cells in the blood and migrate as adherent cells through tissues depending on the ordered expression of specific cell surface adhesion molecule (AM) on a variety of cells.[6] Monocyte adhesion to intact or damaged vascular endothelium requires several families of AM, in particular, the selectin family for the initial contact of monocytes with the endothelium[7] and the integrin receptors for the subsequent firm adhesion and transmigration.[8] On monocyte, these include leukocyte function antigen-1 (LFA-1 or CD11a) and Mac-1 (CD11b) and their endothelial ligand intercellular AM (ICAM-1 or CD45). In addition, a very late antigen-4 (VLA-4 or CD49d) is a monocyte ligand that promotes monocyte adherence to endothelial vascular cell adhesion molecule-1 (VCAM-1) and to connective tissue components.[6] ICAM-1 and VCAM-1 are strongly expressed on sinusoidal lining cells in chronic hepatic inflammation due to HCV infection and play a key role in leukocyte recruitment and extravasation.[9] Moreover, it was found that HCV-infected hepatocytes but not normal hepatocytes express ICAM-1.[10]
Cytokines and chemoattractants direct leukocyte trafficking and positioning within tissues, bring flexibility and specificity to recruitment,[11] initiate and regulate the margination and extravasation of monocytes. Tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) induce leukocyte infiltration by up-regulating the expression of leukocyte AM on vascular endothelial cells.[12] Monocyte chemoattractant protein-1 (MCP-1) plays an important role in the recruitment of monocytes and macrophages from the bloodstream to inflamed tissue. It is also implicated in the pathogenesis of a variety of diseases that are characterized by mononuclear cell infiltration and maintenance of the inflammatory infiltrate.[13]
We aim to analyze the monocyte inflammatory milieu, monocytes adhesion molecules, their endothelial receptors, cytokines and chemokines in patients with HCV induced chronic liver disease, in an attempt to clarify the role of blood monocytes in induction of inflammation and fibrogenesis in chronic hepatitis C liver disease.
Subjects and Methods
Patients: Seventy-five individuals, of whom sixty patients with HCV induced chronic liver disease admitted to the Hepato-Gastroenterology Department, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt and 15 age- and sex-matched healthy adults were enrolled in this study. Patients have M/F ratio of 38/22 and mean age of 45.6 ± 6.4 (range 22-60 years). The controls comprised 10 males and 5 females having a mean age of 42.8 ± 6.6, their liver function tests were within normal range and had no serologic evidence of hepatitis B and/or C viruses. The study was approved by the Institution's Human Research Ethics Committee.
Diagnosis and classification of patients were based on full medical history, thorough clinical examination, liver and kidneys function tests, parasitological examination, abdominal ultrasonography, procto-sigmoidoscopy and liver biopsies. None of the subjects gave history of medication with interferon and ribavirin or drugs known to have influence on the coagulation process within 8 weeks of study time or had a renal impairment based on normal creatinine clearance. Cases with schistosomiasis infection, chronic viral diseases other than HCV, nonalcoholic steatohepatitis, biliary disorders, and malignancies were excluded from the study. All studied patients had CHC infection with circulating anti-HCV antibodies and were categorized into 2 groups: patients with CHC and patients with liver cirrhosis (LC), 30 cases each.
Serological tests: Hepatitis B markers, including hepatitis B surface antigen and anti-HBs antibodies, total and IgM class antibodies against hepatitis B core antigen, hepatitis B antigen, and anti-HBe antibodies, were tested using commercially available enzyme immunoassay kits (Abbott Laboratories; North Chicago, Illinois, USA). Circulating anti-HCV antibodies were detected using Murex enzyme immunoassay kit (Murex anti-HCV, Version V; Murex Diagnostics; Dartford, England). The presence of HCV-RNA in patients' sera was detected by real-time polymerase chain reaction using the Amplicor test (Roche Diagnostic Systems; Meylan, France).
Immunological studies: Serum levels of circulating sE-selectin, sICAM-1, sVCAM-1, TNF-α, IL-1 and MCP-1 were measured using respective ELISA commercial kit (R&D Systems Inc, Minneapolis, USA). The coefficient of variation between duplicate samples was less than 10%. Normal values were indicated for each assay by the purchasing company and were not significant key different from the values obtained from the 15 healthy subjects. The latter values were considered, therefore, as reliable control values.
Flowcytometric analysis: Detection of circulating peripheral blood monocyte surface adhesion molecule antigens expression was performed by analysis of patients venous blood samples using mouse anti-human fluorescein isothiocyanate (FITC) conjugated CD11a (LFA-1) monoclonal antibody and phycoerytrin (PE) conjugated CD11b (Mac-1) and CD49d (VLA-4) monoclonal antibodies (Becton Dickinson, Mountain view, CA, USA) by flow cytometry (Coulter, Coultronic, Margency, France). The IgG1, IgG2a and IgG2b were used as isotype controls. Five thousand gated monocytes were counted. Cells expressing receptor for CD11a, CD11b and CD49d emitted fluorescence signals which would be summated and multiplied in the PMTs and the computer would analyze the data as a signal frequency histogram.
Statistical analysis: The Statistical Package for Social Sciences (SPSS) for Windows (version 11) computer program was used for statistical analysis. Means of different groups were compared using one-way ANOVA. Comparison between percent positive cases was calculated by Chi-square test. A p value <0.05 was considered statistically significant. Pearson correlation coefficient r was used to measure the relationship between 2 variables.
Results
Progressive impairment of liver function tests, such as the increase in the activity of aminotransferases and bilirubin concentrations, were observed to increase together with the advancement of liver cirrhosis (Table 1). A decrease in albumin concentrations concomitant with an increase in prothrombin time was noted in the diseased groups compared to controls, and the alterations in these parameters paralleled the progress of the disease.
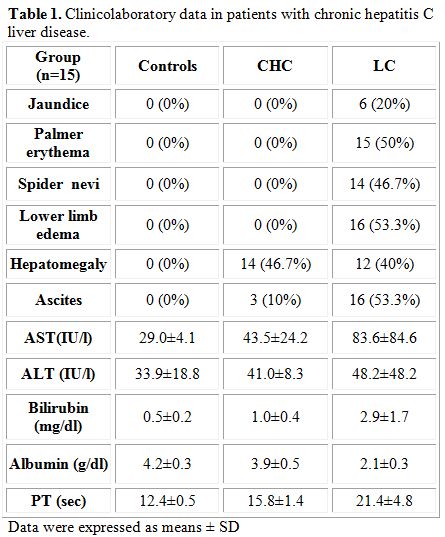
Table 1. Clinicolaboratory data in patients with chronic hepatitis C liver disease
Surface expression of monocyte adhesion molecule: Flow cytometric analysis revealed (Table 2) increased surface antigen expression of LFA-1 (CD11a) and Mac-1 (CD11b) and VLA-4 (CD49d) on circulating peripheral blood monocytes in patients with HCV which match the advancement of the disease. A significant increase in surface expression of CD11a on circulating peripheral blood monocytes was noticed in patients with CHC (p<0.05) and LC (p<0.01) compared to controls. Similarly, an increase in surface expression of CD11b (p<0.05) and CD49 (p<0.01) on circulating peripheral blood monocytes was also found in patients with LC compared to control group. Moreover, a marked increase (p<0.01) in surface expression of CD11b and CD49d on circulating peripheral blood monocytes was detected in patients with LC compared to those with CHC.
Circulating levels of soluble adhesions molecules: Data also revealed (Table 2) a statistically significant increase (p<0.01) in the levels of soluble adhesion molecules sE-selectin, sICAM-1 and sVCAM- both diseased groups compared to controls. Moreover, a statistically significant increase (p<0.01) in the levels of sICAM-1 and sVCAM was detected in patients with LC compared to those with CHC revealing that the increase in the levels of these soluble adhesion molecules parallels the disease progression.
Circulating levels of cytokines: A statistically significant increase (p<0.01) in circulating levels of TNF-α was found in both groups of patients compared to controls and in patients with LC compared to those with CHC (Table 2). Data revealed no statistically significant difference in the levels of IL-1 level in different groups studied. Although an increase in the levels of MCP-1 was found in patients with CHC and LC compared to controls and in LC group compared to patients with CHC, data were comparable.
Correlation analysis: Correlations analyses between different studied parameters were demonstrated (Table 3).
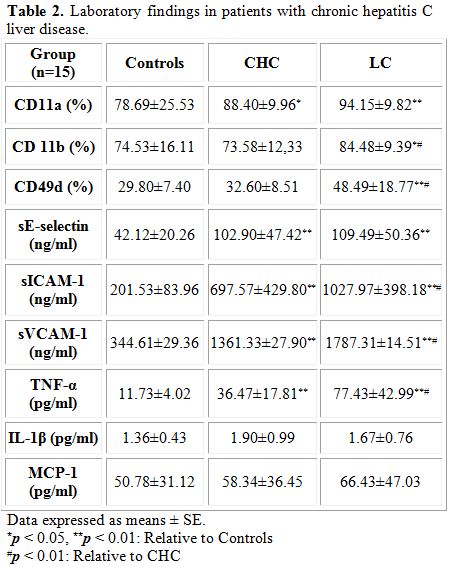
Table 2. Laboratory findings in patients with chronic hepatitis C liver disease
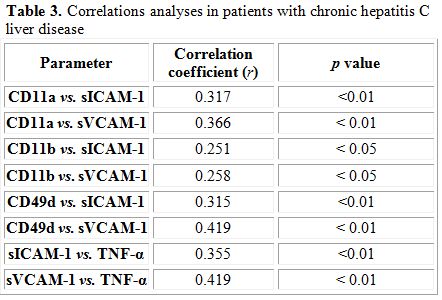
Table 3. Correlations analyses in patients with chronic hepatitis C liver disease
Discussion
The hepatic vascular bed is a unique low-flow environment through which leukocytes are recruited to the liver during homeostatic immune surveillance and in response to infection or injury. The rate of leukocyte recruitment and the nature of cells recruited through the sinusoids in response to inflammatory signals will shape the severity of disease.[11] Infiltrating immune cells were recently demonstrated to be linked to the development of liver fibrosis.[14] The macrophage compartment of the liver, traditionally called 'Kupffer cells', is constantly replenished to a significant extent by blood monocytes[15] and is greatly augmented by an overwhelming number of infiltrating monocytes upon acute or chronic liver injury.[14]
In the present work, a marked increase in the surface expression of CD11a was noticed in both groups of patients with chronic hepatitis C liver disease compared to controls. In addition, a marked increase in the surface expression of CD11b and CD49d was also noticed in cirrhotic patients compared to those with CHC. These findings indicate that the progressive liver damage and the development of LC have a significant impact on integrins by intensifying of their expression on monocyte surfaces. The increase in surface expression provides the mechanism for endothelial transmigration required for hepatic infiltration by monocytes, as documented after inflammation and imply that a cell population of higher function may potentially appear.[16,17] Those monocytes which respond to liver inflammatory environment with strongly increased adhesion properties would accumulate at the site of inflammation. Circulating monocytes are precursors of dendritic cells and macrophages of the liver.[18] Activated by a liver inflammatory environment liver resident macrophages–Kupffer’s cells constitutively show high LFA-1, Mac-1, and VLA-4 expression that enable active participation in the intensification of inflammatory and immunological processes in the liver.[17] Extensive mononuclear cell infiltration was found to be strongly correlated with liver damage in patients with chronic hepatitis B virus infection.[19] Monocytes and activated macrophages contribute to the development and progression of fibrosis via expression of numerous cytokines, such as platelet-derived growth factor and transforming growth factor-β1, or generation of reactive oxygen intermediates.[20] Participation of infiltrating monocytes in the development of liver damage and fibrosis in animal models is proved.[19]
Moreover, data revealed a marked increase in the circulating levels of soluble adhesive molecules sE-selectin, sICAM-1 and sVCAM-1 in both groups of patients with chronic hepatitis C liver disease compared to controls. Similar results are reported in patients with CLD,[21] and CHC[10,22] and the increased level was found to be correlated to the severity and progression of the disease. In addition, up-regulated ICAM-1 and VCAM-1 expression in the hepatic sinusoid cells, vascular endothelial cells, and hepatocytes were reported in chronic liver disease in several studies.[23-25] Circulating adhesion molecules is the proteolytically released form of membrane-bound activated molecule.[26] The activation of endothelial cells has a crucial role in modulation of leukocyte functions and mediating the transmigration of inflammatory cells, which induce inflammation, tissue damage and liver cirrhosis.[27,28] These studies are in accordance with our findings which show that the increased concentrations of each of sICAM-1 and sVCAM-1 in patients with CHC and LC are strongly correlated with the increased surface integrins expression on monocyte in these patients. Endothelial cell incubation with antibodies against integrin receptors inhibits monocyte adhesion.[29] The administration of anti-LFA-1 or anti-ICAM-1 antibodies diminishes significantly the stage of hepatocyte damage.[17] Endothelial soluble adhesive molecules, induced by pro-inflammatory cytokines, are the markers of the functional condition of endothelial cells and probably reflect endothelial activation.[30,31]
Our results also revealed marked elevation in circulating levels of ICAM-1 and VCAM-1 in cirrhotic patients compared to non-cirrhotic cases. Enhanced levels of these adhesion molecules may be the consequence of persistent activation of vascular endothelial cells which are able to produce connective tissue growth factor, a highly profibrogenic molecules involved in several fibrotic disorders, including those of the liver.[32] Such elevation in circulating ICAM-1 and VCAM-1 levels may be also attributed to increased levels of TNF-α which was also reported in our study. Increased peripheral blood monocytes activity and elevated expression of TNF-α which was found to be correlated with liver disease activity (Child-Pugh stage B and C) were reported.[33] TNF-α is a potent mediator of inflammation and sepsis[34] and has a pleiotropic effect on a wide variety of cells including endothelial cells.[35] Furthermore, data revealed the circulating levels of TNF-α in both groups of patients were strongly correlated with each of sICAM-1 and sVCAM-1 values in these patients. These findings indicate that TNF-α might directly induce the expression of ICAM-1 and VCAM-1 in vascular endothelial cells.[36] Moreover, correlation was found between monocyte adhesion molecules CD11a, CD11b and CD49d and each of sICAM-1 and sVCAM-1 which suggest the useful use of these soluble markers as an indicator of enhanced monocyte recruitment to the liver.
Monocyte chemoattractant protein-1 is also implicated in the process of hepatic inflammation, recruiting monocytes and lymphocytes during liver injury. MCP-1 also activates directly hepatic stellate cells, which play a major role in hepatic fibrosis. The decrease in the circulating levels of MCP-1 in patients with CLD may be attributed to its increased utilization or its temporal expression and secretion in sites of inflammations which may suggest that MCP-1 is predominantly locally produced within the liver. In chronic hepatitis, MCP-1 expression was directly correlated with the degree of inflammatory infiltrate in the portal tract, activated stellate cells and monocyte/macrophages. In active cirrhosis, MCP-1 expression was present in the portal tract, epithelial cells of regenerating bile ducts, and the active septa surrounding regenerating nodules.[37] There was a direct relationship between MCP-1 expression and monocyte infiltration after acute liver injury.[38] MCP-1 level was found to be higher in hepatic veins than in peripheral blood and occurred in severe cases of liver diseases.[39] However, our result revealed that the circulating levels of MCP-1 in controls and diseased groups were comparable.
Conclusion
Monocyte/endothelial cell interactions and immune activation are features of chronic HCV infection and suggest their functional contribution to the perpetuation of intrahepatic inflammation and liver cirrhosis. Monocytes and activated macrophages contribute to the development and progression of fibrosis via expression of numerous cytokines. The modulation of monocyte-subset recruitment into the liver via adhesion molecules or chemokines/chemokine receptors and their subsequent differentiation may represent promising approaches for therapeutic interventions in human liver fibrosis. Moreover, the differential release of sE-selectin, sICAM-1 and sVCAM-1 in HCV-induced chronic liver disease suggest that adhesion molecules may influence the nature of the inflammatory cellular infiltrate and affect disease progression. Thus, measurement of serum soluble adhesion molecules may be useful for monitoring progression or regression of liver inflammation and fibrosis during chronic HCV infection especially since ALT values may only reflect hepatocellular necrosis and follow-up liver biopsies are contraindicated.
Hepatitis C virus (HCV) is considered the most common causes of chronic hepatitis in Egypt. Liver fibrosis is the scarring process that represents the liver’s response to injury. Over time this process can result in cirrhosis of the liver, which once have developed the serious complications of liver disease may occur including portal hypertension, liver failure and liver cancer. Cirrhosis and liver cancer are now among the top ten causes of death worldwide, and in many developed countries liver disease is now one of the top five causes of death in middle-age.[1]
Liver damage in chronic hepatitis C (CHC) is commonly attributed to immune-mediated mechanisms.[2-3] Hepatic fibrosis is characterized by abnormal excessive accumulation of extracellular matrix accompanied by exaggerated cytokine release. Recruitment and trans-differentiation of peripheral blood cells, in particular, monocytes into injured liver[4] may play a role in this respect. Chronic viral hepatitis is histologically characterized by predominantly periportal infiltration of mononuclear cells, including lymphocytes and monocytes/macrophages. Intralobular infiltration of these inflammatory cells is an ominous sign of deterioration and a criterion for disease activity.[5]
Monocytes circulate as nonadherent cells in the blood and migrate as adherent cells through tissues depending on the ordered expression of specific cell surface adhesion molecule (AM) on a variety of cells.[6] Monocyte adhesion to intact or damaged vascular endothelium requires several families of AM, in particular, the selectin family for the initial contact of monocytes with the endothelium[7] and the integrin receptors for the subsequent firm adhesion and transmigration.[8] On monocyte, these include leukocyte function antigen-1 (LFA-1 or CD11a) and Mac-1 (CD11b) and their endothelial ligand intercellular AM (ICAM-1 or CD45). In addition, a very late antigen-4 (VLA-4 or CD49d) is a monocyte ligand that promotes monocyte adherence to endothelial vascular cell adhesion molecule-1 (VCAM-1) and to connective tissue components.[6] ICAM-1 and VCAM-1 are strongly expressed on sinusoidal lining cells in chronic hepatic inflammation due to HCV infection and play a key role in leukocyte recruitment and extravasation.[9] Moreover, it was found that HCV-infected hepatocytes but not normal hepatocytes express ICAM-1.[10]
Cytokines and chemoattractants direct leukocyte trafficking and positioning within tissues, bring flexibility and specificity to recruitment,[11] initiate and regulate the margination and extravasation of monocytes. Tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) induce leukocyte infiltration by up-regulating the expression of leukocyte AM on vascular endothelial cells.[12] Monocyte chemoattractant protein-1 (MCP-1) plays an important role in the recruitment of monocytes and macrophages from the bloodstream to inflamed tissue. It is also implicated in the pathogenesis of a variety of diseases that are characterized by mononuclear cell infiltration and maintenance of the inflammatory infiltrate.[13]
We aim to analyze the monocyte inflammatory milieu, monocytes adhesion molecules, their endothelial receptors, cytokines and chemokines in patients with HCV induced chronic liver disease, in an attempt to clarify the role of blood monocytes in induction of inflammation and fibrogenesis in chronic hepatitis C liver disease.
Subjects and Methods
Patients: Seventy-five individuals, of whom sixty patients with HCV induced chronic liver disease admitted to the Hepato-Gastroenterology Department, Theodor Bilharz Research Institute, Imbaba, Giza, Egypt and 15 age- and sex-matched healthy adults were enrolled in this study. Patients have M/F ratio of 38/22 and mean age of 45.6 ± 6.4 (range 22-60 years). The controls comprised 10 males and 5 females having a mean age of 42.8 ± 6.6, their liver function tests were within normal range and had no serologic evidence of hepatitis B and/or C viruses. The study was approved by the Institution's Human Research Ethics Committee.
Diagnosis and classification of patients were based on full medical history, thorough clinical examination, liver and kidneys function tests, parasitological examination, abdominal ultrasonography, procto-sigmoidoscopy and liver biopsies. None of the subjects gave history of medication with interferon and ribavirin or drugs known to have influence on the coagulation process within 8 weeks of study time or had a renal impairment based on normal creatinine clearance. Cases with schistosomiasis infection, chronic viral diseases other than HCV, nonalcoholic steatohepatitis, biliary disorders, and malignancies were excluded from the study. All studied patients had CHC infection with circulating anti-HCV antibodies and were categorized into 2 groups: patients with CHC and patients with liver cirrhosis (LC), 30 cases each.
Serological tests: Hepatitis B markers, including hepatitis B surface antigen and anti-HBs antibodies, total and IgM class antibodies against hepatitis B core antigen, hepatitis B antigen, and anti-HBe antibodies, were tested using commercially available enzyme immunoassay kits (Abbott Laboratories; North Chicago, Illinois, USA). Circulating anti-HCV antibodies were detected using Murex enzyme immunoassay kit (Murex anti-HCV, Version V; Murex Diagnostics; Dartford, England). The presence of HCV-RNA in patients' sera was detected by real-time polymerase chain reaction using the Amplicor test (Roche Diagnostic Systems; Meylan, France).
Immunological studies: Serum levels of circulating sE-selectin, sICAM-1, sVCAM-1, TNF-α, IL-1 and MCP-1 were measured using respective ELISA commercial kit (R&D Systems Inc, Minneapolis, USA). The coefficient of variation between duplicate samples was less than 10%. Normal values were indicated for each assay by the purchasing company and were not significant key different from the values obtained from the 15 healthy subjects. The latter values were considered, therefore, as reliable control values.
Flowcytometric analysis: Detection of circulating peripheral blood monocyte surface adhesion molecule antigens expression was performed by analysis of patients venous blood samples using mouse anti-human fluorescein isothiocyanate (FITC) conjugated CD11a (LFA-1) monoclonal antibody and phycoerytrin (PE) conjugated CD11b (Mac-1) and CD49d (VLA-4) monoclonal antibodies (Becton Dickinson, Mountain view, CA, USA) by flow cytometry (Coulter, Coultronic, Margency, France). The IgG1, IgG2a and IgG2b were used as isotype controls. Five thousand gated monocytes were counted. Cells expressing receptor for CD11a, CD11b and CD49d emitted fluorescence signals which would be summated and multiplied in the PMTs and the computer would analyze the data as a signal frequency histogram.
Statistical analysis: The Statistical Package for Social Sciences (SPSS) for Windows (version 11) computer program was used for statistical analysis. Means of different groups were compared using one-way ANOVA. Comparison between percent positive cases was calculated by Chi-square test. A p value <0.05 was considered statistically significant. Pearson correlation coefficient r was used to measure the relationship between 2 variables.
Results
Progressive impairment of liver function tests, such as the increase in the activity of aminotransferases and bilirubin concentrations, were observed to increase together with the advancement of liver cirrhosis (Table 1). A decrease in albumin concentrations concomitant with an increase in prothrombin time was noted in the diseased groups compared to controls, and the alterations in these parameters paralleled the progress of the disease.

Table 1. Clinicolaboratory data in patients with chronic hepatitis C liver disease
Surface expression of monocyte adhesion molecule: Flow cytometric analysis revealed (Table 2) increased surface antigen expression of LFA-1 (CD11a) and Mac-1 (CD11b) and VLA-4 (CD49d) on circulating peripheral blood monocytes in patients with HCV which match the advancement of the disease. A significant increase in surface expression of CD11a on circulating peripheral blood monocytes was noticed in patients with CHC (p<0.05) and LC (p<0.01) compared to controls. Similarly, an increase in surface expression of CD11b (p<0.05) and CD49 (p<0.01) on circulating peripheral blood monocytes was also found in patients with LC compared to control group. Moreover, a marked increase (p<0.01) in surface expression of CD11b and CD49d on circulating peripheral blood monocytes was detected in patients with LC compared to those with CHC.
Circulating levels of soluble adhesions molecules: Data also revealed (Table 2) a statistically significant increase (p<0.01) in the levels of soluble adhesion molecules sE-selectin, sICAM-1 and sVCAM- both diseased groups compared to controls. Moreover, a statistically significant increase (p<0.01) in the levels of sICAM-1 and sVCAM was detected in patients with LC compared to those with CHC revealing that the increase in the levels of these soluble adhesion molecules parallels the disease progression.
Circulating levels of cytokines: A statistically significant increase (p<0.01) in circulating levels of TNF-α was found in both groups of patients compared to controls and in patients with LC compared to those with CHC (Table 2). Data revealed no statistically significant difference in the levels of IL-1 level in different groups studied. Although an increase in the levels of MCP-1 was found in patients with CHC and LC compared to controls and in LC group compared to patients with CHC, data were comparable.
Correlation analysis: Correlations analyses between different studied parameters were demonstrated (Table 3).

Table 2. Laboratory findings in patients with chronic hepatitis C liver disease

Table 3. Correlations analyses in patients with chronic hepatitis C liver disease
Discussion
The hepatic vascular bed is a unique low-flow environment through which leukocytes are recruited to the liver during homeostatic immune surveillance and in response to infection or injury. The rate of leukocyte recruitment and the nature of cells recruited through the sinusoids in response to inflammatory signals will shape the severity of disease.[11] Infiltrating immune cells were recently demonstrated to be linked to the development of liver fibrosis.[14] The macrophage compartment of the liver, traditionally called 'Kupffer cells', is constantly replenished to a significant extent by blood monocytes[15] and is greatly augmented by an overwhelming number of infiltrating monocytes upon acute or chronic liver injury.[14]
In the present work, a marked increase in the surface expression of CD11a was noticed in both groups of patients with chronic hepatitis C liver disease compared to controls. In addition, a marked increase in the surface expression of CD11b and CD49d was also noticed in cirrhotic patients compared to those with CHC. These findings indicate that the progressive liver damage and the development of LC have a significant impact on integrins by intensifying of their expression on monocyte surfaces. The increase in surface expression provides the mechanism for endothelial transmigration required for hepatic infiltration by monocytes, as documented after inflammation and imply that a cell population of higher function may potentially appear.[16,17] Those monocytes which respond to liver inflammatory environment with strongly increased adhesion properties would accumulate at the site of inflammation. Circulating monocytes are precursors of dendritic cells and macrophages of the liver.[18] Activated by a liver inflammatory environment liver resident macrophages–Kupffer’s cells constitutively show high LFA-1, Mac-1, and VLA-4 expression that enable active participation in the intensification of inflammatory and immunological processes in the liver.[17] Extensive mononuclear cell infiltration was found to be strongly correlated with liver damage in patients with chronic hepatitis B virus infection.[19] Monocytes and activated macrophages contribute to the development and progression of fibrosis via expression of numerous cytokines, such as platelet-derived growth factor and transforming growth factor-β1, or generation of reactive oxygen intermediates.[20] Participation of infiltrating monocytes in the development of liver damage and fibrosis in animal models is proved.[19]
Moreover, data revealed a marked increase in the circulating levels of soluble adhesive molecules sE-selectin, sICAM-1 and sVCAM-1 in both groups of patients with chronic hepatitis C liver disease compared to controls. Similar results are reported in patients with CLD,[21] and CHC[10,22] and the increased level was found to be correlated to the severity and progression of the disease. In addition, up-regulated ICAM-1 and VCAM-1 expression in the hepatic sinusoid cells, vascular endothelial cells, and hepatocytes were reported in chronic liver disease in several studies.[23-25] Circulating adhesion molecules is the proteolytically released form of membrane-bound activated molecule.[26] The activation of endothelial cells has a crucial role in modulation of leukocyte functions and mediating the transmigration of inflammatory cells, which induce inflammation, tissue damage and liver cirrhosis.[27,28] These studies are in accordance with our findings which show that the increased concentrations of each of sICAM-1 and sVCAM-1 in patients with CHC and LC are strongly correlated with the increased surface integrins expression on monocyte in these patients. Endothelial cell incubation with antibodies against integrin receptors inhibits monocyte adhesion.[29] The administration of anti-LFA-1 or anti-ICAM-1 antibodies diminishes significantly the stage of hepatocyte damage.[17] Endothelial soluble adhesive molecules, induced by pro-inflammatory cytokines, are the markers of the functional condition of endothelial cells and probably reflect endothelial activation.[30,31]
Our results also revealed marked elevation in circulating levels of ICAM-1 and VCAM-1 in cirrhotic patients compared to non-cirrhotic cases. Enhanced levels of these adhesion molecules may be the consequence of persistent activation of vascular endothelial cells which are able to produce connective tissue growth factor, a highly profibrogenic molecules involved in several fibrotic disorders, including those of the liver.[32] Such elevation in circulating ICAM-1 and VCAM-1 levels may be also attributed to increased levels of TNF-α which was also reported in our study. Increased peripheral blood monocytes activity and elevated expression of TNF-α which was found to be correlated with liver disease activity (Child-Pugh stage B and C) were reported.[33] TNF-α is a potent mediator of inflammation and sepsis[34] and has a pleiotropic effect on a wide variety of cells including endothelial cells.[35] Furthermore, data revealed the circulating levels of TNF-α in both groups of patients were strongly correlated with each of sICAM-1 and sVCAM-1 values in these patients. These findings indicate that TNF-α might directly induce the expression of ICAM-1 and VCAM-1 in vascular endothelial cells.[36] Moreover, correlation was found between monocyte adhesion molecules CD11a, CD11b and CD49d and each of sICAM-1 and sVCAM-1 which suggest the useful use of these soluble markers as an indicator of enhanced monocyte recruitment to the liver.
Monocyte chemoattractant protein-1 is also implicated in the process of hepatic inflammation, recruiting monocytes and lymphocytes during liver injury. MCP-1 also activates directly hepatic stellate cells, which play a major role in hepatic fibrosis. The decrease in the circulating levels of MCP-1 in patients with CLD may be attributed to its increased utilization or its temporal expression and secretion in sites of inflammations which may suggest that MCP-1 is predominantly locally produced within the liver. In chronic hepatitis, MCP-1 expression was directly correlated with the degree of inflammatory infiltrate in the portal tract, activated stellate cells and monocyte/macrophages. In active cirrhosis, MCP-1 expression was present in the portal tract, epithelial cells of regenerating bile ducts, and the active septa surrounding regenerating nodules.[37] There was a direct relationship between MCP-1 expression and monocyte infiltration after acute liver injury.[38] MCP-1 level was found to be higher in hepatic veins than in peripheral blood and occurred in severe cases of liver diseases.[39] However, our result revealed that the circulating levels of MCP-1 in controls and diseased groups were comparable.
Conclusion
Monocyte/endothelial cell interactions and immune activation are features of chronic HCV infection and suggest their functional contribution to the perpetuation of intrahepatic inflammation and liver cirrhosis. Monocytes and activated macrophages contribute to the development and progression of fibrosis via expression of numerous cytokines. The modulation of monocyte-subset recruitment into the liver via adhesion molecules or chemokines/chemokine receptors and their subsequent differentiation may represent promising approaches for therapeutic interventions in human liver fibrosis. Moreover, the differential release of sE-selectin, sICAM-1 and sVCAM-1 in HCV-induced chronic liver disease suggest that adhesion molecules may influence the nature of the inflammatory cellular infiltrate and affect disease progression. Thus, measurement of serum soluble adhesion molecules may be useful for monitoring progression or regression of liver inflammation and fibrosis during chronic HCV infection especially since ALT values may only reflect hepatocellular necrosis and follow-up liver biopsies are contraindicated.
References
- Bosetti C, Levi F, Zatonski WA, Negri E,
LaVecchia C. Worldwide mortality from cirrhosis: An update to 2002. J
Hepatol 2007; 46: 827-839. http://dx.doi.org/10.1016/j.jhep.2007.01.025 PMid:17336419

- Boyer N, Marcellin P. Pathogenesis, diagnosis and management of hepatitis C. J Hepatol 2000; 32: 98-10l. [PubMed] http://dx.doi.org/10.1016/S0168-8278(00)80419-5

- Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007; 112: 141-55. http://dx.doi.org/10.1042/CS20060171 PMid:17199558

- Ruhnke M, Nussler AK, Ungefroren H, et al.
Human monocyte-derived neohepatocytes: a promising alternative to
primary human hepatocytes for autologous cell therapy. Transplantation
2005; 79: 1097–103. http://dx.doi.org/10.1097/01.TP.0000157362.91322.82 PMid:15880050

- Harvey EC, Post J J, Palladinetti P,
Freeman JA, Ffrench RA, Kumar RK, Marinos G and Lloyd AR. Expression of
the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C
virus infection correlates with histological severity and lobular
inflammation. J Leuk Biol 2003; 74: 360-369. [PubMed] http://dx.doi.org/10.1189/jlb.0303093 PMid:12949239

- Imhof B A, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 2004; 4: 432-444. http://dx.doi.org/10.1038/nri1375 PMid:15173832

- Hajilooi M, Alizadeh AHM, Ranjbar M,
Fallahian F, Alavian SM. E-selectin gene polymorphisms in iranian
chronic hepatitis B patients. Hepatitis Monthly 2007; 7: 211-216.

- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 2002; 33: 2115-2122.
http://dx.doi.org/10.1161/01.STR.0000021902.33129.69

- Ala A, Dhillon AP, Hodgson HJ. Role of cell
adhesion molecules in leukocyte recruitment in the liver and gut. Int J
Exp Pathol 2003; 84: 1-16. http://dx.doi.org/10.1046/j.1365-2613.2003.00235.x PMid:12694483 PMCid:PMC2517541

- El-Bassiouni NE, El Bassiouny AE, El
Sherif NH, Akl MM, Hussein AT, Omran SA. Soluble adhesion molecules in
chronic hepatitis C and cirrhosis: preferential release. J of Hepatol,
Gastroentrol and Infect Dis 1999; 6: 55-66.

- Oo YH, Shetty S, Adams DH. The Role of Chemokines in the Recruitment of Lymphocytes to the Liver. Dig Dis 2010; 28: 31-44. http://dx.doi.org/10.1159/000282062 PMid:20460888 PMCid:PMC2883834

- Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. The FASEB J 1994; 8: 504-512. PMid:8181668

- Takada Y, Hisamatsu T, Kamada N, Kitazume
MT, Honda H, Oshima Y et al. Monocyte chemoattractant protein-1
contributes to gut homeostasis and intestinal inflammation by
composition of IL-10–producing regulatory macrophage subset. J Immunol
2010; 184: 2671-2676. http://dx.doi.org/10.4049/jimmunol.0804012 PMid:20107182

- Karlmark KR, Weiskirchen R, Zimmermann HW
et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset
upon liver injury promotes hepatic fibrosis. Hepatol 2009; 50: 261-274 http://dx.doi.org/10.1002/hep.22950 PMid:19554540

- Heymann F, Trautwein C, Tacke F. Monocytes
and macrophages as cellular targets in liver fibrosis. Inflamm Allergy
Drug Targets 2009; 8: 307–318. http://dx.doi.org/10.2174/187152809789352230 PMid:19534673

- Walzog B, Gaehtgens P. Adhesion Molecules:
the path to a new understanding of acute inflammation. News in
Physiological Sciences 2000; 15: 107-113. PMid:11390891

- Panasiuk A, Zak J, Maciorkowska E,
PanasiukB, Prokopowicz D. Expression of β2-integrin on leukocytes in
liver cirrhosis. World J Gastroenterol 2006; 12: 6193-6197.
PMid:17036394

- Gordon S, Taylor PR. Monocyte and macrophage Heterogeneity. Nat Rev Immunol 2005; 5: 953- 964. http://dx.doi.org/10.1038/nri1733 PMid:16322748

- Zhang J-Y, Zou Z-S, Huang A, Zhang Z, Fu
J-L, et al. Hyper-activated pro-inflammatory CD16+ monocytes correlate
with the severity of liver injury and fibrosis in patients with chronic
hepatitis B. PLoS ONE 2011; 6: e17484. doi:
10.1371/journal.pone.0017484. http://dx.doi.org/10.1371/journal.pone.0017484

- Marra F, Annunziato F. Immunomodulation: a new approach to the therapy of cirrhosis? Gut 2010; 59: 868-869. http://dx.doi.org/10.1136/gut.2009.203109 PMid:20581231

- Girón-González JA, Rodriguez-Ramos C,
Elvira J et al. Serial analysis of serum and ascitic fluid levels of
soluble adhesion molecules and chemokines in patients with spontaneous
bacterial peritonitis. Clin Exp Immunol 2001; 123: 56-64. http://dx.doi.org/10.1046/j.1365-2249.2001.01414.x PMid:11167998 PMCid:PMC1905962

- Kaplaniski G, Farnarier C, Payan MJ,
Bongrang P, Durand JM. Increased levels of soluble adhesion molecules
in the serum of patients with hepatitis C. Correlation with cytokine
concentrations and liver inflammation and fibrosis. Digest Dis Sci
1997; 42: 2277-2284. http://dx.doi.org/10.1023/A:1018818801824

- Panasiuk A, Prokopowicz D, Panasiuk B.
Monocyte chemotactic protein-1 and soluble adhesion molecules as
possible prognostic markers of the efficacy of antiviral treatment in
chronic hepatitis C. World J Gastroenterol 2004; 10: 3639–3642.
PMid:15534921

- Yokomori H, Oda M, Ogi M, et al.
Expression of adhesion molecules on mature cholangiocytes in canal of
Hering and bile ductules in wedge biopsy samples of primary biliary
cirrhosis. World J Gastroenterol 2005; 11: 4382–4389.
PMid:16038038

- Peng Lv, Shelley Chireyath Paul, Yanjv
Xiao, Shiquan Liu, Hesheng Luo. Effects of thalidomide on the
expression of adhesion molecules in rat liver cirrhosis. Mediators
Inflamm 2006; 2006: 93253. http://dx.doi.org/10.1155/MI/2006/93253 PMid:17047296 PMCid:PMC1618940

- Nikolaos V. Sipsas NV, Sfikakis PP.
Expanding role of circulating adhesion molecules in assessing prognosis
and treatment response in human immunodeficiency virus infection. Clin
Diagn Lab Immunol 2004; 11: 996–1001.

- Bruno CM, Sciacca C, Cilio D, et al.
Circulating adhesion molecules in patients with virus-related chronic
diseases of the liver. World J Gastroenterol 2005; 1: 4566-4569.

- Girón-González JA, Martínez-Sierra C,
Rodriguez-Ramos C, et al. Adhesion molecules as a prognostic marker of
liver cirrhosis. Scand J Gastroenterol 2005; 40: 217–224. http://dx.doi.org/10.1080/00365520510011470 PMid:15764154

- Issekutz TB. In vivo blood monocyte
migration to acute inflammatory reactions, IL-1 alpha, TNF-alpha,
IFN-gamma, and ilizes LFA-1, Mac-1, and VLA-4. The relative importance
of each integrin. J Immunol 1995; 154: 6533-6540. PMid:7759886

- Wong J, Johnston B, Lee SS, et al. A
minimal role for selectins in the recruitment of leukocytes into the
inflamed liver microvasculature. J Clin Invest 1997; 99: 2782-2790. http://dx.doi.org/10.1172/JCI119468 PMid:9169509 PMCid:PMC508125

- El-Bassiouni NE, Hallouda MM, yehia HA,
Mahmoud OM, El-Bassiouny AE. Circulating neutrophil proinflammatory
mediators in cirrhotic patients with ascites: relevance to increased
susceptibility to infection. Kasr El Aini Medical Journal 2004; 10:
19-28.

- Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res 2003; 26: 1-9. http://dx.doi.org/10.1016/S1386-6346 (03)00115-3

- Hanck C, Glatzel M, Singer MV, Rossol S.
Gene expression of TNF-receptors in peripheral blood mononuclear cells
of patients with alcoholic cirrhosis. J Hepatol 2000; 32: 51-57. http://dx.doi.org/10.1016/S0168-8278(00)80189-0

- Beutler B, Cerami A. Cachectin: More than a tumor necrosis factor. New Eng J Med 1987; 316: 379-386. http://dx.doi.org/10.1056/NEJM198702123160705 PMid:3543677

- Philippe J, Dooijewaard G, Offner F,
Turion P, Baele G, Leroux-roels G. Granulocyte elastase, tumor necrosis
factor-α and urokinase levels as prognostic markers in severe
infection. Thromb Haemost 1992; 68: 19-23. PMid:1514167

- Chen H, Liu C, Sun S, Mei Y, Tong E.
Cytokine-induced cell surface expression of adhesion molecules in
vascular endothelial cells in vitro. J Tongji Med Univ 2001; 21: 68-71.
http://dx.doi.org/10.1007/BF02888042 PMid:11523254

- Marra F, DeFranco R, Grappone C, et al.
Increased expression of monocyte chemotactic protein-1 during active
hepatic fibrogenesis: correlation with monocyte infiltration. Am J
Pathol 1998; 152: 423–430. PMid:9466568 PMCid:PMC1857964

- Marsillach J, Camps J, Ferré N, et al.
Paraoxonase-1 is related to inflammation, fibrosis and PPAR delta in
experimental liver disease. J BMC Gastroenterol 2009; 9: 3-16. http://dx.doi.org/10.1186/1471-230X-9-3 PMid:19144177 PMCid:PMC2632645

- Fisher NC, Neil DA, Williams A, Adams DH.
Serum concentrations and peripheral secretion of the beta chemokines
monocyte chemoattractant protein 1 and macrophage inflammatory protein
1alpha in alcoholic liver disease. Gut 1999; 45: 416-420. http://dx.doi.org/10.1136/gut.45.3.416 PMid:10446112 PMCid:PMC1727646
