Tuberculosis in Hematopoietic Stem Cell Transplant Recipients
Jéssica Fernandes Ramos1, Marjorie Vieira Batista1, Silvia Figueiredo Costa1,2
1 Group of Infection in immunocompromised patients of Hospital das Clinicas of University of São Paulo, Brazil.
2 Department of Infectious Diseases, Medical School, University of São Paulo, São Paulo, Brazil.
2 Department of Infectious Diseases, Medical School, University of São Paulo, São Paulo, Brazil.
Correspondence
to:
Professor Silvia Figueiredo Costa. Department of Infectious Diseases, Medical School, University of São Paulo, Brazil. E-mail: costasilviaf@ig.com.br and silviacosta@usp.br
Published: November 4, 2013
Received: July 30, 2013
Accepted: October 16, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013061, DOI 10.4084/MJHID.2013.061
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Literature
on tuberculosis (TB) occurring in recipients of Hematopoietic Stem Cell
Transplant (HSCT) is scanty even in countries where TB is common.
Most reports of TB in HSCT patients were from ASIA, in fact the TB
incidence ranging from 0.0014 (USA) to 16% (Pakistan). There are few
reports of TB diagnosis during the first two weeks after HSCT; most of
cases described in the literature occurred after 90 days of HSCT, and
the lung was the organ most involved. The mortality ranged from 0 to
50% and is higher in allogeneic HSCT than in autologous. There is no
consensus regarding the screening with tuberculin skin test or
QuantiFERON-TB gold, primary prophylaxis for latent TB, and whether the
epidemiologic query should be emphasized in developing countries with
high prevalence of TB.
Introduction
Hematopoietic Stem cell transplant (HSCT) recipients have severe impairment in cell-mediated immunity as result of the conditioning regimen, immunosuppressive therapy and graft-versus-host disease (GVHD).[1,2] Accordingly, they are susceptible to bacterial, viral, and fungal infections. Mycobacterial infections can also occur in these patients, although the incidence is not high, even in countries where tuberculosis (TB) is common.[2] TB in this population of patient is mainly due to reactivation of latent infection.[2] However, in country where TB is endemic, pulmonary tuberculosis could be due to a new infection.
Data from the United States showed that the incidence of mycobacterium infection (MBI) among HSCT ranges from 0, 0014 % to 3%.[1,3-5] Countries in which the prevalence of tuberculosis in the general population is higher than it is in the United States have reported incidences varying from 1.6% in Spain[6] and Turkey,[7] 8.57% in Hong Kong and Taiwan to 16% in Pakistan.[8-11]
There are few reports of TB diagnosis during the first two weeks after HSCT (Table 1). Most of cases described in the literature occurred after 90 days of HSCT.[12-28]
The incidence of TB in HSCT ranged from 0, 0014% to 16% (Table 1). The diagnosis of TB varied from +21 to +1410 (median of 257, 2 days) after the HSCT. The lung was the organ most often involved. Most of cases were described in allogeneic HSCT, and the mortality in this type of HSCT was high, varying from to 0% to 50% (Table 1).
There is no consensus regarding the screening with tuberculin skin test or QuantiFERON-TB gold and primary prophylaxis for latent TB.[29-33]
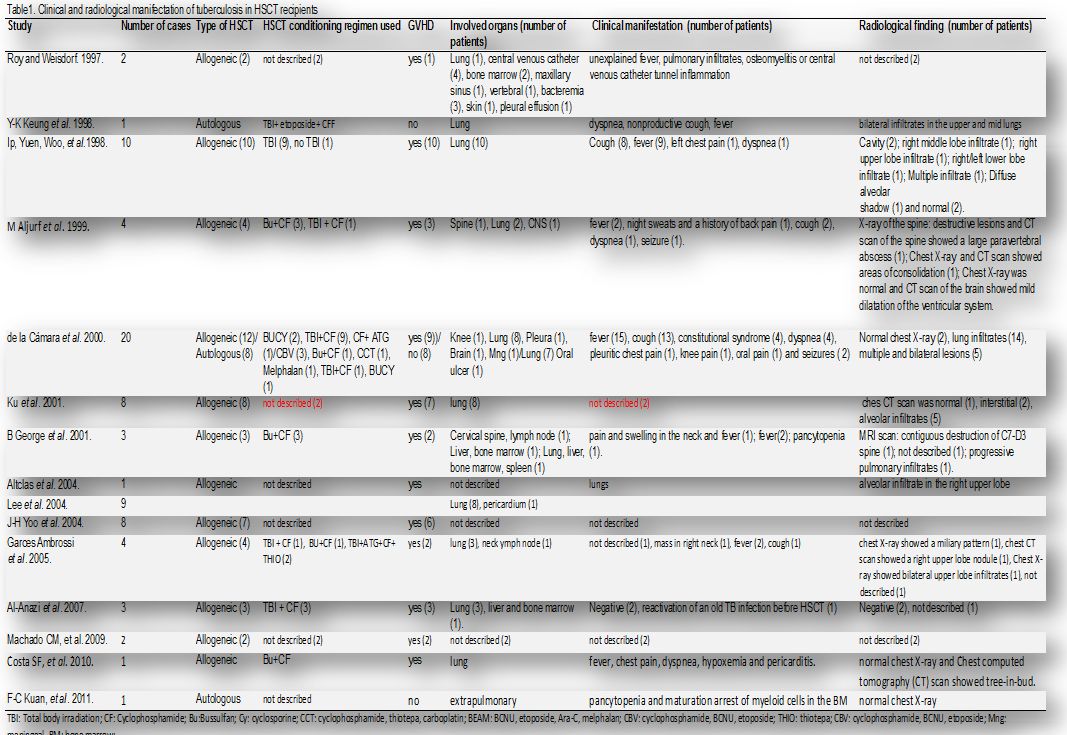
Table 1. Clinical and radiological manifestation of tuberculosis in HSCT recipients
TB Risk Factors
The increased risk of tuberculosis in this setting could be explained by severely impaired cell-mediated immunity as a result of their underlying illness and conditioning chemotherapy. However, there is a lack of prospective studies on TB in HSCT and the clinical, radiological and epidemiological data are based on case reports and retrospective analysis with a small number of patients.[27,34,35,37] Previous study had showed the main risk factors for tuberculosis, comparing to control group, were allogeneic transplantations with unrelated donors and total body irradiation (TBI), with a relative risk (RR) of 23.9 and 4.9 respectively (p<0.05), and chronic GVHD with RR of 3.6 (p<0.05).[34] Another retrospective study observed that the majority of allogeneic patients with post-transplant tuberculosis received corticosteroids for GVHD.[27] Patients with chronic GVHD have a marked delay of T cell subset recovery with low numbers of CD4 cells being present. As this period of impairment of T cell function may be indefinite in the presence of chronic GVHD, it might contribute to an increased predisposition to tuberculosis.[35-37]
Symptoms of TB diseases
The clinical presentation of tuberculosis in patients undergoing HSCT may have a wide range of signs, symptoms and radiological findings (Table 1). Tuberculosis following conventional HSCT usually presents indolent clinical courses. The lung is the most common site of the disease, and the usual manifestations are fever, cough, dyspnea and hypoxemia (Table 1). Although the clinical and radiographic presentation of TB in the HSCT population usually mimics that in the non-transplant population, atypical presentations have been described, such as diffuse alveolar hemorrhage.[34] In immunocompromised host patients, the radiological finding could be mitigated or absent on chest x-ray and on this panel the CT-scan provided additional information to help diagnose pulmonary TB.[38]
In regard to the radiologic features of pulmonary tuberculosis in HSCT recipients, recent studies evaluated chest X-ray[10] and CT-scan.[7] The chest X-ray abnormalities were air-space consolidation (100%) and nodules (80%). On chest CT scans, the most common parenchymal lesions were consolidation (100%), nodules (71%), tree-in-bud appearance (43%), and ground-glass opacity (43%). Cavities were present on CT scans in only 14% of the study patients and lymphadenopathies were noted in 71%.
TB Diagnosis
A suggestive clinical and radiologic finding is not enough to allow initiating specific treatment in patients submitted to HSCT, as recommended in populations of high endemic TB countries. The bacteriological diagnosis is crucial once the incidence is low and differential etiology should be excluded.
Similarly to other scenarios, the main challenge is to obtain a result as fast as possible to start appropriate treatment and establish environmental infection control measures.[2]
Thus, the recommendation is to detect the presence of acid-fast bacilli (AFB) in samples as soon as possible and that these samples should be cultured for identification and antimicrobial susceptibility testing.[39] Some authors had already reported cases of transplanted patients with disseminated non-tuberculosis mycobacteria infection and drug resistant TB, reinforcing the importance of such complimentary tests.[40]
An important obstacle to a fast invasive diagnostic test is the clinical condition characterized by bone marrow suppression (anemia, thrombocytopenia), although some studies showed that investigational procedures like lung CT guide-biopsy are efficacious and safe in this population.[41]
A recently published review of 56 cases of tuberculosis in HSCT, showed that only 55% of cases were diagnosed with culture, whereas acid-fast bacilli (26%) was the second most common approach for diagnosis and histology was responsible for 20,3%.[2]
Molecular methods are increasingly used in diagnosis since they are faster than mycobacterial cultures (3-6 weeks) and have the potential role to determine species. However, there are still some issues in interpreting mycobacterial DNA in respiratory samples (Table 2).[43,44,46] Lee et al.[45] studied real time PCR in CT guided BAL in 99 patients unable to produce sputum samples or with AFB negative result: PCR showed a positive predictive value in 81% out of 27 patients with confirmed TB, although with a lower sensitivity.
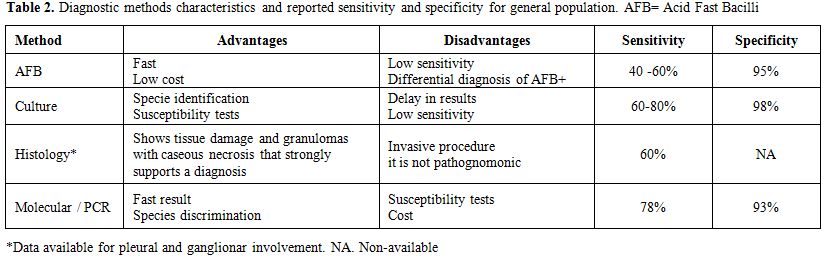
Table 2. Diagnostic methods characteristics and reported sensitivity and specificity for general population. AFB=Acid Fast Bacilli
In general population molecular tests are increasingly used, and several guidelines as UK most recent recommendations (2011) from the National Institute for Health and Clinical Excellence (NICE) on the diagnosis of latent tuberculosis and of active tuberculosis even mention these methods.[47]
The common approach is to use rapid diagnostic tests for Mycobacterium tuberculosis complex (M tuberculosis, M bovis, M africanum) on bronchial specimens of patients only if rapid confirmation would alter the patient’s care or before conducting a large contact tracing initiative. Such tests are not recommended for pleural, cerebrospinal fluid, or urine in order to exclude the diagnosis of TB as they have a high false negative rate.[48]
The Xpert MTB/RIF assay, which enables simultaneous detection of Mycobacterium tuberculosis (MTB) and rifampicin (RIF) resistance, was endorsed by World Health Organization (WHO) in December, 2010. This assay was specifically recommended for use as the initial diagnostic test for suspected drug-resistant or HIV-associated pulmonary tuberculosis. One Xpert MTB/RIF test on sputum detects 90% of pulmonary tuberculosis (99% of smear-positive disease and about 75% of smear-negative disease). Such high sensitivity of Xpert MTB/RIF for rifampicin resistance is accompanied by some false-positive results and confirmatory drug sensitivity testing is needed.[49]
A different test based on antigen detection was already studied in the general population. Rajpal and colleagues studied an India tribal population where nearly 80% suffers from malnutrition. In this group, they compared 41 patients with active TB (90% confirmed with AFB and/or culture positive) with 87 controls to three different diagnostic blood tests: ELISA based TB antigen, ADA and in house IS6110 based PCR. The positivity of PCR assay in TB patients was around 48.78%, which was found to be significant (p < 0.0001) than the control group (2.30%). Mean absorbance of Antigen 85 ELISA in TB group was significantly (p<0.05) higher than non-TB control group. There was no immunocompromised patient (HIV or immunossupressors) in their cohort.[50]
Blood TB PCR is still not standardized, but offers great promise for the rapid and specific diagnosis. Indian investigators recently evaluated a multiplex nested PCR method targeting insertion sequence 6110 and MPB64 sequence in 130 samples. The sensitivity and specificity of the assay was respectively 95.7% and 100% with a negative predictive value of 99.2%.[51]
Molecular techniques such as polymerase chain reaction (PCR) was rarely used for diagnosis in HSCT patients reported (3.7%), and are mainly used in combination with other diagnostic approach.[2]
There are few studies, however, comparing accuracy of different tests in this transplant setting, and most of the data come from general population, mainly from pulmonary TB.[46,57]
Treatment of TB
The use of drugs against TB should be in agreement with local recommendations, based on surveillance of mycobacterial resistance patterns to antimicrobial. Since the first drugs available in 1940s, the subsequent emergence of resistant species led to combination therapy.[53,54] In fact the treatment of TB in HSCT recipients are similar to the general population with two important differences: the duration of therapy, normally longer, and the drug regimen, because interaction was reported between rifamycins (rifampicin and rifabutin) and immunosuppressive drugs like cyclosporine.
The approach recommended by World Health Organization, North American and European guidelines is a 6-month chemotherapy regimen using a combination of 4 drugs (rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months, followed by rifampicin and isoniazid for 4 months) with cure rates of approximately 90% of drug-susceptible cases.[54-56] The Brazilian recommendation is in agreement with other countries (Table 3).[57]
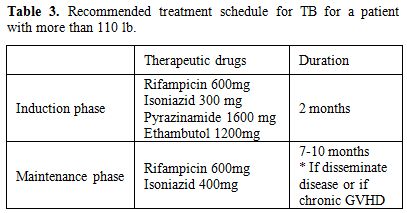
Table 3. Recommended treatment schedule for TB for a patient with more than 110 lb.
Most of the reports with combination therapy in HSCT patients had shown good results. La Camara and colleagues treated nineteen patients with a median of three drugs. Seventeen received more than 3 months of treatment and all were cured. An interesting finding of this study was the interaction between cyclosporine with rifampicin, with three patients presenting a decrease in cyclosporine levels, two of whom with worsening in chronic GVHD. Three patients (18%) developed hepatotoxicity.[56]
In more pronounced immunosuppression state like in unrelated cord blood transplantation there is already TB cases reported[52,53] In this cases the therapy of choice, even with possible drug interaction, was the classic four drugs of therapeutic guidelines.
The treatment of tuberculosis (TB) is complicated by drug-induced hepatotoxicity, one of the leading types of drug-induced liver injury (DILI). Current recommendations are to obtain baseline biochemical testing in all patients being treated for TB, but there is a lack of evidence, these recommendations are based on observational studies and expert opinion. It is important to notice the phenomenon of adaptation (i.e., temporary stress or mild injury to the liver) that occurs in 20% or more of patients treated with anti-TB medications and often results in cessation or interruption of treatment.[62]
Some authors advocate that concerted efforts at identifying biomarkers or hepatic enzymes trending to prevent severe DILI.[63]
Another issue of therapy is the interaction between rifampicin and immunosuppressive drugs used in GVHD prophylaxis. Rifampicin reduces the serum levels of cyclosporine and sirolimus.[56] Rifabutin could be an alternative, because it is a weaker inducer of cytochrome P450, but the clinical experience is limited with just one report.[57] It is common in most of treatment descriptions tapering of cyclosporine and temporary addition of corticosteroids.
In spite of some prescribing practices and poor patient compliance had increased the risk of selection of drug-resistant (DR) strains of Mycobacterium tuberculosis, which are more difficult and more expensive to treat, reports of tuberculosis resistant in HSCT are rare. Cordonnier and colleagues reported 20 cases of tuberculosis. Susceptibility test results were available in 12 cases and none was resistant to any drug tested.[11] Nevertheless a case of MDR-TB (which is resistance to rifampicin and isoniazid) have already been published in this population and was successfully treated with cycloserine 500mg, levofloxacin 500mg, PAS 10g and kanamycin 1g three times a week during 18 months, with no relapse.[64]
Fortunately a portfolio of promising new TB drugs is on the horizon. Eleven new or repurposed tuberculosis drugs are in clinical investigation, 3 in phase 3 trials, which are evaluating the possibility to shorten treatment to 4 months, using strategies like inclusion of a third-generation fluoroquinolone or rifapentine (a semisynthetic rifamycin that has a longer half-life than rifampicin) and compounds as linezolid, probably a limited option in HSCT patients due the myelotoxicity when used for long period.[65]
Some perspectives in TB treatment with adjunct immunotherapies should require caution in HSCT recipients, since the safety regarding GVHD or bone marrow rejection is not well studied.[66]
TB Latent
In some countries the use of tests based on interferon release assays appear to be useful to determine latent TB infection, but further studies are needed to define their utility in immunocompromised host.[42,43]
TB screening using TST or QuantiFERON-TB gold (QFT-GIT) in HSCT is controversial.[29-32] T cells play an important role in protective immunity against tuberculosis. The question arises as to whether TST-specific memory T cells are transferred from the marrow donor to the recipient and persist in the long-term.[12]
The Infectious Diseases Working Party of the European Blood and Marrow Transplant Group survey showed that 51% of centers had a program of vaccination against tuberculosis in their countries, only 10% systematically screening their patient before transplant by TST, and 51% screening in case of suspicious.[11]
A study of 295 HSCT in Korea showed an incidence of TB of 3.1%.[19] Multivariate analysis revealed that only a previous history of TB infection and total Body Irradiation (TBI) increased the risk of TB infection in HSCT patients (relative risk, 4.8 and 12.5, respectively).[18]
Isoniazid prophylaxis in HSCT recipients with only radiological findings suggestive of past inactive TB infection did not significantly alter the incidence of TB infections (P = 0.236).[18] Other study evaluated the frequency of tuberculin skin test (TST) positivity among 26 patients and their donors screened by TST to investigate whether tuberculin positivity of a recipient or donor influenced the rate of tuberculosis disease, transplant-related events, and to evaluate the effectiveness of isoniazid (INAH) prophylaxis administered to those with positive TST.[32] The frequency of TST positivity was 23% (n = 6) among recipients and also 23% (n = 6) among donors. Two recipients and five donors with positive TST received INAH prophylaxis for six months. The transplantation procedure was not postponed for either recipient or donor TST positivity. Despite the high frequency of tuberculosis in their country, they have not detected any case of tuberculosis in their center, either among the TST screened (n = 26) or non-screened (n = 128).[32]
A recent study compared the simultaneous use of QFT-GIT and TST (>5 mm) and found similar frequencies of positive outcomes in the two screening test, although the overall agreement was poor (Kappa=0.08, 95% CI -0.006 to 0.24). However, the study did not have adequate power. The authors evaluated a cohort of 244 patients, 100 autologous and 144 allogeneic recipients during a 28-month period. No prophylaxis for latent tuberculosis was administrated, 201 (82%) patients had Bacillus Calmette-Guérin (BCG) scars or previous vaccination. Two patients developed tuberculosis after HSCT, the incidence of tuberculosis among those with positive QFT-GIT was 2.80 and among positive TST was 0 per 100 patients-years.[33] Thus, further studies are needed to validate the use of QFT-GIT in HSCT patients.
Conclusion
Most of reports of TB in HSCT patients were from ASIA, with TB incidence varying from 0.0014 (USA) to 16% (Pakistan), and the lung was the organ most often involved. The mortality varied from 0 to 50%, and it was higher in allogeneic HSCT than autologous HSCT. Thus, TB is more frequent and with worse outcome among allogeneic HSCT. Risk factors associated with TB in allogeneic HSCT are use of steroid and GVHD. There is no consensus regarding the screening with either TST or QFT-GIT, use of primary prophylaxis for latent TB, and, whether in a developing country with high prevalence of TB, the epidemiologic query should be emphasized.
Hematopoietic Stem cell transplant (HSCT) recipients have severe impairment in cell-mediated immunity as result of the conditioning regimen, immunosuppressive therapy and graft-versus-host disease (GVHD).[1,2] Accordingly, they are susceptible to bacterial, viral, and fungal infections. Mycobacterial infections can also occur in these patients, although the incidence is not high, even in countries where tuberculosis (TB) is common.[2] TB in this population of patient is mainly due to reactivation of latent infection.[2] However, in country where TB is endemic, pulmonary tuberculosis could be due to a new infection.
Data from the United States showed that the incidence of mycobacterium infection (MBI) among HSCT ranges from 0, 0014 % to 3%.[1,3-5] Countries in which the prevalence of tuberculosis in the general population is higher than it is in the United States have reported incidences varying from 1.6% in Spain[6] and Turkey,[7] 8.57% in Hong Kong and Taiwan to 16% in Pakistan.[8-11]
There are few reports of TB diagnosis during the first two weeks after HSCT (Table 1). Most of cases described in the literature occurred after 90 days of HSCT.[12-28]
The incidence of TB in HSCT ranged from 0, 0014% to 16% (Table 1). The diagnosis of TB varied from +21 to +1410 (median of 257, 2 days) after the HSCT. The lung was the organ most often involved. Most of cases were described in allogeneic HSCT, and the mortality in this type of HSCT was high, varying from to 0% to 50% (Table 1).
There is no consensus regarding the screening with tuberculin skin test or QuantiFERON-TB gold and primary prophylaxis for latent TB.[29-33]

Table 1. Clinical and radiological manifestation of tuberculosis in HSCT recipients
TB Risk Factors
The increased risk of tuberculosis in this setting could be explained by severely impaired cell-mediated immunity as a result of their underlying illness and conditioning chemotherapy. However, there is a lack of prospective studies on TB in HSCT and the clinical, radiological and epidemiological data are based on case reports and retrospective analysis with a small number of patients.[27,34,35,37] Previous study had showed the main risk factors for tuberculosis, comparing to control group, were allogeneic transplantations with unrelated donors and total body irradiation (TBI), with a relative risk (RR) of 23.9 and 4.9 respectively (p<0.05), and chronic GVHD with RR of 3.6 (p<0.05).[34] Another retrospective study observed that the majority of allogeneic patients with post-transplant tuberculosis received corticosteroids for GVHD.[27] Patients with chronic GVHD have a marked delay of T cell subset recovery with low numbers of CD4 cells being present. As this period of impairment of T cell function may be indefinite in the presence of chronic GVHD, it might contribute to an increased predisposition to tuberculosis.[35-37]
Symptoms of TB diseases
The clinical presentation of tuberculosis in patients undergoing HSCT may have a wide range of signs, symptoms and radiological findings (Table 1). Tuberculosis following conventional HSCT usually presents indolent clinical courses. The lung is the most common site of the disease, and the usual manifestations are fever, cough, dyspnea and hypoxemia (Table 1). Although the clinical and radiographic presentation of TB in the HSCT population usually mimics that in the non-transplant population, atypical presentations have been described, such as diffuse alveolar hemorrhage.[34] In immunocompromised host patients, the radiological finding could be mitigated or absent on chest x-ray and on this panel the CT-scan provided additional information to help diagnose pulmonary TB.[38]
In regard to the radiologic features of pulmonary tuberculosis in HSCT recipients, recent studies evaluated chest X-ray[10] and CT-scan.[7] The chest X-ray abnormalities were air-space consolidation (100%) and nodules (80%). On chest CT scans, the most common parenchymal lesions were consolidation (100%), nodules (71%), tree-in-bud appearance (43%), and ground-glass opacity (43%). Cavities were present on CT scans in only 14% of the study patients and lymphadenopathies were noted in 71%.
TB Diagnosis
A suggestive clinical and radiologic finding is not enough to allow initiating specific treatment in patients submitted to HSCT, as recommended in populations of high endemic TB countries. The bacteriological diagnosis is crucial once the incidence is low and differential etiology should be excluded.
Similarly to other scenarios, the main challenge is to obtain a result as fast as possible to start appropriate treatment and establish environmental infection control measures.[2]
Thus, the recommendation is to detect the presence of acid-fast bacilli (AFB) in samples as soon as possible and that these samples should be cultured for identification and antimicrobial susceptibility testing.[39] Some authors had already reported cases of transplanted patients with disseminated non-tuberculosis mycobacteria infection and drug resistant TB, reinforcing the importance of such complimentary tests.[40]
An important obstacle to a fast invasive diagnostic test is the clinical condition characterized by bone marrow suppression (anemia, thrombocytopenia), although some studies showed that investigational procedures like lung CT guide-biopsy are efficacious and safe in this population.[41]
A recently published review of 56 cases of tuberculosis in HSCT, showed that only 55% of cases were diagnosed with culture, whereas acid-fast bacilli (26%) was the second most common approach for diagnosis and histology was responsible for 20,3%.[2]
Molecular methods are increasingly used in diagnosis since they are faster than mycobacterial cultures (3-6 weeks) and have the potential role to determine species. However, there are still some issues in interpreting mycobacterial DNA in respiratory samples (Table 2).[43,44,46] Lee et al.[45] studied real time PCR in CT guided BAL in 99 patients unable to produce sputum samples or with AFB negative result: PCR showed a positive predictive value in 81% out of 27 patients with confirmed TB, although with a lower sensitivity.

Table 2. Diagnostic methods characteristics and reported sensitivity and specificity for general population. AFB=Acid Fast Bacilli
In general population molecular tests are increasingly used, and several guidelines as UK most recent recommendations (2011) from the National Institute for Health and Clinical Excellence (NICE) on the diagnosis of latent tuberculosis and of active tuberculosis even mention these methods.[47]
The common approach is to use rapid diagnostic tests for Mycobacterium tuberculosis complex (M tuberculosis, M bovis, M africanum) on bronchial specimens of patients only if rapid confirmation would alter the patient’s care or before conducting a large contact tracing initiative. Such tests are not recommended for pleural, cerebrospinal fluid, or urine in order to exclude the diagnosis of TB as they have a high false negative rate.[48]
The Xpert MTB/RIF assay, which enables simultaneous detection of Mycobacterium tuberculosis (MTB) and rifampicin (RIF) resistance, was endorsed by World Health Organization (WHO) in December, 2010. This assay was specifically recommended for use as the initial diagnostic test for suspected drug-resistant or HIV-associated pulmonary tuberculosis. One Xpert MTB/RIF test on sputum detects 90% of pulmonary tuberculosis (99% of smear-positive disease and about 75% of smear-negative disease). Such high sensitivity of Xpert MTB/RIF for rifampicin resistance is accompanied by some false-positive results and confirmatory drug sensitivity testing is needed.[49]
A different test based on antigen detection was already studied in the general population. Rajpal and colleagues studied an India tribal population where nearly 80% suffers from malnutrition. In this group, they compared 41 patients with active TB (90% confirmed with AFB and/or culture positive) with 87 controls to three different diagnostic blood tests: ELISA based TB antigen, ADA and in house IS6110 based PCR. The positivity of PCR assay in TB patients was around 48.78%, which was found to be significant (p < 0.0001) than the control group (2.30%). Mean absorbance of Antigen 85 ELISA in TB group was significantly (p<0.05) higher than non-TB control group. There was no immunocompromised patient (HIV or immunossupressors) in their cohort.[50]
Blood TB PCR is still not standardized, but offers great promise for the rapid and specific diagnosis. Indian investigators recently evaluated a multiplex nested PCR method targeting insertion sequence 6110 and MPB64 sequence in 130 samples. The sensitivity and specificity of the assay was respectively 95.7% and 100% with a negative predictive value of 99.2%.[51]
Molecular techniques such as polymerase chain reaction (PCR) was rarely used for diagnosis in HSCT patients reported (3.7%), and are mainly used in combination with other diagnostic approach.[2]
There are few studies, however, comparing accuracy of different tests in this transplant setting, and most of the data come from general population, mainly from pulmonary TB.[46,57]
Treatment of TB
The use of drugs against TB should be in agreement with local recommendations, based on surveillance of mycobacterial resistance patterns to antimicrobial. Since the first drugs available in 1940s, the subsequent emergence of resistant species led to combination therapy.[53,54] In fact the treatment of TB in HSCT recipients are similar to the general population with two important differences: the duration of therapy, normally longer, and the drug regimen, because interaction was reported between rifamycins (rifampicin and rifabutin) and immunosuppressive drugs like cyclosporine.
The approach recommended by World Health Organization, North American and European guidelines is a 6-month chemotherapy regimen using a combination of 4 drugs (rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months, followed by rifampicin and isoniazid for 4 months) with cure rates of approximately 90% of drug-susceptible cases.[54-56] The Brazilian recommendation is in agreement with other countries (Table 3).[57]

Table 3. Recommended treatment schedule for TB for a patient with more than 110 lb.
Most of the reports with combination therapy in HSCT patients had shown good results. La Camara and colleagues treated nineteen patients with a median of three drugs. Seventeen received more than 3 months of treatment and all were cured. An interesting finding of this study was the interaction between cyclosporine with rifampicin, with three patients presenting a decrease in cyclosporine levels, two of whom with worsening in chronic GVHD. Three patients (18%) developed hepatotoxicity.[56]
In more pronounced immunosuppression state like in unrelated cord blood transplantation there is already TB cases reported[52,53] In this cases the therapy of choice, even with possible drug interaction, was the classic four drugs of therapeutic guidelines.
The treatment of tuberculosis (TB) is complicated by drug-induced hepatotoxicity, one of the leading types of drug-induced liver injury (DILI). Current recommendations are to obtain baseline biochemical testing in all patients being treated for TB, but there is a lack of evidence, these recommendations are based on observational studies and expert opinion. It is important to notice the phenomenon of adaptation (i.e., temporary stress or mild injury to the liver) that occurs in 20% or more of patients treated with anti-TB medications and often results in cessation or interruption of treatment.[62]
Some authors advocate that concerted efforts at identifying biomarkers or hepatic enzymes trending to prevent severe DILI.[63]
Another issue of therapy is the interaction between rifampicin and immunosuppressive drugs used in GVHD prophylaxis. Rifampicin reduces the serum levels of cyclosporine and sirolimus.[56] Rifabutin could be an alternative, because it is a weaker inducer of cytochrome P450, but the clinical experience is limited with just one report.[57] It is common in most of treatment descriptions tapering of cyclosporine and temporary addition of corticosteroids.
In spite of some prescribing practices and poor patient compliance had increased the risk of selection of drug-resistant (DR) strains of Mycobacterium tuberculosis, which are more difficult and more expensive to treat, reports of tuberculosis resistant in HSCT are rare. Cordonnier and colleagues reported 20 cases of tuberculosis. Susceptibility test results were available in 12 cases and none was resistant to any drug tested.[11] Nevertheless a case of MDR-TB (which is resistance to rifampicin and isoniazid) have already been published in this population and was successfully treated with cycloserine 500mg, levofloxacin 500mg, PAS 10g and kanamycin 1g three times a week during 18 months, with no relapse.[64]
Fortunately a portfolio of promising new TB drugs is on the horizon. Eleven new or repurposed tuberculosis drugs are in clinical investigation, 3 in phase 3 trials, which are evaluating the possibility to shorten treatment to 4 months, using strategies like inclusion of a third-generation fluoroquinolone or rifapentine (a semisynthetic rifamycin that has a longer half-life than rifampicin) and compounds as linezolid, probably a limited option in HSCT patients due the myelotoxicity when used for long period.[65]
Some perspectives in TB treatment with adjunct immunotherapies should require caution in HSCT recipients, since the safety regarding GVHD or bone marrow rejection is not well studied.[66]
TB Latent
In some countries the use of tests based on interferon release assays appear to be useful to determine latent TB infection, but further studies are needed to define their utility in immunocompromised host.[42,43]
TB screening using TST or QuantiFERON-TB gold (QFT-GIT) in HSCT is controversial.[29-32] T cells play an important role in protective immunity against tuberculosis. The question arises as to whether TST-specific memory T cells are transferred from the marrow donor to the recipient and persist in the long-term.[12]
The Infectious Diseases Working Party of the European Blood and Marrow Transplant Group survey showed that 51% of centers had a program of vaccination against tuberculosis in their countries, only 10% systematically screening their patient before transplant by TST, and 51% screening in case of suspicious.[11]
A study of 295 HSCT in Korea showed an incidence of TB of 3.1%.[19] Multivariate analysis revealed that only a previous history of TB infection and total Body Irradiation (TBI) increased the risk of TB infection in HSCT patients (relative risk, 4.8 and 12.5, respectively).[18]
Isoniazid prophylaxis in HSCT recipients with only radiological findings suggestive of past inactive TB infection did not significantly alter the incidence of TB infections (P = 0.236).[18] Other study evaluated the frequency of tuberculin skin test (TST) positivity among 26 patients and their donors screened by TST to investigate whether tuberculin positivity of a recipient or donor influenced the rate of tuberculosis disease, transplant-related events, and to evaluate the effectiveness of isoniazid (INAH) prophylaxis administered to those with positive TST.[32] The frequency of TST positivity was 23% (n = 6) among recipients and also 23% (n = 6) among donors. Two recipients and five donors with positive TST received INAH prophylaxis for six months. The transplantation procedure was not postponed for either recipient or donor TST positivity. Despite the high frequency of tuberculosis in their country, they have not detected any case of tuberculosis in their center, either among the TST screened (n = 26) or non-screened (n = 128).[32]
A recent study compared the simultaneous use of QFT-GIT and TST (>5 mm) and found similar frequencies of positive outcomes in the two screening test, although the overall agreement was poor (Kappa=0.08, 95% CI -0.006 to 0.24). However, the study did not have adequate power. The authors evaluated a cohort of 244 patients, 100 autologous and 144 allogeneic recipients during a 28-month period. No prophylaxis for latent tuberculosis was administrated, 201 (82%) patients had Bacillus Calmette-Guérin (BCG) scars or previous vaccination. Two patients developed tuberculosis after HSCT, the incidence of tuberculosis among those with positive QFT-GIT was 2.80 and among positive TST was 0 per 100 patients-years.[33] Thus, further studies are needed to validate the use of QFT-GIT in HSCT patients.
Conclusion
Most of reports of TB in HSCT patients were from ASIA, with TB incidence varying from 0.0014 (USA) to 16% (Pakistan), and the lung was the organ most often involved. The mortality varied from 0 to 50%, and it was higher in allogeneic HSCT than autologous HSCT. Thus, TB is more frequent and with worse outcome among allogeneic HSCT. Risk factors associated with TB in allogeneic HSCT are use of steroid and GVHD. There is no consensus regarding the screening with either TST or QFT-GIT, use of primary prophylaxis for latent TB, and, whether in a developing country with high prevalence of TB, the epidemiologic query should be emphasized.
References
- Navari RM, Sullivan, KM, Springmeyer SC, et
al. Mycobacterial infections in marrow transplant patients.
Transplantation 1983;36(5):509-13. http://dx.doi.org/10.1097/00007890-198311000-00008 PMid:6356515

- Akan H, Arslan O, Akan OA. Tuberculosis in stem cell transplant patients. J Hosp Infect 2006;62(4):421-6. http://dx.doi.org/10.1016/j.jhin.2005.09.020 PMid:16413085

- Roy V, Weisdorf D. Mycobacterial infections
following bone marrow transplantation: a 20 year retrospective review.
Bone Marrow Transplant 1997;19(5):467-0. http://dx.doi.org/10.1038/sj.bmt.1700686 PMid:9052913

- Keung YK, Nugent K, Jumper C, Cobos E.
Mycobacterium tuberculosis infection masquerading as diffuse alveolar
hemorrhage after autologous stem cell transplant. Bone Marrow
Transplant 1999;23(7):737-8. http://dx.doi.org/10.1038/sj.bmt.1701648 PMid:10218854

- Kurzrock R, Zander A, Vellekoop L, Kanojia
M, Luna, M, Dicke K. Mycobacterial pulmonary infections after
allogeneic bone marrow transplantation. Am J Med 1984;77(1):35-40. http://dx.doi.org/10.1016/0002-9343 (84)90432-7

- de la Câmara R, Martino R, Granados E, et
al. Tuberculosis after hematopoietic stem cell transplantation:
incidence, clinical characteristics and outcome. Spanish Group on
Infectious Complications in Hematopoietic Transplantation. Bone Marrow
Transplant 2000;26(3):291-8. http://dx.doi.org/10.1038/sj.bmt.1702506 PMid:10967568

- Budak-Alpdogan T, Tangün Y,
Kalayoglu-Besisik S, et al. The frequency of tuberculosis in adult
allogeneic stem cell transplant recipients in Turkey. Biol Blood Marrow
Transplant 2000;6(4):370-4. http://dx.doi.org/10.1016/S1083-8791(00)70013-9

- Ip MS, Yuen KY, Woo PC, et al. Risk factors
for pulmonary tuberculosis in bone marrow transplant recipients. Am J
Respir Crit Care Med 1998;158(4):1173-7. http://dx.doi.org/10.1164/ajrccm.158.4.9712072 PMid:9769278

- Ku SC, Tang JL, Hsueh PR, Luh KT, Yu CJ,
Yang PC. Pulmonary tuberculosis in allogeneic hematopoietic stem cell
transplantation. Bone Marrow Transplant 2001;27(12):1293-7. http://dx.doi.org/10.1038/sj.bmt.1703092

- Wang J, Chang Y, Lee L, et al. Diffuse
Pulmonary Infiltrates After Bone Marrow Transplantation: The Role of
Open Lung Biopsy. Ann Thorac Surg 2004;78:267–72 http://dx.doi.org/10.1016/j.athoracsur.2004.03.002 PMid:15223441

- Cordonnier C, Martino R, Trabasso P, et
al. European Blood and Marrow Transplant Group Infectious Diseases
Working Party. Mycobacterial infection: a difficult and late diagnosis
in stem cell transplant recipients. Clin Infect Dis 2004;38(9):1229-36.
http://dx.doi.org/10.1086/383307 PMid:15127333

- Aljurf M, Gyger M, Alrajhi A, et al.
Mycobacterium tuberculosis infection in allogeneic bone marrow
transplantation patients. Bone Marrow Transplant 1999;24(5):551-4 http://dx.doi.org/10.1038/sj.bmt.1701930 PMid:10482941

- Ullah, K., Ahmed, P., Raza, S., et al.
(2007). Allogeneic stem cell transplantation in hematological
disorders: single center experience from Pakistan. Transplant
Proc;39(10):3347-57. http://dx.doi.org/10.1016/j.transproceed.2007.08.099 PMid:18089384

- Altclas J, Lescano A, Salgueira C, et al.
Multidrug-resistant tuberculosis in bone marrow transplant recipient.
Transpl Infect Dis 2005;7(1):45-6. http://dx.doi.org/10.1111/j.1399-3062.2005.00083.x PMid:15984950

- Maeda T, Kusumi E, Kami M, et al. Tokyo
Stem Cell Transplant (SCT) Consortium. Disseminated tuberculosis
following reduced-intensity cord blood transplantation for adult
patients with hematological diseases. Bone Marrow Transplant
2005;35(1):91-7. http://dx.doi.org/10.1038/sj.bmt.1704740 PMid:15516933

- Khan B, Ahmed P, Ullah K, Hussain CA,
Hussain I, Raza S. Frequency of tuberculosis in haematological
malignancies and stem cell transplant recipients. J Coll Physicians
Surg Pak 2005;15(1):30-3. PMid:15670521

- Garces Ambrossi G, Jakubowski A, Feinstein
MB, Weinstock, DM. Active tuberculosis limited to foreign-born patients
after allogeneic hematopoietic stem cell transplant. Bone Marrow
Transplant 2005;36(8):741-3. http://dx.doi.org/10.1038/sj.bmt.1705129 PMid:16113670

- George B, Mathews V, Srivastava A, Chandy
M. Infections among allogeneic bone marrow transplant recipients in
India. Bone Marrow Transplant 2004;33(3):311-15 http://dx.doi.org/10.1038/sj.bmt.1704347 PMid:14647246

- Lee J, Lee MH, Kim WS, et al. Tuberculosis
in hematopoietic stem cell transplant recipients in Korea. Int J
Hematol 2004;79(2):185-8. http://dx.doi.org/10.1532/IJH97.A10219 PMid:15005349

- Erdstein AA, Daas P, Bradstock KF,
Robinson T, Hertzberg MS. Tuberculosis in allogeneic stem cell
transplant recipients: still a problem in the 21st century.Transpl
Infect Dis 2004;6(4):142-6. http://dx.doi.org/10.1111/j.1399-3062.2004.00068.x PMid:15762931

- Yoo JH, Lee DG, Choi SM, et al. Infectious
complications and outcomes after allogeneic hematopoietic stem cell
transplantation in Korea. Bone Marrow Transplant 2004;34(6):497-504. http://dx.doi.org/10.1038/sj.bmt.1704636 PMid:15286689

- Kerridge I, Ethell M, Potter M, Prentice
HG. Mycobacterium tuberculosis infection following allogeneic
peripheral blood stem cell transplantation. Intern Med J
2003;33(12):619-20. http://dx.doi.org/10.1111/j.1445-5994.2003.00451.x PMid:14656242

- Kindler T, Schindel C, Brass U, Fischer T.
Fatal sepsis due to Mycobacterium tuberculosis after allogeneic bone
marrow transplantation. Bone Marrow Transplantation 2001, 27, 217–18 http://dx.doi.org/10.1038/sj.bmt.1702737 PMid:11281394

- Campos A, Vaz CP, Campilho F, et al.
Central nervous system (CNS) tuberculosis following allogeneic stem
cell transplantation. Bone Marrow Transplant 2000;25(5):567-9. http://dx.doi.org/10.1038/sj.bmt.1702163 PMid:10713637

- Ullah K, Raza S, Ahmed P, et al.
Post-transplant infections: single center experience from the
developing world. Int J infect Dis 2008;12:203-14. http://dx.doi.org/10.1016/j.ijid.2007.06.012 PMid:17920999

- Machado CM, Martins TC, Colturato I, et
al. Epidemiology of neglected tropical diseases in transplant
recipients. Review of the literature and experience of a Brazilian HSCT
center. Rev Inst Med Trop Sao Paulo. 2009;51(6):309-24.
PMid:20209266

- Russo RL, Dulley FL, Suganuma L, et al.
Tuberculosis in hematopoietic stem cell transplant patients: case
report and review of the literature. Int J Infect Dis. 2010;14 Suppl
3:e187-91. http://dx.doi.org/10.1016/j.ijid.2009.08.001 PMid:19819176

- Kuan FC, Lin PY, Hwang CE, et al.
Pancytopenia and myeloid maturation arrest in an autologous stem cell
transplant recipient. Bone Marrow Transplant.2011;46(4):610-1. http://dx.doi.org/10.1038/bmt.2010.155 PMid:20622905

- Rouleau M, Senik A, Leroy E, Vernant JP.
Long-term persistence of transferred PPD-reactive T cells after
allogeneic bone marrow transplantation. Transplantation
1993;55(1):72-6. http://dx.doi.org/10.1097/00007890-199301000-00014 PMid:8380510

- Ahmed P, Anwar M, Khan B, et al. Role of
isoniazid prophylaxis for prevention of tuberculosis in haemopoietic
stem cell transplant recipients. J Pak Med Assoc 2005;55(9):378-81.
PMid:16302471

- Gauchon A, André N, Rome A, et al.
Evaluation of a screening strategy after occurence of two simultaneous
contaminating tuberculosis cases in a pediatric oncology department.
Arch Pediatr 2008;15(3):236-44. http://dx.doi.org/10.1016/j.arcped.2008.01.005 PMid:18329257

- Tavil B, Gulhan B, Ozcelik U, et al.
Tuberculin skin test positivity in pediatric allogeneic BMT recipients
and donors in Turkey. Pediatr Transplant 2007, (4):414-18. http://dx.doi.org/10.1111/j.1399-3046.2007.00679.x PMid:17493222

- Moon SM, Lee SO, Choi SH, Kim YS, Woo JH,
Yoon DH, Suh C, Kim DY, Lee JH, Lee JH, Lee KH, Kim SH. Comparison of
the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for
detecting latent tuberculosis infection prior to hematopoietic stem
cell transplantation. Transpl Infect Dis. 2013;15(1):104-9. http://dx.doi.org/10.1111/j.1399- 3062.2012.00765.x PMid:22823749

- Keung YK, Nugent K, Jumper C, et al.
Mycobacterium tuberculosis infection masquerading as diffuse alveolar
hemorrhage after autologous stem cell trans- plant. Bone Marrow
Transplant 1999;23(7):737 – 35. http://dx.doi.org/10.1038/sj.bmt.1701648 PMid:10218854

- Friedrich W, O'Reilly RJ, Koziner B, et
al. T-lymphocyte reconstitution in recipients of bone marrow
transplants with or without GVHD: imbalances on T-cell subpopulation
having unique regulatory and cognitive function. Blood 1982; 59:
696–701. PMid:6800422

- Lum LG, Seigneuret MC, Orcutt-Thordarson
N, et al. The regulation of immunoglobulin synthesis after HLA
identical bone marrow transplantation. Blood 1985; 65: 1422–1433.
PMid:2986745

- Al-Anazi KA, Al-Jasser AM, Evans DA.
Infections caused by mycobacterium tuberculosis in patients with
hematological disorders and in recipients of hematopoietic stem cell
transplant, a twelve year retrospective study. Ann Clin Microbiol
Antimicrob. 2007 16;6:16.

- Jung JI, Lee DG, Kim YJ et al. Pulmonary
tuberculosis after hematopoietic stem cell transplantation: radiologic
findings. J Thorac Imag. 2009;24(1):10-6. http://dx.doi.org/10.1097/RTI.0b013e31818c6b97 PMid:19242297

- Delogu G and Herrmann J-L. Mycobacterium
species. In European Manual of Clinical Microbiology. 1st edition.
ISBN: 978-2-87805-026-4, pages 297-307, 2012.

- Yamazaki R, Mori T, Nakazato T, Okamoto S
et al. Non-Tuberculous Mycobacterial Infection Localized in Small
Intestine Developing after Allogeneic Bone Marrow Transplantation.
Inter Med 49: 1191-1193, 2010 http://dx.doi.org/10.2169/internalmedicine.49.3288 PMid:20558941

- Shi JM, Cai Z, Huang H, Ling MF et al.
Role of CT-guided percutaneous lung biopsy in diagnosis of pulmonary
fungal infection in patients with hematologic diseases. Int J Hematol
(2009) 89:624–627 http://dx.doi.org/10.1007/s12185-009-0351-0 PMid:19468797

- Redelman-Sidi G and Sepkowitz KA.
Interferon Gamma Release Assays in the Diagnosis of Latent Tuberculosis
Infection Among Immunocompromised Adults. Am J Respir Crit Care Med.
2013. First published online doi: 10.1164/rccm/201209-1621CI.

- Pai M, Palamountain K M. New tuberculosis
technologies: challenges for retooling and scale-up. Int J Tuberc Lung
Dis 16 (10): 1281-1290; 2012. 44.

- MMWR. Updated Guidelines for the Use of
Nucleic Acid Amplification Tests in the Diagnosis of Tuberculosis.
January 16, 2009 / 58 (01); 7-10.

- Lee J I, Lee BJ, Roy EY et al. The
diagnostic accuracy of tuberculosis real-time polymerase chain reaction
analysis of computed tomography-guided bronchial wash samples. Diagn
Microb Infec Dis 2011; 71: 51-6 http://dx.doi.org/10.1016/j.diagmicrobio.2010.12.019 PMid:21795005

- Min J-W, Yoon HI, Park KU et al. Real-time
polymerase chain reaction in bronchial aspirate for rapid detection of
sputum smear-negative tuberculosis.Int J Tuberc Lung Dis 2010; 14(7):
852-8 PMid:20550768

- Abubakar I, Griffiths C and Ormerod P.
Diagnosis of active and latent tuberculosis: summary of NICE guidance.
BMJ 2012;345:e6828. http://dx.doi.org/10.1136/bmj.e6828

- Horne DJ, Narita M, Spitters CL et al. Challenging Issues in Tuberculosis in Solid Organ Transplantation. Clin Infect Dis 2013. http://dx.doi.org/10.1093/cid/cit488

- Lawn SD, Mwaba P, Bates M et al. Advances
in tuberculosis diagnostics: the Xpert MTB/RIF assay and future
prospects for a point-of-care test. Lancet Infect Dis 2013; 13: 349–61.
http://dx.doi.org/10.1016/S1473-3099(13)70008-2

- Rajpal S, Kashyap RS, Nayak AR et al.
Laboratory Investigations on the Diagnosis of Tuberculosis in the
Malnourished Tribal Population of Melghat, India. Plos One 2013, 8 (9):
e74652 http://dx.doi.org/10.1371/journal.pone.0074652 PMid:24069327 PMCid:PMC3772098

- Dubey A, Gwal R, Agrawal S. Mycobacterium
tuberculosis detection in blood using multiplex nested polymerase chain
reaction. Int J Tuberc Lung Dis. 2013 17 (10):1341-5. http://dx.doi.org/10.5588/ijtld.12.0536 PMid:24025388

- Bento J, Silva AS, Rodrigues F, Duarte R. Métodos Diagnósticos em Tuberculose. Acta Med Port 2011; 24: 145-154 PMid:21672452

- Hopewell PC, Pai M, Maher D et al. International standards for tuberculosis care. Lancet Infect Dis. 2006;6 (11):710 http://dx.doi.org/10.1016/S1473-3099 (06)70628-4

- Yew WW, Lange C and Leung CC. Treament of tuberculosis: update 2010. Eur Respir J 2011; 37: 441-462. http://dx.doi.org/10.1183/09031936.00033010 PMid:20847074

- World Health Organization. Treatment of Tuberculosis Guidelines; 2009. Report No.: WHO/HTM/TB/2009/420. ISBN: 9789241547833.
- American Thoracic Society: CDC; Council of
the Infectious Disease Society of America. Diagnostic standards and
classification of tuberculosis in adults and children. Am J Respir Crit
Care Med 2000;161:1376-95 http://dx.doi.org/10.1164/ajrccm.161.4.16141 PMid:10764337

- Ministério da Saúde do Brasil. Available in http://portal.saude.gov.br/portal/arquivos/pdf/nota_tecnica_versao_28_de_agosto_v_5.pdf
- La Camara R, Martino R, Granados E et al.
Tuberculosis after hematopoietic stem cell transplantation: incidence,
clinical characteristics and outcome. Bone Marrow Transplantation
(2000) 26, 291–298 http://dx.doi.org/10.1038/sj.bmt.1702506 PMid:10967568

- Maeda T, Kusumi E, Kami M et al.
Disseminated tuberculosis following reduced-intensity cord blood
transplantation for adult patients with hematological diseases. Bone
Marrow Transplantation (2005) 35, 91–97. http://dx.doi.org/10.1038/sj.bmt.1704740 PMid:15516933

- De Assis RA, Kerbauy FR, Rodrigues M et
al. Mycobacterium tuberculosis infection: a rare late complication
after cord blood hematopoietic SCT. Bone Marrow Transplantation (2009)
43, 667–668 http://dx.doi.org/10.1038/bmt.2008.379 PMid:19029968

- Aguado JM, Torre-Cisneros J, Munoz P et
al. Tuberculosis in Solid-Organ Transplant Recipients: Consensus
Statement of the Group for the Study of Infection in Transplant
Recipients (GESITRA) of the Spanish Society of Infectious Diseases and
Clinical Microbiology. Clin Infect Dis 2009; 48:1276–84 http://dx.doi.org/10.1086/597590 PMid:19320593

- Saukkonen JJ, Cohn DL, Jasmer RM et al.
ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis
Therapy Subcommittee. An official ATS statement: hepatotoxicity of
antituberculosis therapy. Am J Respir Crit Care Med 2006;174:935–952. http://dx.doi.org/10.1164/rccm.200510-1666ST PMid:17021358

- Devarbhavi H. Adaptation and Antituberculosis Drug–induced Liver Injury. Am J Respir Crit Car Med 2012; 186:387-388. http://dx.doi.org/10.1164/ajrccm.186.4.387 PMid:22896595

- Altclas J, Lescano A, Palmero D et al.
Multidrug-resistant tuberculosis in bone marrow transplant recipient.
Transpl Infect Dis 2005: 7: 45-46. http://dx.doi.org/10.1111/j.1399-3062.2005.00083.x PMid:15984950

- Lienhardt C, Raviglione M, Spigelman M et
al. New Drugs for the Treatment of Tuberculosis: Needs, Challenges,
Promise, and Prospects for the Future. J Infect Dis 2012; 205:S241–9. http://dx.doi.org/10.1093/infdis/jis034 PMid:22448022

- Uhlin M, Andersson J, Zumla A and Maeurer M. Adjunct Immunotherapies for Tuberculosis. J Infec Dis 2012; 205: S325–34. http://dx.doi.org/10.1093/infdis/jis197 PMid:22457298
