Therapeutic Value of Combined Therapy with Deferasirox and Silymarin on Iron Overload in Children with Beta Thalassemia
Adel A. Hagag1, Mohamed S Elfrargy1, Rana A. Gazar1 and Aml Ezzat Abd El-Lateef2
1 Departments of Pediatrics and 2Clinical Pathology, Faculty of Medicine, Tanta University, Egypt
Correspondence
to:
Adel A. Hagag. Departments of Pediatrics, Faculty of Medicine, Tanta University, Egypt. E-mail: adelhagag20@yahoo.com
Published: November 4, 2013
Received: July 6, 2013
Accepted: September 22, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013065, DOI 10.4084/MJHID.2013.065
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Background:
Beta thalassemia is an inherited hemoglobin disorder resulting in a
severe, chronic anemia requiring life-long blood transfusion that
induces iron overload. Silymarin is a flavonoid complex isolated from
Silybin marianum with a strong antioxidant activity, inducing an
hepatoprotective action, and probably, a protective effect on iron
overload. The aim of this work was to determine the silymarin value in
improving iron chelation in thalassemic patients with iron overload
treated with Deferasirox. Patients and Methods:
This study was conducted on 40 children with beta thalassemia major
under follow-up at Hematology Unit, Pediatric Department, Tanta
University Hospital with serum ferritin level more than 1000 ng/ml and
was divided into two groups. Group IA: Received oral Deferasirox
(Exjade) and silymarin for 6 months. Group IB: Received oral
Deferasirox (Exjade) and placebo for 6 months and 20 healthy children
serving as a control group in the period between April 2011 and August
2012 and was performed after approval from research ethical committee
center in Tanta University Hospital and obtaining an informed written
parental consent from all participants in this study. Results: Serum ferritin levels were markedly decreased in group IA cases compared with group IB (P= 0.001). Conclusion:
From this study we concluded that, silymarin in combination with Exjade
can be safely used in the treatment of iron-loaded thalassemic patients
as it showed good iron chelation with no sign of toxicity. Recommendations:
We recommend extensive multicenter studies in a large number of
patients with longer duration of follow-up and more advanced techniques
of assessment of iron status in order to clarify the exact role of
silymarin in reducing iron overload in children with beta thalassemia.
Introduction
Thalassemias are a heterogeneous group of inherited anemias that collectively represents the most common monogenic disorders. The different forms of b-thalassemia are characterized by reduced or absent production of b-chains of hemoglobin. Patients with the most severe form of b-thalassemia major or Cooley’s present a profound anemia that, if not treated, leads to death in the first few years of life. The only available curative therapy is allogeneic bone marrow transplantation which is available for less than 30% of patients.[1]
The most common treatment for the most serious types of thalassemia is blood transfusion which is necessary, in order to provide the patient with healthy red blood cells containing normal hemoglobin. Repeated blood transfusion leads to iron overload.[2] In iron overload excess iron accumulates in the body which is deposited in body organs as heart, liver and endocrine glands causing organ damage. Probably, iron saturates the liver firstly, and then accumulates in other organs. Excess iron accumulation is a leading cause of clinical deterioration and often death.[3]
The emergence of new iron chelators has a major impact on the treatment of thalassemia major. Moreover, the availability of more than one iron chelators opens up the possibility of reducing iron overload of specific organs while enhancing its overall excretion.[4]
Deferasirox is a triazole compound with two molecules of it are needed to bind one molecule of iron fully (tridentate chelator). It has high affinity to iron, with minimal binding to copper and zinc. It is supplied as orally dispersible tablets that are dissolved in water or juice and administered best on an empty stomach. Deferasirox- iron complex is excreted almost exclusively in the feces, with minimal urinary excretion.[5] Deferasirox has been implemented as an alternative to the gold standard chelator, desferrioxamine.[6] Deferasirox is administered, once-daily with a good safety and efficacy profile.[7] It is marketed as Exjade and is mainly used is to reduce iron overload in patients who are receiving long-term blood transfusions as beta-thalassemia and other chronic anemias.[8]
Silymarinis is an herbal remedy used for the treatment of liver and gall bladder disorders. Silymarin is a flavonoid complex extracted from milk thistle (Silybum marianum).[9] There are some studies designed to investigate the therapeutic activity of orally administered silymarin in patients with thalassemia major under conventional iron chelation therapy.[10]
Aim of the Work
The aim of this work was to compare the iron chelating efficacy of oral Deferasirox compared with combination therapy of oral Deferasirox and silymarin in children with beta thalassemia major with iron overload.
Patient and Methods
This prospective, study was conducted on 40 children with beta thalassemia major under follow-up at Hematology Unit of Pediatric Department, Tanta University Hospital having serum ferritin level more than 1000 ng/ml and 20 healthy children serving as a control group in the period between April 2011 and August 2012 and was performed after approval from research ethical committee center in Tanta University Hospital and obtaining an informed written parental consent from all participants in this research.
Study design. Thalassemic patients included in the study (group I) were divided into two subgroups Group IA and Group IB by simple random allocation, Group IA received combination of oral Deferasirox 20-40 mg/kg/day supplied in orally dispersible tablets that are dissolved in water or juice and administered best on an empty stomach[5] with oral silymarin in the form of Legalon tablets 140 mg, one hour before each meal (3 times daily) for 6 months[11] while group IB received oral Deferasirox 20-40 mg/kg/day and placebo. Group II: included 20 healthy children matched in age and sex serving as a control group.
Inclusion criteria will be: Children with β- thalassemia with serum ferritin > 1000 ng/ml who did not received any iron chelation therapy before the start of this study.
Exclusion criteria will be: Children with β-thalassemia with serum ferritin < 1000 ng/ml or who received any iron chelation therapy before the start of this study.
All the children in both groups will be subjected to the following:
1-Complete history taking with especial account on onset of thalassemia, chelation therapy, frequency of blood transfusion.
2-Through clinical examination with especial account on: pallor, jaundice, mongloid facies, splenomegaly, hepatomegaly and splenectomy.
3-Investigations including:
• Complete blood count.
• Hemoglobin electrophoresis.
• Liver functions including bilirubin level, alanine transferase (ALT) and aspartate transferase (AST).
• Renal function tests including blood urea and serum creatinine.
• Assessment of serum iron status including serum ferritin, serum iron and iron binding capacity. Assessment of liver function tests, renal function tests and serum iron status were done two times in group I; one time before the start of chelation therapy and one time after chelation therapy but only one time in control group.
Specimen collection and handling: blood specimens were collected in a plain tube using sterile needles through gentle venipuncture after sterilization of site of puncture by alchol, and collected samples were allowed to clot for 4 minutes then centrifuged to separate clear non hemolysed serum.[12]
Determination of serum iron: The iron dissociated from transferrin-iron complex by a solution of guanidine acetate and reduced by ascorbic acid reacts with ferrozine to give a pink complex (according to procedure recommended by the serum iron from Biomaghreb company).[13]
Determination of serum total iron binding capacity (TIBC): An excess of iron is added to the serum to saturate the transferrin. The unbound iron is precipitated with basic magnesium carbonate (according to procedure recommended by the serum total iron binding capacity from Biomaghreb company).[14]
Serum ferritin test: Serum level of ferritin by ELIZA [DRGŪ Ferritin ELISA (EIA-4292)].[15]
Principle of the test. Anti-human-ferritin antibodies are bound to microwells. Ferritin, if present in diluted serum or plasma, bind to the respective antibody. Washing of the microwells removes unspecific serum and plasma components. Horseradish peroxidase (HRP) conjugated anti-human ferritin immunologically detects the bound specimen sample ferritin forming a conjugate/ferritin/antibody complex. Washing of the microwells removes unbound conjugate. An enzyme substrate in the presence of bound conjugate hydrolyzes to form a blue color. The addition of an acid stops the reaction forming a yellow end-product. The intensity of this yellow color is measured photometrically at 450 nm. The amount of color is directly proportional to the concentration of ferritin present in the original sample.[15]
Statistics
Statistical presentation and analysis of the present study was conducted, using the mean, standard deviation and chi-square test by SPSS Version 16.[16]
Results
Table 1 shows no significant difference between group IA and group IB regarding age, sex distribution, age of onset of beta thalassemia, frequency of blood transfusion and clinical manifestations.
Table 2 show no significant difference between group IA and group IB regarding CBC, significant lower RBCs indices in patients than control groups, significant higher T.L.C. and platelet count in patients group than control group and significant higher reticulocyte count in patient groups than control group. Significant (p < 0.05), highly significant (p < 0.01), t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control.
Table 3 shows no statistically significant difference in serum creatinine, blood urea, AST and ALT between Group IA and Group IB before and after chelation therapy and no significant difference between patients groups and control group. No statistically significant difference in serum biliruben level between Group IA and Group IB before and after chelation therapy but there was significantly higher serum bilirubin level in patient groups than control group. t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control and t4 comparison between the same group (as group IA before and after chelation therapy).
Table 4 shows that serum ferritin and serum iron levels is significantly higher in patients than control group and there were no statistically significant difference in serum ferritin and serum iron levels between group IA and group IB before start of chelation therapy while there was statistically significant difference between group IA and group IB after chelation therapy with lower level of serum ferritin and serum iron in group IA. Serum TIBC level is significantly lower in patients than control group and there was no statistically significant difference in serum TIBC level between group IA and group IB before start of chelation therapy while there was statistically significant differences between group IA and group IB after chelation therapy with higher level of serum TIBC group IA. t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control and t4 comparison between the same group (as group IA before and after chelation therapy.
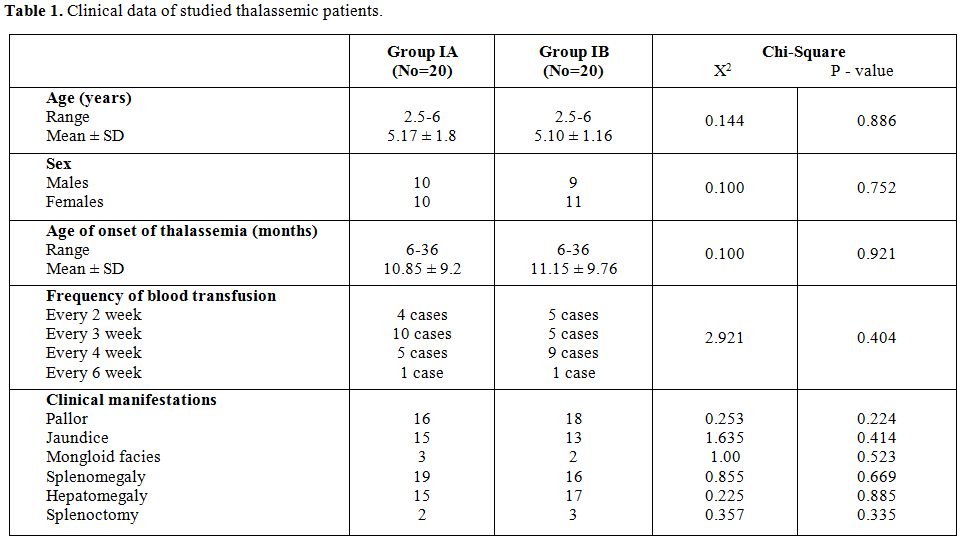
Table 1. Clinical data of studied thalassemic patients.
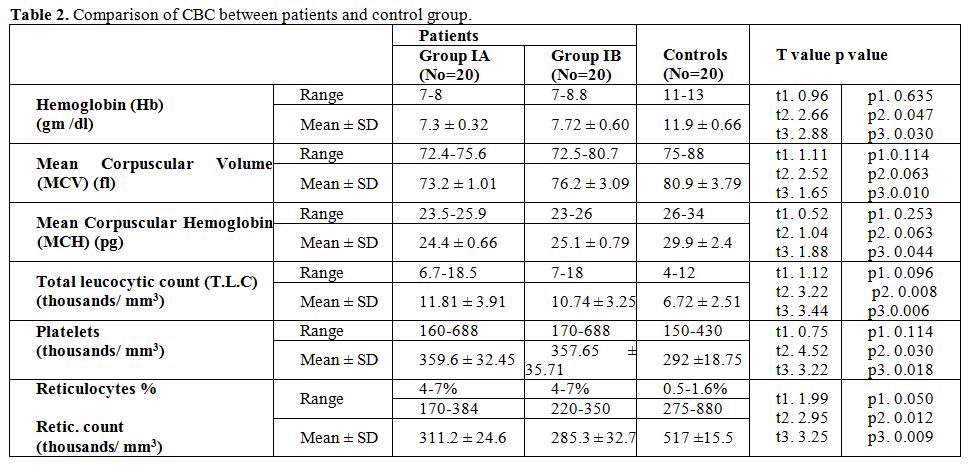
Table 2. Comparison of CBC between patients and control group.
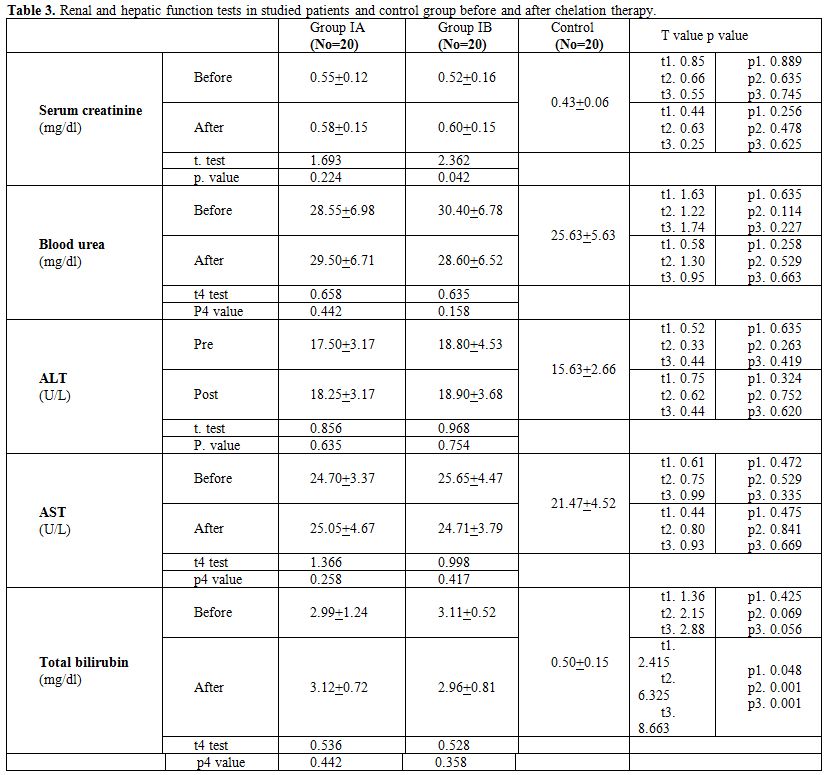
Table 3. Renal and hepatic function tests in studied patients and control group before and after chelation therapy.
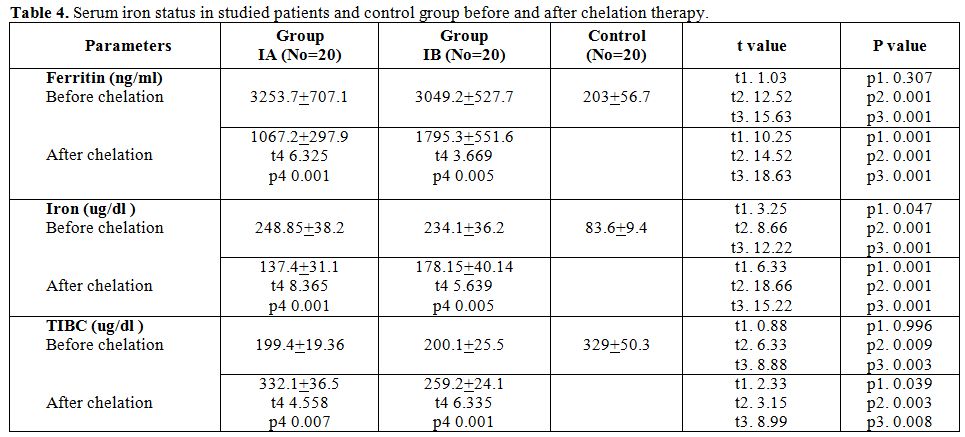
Table 4. Serum iron status in studied patients and control group before and after chelation therapy.
Discussion
Thalassemias are one of the most common genetic disorders worldwide. It is the commonest cause of chronic hemolytic anemia in Middle East.[17] In beta thalassemia major, impaired biosynthesis of beta-globin leads to accumulation of unpaired alpha-globin chain, shortened red cell life span and iron overload causing functional and physiological abnormalities in various organ systems.[18]
Silymarin is a flavonoid agent with antioxidant and free radical scavenging abilities. Silymarin also acts as an iron chelator by binding Fe (III). Despite the iron chelating activity of silymarin suggests its possible application in chelation therapy of iron overload; the biological effects of silymarin are different from other iron chelators, probably due to antioxidant activity of silymarin, which causes pro-oxidant effect via iron-catalyzed oxidation with subsequent generation of reactive oxygen species.[11]
This study was carried out on 40 children with β-thalassemia major under follow up in outpatient’s clinic of Pediatric Hematology Unit, Tanta University Hospital and 20 healthy children serving as a control group in the period between April 2011 and August 2012. Patients included in the study (group I) were subdivided into two subgroups (group IA and group IB) by simple random allocation, group IA received combination of exjade with silymarin while group IB used exjade and placebo.
This study shows that; the age of onset of β-thalassemia in studied cases ranged from 6 – 36 months with non-significant difference (P.value=0.886) in age of onset between group IA and group IB. These results are in agreement with Kattamis,[19] who said that symptoms of β- thalassemia appear usually in the first year of life, at the time when the synthesis of γ-chains is not replaced by the synthesis of β-chains, with the mean age at presentation of 13 months and Cao et al.[20] who found that beta thalassemia was recognized at age around 8 months in patients with transfusion-dependent beta thalassemia but at age of 2 years in non–transfusion dependent thalassemic children.
In our study; pallor and jaundice represent the most common presenting symptoms in studied cases of both groups while hepatomegaly and splenomegaly represent the most common presenting signs. This data are in agreement with Galanello and Origa,[21] who noted that these clinical finding are due to chronic hemolysis, extramedullary erythropoiesis and iron overload.
In this study, serum ferritin level is significantly higher in patients than control groups. This is in agreement with Hershko,[22] who demonstrated that; in thalassemia major, iron overload is the main outcome of multiple blood transfusions and inappropriately increased iron absorption associated with ineffective erythropoiesis.
In the current study, there were no significant differences in the initial serum ferritin levels between group IA and group IB but after regular chelation therapy, serum ferritin level is significantly lowered in group IA than group IB. This is in agreement with Gharagozloo et al.,[10] who assessed the efficacy of silymarin and desferroxamine compared with desferroxamine alone in removing excess iron by serial measurement of serum ferritin levels in 48 patients and found that, at the beginning of the trial, all patients had comparable mean serum ferritin levels but after receiving silymarin plus desferrioxamine, the drop in serum ferritin was higher than in desferrioxamine treated patients, indicating that the combined therapy depleted iron stores more successfully than desferroxamine alone. Our study is not in agreement with Adibi et al.,[23] who found that silymarin did not cause significant changes in liver iron concentration and concluded that evaluating a longer course of treatment with this drug is thus suggested. Variation in results could be explained by different mode of evaluating iron reduction (serum ferritin in our study versus liver iron concentration in Adibi et al., study and variation in severity of iron over load in both studies.
In this study, serum iron level is significantly higher in patient groups than control group while TIBC level is significantly lower in patient groups than control group. This is in agreement with Ghone et al.,[24] who stated that, the majority of the beta thalassemia major patients had significant increase of serum iron with significant decrease of total iron binding capacity and have severe anemia due to ineffective erythropoiesis which is a primary reason for iron overload. Thus, increased iron may increase the potential of oxidative injury to erythrocyte and cell organelles.
In the current study, there were no significant differences in the initial serum iron and TIBC levels in studied patients but after regular administration of chelation therapy, serum iron level is significantly lowered in group IA than group IB and TIBC is significantly increased in group IA more than group IB. This is in agreement with Wood,[25] who stated that serum iron is increased and TIBC is decreased in cases of beta thalassemia.
In this study, there were no significant differences in pre and post values of renal and liver function tests in patients groups before and after chelation therapy. This data is in agreement with Gharagozloo, et al.,[10] who demonstrated that; thalassemic patients with severe iron overload can be safely treated with a combination of silymarin and desferrioxamine with no detectable abnormalities in complete blood count, liver or renal functions due to silymarin use.
Conclusion
From this study we concluded that, silymarin in combination with exjade can be safely used in treatment of iron-loaded thalassemic patients. It showed good iron chelation with no sign of toxicity.
Recommendations
Extensive multicenter studies in large number of patients with longer duration of follow up and more advanced methods of assessment of iron status is recommended to clarify the exact role of silymarin in reduction of iron over load in children with beta thalassemia.
Thalassemias are a heterogeneous group of inherited anemias that collectively represents the most common monogenic disorders. The different forms of b-thalassemia are characterized by reduced or absent production of b-chains of hemoglobin. Patients with the most severe form of b-thalassemia major or Cooley’s present a profound anemia that, if not treated, leads to death in the first few years of life. The only available curative therapy is allogeneic bone marrow transplantation which is available for less than 30% of patients.[1]
The most common treatment for the most serious types of thalassemia is blood transfusion which is necessary, in order to provide the patient with healthy red blood cells containing normal hemoglobin. Repeated blood transfusion leads to iron overload.[2] In iron overload excess iron accumulates in the body which is deposited in body organs as heart, liver and endocrine glands causing organ damage. Probably, iron saturates the liver firstly, and then accumulates in other organs. Excess iron accumulation is a leading cause of clinical deterioration and often death.[3]
The emergence of new iron chelators has a major impact on the treatment of thalassemia major. Moreover, the availability of more than one iron chelators opens up the possibility of reducing iron overload of specific organs while enhancing its overall excretion.[4]
Deferasirox is a triazole compound with two molecules of it are needed to bind one molecule of iron fully (tridentate chelator). It has high affinity to iron, with minimal binding to copper and zinc. It is supplied as orally dispersible tablets that are dissolved in water or juice and administered best on an empty stomach. Deferasirox- iron complex is excreted almost exclusively in the feces, with minimal urinary excretion.[5] Deferasirox has been implemented as an alternative to the gold standard chelator, desferrioxamine.[6] Deferasirox is administered, once-daily with a good safety and efficacy profile.[7] It is marketed as Exjade and is mainly used is to reduce iron overload in patients who are receiving long-term blood transfusions as beta-thalassemia and other chronic anemias.[8]
Silymarinis is an herbal remedy used for the treatment of liver and gall bladder disorders. Silymarin is a flavonoid complex extracted from milk thistle (Silybum marianum).[9] There are some studies designed to investigate the therapeutic activity of orally administered silymarin in patients with thalassemia major under conventional iron chelation therapy.[10]
Aim of the Work
The aim of this work was to compare the iron chelating efficacy of oral Deferasirox compared with combination therapy of oral Deferasirox and silymarin in children with beta thalassemia major with iron overload.
Patient and Methods
This prospective, study was conducted on 40 children with beta thalassemia major under follow-up at Hematology Unit of Pediatric Department, Tanta University Hospital having serum ferritin level more than 1000 ng/ml and 20 healthy children serving as a control group in the period between April 2011 and August 2012 and was performed after approval from research ethical committee center in Tanta University Hospital and obtaining an informed written parental consent from all participants in this research.
Study design. Thalassemic patients included in the study (group I) were divided into two subgroups Group IA and Group IB by simple random allocation, Group IA received combination of oral Deferasirox 20-40 mg/kg/day supplied in orally dispersible tablets that are dissolved in water or juice and administered best on an empty stomach[5] with oral silymarin in the form of Legalon tablets 140 mg, one hour before each meal (3 times daily) for 6 months[11] while group IB received oral Deferasirox 20-40 mg/kg/day and placebo. Group II: included 20 healthy children matched in age and sex serving as a control group.
Inclusion criteria will be: Children with β- thalassemia with serum ferritin > 1000 ng/ml who did not received any iron chelation therapy before the start of this study.
Exclusion criteria will be: Children with β-thalassemia with serum ferritin < 1000 ng/ml or who received any iron chelation therapy before the start of this study.
All the children in both groups will be subjected to the following:
1-Complete history taking with especial account on onset of thalassemia, chelation therapy, frequency of blood transfusion.
2-Through clinical examination with especial account on: pallor, jaundice, mongloid facies, splenomegaly, hepatomegaly and splenectomy.
3-Investigations including:
• Complete blood count.
• Hemoglobin electrophoresis.
• Liver functions including bilirubin level, alanine transferase (ALT) and aspartate transferase (AST).
• Renal function tests including blood urea and serum creatinine.
• Assessment of serum iron status including serum ferritin, serum iron and iron binding capacity. Assessment of liver function tests, renal function tests and serum iron status were done two times in group I; one time before the start of chelation therapy and one time after chelation therapy but only one time in control group.
Specimen collection and handling: blood specimens were collected in a plain tube using sterile needles through gentle venipuncture after sterilization of site of puncture by alchol, and collected samples were allowed to clot for 4 minutes then centrifuged to separate clear non hemolysed serum.[12]
Determination of serum iron: The iron dissociated from transferrin-iron complex by a solution of guanidine acetate and reduced by ascorbic acid reacts with ferrozine to give a pink complex (according to procedure recommended by the serum iron from Biomaghreb company).[13]
Determination of serum total iron binding capacity (TIBC): An excess of iron is added to the serum to saturate the transferrin. The unbound iron is precipitated with basic magnesium carbonate (according to procedure recommended by the serum total iron binding capacity from Biomaghreb company).[14]
Serum ferritin test: Serum level of ferritin by ELIZA [DRGŪ Ferritin ELISA (EIA-4292)].[15]
Principle of the test. Anti-human-ferritin antibodies are bound to microwells. Ferritin, if present in diluted serum or plasma, bind to the respective antibody. Washing of the microwells removes unspecific serum and plasma components. Horseradish peroxidase (HRP) conjugated anti-human ferritin immunologically detects the bound specimen sample ferritin forming a conjugate/ferritin/antibody complex. Washing of the microwells removes unbound conjugate. An enzyme substrate in the presence of bound conjugate hydrolyzes to form a blue color. The addition of an acid stops the reaction forming a yellow end-product. The intensity of this yellow color is measured photometrically at 450 nm. The amount of color is directly proportional to the concentration of ferritin present in the original sample.[15]
Statistics
Statistical presentation and analysis of the present study was conducted, using the mean, standard deviation and chi-square test by SPSS Version 16.[16]
Results
Table 1 shows no significant difference between group IA and group IB regarding age, sex distribution, age of onset of beta thalassemia, frequency of blood transfusion and clinical manifestations.
Table 2 show no significant difference between group IA and group IB regarding CBC, significant lower RBCs indices in patients than control groups, significant higher T.L.C. and platelet count in patients group than control group and significant higher reticulocyte count in patient groups than control group. Significant (p < 0.05), highly significant (p < 0.01), t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control.
Table 3 shows no statistically significant difference in serum creatinine, blood urea, AST and ALT between Group IA and Group IB before and after chelation therapy and no significant difference between patients groups and control group. No statistically significant difference in serum biliruben level between Group IA and Group IB before and after chelation therapy but there was significantly higher serum bilirubin level in patient groups than control group. t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control and t4 comparison between the same group (as group IA before and after chelation therapy).
Table 4 shows that serum ferritin and serum iron levels is significantly higher in patients than control group and there were no statistically significant difference in serum ferritin and serum iron levels between group IA and group IB before start of chelation therapy while there was statistically significant difference between group IA and group IB after chelation therapy with lower level of serum ferritin and serum iron in group IA. Serum TIBC level is significantly lower in patients than control group and there was no statistically significant difference in serum TIBC level between group IA and group IB before start of chelation therapy while there was statistically significant differences between group IA and group IB after chelation therapy with higher level of serum TIBC group IA. t1 comparison between group IA and group IB, t2 comparison between group IA and control, t3 comparison between group IB and control and t4 comparison between the same group (as group IA before and after chelation therapy.

Table 1. Clinical data of studied thalassemic patients.

Table 2. Comparison of CBC between patients and control group.

Table 3. Renal and hepatic function tests in studied patients and control group before and after chelation therapy.

Table 4. Serum iron status in studied patients and control group before and after chelation therapy.
Discussion
Thalassemias are one of the most common genetic disorders worldwide. It is the commonest cause of chronic hemolytic anemia in Middle East.[17] In beta thalassemia major, impaired biosynthesis of beta-globin leads to accumulation of unpaired alpha-globin chain, shortened red cell life span and iron overload causing functional and physiological abnormalities in various organ systems.[18]
Silymarin is a flavonoid agent with antioxidant and free radical scavenging abilities. Silymarin also acts as an iron chelator by binding Fe (III). Despite the iron chelating activity of silymarin suggests its possible application in chelation therapy of iron overload; the biological effects of silymarin are different from other iron chelators, probably due to antioxidant activity of silymarin, which causes pro-oxidant effect via iron-catalyzed oxidation with subsequent generation of reactive oxygen species.[11]
This study was carried out on 40 children with β-thalassemia major under follow up in outpatient’s clinic of Pediatric Hematology Unit, Tanta University Hospital and 20 healthy children serving as a control group in the period between April 2011 and August 2012. Patients included in the study (group I) were subdivided into two subgroups (group IA and group IB) by simple random allocation, group IA received combination of exjade with silymarin while group IB used exjade and placebo.
This study shows that; the age of onset of β-thalassemia in studied cases ranged from 6 – 36 months with non-significant difference (P.value=0.886) in age of onset between group IA and group IB. These results are in agreement with Kattamis,[19] who said that symptoms of β- thalassemia appear usually in the first year of life, at the time when the synthesis of γ-chains is not replaced by the synthesis of β-chains, with the mean age at presentation of 13 months and Cao et al.[20] who found that beta thalassemia was recognized at age around 8 months in patients with transfusion-dependent beta thalassemia but at age of 2 years in non–transfusion dependent thalassemic children.
In our study; pallor and jaundice represent the most common presenting symptoms in studied cases of both groups while hepatomegaly and splenomegaly represent the most common presenting signs. This data are in agreement with Galanello and Origa,[21] who noted that these clinical finding are due to chronic hemolysis, extramedullary erythropoiesis and iron overload.
In this study, serum ferritin level is significantly higher in patients than control groups. This is in agreement with Hershko,[22] who demonstrated that; in thalassemia major, iron overload is the main outcome of multiple blood transfusions and inappropriately increased iron absorption associated with ineffective erythropoiesis.
In the current study, there were no significant differences in the initial serum ferritin levels between group IA and group IB but after regular chelation therapy, serum ferritin level is significantly lowered in group IA than group IB. This is in agreement with Gharagozloo et al.,[10] who assessed the efficacy of silymarin and desferroxamine compared with desferroxamine alone in removing excess iron by serial measurement of serum ferritin levels in 48 patients and found that, at the beginning of the trial, all patients had comparable mean serum ferritin levels but after receiving silymarin plus desferrioxamine, the drop in serum ferritin was higher than in desferrioxamine treated patients, indicating that the combined therapy depleted iron stores more successfully than desferroxamine alone. Our study is not in agreement with Adibi et al.,[23] who found that silymarin did not cause significant changes in liver iron concentration and concluded that evaluating a longer course of treatment with this drug is thus suggested. Variation in results could be explained by different mode of evaluating iron reduction (serum ferritin in our study versus liver iron concentration in Adibi et al., study and variation in severity of iron over load in both studies.
In this study, serum iron level is significantly higher in patient groups than control group while TIBC level is significantly lower in patient groups than control group. This is in agreement with Ghone et al.,[24] who stated that, the majority of the beta thalassemia major patients had significant increase of serum iron with significant decrease of total iron binding capacity and have severe anemia due to ineffective erythropoiesis which is a primary reason for iron overload. Thus, increased iron may increase the potential of oxidative injury to erythrocyte and cell organelles.
In the current study, there were no significant differences in the initial serum iron and TIBC levels in studied patients but after regular administration of chelation therapy, serum iron level is significantly lowered in group IA than group IB and TIBC is significantly increased in group IA more than group IB. This is in agreement with Wood,[25] who stated that serum iron is increased and TIBC is decreased in cases of beta thalassemia.
In this study, there were no significant differences in pre and post values of renal and liver function tests in patients groups before and after chelation therapy. This data is in agreement with Gharagozloo, et al.,[10] who demonstrated that; thalassemic patients with severe iron overload can be safely treated with a combination of silymarin and desferrioxamine with no detectable abnormalities in complete blood count, liver or renal functions due to silymarin use.
Conclusion
From this study we concluded that, silymarin in combination with exjade can be safely used in treatment of iron-loaded thalassemic patients. It showed good iron chelation with no sign of toxicity.
Recommendations
Extensive multicenter studies in large number of patients with longer duration of follow up and more advanced methods of assessment of iron status is recommended to clarify the exact role of silymarin in reduction of iron over load in children with beta thalassemia.
References
- Miccio A, Cesari R, Lotti F, et al. The cure of thalassemia by bone marrow transplantation. Blood Rev 2007; 16: 81-85.

- Pathare A, Taher A, Daar S. Practical chelation protocol based on stratification of thalassemia patients. 2010;89(4):405-409.

- Aessopos A, Berdoukas V, Tsironi M. The
heart in transfusion dependent homozygous thalassaemia today:
Prediction, prevention and management. Eur J Haematol 2008; 80 (1):
93-106. PMid:18081719 PMCid:PMC2253710

- Kattamis A, Ladis V, Berdousi H, et al.
Iron chelation treatment with combined therapy with deferiprone and
deferroxamine: A 12-month trial. Blood Cells Mol Dis 2006; 36: 21-25. http://dx.doi.org/10.1016/j.bcmd.2005.11.002 PMid:16386928

- Piga A, Gagliot C, Fogliacco E, et al.
Comparative effects of deperiprone and deferoxamine on survival and
cardiac disease in patients with thalassemia major: A retrospective
analysis. Haematologica 2003; 88:489-496. PMid:12745268

- Goldie YL, Samuel JFord, Chris T Selepis,
et al. The Iron Chelator, Deferasirox, as a Novel Strategy for Cancer
Treatment: Oral Activity against Human Lung Tumor Xenografts and
Molecular Mechanism of Action. Molecular Pharmacology January 2013; 83
(1):179-190. http://dx.doi.org/10.1124/mol.112.081893 PMid:23074173

- Rebecca LC Adams and Robert J Bird. Safety
and efficacy of deferasirox in the management of transfusion-dependent
patients with myelodysplastic syndrome and aplastic anaemia: A
perspective review. Therapeutic Advances in Hematology April 2013;
4(2):93-102 http://dx.doi.org/10.1177/2040620712472355 PMid:23610617 PMCid:PMC3629757

- Choudhry VP and Naithani R. Current status
of iron overload and chelation with deferasirox. Indian J Pediatr 2007;
74 (8): 759–64. http://dx.doi.org/10.1007/s12098-007-0134-7 PMid:17785900

- Valenzuela A and Garrido A. Biochemical
bases of the pharmacological action of the flavonoid silymarin and of
its structural isomer silibinin. Biol Res 2002; 27: 105-112.

- Gharagozloo M, Moayedi B, Zakerinia M, et
al. Combined therapy of silymarin and desferrioxamine in patients with
beta-thalassemia major: A randomized double-blind clinical trial.
Fundam Clin Pharmacol. 2009; 23(3):359-65. http://dx.doi.org/10.1111/j.1472-8206.2009.00681.x PMid:19453758

- Gharagozloo M, Khoshdel Z, Amirghofran Z.
The effect of an iron chelator, silybin, on the proliferation and cell
cycle of Jurkat cells: A comparison with desferrioxamine. Eur J
Pharmacol 2008; 589(1-3): 1-7. http://dx.doi.org/10.1016/j.ejphar.2008.03.059 PMid:18619590

- Dawson DW. The accuracy and clinical interpretation of serum ferritin assays. Clin Lab Haematol 1992; 14(1):47-52. PMid:1600693

- Bishop NL, Fudy EP, Schoeff L. Priniciples and correlations In: Harris N. and Winter W (eds.) Clinical chemistry, 5th edition, Lippinocott Williams and Wilkins, Philadelphia 2000; 180-220.
- Muntzel M, Thierry H, Bernard L, et al.
Effect of erythropoietin on hematocrit and blood pressure in
normotensive and hypertensive rats. J Am Soc Nephrol 1992; 3:182-187.
PMid:1391718

- Beard J L. Iron Biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001; 131:568-580.

- Levesque R. SPSS programming and data management: A guide for SPSS and SAS Users, 4th edition, SPSS Inc, Chicago, 2007.
- Khalifa AS, Baffico M, Heshmat NM, et al.
Relationship between the hematological phenotype and type of beta
thalassemic mutation in Egyption Children. Egy J Hematol 1995; 20(2):
103-137.

- Sadeghi-Bojd S, Hashemi M, Karimi M. Renal
tubular function in patients with B–thalassemia major in Zahedan,
Southeast Iran. Singapore Med J 2008; 49(5): 410-412. PMid:18465053

- Kattamis CA. Management of thalassemias:
Growth and development, Hormone substitution, vitamin supplementation,
and vaccination. Sem Hematol 1995; 32: 269. PMid:8560284

- Cao A, Galanello R, Rosatti C. Clinical experience of mangement of thalassemia.Semin Hematol 1996; 33: 66-75. PMid:8714586

- Galanello R and Origa R. Beta-thalassemia Galanello and Origa Orphanet Journal of Rare Diseases 2010; 5:11-15. http://dx.doi.org/10.1186/1750-1172-5-11 PMid:20492708 PMCid:PMC2893117

- Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Annals of New York Academy of Science 2010, 1202:1-9. http://dx.doi.org/10.1111/j.1749-6632.2010.05544.x PMid:20712765

- Adibi A, Azin SH, Behjat SM et al.
Therapeutic effects of deferoxamine and silymarin versus deferoxamine
alone in β-thalassemia major based on findings of liver MRI. Journal of
Research in Medical Sciences 2012; (1): 73-78

- Ghone RA, Kumbar KM, Suryakar AN, et al.
Oxidative stress and disturbance in antioxidant balance in beta
thalassemia major. Indian J Clin Biochemistry 2008; 23(4):337-340. http://dx.doi.org/10.1007/s12291-008-0074-7 PMid:23105782 PMCid:PMC3453139

- Wood JC. Diagnosis and management of transfusion iron overload: The role of imaging. Am J Hematol 2007; 82(12):1132-1135. http://dx.doi.org/10.1002/ajh.21099 PMid:17963249 PMCid:PMC2892928
