Bacterial Profile, Antibiotic Sensitivity and Resistance of Lower Respiratory Tract Infections in Upper Egypt
Gamal Agmy1, Sherif Mohamed1, Yaser Gad1, Esam Farghally2, Hamdy Mohammedin3 and Hebba Rashed4
1 Department of Chest Diseases, Faculty of Medicine, Assiut University, Egypt
2 Department of Chest Diseases, Faculty of Medicine, El-Minia University, Egypt
3 Department of Chest Diseases, Faculty of Medicine, Sohag University, Egypt
4 Department of Clinical Pathology, Faculty of Medicine, Assiut University, Egypt
2 Department of Chest Diseases, Faculty of Medicine, El-Minia University, Egypt
3 Department of Chest Diseases, Faculty of Medicine, Sohag University, Egypt
4 Department of Clinical Pathology, Faculty of Medicine, Assiut University, Egypt
Correspondence
to:
Sherif Mohamed, MD. Lecturer of Chest Diseases, Department of Chest
Diseases, Faculty of Medicine, Assiut University, 71516, Assiut, Egypt.
Tel: +20882413713, Fax: +208823233327. E-mail: saawm20@yahoo.com
Published: September 2, 2013
Received: May 21, 2013
Accepted: August 2, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013056, DOI 10.4084/MJHID.2013.056
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Background:
Lower respiratory tract infections (LRTI) account for a considerable
proportion of morbidity and antibiotic use. We aimed to identify the
causative bacteria, antibiotic sensitivity and resistance of
hospitalized adult patients due to LRTI in Upper Egypt. Methods: A
multicentre prospective study was performed at 3 University Hospitals
for 3 years. Samples included sputum or bronchoalveolar lavage (BAL)
for staining and culture, and serum for serology. Samples were cultured
on 3 bacteriological media (Nutrient, Chocolate ,MacConkey's agars).
Colonies were identified via MicroScan WalkAway-96. Pneumoslide IgM kit
was used for detection of atypical pathogens via indirect
immunofluorescent assay.
Results: The predominant isolates in 360 patients with CAP were S. pneumoniae (36%), C. pneumoniae (18%), and M. pneumoniae (12%). A higher sensitivity was recorded for moxifloxacin, levofloxacin, macrolides, and cefepime. A higher of resistance was recorded for doxycycline, cephalosporins, and β-lactam-β-lactamase inhibitors. The predominant isolates in 318 patients with HAP were, methicillin-resistant Staphylococcus aureus; MRSA (23%), K. pneumoniae (14%), and polymicrobial in 12%. A higher sensitivity was recorded for vancomycin, ciprofloxacin, and moxifloxacin. Very high resistance was recorded for β-lactam-β-lactamase inhibitors and cephalosporins. The predominant organisms in 376 patients with acute exacerbation of chronic obstructive pulmonary diseases (AECOPD) were H. influnzae (30%), S. pneumoniae (25%), and M. catarrhalis (18%). A higher sensitivity was recorded for moxifloxacin, macrolides and cefepime. A higher rate of resistance was recorded for aminoglycosides and cephalosporins.
Conclusions: The most predominant bacteria for CAP in Upper Egypt are S. pneumoniae and atypical organisms, while that for HAP are MRSA and Gram negative bacteria. For acute exacerbation of COPD, H. influnzae was the commonest organism. Respiratory quinolones, macrolides, and cefepime are the most efficient antibiotics in treatment of LRTI in our locality.
Results: The predominant isolates in 360 patients with CAP were S. pneumoniae (36%), C. pneumoniae (18%), and M. pneumoniae (12%). A higher sensitivity was recorded for moxifloxacin, levofloxacin, macrolides, and cefepime. A higher of resistance was recorded for doxycycline, cephalosporins, and β-lactam-β-lactamase inhibitors. The predominant isolates in 318 patients with HAP were, methicillin-resistant Staphylococcus aureus; MRSA (23%), K. pneumoniae (14%), and polymicrobial in 12%. A higher sensitivity was recorded for vancomycin, ciprofloxacin, and moxifloxacin. Very high resistance was recorded for β-lactam-β-lactamase inhibitors and cephalosporins. The predominant organisms in 376 patients with acute exacerbation of chronic obstructive pulmonary diseases (AECOPD) were H. influnzae (30%), S. pneumoniae (25%), and M. catarrhalis (18%). A higher sensitivity was recorded for moxifloxacin, macrolides and cefepime. A higher rate of resistance was recorded for aminoglycosides and cephalosporins.
Conclusions: The most predominant bacteria for CAP in Upper Egypt are S. pneumoniae and atypical organisms, while that for HAP are MRSA and Gram negative bacteria. For acute exacerbation of COPD, H. influnzae was the commonest organism. Respiratory quinolones, macrolides, and cefepime are the most efficient antibiotics in treatment of LRTI in our locality.
Introduction
Acute respiratory tract infections, such as bacterial pneumonia and acute exacerbations of chronic bronchitis, account for a considerable proportion of morbidity and antibiotic use. Moreover, these infections result in high mortality rates.[1] Unfortunately, the three major bacterial respiratory pathogens; Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae; have a worldwide increasing prevalence of antibiotic resistance.[2-4] The importance of monitoring the progress of such resistance has led to numerous international, regional and national surveillance programmes. However, results from surveillance studies show wide variations in susceptibility rates, both geographically and over time.[1,5] Prevalent flora and antimicrobial resistance pattern may vary from region to region depending upon the antibiotic pressure in that locality.[6] Thus, there is a great need for local resistance prevalence data in order to guide empirical prescription and to identify areas in which medical need for new agents is greater. Therefore, the present study was designed to identify the bacterial profile of lower respiratory tract infections (LRTIs) in Upper Egypt and to determine the antibiotic susceptibility and resistance patterns among these pathogens in our locality.
Materials and Methods
Patients: The present multicentre prospective study was performed at three University Hospitals in Upper Egypt (Assiut, El-Minia and Sohag University Hospitals) for 3 consecutive years; from July 2009 to June 2012; aiming to identify the causative bacteria, antibiotic sensitivity and antibiotic resistance in hospitalized adult patients ( > 15 years old) due to lower respiratory tract infections in Upper Egypt. The study included 360 patients with community-acquired pneumonia (CAP), 318 patient with hospital-acquired pneumonia (HAP) and 376 patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). The later patients had mild or moderate exacerbations.3 All patients were subjected to full clinical, radiological and relevant laboratory examinations. All patients were non-ICU in-patients. CAP was defined as pneumonia acquired outside the hospital setting.[7] HAP was defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission.[8] AECOPD were defined according to the GOLD guidelines.3 Indications to hospitalize patients with CAP or AECOPD were according to the relevant international guidelines for each category,[3,4] respectively. Samples collected from all patients were sent to the Department of Clinical Pathology, Faculty of Medicine, Assiut University. The study was approved by the local ethical committee of the Faculty of Medicine, Assiut University and a written informed consent was obtained from all patients for publication of this study.
Sample collection: Samples included sputum and /or bronchoalveolar lavage (BAL), for Gram stain and culture, blood samples for blood cultures and serum sample for serology. One morning spontaneously produced or induced sputum sample was obtained from the majority of patients. The valid sputum originating from the lower respiratory tract was defined as that containing squamous epithelial cell less than 10/high power field and polymorphonucleocytes more than 25/high power field. One BAL was taken from each patient under anesthesia. BAL was obtained to diagnose if there was other hidden pathology. We would not normally recommend this as a routine investigation for culture in LRTIs. For blood samples; 5to10 ml of venous blood was collected from each patient using sterile syringes. Blood samples were inoculated immediately under complete aseptic conditions into bottles containing 50 ml of brain heart infusion broth.[9]
Processing of samples: Such validated sputum as well as BAL samples were cultured on three bacteriological media (Nutrient, Chocolate and MacConkey's) agar plates. Sputa or BAL were mechanically liquefied and homogenized by vortexing for 1 minute with glass beads followed by centrifuging at 3.000 revolutions per minute for 10 minutes. Specimens were then serially diluted in sterile 0.9 % saline solutions with final concentrations of 10-4, 10-5, 10-6 and the colonies were counted as number of colonies per plate X 100 (dilution factor). The valid sputum culture defined as that had quantitative culture ≥105 CFU/ml and the valid BAL culture that had ≥104 CFU/ml.[9] Plates were incubated aerobically at 37°C with 5% CO2 for 24-48 hours. For blood samples; the blood culture bottles were incubated aerobically at 37°C for 7 days. The bottles were examined daily for evidence of bacterial growth as haemolysis, gas production or turbidity above the red cell line. Subcultures using sterile syringes were done on blood agar, chocolate agar, MacConkey's agar and Bile Esculin Azide agar daily for 7 days before reporting blood cultures as negative.[9] Isolation of anaerobes was not considered.
Identification of bacterial isolates: Bacterial isolates were identified by their biochemical characteristics via the automated Microscan WalkAway system (Siemens-France).[10] Isolates from repeat culture of previously recruited patients and isolates identified as commensals or contaminants were excluded.
Identification of staphylococcal isolates: According to Louie et al.,[11] staphylococci were identified by standard methods including the gram stain, catalase test and tube coagulase test. Samples were cultured on Mannitol Salt Agar (Oxoid, UK), where S. aureus produces yellow colonies (1 mm in diameter) surrounded by a yellow medium.[11] Isolates which showed positive growth on Mannitol Salt Agar plates were subcultured on an Oxacillin Resistant Screening Agar Base medium (Oxoid, UK) for detection of oxacillin resistance.
Serology: PNEUMOSLIDE IgM which is an indirect immunofluorescent assay (IFA) kit (VIRCELL PNEUMOSLIDE; VIRCELL, GRANADA, Spain) for the simultaneous diagnosis in human serum of IgM antibodies of the main infectious agents of the respiratory tract was used for detection of atypical pathogens.[12] Antibodies to Legionella pneumophila serogroup 1, Mycoplasma pneumoniae, Coxiella burnetii, Chlamydophila pneumoniae, adenovirus, respiratory syncytial virus, influenza A, influenza B and parainfluenza serotypes 1, 2 and 3 are used in this kit.[12] However, because the aim of this study was to address the bacterial profile of LRTIs; only results for bacterial pathogens were considered and interpreted.
Antimicrobial Susceptibility testing: Susceptibility testing was done by Disc diffusion method.[13] The following antibiotics were tested: B-lactams (Ampicillin-sulbactam, Amoxicillin/clavulanic acid, Oxacillin), Cephalosporins (Ceftriaxone, Cefuroxime, Cefotaxime, Ceftazidime, Cefepime), Carbepenems (Imipenem), Macrolides (Erythromycin, Azithromycin, Clarithromycin), Aminoglycosides (Amikacin, Gentamicin), Quinolones (Ciprofloxacin, Levofloxacin, Moxifloxacin), and others (Vancomycin, Doxycycline). Zone diameter was measured and interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.[13]
Statistic alanalysis: Statistical analysis was carried out using the SPSS software (Ver.16).
Results
Patients with CAP: The predominant isolates in 360 patients with CAP were S. pneumoniae (36%), C. pneumoniae (18%), M. pneumoniae (12%) and K. peumoniae (10%). (Table 1) The sensitivity and resistance rates of S. pneumoniae and K. peumoniae against tested antibiotics are depicted in Table 2. A higher sensitivity was recorded for moxifloxacin, levofloxacin, macrolides, and cefepime; whereas, a higher rate of resistance was recorded for doxycycline, cephalosporins, ampicillin-sulbactam, and amoxicillin-clavulinate.
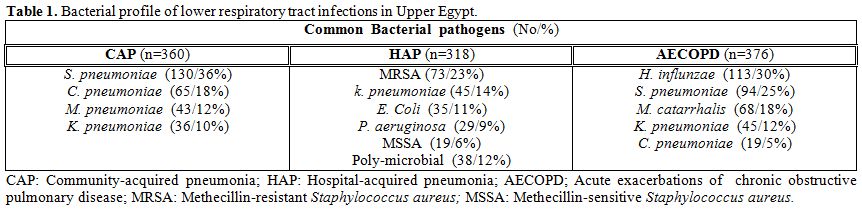
Table 1. Bacterial profile of lower respiratory tract infections in Upper Egypt
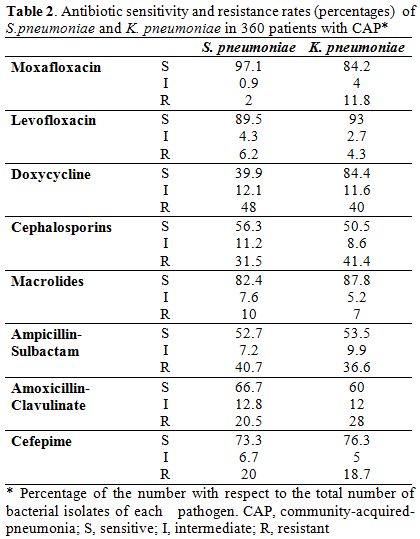
Table 2. Antibiotic sensitivity and resistance rates (percentages) of S. pneumoniae and K. pneumoniae in 360 patients with CAP*
Patients with HAP: The predominant isolates in 318 patients with HAP were, methicillin-resistant Staphylococcus aureus; MRSA (23%), K. pneumoniae (14%), E. coli (11%), P. aeruginosa (9%), methicillin-sensitive Staphylococcus aureus; MSSA (6%), and poly-microbial in 12%. (Table 1) No growth was demonstrated in 25%. Table 3 shows the sensitivity and resistance rates of common pathogens causative of HAP against tested antibiotics. Higher sensitivity rates were recorded for vancomycin, amikacin, moxifloxacin, levofloxacin, and cefepime.
Characteristically, MRSA showed an absolute resistance (100%) for β-lactam-β-lactamase inhibitors, and high resistance rate (92%) for cephalosporins. Patients with AECOPD. The predominant isolates in 376 patients with AECOPD were H. influenzae (30%), S. pneumoniae (25%), M. catarrhalis (18%), and K. pneumoniae (12%). (Table 1) The sensitivity and resistance rates of common pathogens causative of AECOPD against tested antibiotics are demonstrated in Table 4. A higher sensitivity was noted for respiratory quinolones, macrolides and cefepime, while a higher rate of resistance was recorded for aminoglycosides, cephalosporins, and doxycycline.
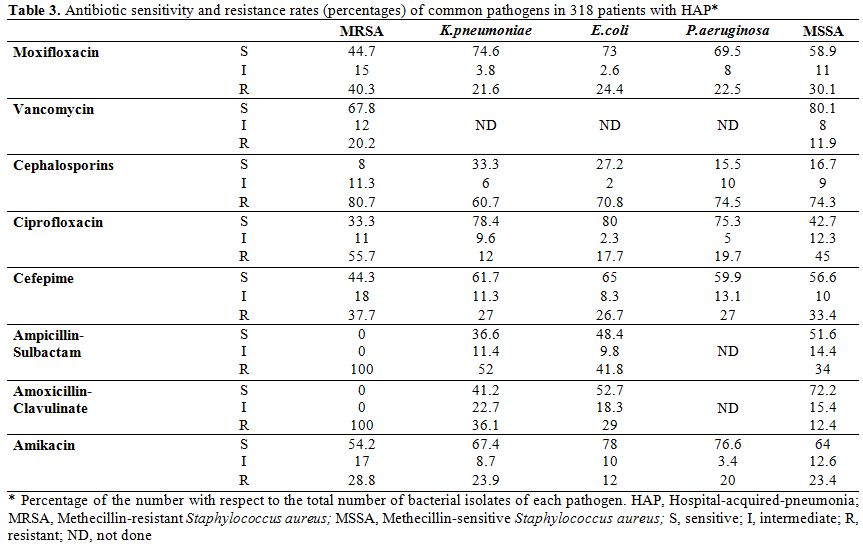
Table 3. Antibiotic sensitivity and resistance rates (percentages) of common pathogens in 318 patients with HAP*
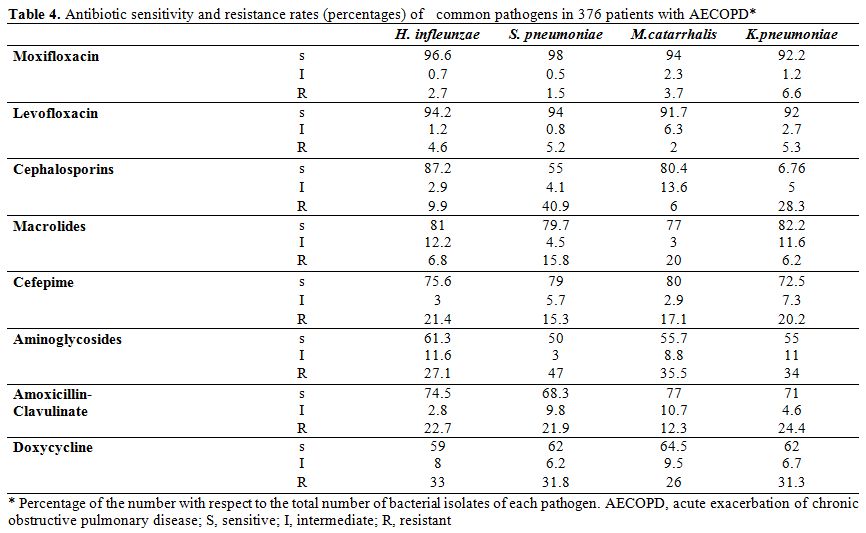
Table 4. Antibiotic sensitivity and resistance rates (percentages) of common pathogens in 376 patients with AECOPD*
Discussion
The increasing antibiotic resistance problems, largely due to wide spread and irrational use of antimicrobial agents in hospitals and community, is of great concern, especially in developing countries. Reliable statistics on antibiotic resistance that are mandatory to control spread of resistant pathogens are available from the developed nations. These data are generated by large surveillance studies in countries such as the USA, Europe, Australia, etc.[2] However such data are sparse in developing countries - like Egypt - due to the lack of large-scale meta-analytic studies. Hospital antibiograms are commonly used to help guide empiric antimicrobial therapy and are an important component of detecting and monitoring trends in antimicrobial resistance.[6] It would be ideal, through multicenter studies, to generate nationwide or more appropriately region-specific antibiograms.
This work represents a multicentre prospective study performed at three University Hospitals in Upper Egypt; aiming to identify the causative bacteria, antibiotic sensitivity and antibiotic resistance of LRTIs in Upper Egypt; considering this an important issue to be investigated in our locality. To the best of our knowledge, this is the first multicentre study addressing the problems of microbial aetiology, antimicrobial sensitivity and resistance of three patterns of LRTIs in Upper Egypt. International guidelines for CAP strongly recommend that locally adapted guidelines should be implemented to improve process of care variables and relevant clinical outcomes.[4]
For patients with CAP, our results showed similar bacterial profiles to those reported by the international,[4] and local[10] studies. However, our results showed higher prevalence of the so-called atypical organisms. This "local" pattern of predominance should be taken into consideration upon prescribing antimicrobials in our locality. Fortunately, this higher prevalence was closely-related to the susceptibility pattern; hence we found the highest rates for respiratory quinolones and macrolides. Over the past 3 decades, antimicrobial resistance among S. pneumoniae has escalated dramatically worldwide. By the early 1990s, penicillin-resistant clones of S. pneumoniae spread rapidly across Europe and globally. Additionally, resistance to macrolides and other antibiotic classes escalated in tandem with penicillin resistance. Recently, it was reported that 15 to 30% of S. pneumoniae worldwide are multidrug-resistant (MDR).[14]
Our data revealed high resistance rates for doxycycline, cephalosporins, and the β-lactam-β-lactamase inhibitors. These findings are in agreement with the increasing prevalence of resistance of S. pneumoniae to those antimicrobial groups, demonstrated by Egyptian,[15] regional,[5,16] and world-wide[4,5] studies. Moreover, our results highlight the increasing problem of MDR S. pneumoniae in CAP, a problem that was extensively addressed in the literature.[14-16] This, alarms us for the need for judicious use of different antimicrobial groups, particularly in our resource-limited country. Moreover, this calls for a greater focus on the identification of relevant drivers of resistance and on the implementation of effective strategies to combat the problems of resistance and multi-drug resistance.
With regards to patients with HAP, the problem of antibiotic resistance seems to be more important; hence the situation is more complicated than that in CAP. Nosocomial pneumonias result in high morbidity and mortality especially among ICU patients.[8] In most clinical situations, there is a need to initiate empirical antimicrobial therapy before obtaining the microbial results. However, the situation is further complicated by the emergence of multiple beta lactamase producers and MDR pathogens.[16,17] Obviously, there is a great need for obtaining data on prevalent strains in HAP; along with the susceptibility pattern, to help in revising antibiotic policy and guiding clinicians for the better management of patients with HAP; particularly in developing countries.
The current study revealed the predominance of MRSA, Gram-negative organisms, and P. aeruginosa among patients with HAP. This is clearly different form the results obtained by Goel and co-workers[17] and even from those obtained by the "local" studies of Ahmed, et al.[18] and Agmy, et al..[19] Although the later study addressed the problem of HAP in 75 cases of ICU patients at Assiut University Hospital, the predominant pathogens were S. aureus (32%), P. aeruginosa (30%), and S. pneumoniae (15%). Obviously, this "local" difference explains the changing pattern of causative pathogens over time, even at the same hospital. This confirms the importance of implementing continued local surveillance programmes.[1] However, in the study of Agmy et al.,[19] factors such as differences in patients' numbers and demographics, research methodologies, and being ICU patients should be taken into consideration. Our data show an alarming high prevalence of MRSA. This coincides with the recent report by Borg et al. who observed that the prevalence of MRSA in invasive isolates from blood cultures from nine hospitals in Egypt was 52%.[20] Interestingly, our data showed polymicrobial aetiology in 12% of cases; that was concordant to that reported by other studies.[17,21] Our results revealed very high rates of resistance for β-lactam-β-lactamase inhibitors and cephalosporins. Goel and co-workers observed 100% and 96.9% resistance to ceftazidime against A. baumannii, and Klebsiella spp., respectively.[17] This, again adds to the complex scenario of antimicrobial resistance found usually in nosocomial infections; particularly in developing countries.[17,18] High resistance to β-lactam-β-lactamase inhibitors and cephalosporins at our locality might be due to the selective influence of overuse of these drugs. On the other hand, high susceptibility rates for respiratory quinolones still confirms the importance of these agents for management of HAP in our locality.[17] Because MDR pathogens are usually difficult to treat and associated with increased mortality,[8,14-16] it was recently[22] recommended that empiric therapy of HAP requires the use of multidrug empirical treatment regimens.
Morbidity and mortality in COPD patients are, for the most part, related to their acute exacerbations, which occur one to three times a year on average.[3] The most common causes of these exacerbations are infection of the tracheobronchial tree and air pollution.[23] There has been a controversy regarding whether bacteria play a role in AECOPD, and thus, whether antibiotics play a role in disease management. Several studies have shown an association between the presence of certain bacterial species, such as S. pneumoniae, M. catarrhalis and H. influenzae, and AECOPD.[24] However, these potential pathogenic microorganisms (PPMO) were also present in sputa obtained from COPD patients with stable disease.[25] Conversely, bronchoscopic studies have shown that at least 50% of COPD patients have bacteria in high concentrations in their lower airways during exacerbations.[26]
The profile of causative pathogens observed in the current study is very similar to that published in the literature.[3] Recently, however, Huang and co-workers[27] addressed the profile of airway bacterial communities using a culture-independent microarray, and concluded that bacterial community diversity in COPD airways is substantially greater than previously recognized. Further studies are needed to comprehensively profile the bacterial pathogens in AECOPD. Again, very high susceptibility rates for the respiratory quinolones confirm the importance of using such agents for AECOPD in our locality. It also represents an agreement with the international recommendations for antibiotics indicated for mild and moderate COPD exacerbations.[3] Moreover, our reported resistance rates for aminoglycosides, cephalosporins, and doxycycline further encourage using respiratory quinolones for AECOPD in our locality. However, we still recommend the judicious use of respiratory quinolones to prevent emerging resistance to such agents.
At the end, our results in three patterns of lower respiratory tract infections have many similarities and differences to other studies. Continued surveillance; particularly based on local data is obviously needed to clarify the problems of antimicrobial resistance and to prevent further spread of such resistance.
To conclude, data from this study can be very useful. A master antibiogram for our region would allow tertiary care institutions to consider resistance patterns in hospitals referring patients and to select appropriate antimicrobial therapy or change drugs in non-responding patients. The concept of "presumptive antimicrobial therapy" could replace that of "empiric antimicrobial therapy"; based on common pathogens, known susceptibility patterns and host factors in any given region. Then, implementing continued local surveillance programmes for antibiotic resistance is essentially important. Also further local studies should be carried out to elucidate the mechanisms of resistance of different pathogens in LRTIs. Judicious use of antimicrobials is essential to prevent the emergence of resistant and/or MDR bacteria in LRTIs.
Conclusions
The most predominant bacteria for CAP in Upper Egypt are S. pneumoniae and atypical organisms, while that for HAP are MRSA and Gram negative bacteria. For acute exacerbation of COPD, H. influenzae and S. pneumoniae were the commonest responsible organisms. Respiratory quinolones, macrolides, and cefepime are the most efficient antibiotics in treatment of lower bacterial respiratory tract infections in our locality.
List of Abbreviations
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; BAL, bronchoalveolar lavage CAP, community-acquired pneumonia; CFU, colony forming unit; CLSI, Clinical and Laboratory Standards Institute; COPD, chronic obstructive pulmonary disease; GOLD, The Global Initiative for Chronic Obstructive Lung Disease; HAP, hospital-acquired pneumonia; IFA, immunofluorescent assay LRTI, lower respiratory tract infections; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PPMO, potential pathogenic microorganisms.
Acute respiratory tract infections, such as bacterial pneumonia and acute exacerbations of chronic bronchitis, account for a considerable proportion of morbidity and antibiotic use. Moreover, these infections result in high mortality rates.[1] Unfortunately, the three major bacterial respiratory pathogens; Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae; have a worldwide increasing prevalence of antibiotic resistance.[2-4] The importance of monitoring the progress of such resistance has led to numerous international, regional and national surveillance programmes. However, results from surveillance studies show wide variations in susceptibility rates, both geographically and over time.[1,5] Prevalent flora and antimicrobial resistance pattern may vary from region to region depending upon the antibiotic pressure in that locality.[6] Thus, there is a great need for local resistance prevalence data in order to guide empirical prescription and to identify areas in which medical need for new agents is greater. Therefore, the present study was designed to identify the bacterial profile of lower respiratory tract infections (LRTIs) in Upper Egypt and to determine the antibiotic susceptibility and resistance patterns among these pathogens in our locality.
Materials and Methods
Patients: The present multicentre prospective study was performed at three University Hospitals in Upper Egypt (Assiut, El-Minia and Sohag University Hospitals) for 3 consecutive years; from July 2009 to June 2012; aiming to identify the causative bacteria, antibiotic sensitivity and antibiotic resistance in hospitalized adult patients ( > 15 years old) due to lower respiratory tract infections in Upper Egypt. The study included 360 patients with community-acquired pneumonia (CAP), 318 patient with hospital-acquired pneumonia (HAP) and 376 patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD). The later patients had mild or moderate exacerbations.3 All patients were subjected to full clinical, radiological and relevant laboratory examinations. All patients were non-ICU in-patients. CAP was defined as pneumonia acquired outside the hospital setting.[7] HAP was defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission.[8] AECOPD were defined according to the GOLD guidelines.3 Indications to hospitalize patients with CAP or AECOPD were according to the relevant international guidelines for each category,[3,4] respectively. Samples collected from all patients were sent to the Department of Clinical Pathology, Faculty of Medicine, Assiut University. The study was approved by the local ethical committee of the Faculty of Medicine, Assiut University and a written informed consent was obtained from all patients for publication of this study.
Sample collection: Samples included sputum and /or bronchoalveolar lavage (BAL), for Gram stain and culture, blood samples for blood cultures and serum sample for serology. One morning spontaneously produced or induced sputum sample was obtained from the majority of patients. The valid sputum originating from the lower respiratory tract was defined as that containing squamous epithelial cell less than 10/high power field and polymorphonucleocytes more than 25/high power field. One BAL was taken from each patient under anesthesia. BAL was obtained to diagnose if there was other hidden pathology. We would not normally recommend this as a routine investigation for culture in LRTIs. For blood samples; 5to10 ml of venous blood was collected from each patient using sterile syringes. Blood samples were inoculated immediately under complete aseptic conditions into bottles containing 50 ml of brain heart infusion broth.[9]
Processing of samples: Such validated sputum as well as BAL samples were cultured on three bacteriological media (Nutrient, Chocolate and MacConkey's) agar plates. Sputa or BAL were mechanically liquefied and homogenized by vortexing for 1 minute with glass beads followed by centrifuging at 3.000 revolutions per minute for 10 minutes. Specimens were then serially diluted in sterile 0.9 % saline solutions with final concentrations of 10-4, 10-5, 10-6 and the colonies were counted as number of colonies per plate X 100 (dilution factor). The valid sputum culture defined as that had quantitative culture ≥105 CFU/ml and the valid BAL culture that had ≥104 CFU/ml.[9] Plates were incubated aerobically at 37°C with 5% CO2 for 24-48 hours. For blood samples; the blood culture bottles were incubated aerobically at 37°C for 7 days. The bottles were examined daily for evidence of bacterial growth as haemolysis, gas production or turbidity above the red cell line. Subcultures using sterile syringes were done on blood agar, chocolate agar, MacConkey's agar and Bile Esculin Azide agar daily for 7 days before reporting blood cultures as negative.[9] Isolation of anaerobes was not considered.
Identification of bacterial isolates: Bacterial isolates were identified by their biochemical characteristics via the automated Microscan WalkAway system (Siemens-France).[10] Isolates from repeat culture of previously recruited patients and isolates identified as commensals or contaminants were excluded.
Identification of staphylococcal isolates: According to Louie et al.,[11] staphylococci were identified by standard methods including the gram stain, catalase test and tube coagulase test. Samples were cultured on Mannitol Salt Agar (Oxoid, UK), where S. aureus produces yellow colonies (1 mm in diameter) surrounded by a yellow medium.[11] Isolates which showed positive growth on Mannitol Salt Agar plates were subcultured on an Oxacillin Resistant Screening Agar Base medium (Oxoid, UK) for detection of oxacillin resistance.
Serology: PNEUMOSLIDE IgM which is an indirect immunofluorescent assay (IFA) kit (VIRCELL PNEUMOSLIDE; VIRCELL, GRANADA, Spain) for the simultaneous diagnosis in human serum of IgM antibodies of the main infectious agents of the respiratory tract was used for detection of atypical pathogens.[12] Antibodies to Legionella pneumophila serogroup 1, Mycoplasma pneumoniae, Coxiella burnetii, Chlamydophila pneumoniae, adenovirus, respiratory syncytial virus, influenza A, influenza B and parainfluenza serotypes 1, 2 and 3 are used in this kit.[12] However, because the aim of this study was to address the bacterial profile of LRTIs; only results for bacterial pathogens were considered and interpreted.
Antimicrobial Susceptibility testing: Susceptibility testing was done by Disc diffusion method.[13] The following antibiotics were tested: B-lactams (Ampicillin-sulbactam, Amoxicillin/clavulanic acid, Oxacillin), Cephalosporins (Ceftriaxone, Cefuroxime, Cefotaxime, Ceftazidime, Cefepime), Carbepenems (Imipenem), Macrolides (Erythromycin, Azithromycin, Clarithromycin), Aminoglycosides (Amikacin, Gentamicin), Quinolones (Ciprofloxacin, Levofloxacin, Moxifloxacin), and others (Vancomycin, Doxycycline). Zone diameter was measured and interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.[13]
Statistic alanalysis: Statistical analysis was carried out using the SPSS software (Ver.16).
Results
Patients with CAP: The predominant isolates in 360 patients with CAP were S. pneumoniae (36%), C. pneumoniae (18%), M. pneumoniae (12%) and K. peumoniae (10%). (Table 1) The sensitivity and resistance rates of S. pneumoniae and K. peumoniae against tested antibiotics are depicted in Table 2. A higher sensitivity was recorded for moxifloxacin, levofloxacin, macrolides, and cefepime; whereas, a higher rate of resistance was recorded for doxycycline, cephalosporins, ampicillin-sulbactam, and amoxicillin-clavulinate.

Table 1. Bacterial profile of lower respiratory tract infections in Upper Egypt

Table 2. Antibiotic sensitivity and resistance rates (percentages) of S. pneumoniae and K. pneumoniae in 360 patients with CAP*
Patients with HAP: The predominant isolates in 318 patients with HAP were, methicillin-resistant Staphylococcus aureus; MRSA (23%), K. pneumoniae (14%), E. coli (11%), P. aeruginosa (9%), methicillin-sensitive Staphylococcus aureus; MSSA (6%), and poly-microbial in 12%. (Table 1) No growth was demonstrated in 25%. Table 3 shows the sensitivity and resistance rates of common pathogens causative of HAP against tested antibiotics. Higher sensitivity rates were recorded for vancomycin, amikacin, moxifloxacin, levofloxacin, and cefepime.
Characteristically, MRSA showed an absolute resistance (100%) for β-lactam-β-lactamase inhibitors, and high resistance rate (92%) for cephalosporins. Patients with AECOPD. The predominant isolates in 376 patients with AECOPD were H. influenzae (30%), S. pneumoniae (25%), M. catarrhalis (18%), and K. pneumoniae (12%). (Table 1) The sensitivity and resistance rates of common pathogens causative of AECOPD against tested antibiotics are demonstrated in Table 4. A higher sensitivity was noted for respiratory quinolones, macrolides and cefepime, while a higher rate of resistance was recorded for aminoglycosides, cephalosporins, and doxycycline.

Table 3. Antibiotic sensitivity and resistance rates (percentages) of common pathogens in 318 patients with HAP*

Table 4. Antibiotic sensitivity and resistance rates (percentages) of common pathogens in 376 patients with AECOPD*
Discussion
The increasing antibiotic resistance problems, largely due to wide spread and irrational use of antimicrobial agents in hospitals and community, is of great concern, especially in developing countries. Reliable statistics on antibiotic resistance that are mandatory to control spread of resistant pathogens are available from the developed nations. These data are generated by large surveillance studies in countries such as the USA, Europe, Australia, etc.[2] However such data are sparse in developing countries - like Egypt - due to the lack of large-scale meta-analytic studies. Hospital antibiograms are commonly used to help guide empiric antimicrobial therapy and are an important component of detecting and monitoring trends in antimicrobial resistance.[6] It would be ideal, through multicenter studies, to generate nationwide or more appropriately region-specific antibiograms.
This work represents a multicentre prospective study performed at three University Hospitals in Upper Egypt; aiming to identify the causative bacteria, antibiotic sensitivity and antibiotic resistance of LRTIs in Upper Egypt; considering this an important issue to be investigated in our locality. To the best of our knowledge, this is the first multicentre study addressing the problems of microbial aetiology, antimicrobial sensitivity and resistance of three patterns of LRTIs in Upper Egypt. International guidelines for CAP strongly recommend that locally adapted guidelines should be implemented to improve process of care variables and relevant clinical outcomes.[4]
For patients with CAP, our results showed similar bacterial profiles to those reported by the international,[4] and local[10] studies. However, our results showed higher prevalence of the so-called atypical organisms. This "local" pattern of predominance should be taken into consideration upon prescribing antimicrobials in our locality. Fortunately, this higher prevalence was closely-related to the susceptibility pattern; hence we found the highest rates for respiratory quinolones and macrolides. Over the past 3 decades, antimicrobial resistance among S. pneumoniae has escalated dramatically worldwide. By the early 1990s, penicillin-resistant clones of S. pneumoniae spread rapidly across Europe and globally. Additionally, resistance to macrolides and other antibiotic classes escalated in tandem with penicillin resistance. Recently, it was reported that 15 to 30% of S. pneumoniae worldwide are multidrug-resistant (MDR).[14]
Our data revealed high resistance rates for doxycycline, cephalosporins, and the β-lactam-β-lactamase inhibitors. These findings are in agreement with the increasing prevalence of resistance of S. pneumoniae to those antimicrobial groups, demonstrated by Egyptian,[15] regional,[5,16] and world-wide[4,5] studies. Moreover, our results highlight the increasing problem of MDR S. pneumoniae in CAP, a problem that was extensively addressed in the literature.[14-16] This, alarms us for the need for judicious use of different antimicrobial groups, particularly in our resource-limited country. Moreover, this calls for a greater focus on the identification of relevant drivers of resistance and on the implementation of effective strategies to combat the problems of resistance and multi-drug resistance.
With regards to patients with HAP, the problem of antibiotic resistance seems to be more important; hence the situation is more complicated than that in CAP. Nosocomial pneumonias result in high morbidity and mortality especially among ICU patients.[8] In most clinical situations, there is a need to initiate empirical antimicrobial therapy before obtaining the microbial results. However, the situation is further complicated by the emergence of multiple beta lactamase producers and MDR pathogens.[16,17] Obviously, there is a great need for obtaining data on prevalent strains in HAP; along with the susceptibility pattern, to help in revising antibiotic policy and guiding clinicians for the better management of patients with HAP; particularly in developing countries.
The current study revealed the predominance of MRSA, Gram-negative organisms, and P. aeruginosa among patients with HAP. This is clearly different form the results obtained by Goel and co-workers[17] and even from those obtained by the "local" studies of Ahmed, et al.[18] and Agmy, et al..[19] Although the later study addressed the problem of HAP in 75 cases of ICU patients at Assiut University Hospital, the predominant pathogens were S. aureus (32%), P. aeruginosa (30%), and S. pneumoniae (15%). Obviously, this "local" difference explains the changing pattern of causative pathogens over time, even at the same hospital. This confirms the importance of implementing continued local surveillance programmes.[1] However, in the study of Agmy et al.,[19] factors such as differences in patients' numbers and demographics, research methodologies, and being ICU patients should be taken into consideration. Our data show an alarming high prevalence of MRSA. This coincides with the recent report by Borg et al. who observed that the prevalence of MRSA in invasive isolates from blood cultures from nine hospitals in Egypt was 52%.[20] Interestingly, our data showed polymicrobial aetiology in 12% of cases; that was concordant to that reported by other studies.[17,21] Our results revealed very high rates of resistance for β-lactam-β-lactamase inhibitors and cephalosporins. Goel and co-workers observed 100% and 96.9% resistance to ceftazidime against A. baumannii, and Klebsiella spp., respectively.[17] This, again adds to the complex scenario of antimicrobial resistance found usually in nosocomial infections; particularly in developing countries.[17,18] High resistance to β-lactam-β-lactamase inhibitors and cephalosporins at our locality might be due to the selective influence of overuse of these drugs. On the other hand, high susceptibility rates for respiratory quinolones still confirms the importance of these agents for management of HAP in our locality.[17] Because MDR pathogens are usually difficult to treat and associated with increased mortality,[8,14-16] it was recently[22] recommended that empiric therapy of HAP requires the use of multidrug empirical treatment regimens.
Morbidity and mortality in COPD patients are, for the most part, related to their acute exacerbations, which occur one to three times a year on average.[3] The most common causes of these exacerbations are infection of the tracheobronchial tree and air pollution.[23] There has been a controversy regarding whether bacteria play a role in AECOPD, and thus, whether antibiotics play a role in disease management. Several studies have shown an association between the presence of certain bacterial species, such as S. pneumoniae, M. catarrhalis and H. influenzae, and AECOPD.[24] However, these potential pathogenic microorganisms (PPMO) were also present in sputa obtained from COPD patients with stable disease.[25] Conversely, bronchoscopic studies have shown that at least 50% of COPD patients have bacteria in high concentrations in their lower airways during exacerbations.[26]
The profile of causative pathogens observed in the current study is very similar to that published in the literature.[3] Recently, however, Huang and co-workers[27] addressed the profile of airway bacterial communities using a culture-independent microarray, and concluded that bacterial community diversity in COPD airways is substantially greater than previously recognized. Further studies are needed to comprehensively profile the bacterial pathogens in AECOPD. Again, very high susceptibility rates for the respiratory quinolones confirm the importance of using such agents for AECOPD in our locality. It also represents an agreement with the international recommendations for antibiotics indicated for mild and moderate COPD exacerbations.[3] Moreover, our reported resistance rates for aminoglycosides, cephalosporins, and doxycycline further encourage using respiratory quinolones for AECOPD in our locality. However, we still recommend the judicious use of respiratory quinolones to prevent emerging resistance to such agents.
At the end, our results in three patterns of lower respiratory tract infections have many similarities and differences to other studies. Continued surveillance; particularly based on local data is obviously needed to clarify the problems of antimicrobial resistance and to prevent further spread of such resistance.
To conclude, data from this study can be very useful. A master antibiogram for our region would allow tertiary care institutions to consider resistance patterns in hospitals referring patients and to select appropriate antimicrobial therapy or change drugs in non-responding patients. The concept of "presumptive antimicrobial therapy" could replace that of "empiric antimicrobial therapy"; based on common pathogens, known susceptibility patterns and host factors in any given region. Then, implementing continued local surveillance programmes for antibiotic resistance is essentially important. Also further local studies should be carried out to elucidate the mechanisms of resistance of different pathogens in LRTIs. Judicious use of antimicrobials is essential to prevent the emergence of resistant and/or MDR bacteria in LRTIs.
Conclusions
The most predominant bacteria for CAP in Upper Egypt are S. pneumoniae and atypical organisms, while that for HAP are MRSA and Gram negative bacteria. For acute exacerbation of COPD, H. influenzae and S. pneumoniae were the commonest responsible organisms. Respiratory quinolones, macrolides, and cefepime are the most efficient antibiotics in treatment of lower bacterial respiratory tract infections in our locality.
List of Abbreviations
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; BAL, bronchoalveolar lavage CAP, community-acquired pneumonia; CFU, colony forming unit; CLSI, Clinical and Laboratory Standards Institute; COPD, chronic obstructive pulmonary disease; GOLD, The Global Initiative for Chronic Obstructive Lung Disease; HAP, hospital-acquired pneumonia; IFA, immunofluorescent assay LRTI, lower respiratory tract infections; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PPMO, potential pathogenic microorganisms.
References
- Alpuche C, Garau J, and Lim V. Global and
local variations in antimicrobial susceptibilities and resistance
development in the major respiratory pathogens. Int J Antimicrob Agents
2007; 30 Suppl 2: 135-8. http://dx.doi.org/10.1016/j.ijantimicag.2007.07.035 PMid:17945468

- Pfaller MA, Jones RN, Doren GV, Kugler K,
and The SENTRY Participants Group. Bacterial Pathogens Isolated from
Patients with Bloodstream Infection: Frequencies of Occurrence and
Antimicrobial Susceptibility Patterns from the SENTRY Antimicrobial
Surveillance Program (United States and Canada, 1997). Antimicrob
Agents Chemother 1998; 42: 1762-1770. PMid:9661018 PMCid:PMC105680

- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy For The Diagnosis And Prevention Of Chronic Obstructive Pulmonary Disease. Updated 2009.
- Mandell LA, Wunderink RG, Anzueto A,
Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM,
Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of
America/American Thoracic Society Consensus Guidelines on the
Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis
2007; 44 Suppl 2: S27–72. http://dx.doi.org/10.1086/511159 PMid:17278083

- Garcı´a-Rey C, Aguilar L, Baquero F, Casal
J, Dal-Ré R. Importance of Local Variations in Antibiotic Consumption
and Geographical Differences of Erythromycin and Penicillin Resistance
in Streptococcus pneumoniae. J Clin Microbiol 2002; 40: 159–164. http://dx.doi.org/10.1128/JCM.40.1.159-164.2002 PMCid:PMC120130

- Lakshmi V. Need for national/regional
guidelines and policies in India to combat antibiotic resistance.
Indian J of Medical Microbiology 2008; 26: 105-7. http://dx.doi.org/10.4103/0255-0857.40521

- Gordon RC. Community acquired pneumonia in adolescents. Adoles Med 2000; 11: 681-695. PMid:11060562

- American Thoracic Society; Infectious
Diseases Society of America. Guidelines for the Management of Adults
with Hospital-acquired, Ventilator- associated, and
Healthcare-associated Pneumonia. Am J Respir Crit Care Med 2005; 171:
388–416. http://dx.doi.org/10.1164/rccm.200405-644ST PMid:15699079

- Koneman EW, Allen SD, Janda WM,
Schreckenberger RC and Winn WC. Introduction to microbiology, part II:
reporting of cultures from specific specimen sources. In Koneman EW,
Allen SD, Janda WM, Schreckenberger RC, Winn WC, editors. Color Atlas
and text book of diagnostic microbiology. Lippincott-Raven, Philadephia
121–171; 1997.
- El Sayed Zaki M and Goda T.
Clinico-pathological study of atypical pathogens in community-acquired
pneumonia: a prospective study. J Infect Dev Ctries 2009; 3: 199-205. http://dx.doi.org/10.3855/jidc.36 PMid:19759475

- Louie L, Goodfellow J, Mathieu P, Glatt A,
Louie M, Simor AE. Rapid detection of methicillin-resistant
staphylococci from blood culture bottles by using a multiplex PCR
assay. J Clin Microbiol 2002; 40: 2786–90. http://dx.doi.org/10.1128/JCM.40.8.2786-2790.2002 PMid:12149330 PMCid:PMC120630

- El-Sahrigy S, Abdel-Rahman A, Abou Shady
E, Attia and Goma. Pneumoslide- M Technique for Rapid Detection of
Atypical Pathogens in Critically ill Children with Lower Respiratory
Tract Infections. J Med Sci 2006; 6: 793-799. http://dx.doi.org/10.3923/jms.2006.793.799

- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 17th informational supplement. CLSI; M100-S17. Wayne, PA; 2007.
- Lynch JP 3rd, Zhanel GG. Streptococcus
pneumoniae: does antimicrobial resistance matter? Semin Respir Crit
Care Med. 2009; 30: 210-38. http://dx.doi.org/10.1055/s-0029-1202939 PMid:19296420

- Wasfy MO, Pimentel G, Abdel-Maksoud M,
Russell KL, Barrozo CP, Klena JD, Earhart K, Hajjeh R. Antimicrobial
susceptibility and serotype distribution of Streptococcus pneumoniae
causing meningitis in Egypt, 1998–2003. J Antimicrob Chemother 2005;
55: 958–964. http://dx.doi.org/10.1093/jac/dki101 PMid:15820983

- Borg MA, Tiemersma E, Scicluna E, van de
Sande-Bruinsma N, de Kraker M, Monen J, Grundmann H; ARMed Project
members and collaborators. Prevalence of penicillin and erythromycin
resistance among invasive Streptococcus pneumoniae isolates reported by
laboratories in the southern and eastern Mediterranean region. Clin
Microbiol Infect 2009; 15: 232–237. http://dx.doi.org/10.1111/j.1469-0691.2008.02651.x PMid:19154490

- Goel N, Chaudhary U, Aggarwal R, Bala K.
Antibiotic sensitivity pattern of gram negative bacilli isolated from
the lower respiratory tract of ventilated patients in the intensive
care unit. Indian J Crit Care Med 2009; 13: 148-151. http://dx.doi.org/10.4103/0972-5229.58540 PMid:20040812 PMCid:PMC2823096

- Ahmed SH, Daef EA, Badary MS, Mahmoud MA,
Abd-Elsayed AA. Nosocomial blood stream infection in intensive care
units at Assiut University Hospitals (Upper Egypt) with special
reference to extended spectrum b-lactamase producing organisms. BMC
Research Notes 2009; 2: 76-87. http://dx.doi.org/10.1186/1756-0500-2-76 PMid:19419535 PMCid:PMC2694819

- Agmy GM, Sayed SS, Mohamed EA. Nosocomial
pneumonia in intensive care units at Assiut University Hospital
[abstract]. In Proceedings of the Annual Congress of The European
Respiratory Society, Copenhagen; 2005. PMid:19498617

- Borg MA, de Kraker M, Scicluna E, van de
Sande-Bruinsma N, Tiemersma E, Monen J, Grundmann H; ARMed Project
Members and Collaborators. Prevalence of methicillin-resistant
Staphylococcus aureus (MRSA) in invasive isolates from southern and
eastern Mediterranean countries. J Antimicrob Chemoth 2007; 60:
1310–1315. http://dx.doi.org/10.1093/jac/dkm365 PMid:17913724

- Singh AK, Sen MR, Anupurba S, Bhattacharya
P. Antibiotic sensitivity pattern of the bacteria isolated from
nosocomial infection in ICU. J Commun Dis 2002; 34: 257-63.
PMid:14710856

- Jones RN. Microbial Etiologies of
Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated
Bacterial Pneumonia. Clin Infect Dis 2010; 51 Suppl 1: 81–87. http://dx.doi.org/10.1086/653053 PMid:20597676

- White AJ, Gompertz S, Stockley RA. Chronic
obstructive pulmonary disease. 6: The aetiology of exacerbations of
chronic obstructive pulmonary disease. Thorax 2003; 58: 73-80. http://dx.doi.org/10.1136/thorax.58.1.73 PMid:12511727 PMCid:PMC1746462

- Diederen BM, van der Valk PD, Kluytmans
JA, Peeters MF, Hendrix R.The role of atypical respiratory pathogens in
exacerbations of chronic obstructive pulmonary disease. Eur Respir J
2007; 30: 240–244. http://dx.doi.org/10.1183/09031936.00012707 PMid:17459899

- Hirschmann JV. Do bacteria cause exacerbations of COPD? Chest 2000; 118: 193–203. http://dx.doi.org/10.1378/chest.118.1.193 PMid:10893379

- Pela R, Marchesani F, Agostinelli C,
Staccioli D, Cecarini L, Bassotti C, Sanguinetti CM. Airways microbial
flora in COPD patients in stable clinical conditions and during
exacerbations: a bronchoscopic investigation. Monaldi Arch Chest Dis
1998; 53: 262-7. PMid:9785808

- Huang YJ, Kim E, Cox MJ, Brodie EL, Brown
R, Wiener-Kronish JP, Lynch SV. A persistent and diverse airway
microbiota present during chronic obstructive pulmonary disease
exacerbations. OMICS 2010; 14: 9-59. http://dx.doi.org/10.1089/omi.2009.0100 PMid:20141328 PMCid:PMC3116451
