Von Willebrand Factor, Angiodysplasia and Angiogenesis
Anna M. Randi1, Mike A. Laffan2 and Richard D. Starke1
1
Cardiovascular Sciences, National Heart and Lung Institute, Faculty of
Medicine, Hammersmith Campus, Imperial College London, London, United
Kingdom.
2 Department of Haematology, Hammersmith Campus, Imperial College London, London, United Kingdom.
2 Department of Haematology, Hammersmith Campus, Imperial College London, London, United Kingdom.
Correspondence
to:
Anna M. Randi MD PhD. Imperial College London, NHLI Vascular Sciences,
Hammersmith Hospital, Du Cane Rd, London W12 0NN, United Kingdom.
E-mail: a.randi@imperial.ac.uk
Published: September 2, 2013
Received: July 17, 2013
Accepted: August 20, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013060, DOI 10.4084/MJHID.2013.060
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
The
large multimeric glycoprotein Von Willebrand factor (VWF) is best known
for its role in haemostasis; however in recent years other functions of
VWF have been identified, indicating that this protein is involved in
multiple vascular processes. We recently described a new role for VWF
in controlling angiogenesis, which may have significant clinical
implications for patients with Von Willebrand disease (VWD), a genetic
or acquired condition caused by the deficiency or dysfunction of VWF.
VWD can be associated with angiodysplasia, a condition of degenerative
blood vessels often present in the gastrointestinal tract, linked to
dysregulated angiogenesis. Angiodysplasia can cause severe intractable
bleeding, often refractory to conventional VWD treatments. In this
review we summarise the evidence showing that VWF controls
angiogenesis, and review the angiogenic pathways which have been
implicated in this process. We discuss the possible mechanisms though
which VWF regulates angiopoietin-2 (Ang-2) and integrin αvβ3, leading
to signalling through vascular endothelial growth factor receptor-2
(VEGFR2), one of the most potent activators of angiogenesis. We also
review the evidence that links VWF with angiodysplasia, and how the
newly identified function of VWF in controlling angiogenesis may pave
the way for the development of novel therapies for the treatment of
angiodysplasia in congenital VWD and in acquired conditions such as
Heyde syndrome.
Introduction
The presence of vascular abnormalities in von Willebrand disease (VWD) was first described in the 1960s, when Armand J. Quick, one of the pioneers in the study of coagulation, reported the presence of telangectasias, defined as skin and mucous lesions consisting of dilated small blood vessels that tend to bleed (rev in[1]). Since then, several groups have reported the presence of vascular malformation in VWD patients in various localizations, including nail bed,[2] skin, prostate and most frequently angiodysplasia of the gastrointestinal tract.[3] These lesions can be responsible for severe, intractable bleeding which is often not responsive to VWF replacement therapy and thus represent a significant unmet clinical challenge. Until recently, the pathological mechanism underlying vascular malformations in VWD was unexplained. However the recent discovery that von Willebrand factor (VWF) regulates blood vessel formation[4] has shed new light on this syndrome and opened new avenues for the treatment of angiodysplasia. In this review we will summarise the process that led to this discovery, its implications for vascular biology and for the treatment of patients with VWD.
The Cellular and Molecular Basis of Angiogenesis
Angiogenesis (the formation of new blood vessels from pre-existing ones) is a complex process which involves a cascade of events that require fine spatial and temporal coordination (rev in[5]). The initial pro-angiogenic stimulus, often a growth factor produced in response to hypoxia, activates selected endothelial cells (EC) in the pre-existing vascular plexus to undergo changes in polarity and cytoskeletal remodelling, inducing migration towards the source of the pro-angiogenic stimulus. These cells, named tip cells, maintain contact with the adjacent EC, called stalk cells, which acquire a different phenotype.[6] Stalk cells proliferate to support the elongation of the new sprout. Eventually tip cells come into contact with other tip cells and through their thin finger-like protrusions (filopodia) engage in a cell fusion process, which is facilitated by tissue macrophages.[7] Blood flow eventually completes canalisation of the new vascular sprout (rev in[8]). In order to become functional, blood vessels undergo stabilization and maturation, with active remodelling of the newly formed network, recruitment of mural cells and deposition of extracellular matrix.[9] The process requires coordination between EC and other vascular cells, in particular pericytes and smooth muscle cells.
Growth factors driving the initiation of angiogenesis: Vascular endothelial growth factor (VEGF). A large and growing number of molecules involved in regulating angiogenesis have been identified. Some are crucial for the initiation and/or progression of the process and their deficiency or dysregulation is incompatible with vascular development. Many other regulators, however, contribute to downstream steps in this complex process; their defect may give rise to dysfunctional vessels rather than complete disruption of the vasculature (rev in[5,10]). The best characterised pro-angiogenic endothelial growth factor is vascular endothelial growth factor (VEGF), a major regulator of vasculogenesis and physiological angiogenesis during embryogenesis, as well as physiological and pathological angiogenesis in the adult (rev in[5,11]). The VEGF system is also required for lymphangiogenesis (rev in[12]). VEGF-A is the best characterised member of a family which also includes VEGF-B, VEGF-C, VEGF-D and placental-derived growth factor. These bind to the VEGF receptors (R), of which 3 members (VEGF-R1, -R2 and -R3) have been identified. The complexity of the network is further enhanced by splicing and proteolytic cleavage of the ligands (rev in[13]). The main receptor for VEGF in the vascular endothelium is VEGFR2, which is critical for vascular development as well as adult angiogenesis (rev in[14]). VEGF exerts many effects on the vascular endothelium, including promoting proliferation, migration and survival as well as increased permeability (rev in[14]). Binding of VEGF-A to VEGF-R2 on EC stimulates dimerization of the receptor and autophosphorylation of specific intracellular tyrosine residues, leading to activation of intracellular signalling cascades, which lead to cell survival, permeability, migration and/or proliferation.[14] In vivo, VEGF promotes angiogenesis; however overexpression of VEGF leads to the formation of fragile capillaries, with a disrupted structure, reminiscent of angiomas or angiodysplasia.[15,16]
Growth factors controlling quiescence and vascular stability: the Angiopoietins and Tie-2 system. Whilst VEGF controls the early phases of the formation of a new blood vessel, the system most clearly involved in controlling the maturation and stability of new blood vessels is that of Angiopoietins and the Tie-2 receptor. Angiopoietin (Ang)-1 is produced by non-EC, such as pericytes and mural cells that contribute to vascular stability. Ang-1 binds to the tyrosine kinase receptor Tie-2, which is mainly expressed on EC; Ang-1 signalling through Tie2 receptor promotes survival, quiescence and stability of blood vessels. Ang-1 also has anti-permeability and anti-inflammatory functions (rev in[17]). As ever, the picture is complicated by the fact that in some experimental models Ang-1 has been shown to promote cell migration and angiogenesis, in apparent conflict with its pro-quiescence properties. An interesting model has been put forward which proposes that differences in the localization of Tie-2 receptors on EC and their cell surface partners determines whether this signalling pathway supports quiescence or angiogenesis.[18,19]
VEGF and Ang-1 play essential and complementary roles in vascular development and angiogenesis. During embryogenesis, VEGF is required for the formation of the initial vascular plexus, whilst Ang-1 is necessary for the remodelling of this early vascular network into mature blood vessels.[20] A similar interplay between these two systems seems to take place during adult angiogenesis: both VEGF and Ang-1 are able to promote angiogenesis in vivo;[21] however VEGF causes vascular permeability and tissue oedema, whilst Ang-1 contributes to the stabilization and the maturation of growing blood vessels.[22,23] Furthermore, Ang-1 administration or overexpression in the dermal compartment can protect from the potentially lethal actions of VEGF as a consequence of uncontrolled plasma leakage.[24] Co-expression of VEGF and Ang-1 has recently been proposed as a strategy to generate more stable new vessels.[25]
Another crucial regulator of the quiescence/angiogenesis balance is Ang-2. Ang-2 is an antagonistic ligand of Tie-2, which competitively inhibits binding of Ang-1, priming the endothelium for activation and vascular destabilisation. Ang-2 appears to act synergistically with VEGF to promote angiogenesis.[26] Contrary to Ang-1, Ang-2 is synthesised by EC and stored in organelles called Weibel Palade Bodies (WPB), from where it can be rapidly released upon cellular activation.[27] So whilst Ang-1 acts as an agonist of Tie-2, promoting structural integrity of blood vessels, Ang-2 acts as a naturally occurring antagonist, promoting vessel destabilisation and growth, as well as inflammation.[28] Depending on the levels of other growth factors, such as VEGF-A, Ang-2 can also promote vessel regression (rev in[29]). The angiopoietin-Tie-2 system is also an area of intensive research for the development of modulatory drugs (rev in[30]).
Extracellular cues and cell adhesion receptors controlling angiogenesis: integrin αvβ3. Molecular interactions mediated by several adhesion receptors and signalling complexes between cells need to be coordinated to maintain the integrity of the vessel and ultimately to stabilise the newly formed capillary. The extracellular environment is crucial for physiological development of the nascent sprout interaction; cell surface receptors of the integrin family mediate adhesion to and signalling by the extracellular matrix (ECM). Integrins are heterodimeric transmembrane proteins involved in the interaction of cells with their extracellular environment. In response to extracellular cues, integrins are able to transmit so called “outside-in” signals to the cell leading to the activation of signalling cascades via various pathways including those of cellular adhesion and migration. The extracellular conformation of integrins can also be modulated by intracellular processes and transmit so called “inside-out” signals leading to changes in the way the receptor interacts with its extracellular matrix environment and modulation of protease activity (rev in[31]). One integrin receptor in particular, αvβ3, which is expressed on EC and is the best characterised endothelial receptor for VWF, has been shown to play a crucial role in angiogenesis and is a therapeutic target for cancer. The expression of αvβ3 is up-regulated in tumour associated blood vessels[32] and drugs targeting αvβ3 have shown some success in clinical trials (rev in[33]); however its role appears quite complex, since deficiency of this integrin in the mouse has been linked with increased VEGFR2-dependent angiogenesis.[34] Interestingly αvβ3 can associate with VEGFR2 and crosstalk between these receptors can stimulate reciprocal activation (rev in[35]). Ang-1 and -2 have been shown to be able to regulate integrin mediated cell adhesion[36] and Ang-2 can modulate αvβ3 integrin signalling.[19,37]
Angiodysplasia: Vascular Lesions Linked to Abnormal Angiogenesis
Angiogenesis plays a crucial role during embryonic development and in specific processes during adulthood, such as wound healing and the menstrual cycle. Excessive or insufficient angiogenesis has been linked to a growing number of diseases (rev in[38]), and over the last few decades major progress in the understanding of the cellular and molecular basis of the process has been achieved. In parallel to the scientific progress, there has also been intense drug development activity in the search for inhibitors or activators. The area of vascular malformations, however, has received less attention and the links with the pathways controlling angiogenesis are poorly understood. The term angiodysplasia defines vascular malformation, also named ectasia, which affects submucosal veins, mucosal venules and capillaries. The abnormal vascular plexus is fragile and the architecture is disrupted, with possible arteriovenous communications. Angiodysplastic lesions are most commonly observed in the gastrointestinal (GI) tract and are the most common cause of occult GI bleeding in subjects over 65. A firm diagnosis of angiodysplasia may be difficult to achieve, partly because bleeding may be intermittent and partly because not all lesions are accessible to endoscopy. Although angiodysplasia is most frequently located in the proximal large colon (80% of lesions) which is visible by conventional methods, 15% of lesions are located in the small bowel and these may be either missed or require capsule endoscopy, which is not universally available. However, the use of capsule endoscopy has increased the diagnostic yield in patients with obscure GI bleeding to over 60% and as high as 93% in some series, depending on patient selection. This is a significant improvement over push enteroscopy, but in a small number of cases the diagnosis is one of exclusion based on the clinical picture of recurrent GI blood loss.[39]
Despite the limited number of studies on the cellular and molecular basis of angiodysplasia, a link between angiodysplastic lesions and angiogenesis has been identified. The expression of the angiogenic growth factors VEGF and bFGF was found to be increased in samples of angiodysplastic tissue isolated from patients presenting with angiodysplasia.[40,41] Also, increased plasma levels of VEGF have been reported in patients with hereditary haemorrhagic telangectasia (HHT), who present with multiple angiodysplastic lesions,[42] and patients with genetic or acquired VWD[43] (see below).
Von Willebrand Factor as a new Regulator of Angiogenesis
Von Willebrand factor (VWF) is a large multimeric plasma glycoprotein well known for its crucial role in haemostasis, where it mediates platelet adhesion to the endothelium and the sub-endothelial matrix, and acts as a carrier for coagulation factor VIII (FVIII) in plasma. Deficiency or dysfunction of VWF causes von Willebrand disease (VWD), the most common genetic bleeding disorder in man.
VWF is produced by EC and megakaryocytes; in EC, VWF can be constitutively secreted or stored in intracellular organelles called WPB, from where it can be secreted in response to various stimuli (rev in[44]). Although platelets contain VWF, plasma VWF levels have been shown to depend almost entirely on VWF from endothelial cells.[45] The pathways of VWF synthesis, storage and secretion have been extensively investigated (rev in[46]). VWF drives the formation of WPB, which contain numerous proteins (rev in[47]). A proteomic approach has recently identified more WPB proteins.[48] The list of known and newly discovered WPB molecules, shown in table 1, includes several molecules which play a role in angiogenesis.[47-50] Because VWF is essential for WPB formation, these proteins are dependent on VWF for their storage and regulated secretion (see below).
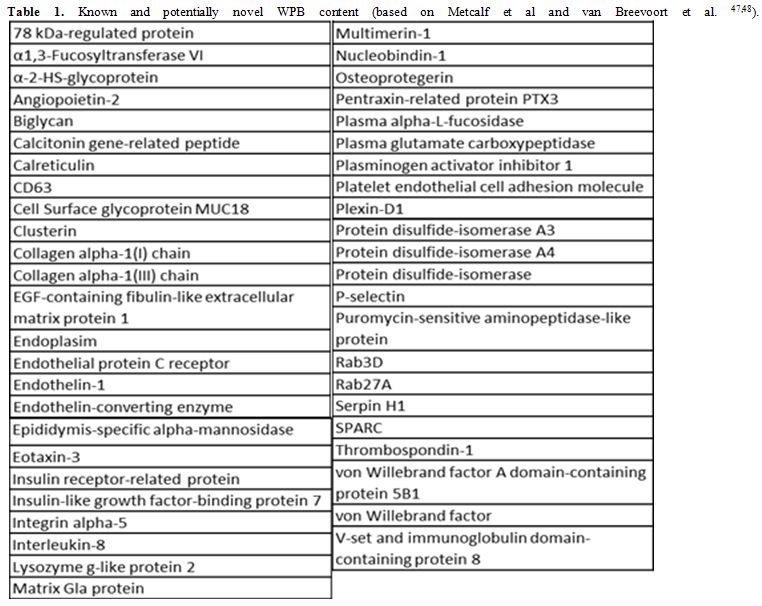
Table 1. Known and potentially novel WPB content (based on Metcalf et al and van Breevoort et al. [47,48]).
In recent years, it has become evident that VWF plays multiple roles in the vasculature. VWF has been shown to control smooth muscle cell proliferation, vascular inflammation, cell death and tumour metastasis (rev in[51]). The large, complex structure of VWF protein supports multiple interactions with cell surface receptors and extracellular matrix proteins; in a recent review by Lenting et al.,[51] VWF has been described as a “molecular bus”, which can interact with 20 other partners. The list of VWF interacting molecules is likely to expand, and with this the understanding of its multiple complex functions.
Recently, our group demonstrated a novel function for VWF in the control of blood vessel formation.[4] Inhibition of VWF expression in EC in vitro was found to cause an increase in proliferation, migration and tube formation, all assays related to angiogenesis. Importantly, these findings were replicated in EC from patients with type 1 or type 2 VWD, which were isolated through a novel technique that uses circulating endothelial progenitors expanded in culture. These cells, called blood outgrowth endothelial cells or BOEC, have allowed for the first time access to EC from the patients, thus opening a new window on the cellular mechanisms controlling VWD. In line with these findings, both vascular development and adult angiogenesis were found to be increased in vivo, in VWF deficient mice. The mechanism of action of VWF in the control of angiogenesis involves enhanced signalling from the growth factor receptor VEGFR2, since an inhibitor to VEGFR2 restored in vitro migration[4] and proliferation (Starke, Randi et al., in preparation) to normal. More recently, a similar result was observed following ablation of VEGFR2 expression in EC in vitro by silencing RNA (Starke, Randi et al., in preparation).
How does VWF control VEGFR2 signalling? The data indicate that this may occur through multiple mechanisms (Figure 2 and[4]). VWF was found to regulate two pathways, possibly linked, which may be controlling angiogenesis: an extracellular pathway involving integrin αvβ3 and an intracellular pathway involving Ang-2 storage in WPB. Both these pathways have been shown to influence VEGF signalling.[28,34]
Integrin αvβ3 is the main endothelial receptor for VWF.[52] αvβ3 is clearly implicated in angiogenesis, although there is some controversy as to its exact role. As discussed above, αvβ3 has been shown to both promote[53,54] and repress angiogenesis.[34] It is likely that the role of αvβ3 on the angiogenic process may depend on the cellular and extracellular context, interacting partners and/or the phase of angiogenesis (rev in[55]). Thus VWF may be modulating angiogenesis partly through interaction with αvβ3 on the endothelial cell surface. Interestingly, αvβ3 levels, function and trafficking were decreased in VWF-deficient EC,[4] suggesting that VWF may regulate αvβ3 activity in multiple ways.
VWF may also control angiogenesis through an intracellular pathway which involves Ang-2. Ang-2 is normally stored WPB with VWF (Figure 1 and [27]). In the absence of VWF, no WPB are formed; therefore Ang-2 may be constitutively released from the cells and presumably acts as a destabilizing, pro-angiogenic agent, as described above. Indeed our studies showed that in VWF-deficient EC in vitro, release of Ang-2 was increased.[4] More recent preliminary data from BOEC confirmed these observations, since Ang-2 release from type 1 and type 3 VWD patients was found to be increased compared to control (Starke, Randi et al., in preparation). Interestingly, Ang-2 has been reported to stimulate the internalisation and degradation of αvβ3[37], which may link the two pathways controlled by VWF.
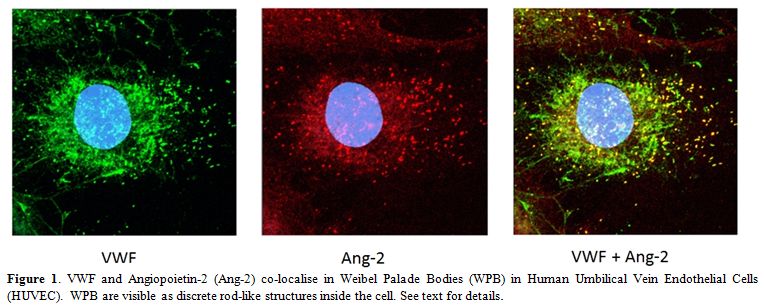
Figure 1. . VWF and Angiopoietin-2 (Ang-2) co-localise in Weibel Palade Bodies (WPB) in Human Umbilical Vein Endothelial Cells (HUVEC). WPB are visible as discrete rod-like structures inside the cell. See text for details.
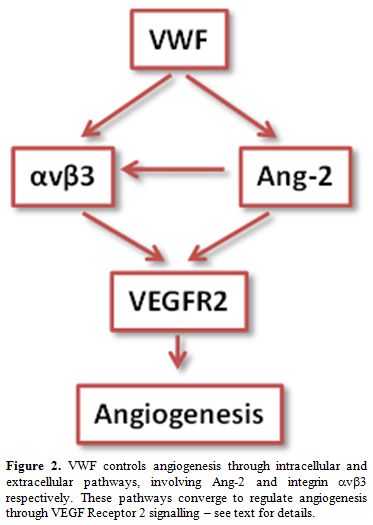
Figure 2. VWF controls angiogenesis through intracellular and extracellular pathways, involving Ang-2 and integrin αvβ3 respectively. These pathways converge to regulate angiogenesis through VEGF Receptor 2 signalling – see text for details.
Besides Ang-2, VWF interacts with or regulates the storage of several proteins which have been implicated in the control of angiogenesis, including interleukin-8,[50] galectin-1[56,57] and galectin-3,[57,58] connective tissue growth factor[59] and insulin-like growth factor binding protein-7.[48,60] Future studies will determine the relative importance of all these pathways in the control of vascular function and angiogenesis by VWF.
These studies suggest that VWF controls stability and quiescence through an intracellular pathway, by directing the formation of WPB and hence the storage of Ang-2 (and possibly other angiogenic regulators), and extracellular pathway, by stabilizing αvβ3 on the cell surface and regulating its levels and activity. In the absence of VWF, these pathways are perturbed and result in enhanced VEGF signalling and as a consequence enhanced proliferation, migration and angiogenesis (see model in Figure 2). Interestingly, preliminary data from BOEC from patients with type 1 & 3 vs type 2 VWD suggest that different types may control angiogenesis through different mechanisms, since Ang-2 storage was normal in type 2 VWD patients (Starke, Randi et al., in preparation).
Von Willebrand Disease, Angiogenesis and Angiodysplasia: Clinical Implications
Many investigators have described an association between VWD and angiodysplasia, particularly in the GI tract (rev in[1,61-63]); severe GI bleeding, which is often not resolved by conventional treatments, remains one of the most serious unmet clinical needs in VWD. Our data suggest that disturbed angiogenesis is linked to the development of angiodysplastic lesions in these patients. Angiodysplasia is most often observed in VWD patients lacking high molecular weight VWF multimers. The survey carried out by Fressinaud and Meyer reviewed histories from 4503 patients with VWD and found the incidence of angiodysplasia to vary with the VWD subtype. Angiodysplasia was most frequently associated with loss of VWF high molecular weight multimers (HMWM), being found in 2% of type 2 and 4.5% of type 3 respectively. In this study, no angiodysplasia in type 1 VWD was reported. Another study found a particular association with the VWD Type 2A mutation S1506L.[64] Interestingly, vascular malformations and GI bleeding are also associated with acquired VWD, often in combination with aortic stenosis, in a triad that has been named Heyde syndrome (rev in[65]), which is also associated with loss of VWF HMWM. Heyde syndrome typically responds to aortic valve replacement with restoration of the normal multimer pattern and cessation of bleeding. For many years it was unclear whether this relationship was one of enhanced detection due to low levels of VWF or whether there was a causal relationship between VWF and GI bleeding. The finding that VWF can directly control vascular stability and angiogenesis provides the first mechanistic link and opens the way to possible novel therapeutic approaches to GI bleeding in VWD. So far, no evidence for a specific role of HMWM has been described in the molecular and cellular models in angiogenesis. However the molecular studies have identified both extracellular and intracellular pathways in the control of angiogenesis; thus it is possible that HMWM may affect the interaction of VWF with EC. Future studies will be required to determine the role of VWF multimers in angiogenesis.
Initial treatment of GI blood loss in patients with VWD is logically carried out with VWF replacement therapy, which can reduce the incidence and severity of recurrent bleeding. However, the von Willebrand Disease Prophylaxis Network (VWD PN) study showed that prophylaxis was less successful at reducing GI blood loss than it was in reducing joint bleeding or menorrhagia.[66] Moreover, it is well recognised that a subgroup of patients continue to have significant blood loss despite otherwise adequate replacement therapy. The failure of VWF replacement coupled with increased understanding of angiogenesis has prompted exploration of alternative therapies for this problem. Some success has been reported with thalidomide in angiodysplasia with or without VWD but this agent has a high incidence of side effects.[67,68] Most recently striking successes have been reported using atorvastatin which has been utilised for its anti-angiogenic effect, but further trials will be required to determine whether this is reproducible.[69,70] Moreover, the characterisation of the molecular pathways through which VWF regulates angiogenesis will provide novel therapeutic targets for the treatment of angiodysplastic GI bleeding.
Conclusions
The finding that VWF regulates angiogenesis clearly has a number of important implications. Firstly, it provides a novel link between VWD and angiodysplasia, which is likely to have therapeutic implications for the future. Secondly, it points the way to investigating the role of VWF in normal development and healing but also in pathological processes such as tumour growth, all of which depend on angiogenesis. We anticipate that these investigations will lead to novel agents to modulate angiogenesis for therapeutic benefit. A critical question for both of these problems will be determining the relative roles of intra- and extra-cellular VWF in regulation of angiogenesis. We therefore remain some way from translation of these exciting findings into clinical practice. Experience to date suggests that replacement therapy does not always correct the defect in angiodysplasia and it is unlikely that simple infusion of VWF will be a panacea for abnormal vasculature.
Acknowledgements
We would like to thank Dr. Koralia Paschalaki for her major contribution on BOEC cultures and for her support throughout the studies. We also thank Dr. Elspeth Payne for her contribution in establishing the BOEC technique in the laboratory.
The presence of vascular abnormalities in von Willebrand disease (VWD) was first described in the 1960s, when Armand J. Quick, one of the pioneers in the study of coagulation, reported the presence of telangectasias, defined as skin and mucous lesions consisting of dilated small blood vessels that tend to bleed (rev in[1]). Since then, several groups have reported the presence of vascular malformation in VWD patients in various localizations, including nail bed,[2] skin, prostate and most frequently angiodysplasia of the gastrointestinal tract.[3] These lesions can be responsible for severe, intractable bleeding which is often not responsive to VWF replacement therapy and thus represent a significant unmet clinical challenge. Until recently, the pathological mechanism underlying vascular malformations in VWD was unexplained. However the recent discovery that von Willebrand factor (VWF) regulates blood vessel formation[4] has shed new light on this syndrome and opened new avenues for the treatment of angiodysplasia. In this review we will summarise the process that led to this discovery, its implications for vascular biology and for the treatment of patients with VWD.
The Cellular and Molecular Basis of Angiogenesis
Angiogenesis (the formation of new blood vessels from pre-existing ones) is a complex process which involves a cascade of events that require fine spatial and temporal coordination (rev in[5]). The initial pro-angiogenic stimulus, often a growth factor produced in response to hypoxia, activates selected endothelial cells (EC) in the pre-existing vascular plexus to undergo changes in polarity and cytoskeletal remodelling, inducing migration towards the source of the pro-angiogenic stimulus. These cells, named tip cells, maintain contact with the adjacent EC, called stalk cells, which acquire a different phenotype.[6] Stalk cells proliferate to support the elongation of the new sprout. Eventually tip cells come into contact with other tip cells and through their thin finger-like protrusions (filopodia) engage in a cell fusion process, which is facilitated by tissue macrophages.[7] Blood flow eventually completes canalisation of the new vascular sprout (rev in[8]). In order to become functional, blood vessels undergo stabilization and maturation, with active remodelling of the newly formed network, recruitment of mural cells and deposition of extracellular matrix.[9] The process requires coordination between EC and other vascular cells, in particular pericytes and smooth muscle cells.
Growth factors driving the initiation of angiogenesis: Vascular endothelial growth factor (VEGF). A large and growing number of molecules involved in regulating angiogenesis have been identified. Some are crucial for the initiation and/or progression of the process and their deficiency or dysregulation is incompatible with vascular development. Many other regulators, however, contribute to downstream steps in this complex process; their defect may give rise to dysfunctional vessels rather than complete disruption of the vasculature (rev in[5,10]). The best characterised pro-angiogenic endothelial growth factor is vascular endothelial growth factor (VEGF), a major regulator of vasculogenesis and physiological angiogenesis during embryogenesis, as well as physiological and pathological angiogenesis in the adult (rev in[5,11]). The VEGF system is also required for lymphangiogenesis (rev in[12]). VEGF-A is the best characterised member of a family which also includes VEGF-B, VEGF-C, VEGF-D and placental-derived growth factor. These bind to the VEGF receptors (R), of which 3 members (VEGF-R1, -R2 and -R3) have been identified. The complexity of the network is further enhanced by splicing and proteolytic cleavage of the ligands (rev in[13]). The main receptor for VEGF in the vascular endothelium is VEGFR2, which is critical for vascular development as well as adult angiogenesis (rev in[14]). VEGF exerts many effects on the vascular endothelium, including promoting proliferation, migration and survival as well as increased permeability (rev in[14]). Binding of VEGF-A to VEGF-R2 on EC stimulates dimerization of the receptor and autophosphorylation of specific intracellular tyrosine residues, leading to activation of intracellular signalling cascades, which lead to cell survival, permeability, migration and/or proliferation.[14] In vivo, VEGF promotes angiogenesis; however overexpression of VEGF leads to the formation of fragile capillaries, with a disrupted structure, reminiscent of angiomas or angiodysplasia.[15,16]
Growth factors controlling quiescence and vascular stability: the Angiopoietins and Tie-2 system. Whilst VEGF controls the early phases of the formation of a new blood vessel, the system most clearly involved in controlling the maturation and stability of new blood vessels is that of Angiopoietins and the Tie-2 receptor. Angiopoietin (Ang)-1 is produced by non-EC, such as pericytes and mural cells that contribute to vascular stability. Ang-1 binds to the tyrosine kinase receptor Tie-2, which is mainly expressed on EC; Ang-1 signalling through Tie2 receptor promotes survival, quiescence and stability of blood vessels. Ang-1 also has anti-permeability and anti-inflammatory functions (rev in[17]). As ever, the picture is complicated by the fact that in some experimental models Ang-1 has been shown to promote cell migration and angiogenesis, in apparent conflict with its pro-quiescence properties. An interesting model has been put forward which proposes that differences in the localization of Tie-2 receptors on EC and their cell surface partners determines whether this signalling pathway supports quiescence or angiogenesis.[18,19]
VEGF and Ang-1 play essential and complementary roles in vascular development and angiogenesis. During embryogenesis, VEGF is required for the formation of the initial vascular plexus, whilst Ang-1 is necessary for the remodelling of this early vascular network into mature blood vessels.[20] A similar interplay between these two systems seems to take place during adult angiogenesis: both VEGF and Ang-1 are able to promote angiogenesis in vivo;[21] however VEGF causes vascular permeability and tissue oedema, whilst Ang-1 contributes to the stabilization and the maturation of growing blood vessels.[22,23] Furthermore, Ang-1 administration or overexpression in the dermal compartment can protect from the potentially lethal actions of VEGF as a consequence of uncontrolled plasma leakage.[24] Co-expression of VEGF and Ang-1 has recently been proposed as a strategy to generate more stable new vessels.[25]
Another crucial regulator of the quiescence/angiogenesis balance is Ang-2. Ang-2 is an antagonistic ligand of Tie-2, which competitively inhibits binding of Ang-1, priming the endothelium for activation and vascular destabilisation. Ang-2 appears to act synergistically with VEGF to promote angiogenesis.[26] Contrary to Ang-1, Ang-2 is synthesised by EC and stored in organelles called Weibel Palade Bodies (WPB), from where it can be rapidly released upon cellular activation.[27] So whilst Ang-1 acts as an agonist of Tie-2, promoting structural integrity of blood vessels, Ang-2 acts as a naturally occurring antagonist, promoting vessel destabilisation and growth, as well as inflammation.[28] Depending on the levels of other growth factors, such as VEGF-A, Ang-2 can also promote vessel regression (rev in[29]). The angiopoietin-Tie-2 system is also an area of intensive research for the development of modulatory drugs (rev in[30]).
Extracellular cues and cell adhesion receptors controlling angiogenesis: integrin αvβ3. Molecular interactions mediated by several adhesion receptors and signalling complexes between cells need to be coordinated to maintain the integrity of the vessel and ultimately to stabilise the newly formed capillary. The extracellular environment is crucial for physiological development of the nascent sprout interaction; cell surface receptors of the integrin family mediate adhesion to and signalling by the extracellular matrix (ECM). Integrins are heterodimeric transmembrane proteins involved in the interaction of cells with their extracellular environment. In response to extracellular cues, integrins are able to transmit so called “outside-in” signals to the cell leading to the activation of signalling cascades via various pathways including those of cellular adhesion and migration. The extracellular conformation of integrins can also be modulated by intracellular processes and transmit so called “inside-out” signals leading to changes in the way the receptor interacts with its extracellular matrix environment and modulation of protease activity (rev in[31]). One integrin receptor in particular, αvβ3, which is expressed on EC and is the best characterised endothelial receptor for VWF, has been shown to play a crucial role in angiogenesis and is a therapeutic target for cancer. The expression of αvβ3 is up-regulated in tumour associated blood vessels[32] and drugs targeting αvβ3 have shown some success in clinical trials (rev in[33]); however its role appears quite complex, since deficiency of this integrin in the mouse has been linked with increased VEGFR2-dependent angiogenesis.[34] Interestingly αvβ3 can associate with VEGFR2 and crosstalk between these receptors can stimulate reciprocal activation (rev in[35]). Ang-1 and -2 have been shown to be able to regulate integrin mediated cell adhesion[36] and Ang-2 can modulate αvβ3 integrin signalling.[19,37]
Angiodysplasia: Vascular Lesions Linked to Abnormal Angiogenesis
Angiogenesis plays a crucial role during embryonic development and in specific processes during adulthood, such as wound healing and the menstrual cycle. Excessive or insufficient angiogenesis has been linked to a growing number of diseases (rev in[38]), and over the last few decades major progress in the understanding of the cellular and molecular basis of the process has been achieved. In parallel to the scientific progress, there has also been intense drug development activity in the search for inhibitors or activators. The area of vascular malformations, however, has received less attention and the links with the pathways controlling angiogenesis are poorly understood. The term angiodysplasia defines vascular malformation, also named ectasia, which affects submucosal veins, mucosal venules and capillaries. The abnormal vascular plexus is fragile and the architecture is disrupted, with possible arteriovenous communications. Angiodysplastic lesions are most commonly observed in the gastrointestinal (GI) tract and are the most common cause of occult GI bleeding in subjects over 65. A firm diagnosis of angiodysplasia may be difficult to achieve, partly because bleeding may be intermittent and partly because not all lesions are accessible to endoscopy. Although angiodysplasia is most frequently located in the proximal large colon (80% of lesions) which is visible by conventional methods, 15% of lesions are located in the small bowel and these may be either missed or require capsule endoscopy, which is not universally available. However, the use of capsule endoscopy has increased the diagnostic yield in patients with obscure GI bleeding to over 60% and as high as 93% in some series, depending on patient selection. This is a significant improvement over push enteroscopy, but in a small number of cases the diagnosis is one of exclusion based on the clinical picture of recurrent GI blood loss.[39]
Despite the limited number of studies on the cellular and molecular basis of angiodysplasia, a link between angiodysplastic lesions and angiogenesis has been identified. The expression of the angiogenic growth factors VEGF and bFGF was found to be increased in samples of angiodysplastic tissue isolated from patients presenting with angiodysplasia.[40,41] Also, increased plasma levels of VEGF have been reported in patients with hereditary haemorrhagic telangectasia (HHT), who present with multiple angiodysplastic lesions,[42] and patients with genetic or acquired VWD[43] (see below).
Von Willebrand Factor as a new Regulator of Angiogenesis
Von Willebrand factor (VWF) is a large multimeric plasma glycoprotein well known for its crucial role in haemostasis, where it mediates platelet adhesion to the endothelium and the sub-endothelial matrix, and acts as a carrier for coagulation factor VIII (FVIII) in plasma. Deficiency or dysfunction of VWF causes von Willebrand disease (VWD), the most common genetic bleeding disorder in man.
VWF is produced by EC and megakaryocytes; in EC, VWF can be constitutively secreted or stored in intracellular organelles called WPB, from where it can be secreted in response to various stimuli (rev in[44]). Although platelets contain VWF, plasma VWF levels have been shown to depend almost entirely on VWF from endothelial cells.[45] The pathways of VWF synthesis, storage and secretion have been extensively investigated (rev in[46]). VWF drives the formation of WPB, which contain numerous proteins (rev in[47]). A proteomic approach has recently identified more WPB proteins.[48] The list of known and newly discovered WPB molecules, shown in table 1, includes several molecules which play a role in angiogenesis.[47-50] Because VWF is essential for WPB formation, these proteins are dependent on VWF for their storage and regulated secretion (see below).

Table 1. Known and potentially novel WPB content (based on Metcalf et al and van Breevoort et al. [47,48]).
In recent years, it has become evident that VWF plays multiple roles in the vasculature. VWF has been shown to control smooth muscle cell proliferation, vascular inflammation, cell death and tumour metastasis (rev in[51]). The large, complex structure of VWF protein supports multiple interactions with cell surface receptors and extracellular matrix proteins; in a recent review by Lenting et al.,[51] VWF has been described as a “molecular bus”, which can interact with 20 other partners. The list of VWF interacting molecules is likely to expand, and with this the understanding of its multiple complex functions.
Recently, our group demonstrated a novel function for VWF in the control of blood vessel formation.[4] Inhibition of VWF expression in EC in vitro was found to cause an increase in proliferation, migration and tube formation, all assays related to angiogenesis. Importantly, these findings were replicated in EC from patients with type 1 or type 2 VWD, which were isolated through a novel technique that uses circulating endothelial progenitors expanded in culture. These cells, called blood outgrowth endothelial cells or BOEC, have allowed for the first time access to EC from the patients, thus opening a new window on the cellular mechanisms controlling VWD. In line with these findings, both vascular development and adult angiogenesis were found to be increased in vivo, in VWF deficient mice. The mechanism of action of VWF in the control of angiogenesis involves enhanced signalling from the growth factor receptor VEGFR2, since an inhibitor to VEGFR2 restored in vitro migration[4] and proliferation (Starke, Randi et al., in preparation) to normal. More recently, a similar result was observed following ablation of VEGFR2 expression in EC in vitro by silencing RNA (Starke, Randi et al., in preparation).
How does VWF control VEGFR2 signalling? The data indicate that this may occur through multiple mechanisms (Figure 2 and[4]). VWF was found to regulate two pathways, possibly linked, which may be controlling angiogenesis: an extracellular pathway involving integrin αvβ3 and an intracellular pathway involving Ang-2 storage in WPB. Both these pathways have been shown to influence VEGF signalling.[28,34]
Integrin αvβ3 is the main endothelial receptor for VWF.[52] αvβ3 is clearly implicated in angiogenesis, although there is some controversy as to its exact role. As discussed above, αvβ3 has been shown to both promote[53,54] and repress angiogenesis.[34] It is likely that the role of αvβ3 on the angiogenic process may depend on the cellular and extracellular context, interacting partners and/or the phase of angiogenesis (rev in[55]). Thus VWF may be modulating angiogenesis partly through interaction with αvβ3 on the endothelial cell surface. Interestingly, αvβ3 levels, function and trafficking were decreased in VWF-deficient EC,[4] suggesting that VWF may regulate αvβ3 activity in multiple ways.
VWF may also control angiogenesis through an intracellular pathway which involves Ang-2. Ang-2 is normally stored WPB with VWF (Figure 1 and [27]). In the absence of VWF, no WPB are formed; therefore Ang-2 may be constitutively released from the cells and presumably acts as a destabilizing, pro-angiogenic agent, as described above. Indeed our studies showed that in VWF-deficient EC in vitro, release of Ang-2 was increased.[4] More recent preliminary data from BOEC confirmed these observations, since Ang-2 release from type 1 and type 3 VWD patients was found to be increased compared to control (Starke, Randi et al., in preparation). Interestingly, Ang-2 has been reported to stimulate the internalisation and degradation of αvβ3[37], which may link the two pathways controlled by VWF.

Figure 1. . VWF and Angiopoietin-2 (Ang-2) co-localise in Weibel Palade Bodies (WPB) in Human Umbilical Vein Endothelial Cells (HUVEC). WPB are visible as discrete rod-like structures inside the cell. See text for details.

Figure 2. VWF controls angiogenesis through intracellular and extracellular pathways, involving Ang-2 and integrin αvβ3 respectively. These pathways converge to regulate angiogenesis through VEGF Receptor 2 signalling – see text for details.
Besides Ang-2, VWF interacts with or regulates the storage of several proteins which have been implicated in the control of angiogenesis, including interleukin-8,[50] galectin-1[56,57] and galectin-3,[57,58] connective tissue growth factor[59] and insulin-like growth factor binding protein-7.[48,60] Future studies will determine the relative importance of all these pathways in the control of vascular function and angiogenesis by VWF.
These studies suggest that VWF controls stability and quiescence through an intracellular pathway, by directing the formation of WPB and hence the storage of Ang-2 (and possibly other angiogenic regulators), and extracellular pathway, by stabilizing αvβ3 on the cell surface and regulating its levels and activity. In the absence of VWF, these pathways are perturbed and result in enhanced VEGF signalling and as a consequence enhanced proliferation, migration and angiogenesis (see model in Figure 2). Interestingly, preliminary data from BOEC from patients with type 1 & 3 vs type 2 VWD suggest that different types may control angiogenesis through different mechanisms, since Ang-2 storage was normal in type 2 VWD patients (Starke, Randi et al., in preparation).
Von Willebrand Disease, Angiogenesis and Angiodysplasia: Clinical Implications
Many investigators have described an association between VWD and angiodysplasia, particularly in the GI tract (rev in[1,61-63]); severe GI bleeding, which is often not resolved by conventional treatments, remains one of the most serious unmet clinical needs in VWD. Our data suggest that disturbed angiogenesis is linked to the development of angiodysplastic lesions in these patients. Angiodysplasia is most often observed in VWD patients lacking high molecular weight VWF multimers. The survey carried out by Fressinaud and Meyer reviewed histories from 4503 patients with VWD and found the incidence of angiodysplasia to vary with the VWD subtype. Angiodysplasia was most frequently associated with loss of VWF high molecular weight multimers (HMWM), being found in 2% of type 2 and 4.5% of type 3 respectively. In this study, no angiodysplasia in type 1 VWD was reported. Another study found a particular association with the VWD Type 2A mutation S1506L.[64] Interestingly, vascular malformations and GI bleeding are also associated with acquired VWD, often in combination with aortic stenosis, in a triad that has been named Heyde syndrome (rev in[65]), which is also associated with loss of VWF HMWM. Heyde syndrome typically responds to aortic valve replacement with restoration of the normal multimer pattern and cessation of bleeding. For many years it was unclear whether this relationship was one of enhanced detection due to low levels of VWF or whether there was a causal relationship between VWF and GI bleeding. The finding that VWF can directly control vascular stability and angiogenesis provides the first mechanistic link and opens the way to possible novel therapeutic approaches to GI bleeding in VWD. So far, no evidence for a specific role of HMWM has been described in the molecular and cellular models in angiogenesis. However the molecular studies have identified both extracellular and intracellular pathways in the control of angiogenesis; thus it is possible that HMWM may affect the interaction of VWF with EC. Future studies will be required to determine the role of VWF multimers in angiogenesis.
Initial treatment of GI blood loss in patients with VWD is logically carried out with VWF replacement therapy, which can reduce the incidence and severity of recurrent bleeding. However, the von Willebrand Disease Prophylaxis Network (VWD PN) study showed that prophylaxis was less successful at reducing GI blood loss than it was in reducing joint bleeding or menorrhagia.[66] Moreover, it is well recognised that a subgroup of patients continue to have significant blood loss despite otherwise adequate replacement therapy. The failure of VWF replacement coupled with increased understanding of angiogenesis has prompted exploration of alternative therapies for this problem. Some success has been reported with thalidomide in angiodysplasia with or without VWD but this agent has a high incidence of side effects.[67,68] Most recently striking successes have been reported using atorvastatin which has been utilised for its anti-angiogenic effect, but further trials will be required to determine whether this is reproducible.[69,70] Moreover, the characterisation of the molecular pathways through which VWF regulates angiogenesis will provide novel therapeutic targets for the treatment of angiodysplastic GI bleeding.
Conclusions
The finding that VWF regulates angiogenesis clearly has a number of important implications. Firstly, it provides a novel link between VWD and angiodysplasia, which is likely to have therapeutic implications for the future. Secondly, it points the way to investigating the role of VWF in normal development and healing but also in pathological processes such as tumour growth, all of which depend on angiogenesis. We anticipate that these investigations will lead to novel agents to modulate angiogenesis for therapeutic benefit. A critical question for both of these problems will be determining the relative roles of intra- and extra-cellular VWF in regulation of angiogenesis. We therefore remain some way from translation of these exciting findings into clinical practice. Experience to date suggests that replacement therapy does not always correct the defect in angiodysplasia and it is unlikely that simple infusion of VWF will be a panacea for abnormal vasculature.
Acknowledgements
We would like to thank Dr. Koralia Paschalaki for her major contribution on BOEC cultures and for her support throughout the studies. We also thank Dr. Elspeth Payne for her contribution in establishing the BOEC technique in the laboratory.
References
- Quick AJ. Telangiectasia: its relationship to the Minot-von Willebrand syndrome. Am.J Med Sci. 1967;254:585-601. http://dx.doi.org/10.1097/00000441-196711000-00002 PMid:4862041

- Koscielny JK, Latza R, Mursdorf S et al.
Capillary microscopic and rheological dimensions for the diagnosis of
von Willebrand disease in comparison to other haemorrhagic diatheses.
Thromb. Haemost. 2000;84:981-988. PMid:11154145

- Duray PH, Marcal JM, Jr., LiVolsi VA et al.
Gastrointestinal angiodysplasia: a possible component of von
Willebrand's disease. Hum. Pathol. 1984;15:539-544. http://dx.doi.org/10.1016/S0046-8177(84)80007-6

- Starke RD, Ferraro F, Paschalaki KE et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011;117:1071-1080. http://dx.doi.org/10.1182/blood-2010-01-264507 PMid:21048155 PMCid:PMC3035068

- Carmeliet P. Angiogenesis in health and disease. Nat.Med. 2003;9:653-660. http://dx.doi.org/10.1038/nm0603-653 PMid:12778163

- Gerhardt H, Golding M, Fruttiger M et al.
VEGF guides angiogenic sprouting utilizing endothelial tip cell
filopodia. J Cell Biol 2003;161:1163-1177. http://dx.doi.org/10.1083/jcb.200302047 PMid:12810700 PMCid:PMC2172999

- Fantin A, Vieira JM, Gestri G et al. Tissue
macrophages act as cellular chaperones for vascular anastomosis
downstream of VEGF-mediated endothelial tip cell induction. Blood
2010;116:829-840. http://dx.doi.org/10.1182/blood-2009-12-257832 PMid:20404134 PMCid:PMC2938310

- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr.Opin.Cell Biol 2010;22:617-625. http://dx.doi.org/10.1016/j.ceb.2010.08.010 PMid:20817428

- Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685-693. http://dx.doi.org/10.1038/nm0603-685 PMid:12778167

- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873-887. http://dx.doi.org/10.1016/j.cell.2011.08.039 PMid:21925313

- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 2009;20:158-163. PMid:20167554

- Lohela M, Bry M, Tammela T, Alitalo K.
VEGFs and receptors involved in angiogenesis versus lymphangiogenesis.
Curr. Opin. Cell Biol 2009;21:154-165. http://dx.doi.org/10.1016/j.ceb.2008.12.012 PMid:19230644

- Ladomery MR, Harper SJ, Bates DO.
Alternative splicing in angiogenesis: the vascular endothelial growth
factor paradigm. Cancer Lett. 2007;249:133-142. http://dx.doi.org/10.1016/j.canlet.2006.08.015 PMid:17027147

- Olsson AK, Dimberg A, Kreuger J,
Claesson-Welsh L. VEGF receptor signalling - in control of vascular
function. Nat. Rev. Mol. Cell Biol 2006;7:359-371. http://dx.doi.org/10.1038/nrm1911 PMid:16633338

- Springer ML, Chen AS, Kraft PE, Bednarski
M, Blau HM. VEGF gene delivery to muscle: potential role for
vasculogenesis in adults. Mol. Cell 1998;2:549-558. http://dx.doi.org/10.1016/S1097-2765(00)80154-9

- Schwarz ER, Speakman MT, Patterson M et
al. Evaluation of the effects of intramyocardial injection of DNA
expressing vascular endothelial growth factor (VEGF) in a myocardial
infarction model in the rat--angiogenesis and angioma formation. J. Am.
Coll. Cardiol. 2000;35:1323-1330. http://dx.doi.org/10.1016/S0735-1097(00)00522-2

- Eklund L, Olsen BR. Tie receptors and
their angiopoietin ligands are context-dependent regulators of vascular
remodeling. Exp. Cell Res. 2006;312:630-641. http://dx.doi.org/10.1016/j.yexcr.2005.09.002 PMid:16225862

- Fukuhara S, Sako K, Minami T et al.
Differential function of Tie2 at cell-cell contacts and cell-substratum
contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513-526. http://dx.doi.org/10.1038/ncb1714 PMid:18425120

- Felcht M, Luck R, Schering A et al.
Angiopoietin-2 differentially regulates angiogenesis through TIE2 and
integrin signaling. J. Clin. Invest 2012;122:1991-2005. http://dx.doi.org/10.1172/JCI58832 PMid:22585576 PMCid:PMC3366398

- Suri C, Jones PF, Patan S et al. Requisite
role of angiopoietin-1, a ligand for the TIE2 receptor, during
embryonic angiogenesis. Cell 1996;87:1171-1180. http://dx.doi.org/10.1016/S0092-8674(00)81813-9

- Suri C, McClain J, Thurston G et al. Increased vascularization in mice overexpressing angiopoietin-1. Science 1998;282:468-471. http://dx.doi.org/10.1126/science.282.5388.468 PMid:9774272

- Senger DR, Galli SJ, Dvorak AM et al.
Tumor cells secrete a vascular permeability factor that promotes
accumulation of ascites fluid. Science 1983;219:983-985. http://dx.doi.org/10.1126/science.6823562 PMid:6823562

- Thurston G, Suri C, Smith K et al.
Leakage-resistant blood vessels in mice transgenically overexpressing
angiopoietin-1. Science 1999;286:2511-2514. http://dx.doi.org/10.1126/science.286.5449.2511 PMid:10617467

- Thurston G, Rudge JS, Ioffe E et al.
Angiopoietin-1 protects the adult vasculature against plasma leakage.
Nat. Med. 2000;6:460-463. http://dx.doi.org/10.1038/74725 PMid:10742156

- Tao Z, Chen B, Tan X et al. Coexpression
of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte
proliferation reduces apoptosis in porcine myocardial infarction (MI)
heart. Proc. Natl. Acad. Sci. U.S.A 2011;108:2064-2069. http://dx.doi.org/10.1073/pnas.1018925108 PMid:21245320 PMCid:PMC3033313

- Daly C, Eichten A, Castanaro C et al.
Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it
limits the effects of VEGF inhibition. Cancer Res. 2013;73:108-118. http://dx.doi.org/10.1158/0008-5472.CAN-12-2064 PMid:23149917

- Fiedler U, Scharpfenecker M, Koidl S et
al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released
upon stimulation from endothelial cell Weibel-Palade bodies. Blood
2004;103:4150-4156. http://dx.doi.org/10.1182/blood-2003-10-3685 PMid:14976056

- Lobov IB, Brooks PC, Lang RA.
Angiopoietin-2 displays VEGF-dependent modulation of capillary
structure and endothelial cell survival in vivo. Proceedings of the
National Academy of Sciences of the United States of America
2002;99:11205-11210. http://dx.doi.org/10.1073/pnas.172161899 PMid:12163646 PMCid:PMC123234

- Holash J, Wiegand SJ, Yancopoulos GD. New
model of tumor angiogenesis: dynamic balance between vessel regression
and growth mediated by angiopoietins and VEGF. Oncogene
1999;18:5356-5362. http://dx.doi.org/10.1038/sj.onc.1203035 PMid:10498889

- Augustin HG, Koh GY, Thurston G, Alitalo
K. Control of vascular morphogenesis and homeostasis through the
angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol 2009;10:165-177. http://dx.doi.org/10.1038/nrm2639 PMid:19234476

- Desgrosellier JS, Cheresh DA. Integrins in
cancer: biological implications and therapeutic opportunities. Nat Rev
Cancer 2010;10:9-22. http://dx.doi.org/10.1038/nrc2748 PMid:20029421

- Gladson CL, Cheresh DA. Glioblastoma
expression of vitronectin and the alpha v beta 3 integrin. Adhesion
mechanism for transformed glial cells. J. Clin. Invest
1991;88:1924-1932. http://dx.doi.org/10.1172/JCI115516 PMid:1721625 PMCid:PMC295768

- Scaringi C, Minniti G, Caporello P, Enrici
RM. Integrin inhibitor cilengitide for the treatment of glioblastoma: a
brief overview of current clinical results. Anticancer Res.
2012;32:4213-4223. PMid:23060541

- Reynolds LE, Wyder L, Lively JC et al.
Enhanced pathological angiogenesis in mice lacking beta3 integrin or
beta3 and beta5 integrins. Nat. Med. 2002;8:27-34. http://dx.doi.org/10.1038/nm0102-27 PMid:11786903

- Somanath PR, Malinin NL, Byzova TV.
Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis.
Angiogenesis. 2009;12:177-185. http://dx.doi.org/10.1007/s10456-009-9141-9 PMid:19267251 PMCid:PMC2863048

- Carlson TR, Feng Y, Maisonpierre PC,
Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated
by integrins. J. Biol. Chem. 2001;276:26516-26525. http://dx.doi.org/10.1074/jbc.M100282200 PMid:11346644

- Thomas M, Felcht M, Kruse K et al.
Angiopoietin-2 stimulation of endothelial cells induces aVb3 integrin
internalization and degradation. Journal of Biological Chemistry 2010 http://dx.doi.org/10.1074/jbc.M109.097543

- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005;438:932-936. http://dx.doi.org/10.1038/nature04478 PMid:16355210

- Regula J, Wronska E, Pachlewski J.
Vascular lesions of the gastrointestinal tract. Best. Pract. Res. Clin.
Gastroenterol. 2008;22:313-328. http://dx.doi.org/10.1016/j.bpg.2007.10.026 PMid:18346686

- Tan H, Chen H, Xu C et al. Role of
vascular endothelial growth factor in angiodysplasia: an interventional
study with thalidomide. J. Gastroenterol. Hepatol. 2012;27:1094-1101. http://dx.doi.org/10.1111/j.1440-1746.2011.06967.x PMid:22098296

- Junquera F, Saperas E, de T, I, Vidal MT,
Malagelada JR. Increased expression of angiogenic factors in human
colonic angiodysplasia. Am. J. Gastroenterol. 1999;94:1070-1076. http://dx.doi.org/10.1111/j.1572-0241.1999.01017.x PMid:10201485

- Cirulli A, Liso A, D'Ovidio F et al.
Vascular endothelial growth factor serum levels are elevated in
patients with hereditary hemorrhagic telangiectasia. Acta Haematol.
2003;110:29-32. http://dx.doi.org/10.1159/000072411 PMid:12975554

- Gritti G, Cortelezzi A, Bucciarelli P et
al. Circulating and progenitor endothelial cells are abnormal in
patients with different types of von Willebrand disease and correlate
with markers of angiogenesis. Am. J. Hematol. 2011;86:650-656. http://dx.doi.org/10.1002/ajh.22070 PMid:21630316

- Rondaij MG, Bierings R, Kragt A, van
Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies
in endothelial cells. Arterioscler.Thromb. Vasc. Biol.
2006;26:1002-1007. http://dx.doi.org/10.1161/01.ATV.0000209501.56852.6c PMid:16469951

- Kanaji S, Fahs SA, Shi Q, Haberichter SL,
Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet
adhesion and hemostasis. J.Thromb.Haemost. 2012;10:1646-1652. http://dx.doi.org/10.1111/j.1538-7836.2012.04797.x PMid:22642380 PMCid:PMC3419786

- Michaux G, Cutler DF. How to roll an endothelial cigar: the biogenesis of Weibel-Palade bodies. Traffic. 2004;5:69-78. http://dx.doi.org/10.1111/j.1600-0854.2004.00157.x

- Metcalf DJ, Nightingale TD, Zenner HL,
Lui-Roberts WW, Cutler DF. Formation and function of Weibel-Palade
bodies. J. Cell Sci. 2008;121:19-27. http://dx.doi.org/10.1242/jcs.03494 PMid:18096688

- van Breevoort D, van Agtmaal EL, Dragt BS
et al. Proteomic screen identifies IGFBP7 as a novel component of
endothelial cell-specific Weibel-Palade bodies. J. Proteome. Res.
2012;11:2925-2936. http://dx.doi.org/10.1021/pr300010r PMid:22468712

- Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125-137. http://dx.doi.org/10.1007/s10456-009-9147-3 PMid:19449109

- Petreaca ML, Yao M, Liu Y, Defea K,
Martins-Green M. Transactivation of vascular endothelial growth factor
receptor-2 by interleukin-8 (IL-8/CXCL8) is required for
IL-8/CXCL8-induced endothelial permeability. Mol. Biol. Cell
2007;18:5014-5023. http://dx.doi.org/10.1091/mbc.E07-01-0004 PMid:17928406 PMCid:PMC2096609

- Lenting PJ, Casari C, Christophe OD, Denis
CV. von Willebrand factor: the old, the new and the unknown. J. Thromb.
Haemost. 2012;10:2428-2437. http://dx.doi.org/10.1111/jth.12008 PMid:23020315

- Cheresh DA. Human endothelial cells
synthesize and express an Arg-Gly-Asp-directed adhesion receptor
involved in attachment to fibrinogen and von Willebrand factor. Proc.
Natl. Acad. Sci. U.S.A 1987;84:6471-6475. http://dx.doi.org/10.1073/pnas.84.18.6471 PMid:2442758 PMCid:PMC299099

- Brooks PC, Montgomery AM, Rosenfeld M et
al. Integrin alpha v beta 3 antagonists promote tumor regression by
inducing apoptosis of angiogenic blood vessels. Cell 1994;79:1157-1164.
http://dx.doi.org/10.1016/0092-8674(94)90007-8

- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569-571. http://dx.doi.org/10.1126/science.7512751 PMid:7512751

- Robinson SD, Hodivala-Dilke KM. The role
of beta3-integrins in tumor angiogenesis: context is everything. Curr.
Opin. Cell Biol. 2011;23:630-637. http://dx.doi.org/10.1016/j.ceb.2011.03.014 PMid:21565482

- Thijssen VL, Postel R, Brandwijk RJ et al.
Galectin-1 is essential in tumor angiogenesis and is a target for
antiangiogenesis therapy. Proc. Natl. Acad. Sci. U.S.A
2006;103:15975-15980. http://dx.doi.org/10.1073/pnas.0603883103 PMid:17043243 PMCid:PMC1635112

- Saint-Lu N, Oortwijn BD, Pegon JN et al.
Identification of galectin-1 and galectin-3 as novel partners for von
Willebrand factor. Arterioscler. Thromb. Vasc. Biol. 2012;32:894-901. http://dx.doi.org/10.1161/ATVBAHA.111.240309 PMid:22267483

- Markowska AI, Liu FT, Panjwani N.
Galectin-3 is an important mediator of VEGF- and bFGF-mediated
angiogenic response. J. Exp. Med. 2010;207:1981-1993. http://dx.doi.org/10.1084/jem.20090121 PMid:20713592 PMCid:PMC2931172

- Pi L, Shenoy AK, Liu J et al. CCN2/CTGF
regulates neovessel formation via targeting structurally conserved
cystine knot motifs in multiple angiogenic regulators. FASEB J.
2012;26:3365-3379. http://dx.doi.org/10.1096/fj.11-200154 PMid:22611085 PMCid:PMC3405264

- Tamura K, Hashimoto K, Suzuki K et al.
Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular
endothelial cell growth factor (VEGF)-induced angiogenesis in human
vascular endothelial cells. Eur. J. Pharmacol. 2009;610:61-67. http://dx.doi.org/10.1016/j.ejphar.2009.01.045 PMid:19374835

- Warkentin TE, Moore JC, Anand SS, Lonn EM,
Morgan DG. Gastrointestinal bleeding, angiodysplasia, cardiovascular
disease, and acquired von Willebrand syndrome. Transfus. Med. Rev.
2003;17:272-286. http://dx.doi.org/10.1016/S0887-7963(03)00037-3

- Makris M. Gastrointestinal bleeding in von Willebrand disease. Thromb.Res. 2006;118 Suppl 1:S13-S17. http://dx.doi.org/10.1016/j.thromres.2006.01.022 PMid:16542710

- Fressinaud E, Meyer D. International
survey of patients with von Willebrand disease and angiodysplasia.
Thromb.Haemost. 1993;70:546. PMid:8259565

- Castaman G, Federici AB, Tosetto A et al.
Different bleeding risk in type 2A and 2M von Willebrand disease: a
2-year prospective study in 107 patients. J.Thromb.Haemost.
2012;10:632-638. http://dx.doi.org/10.1111/j.1538-7836.2012.04661.x PMid:22329792

- Massyn MW, Khan SA. Heyde syndrome: a
common diagnosis in older patients with severe aortic stenosis. Age
Ageing 2009;38:267-270. http://dx.doi.org/10.1093/ageing/afp019 PMid:19276092

- Abshire TC, Federici AB, Alvarez MT et al.
Prophylaxis in severe forms of von Willebrand's disease: results from
the von Willebrand Disease Prophylaxis Network (VWD PN). Haemophilia.
2013;19:76-81. http://dx.doi.org/10.1111/j.1365-2516.2012.02916.x PMid:22823000

- Nomikou E, Tsevrenis V, Gafou A, Bellia M,
Theodossiades G. Type IIb von Willebrand disease with angiodysplasias
and refractory gastrointestinal bleeding successfully treated with
thalidomide. Haemophilia. 2009;15:1340-1342. http://dx.doi.org/10.1111/j.1365-2516.2009.02085.x PMid:19702883

- Bauditz J, Schachschal G, Wedel S, Lochs H. Thalidomide for treatment of severe intestinal bleeding. Gut 2004;53:609-612. http://dx.doi.org/10.1136/gut.2003.029710 PMid:15016759 PMCid:PMC1774015

- Sohal M, Laffan M. Von Willebrand disease and angiodysplasia responding to atorvastatin. Br. J. Haematol. 2008;142:308-309. http://dx.doi.org/10.1111/j.1365-2141.2008.07005.x PMid:18510690

- Alikhan R, Keeling D. Von Willebrand disease, angiodysplasia and atorvastatin. Br. J. Haematol. 2010;149:159-160. http://dx.doi.org/10.1111/j.1365-2141.2009.08031.x PMid:19995387
