Autoimmune Cytopenias in Chronic Lymphocytic Leukemia, Facts and Myths
Pavankumar Tandra1, Jairam Krishnamurthy1, Vijaya Raj Bhatt1, Kam Newman2, James O Armitage1 and Mojtaba Akhtari1
1 Division of Hematology and Oncology, University of Nebraska Medical Center, Omaha, NE 68198, USA.
2 Dr. Kam Newman, Section of Transfusion Medicine, Cleveland Clinic Foundation, 9500 Euclid Avenue 6-1, Cleveland, OH 44195,USA.
2 Dr. Kam Newman, Section of Transfusion Medicine, Cleveland Clinic Foundation, 9500 Euclid Avenue 6-1, Cleveland, OH 44195,USA.
Correspondence
to:
Mojtaba Akhtari, MD, UNMC Hematology and Oncology Division, 987680
Nebraska Medical Center, Omaha, NE 681980-7680. Tel: 402-559-3834, Fax:
402-559-6520. E-mail: Mojtaba.akhtari@unmc.edu
Published: November 4, 2013
Received: August 8, 2013
Accepted: October 31, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013068, DOI 10.4084/MJHID.2013.068
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
CLL
has been defined as presence of more than 5000 small mature appearing
monoclonal B lymphocytes with a specific immunophenotype in peripheral
blood. It is a well-known fact that CLL is associated with autoimmune
cytopenias. CLL cells are CD5+ B lymphocytes, and usually are not the
“guilty” cells which produce autoantibodies. T cell defect is another
characteristic of CLL and the total number of T cells is increased, and
there is inversion of the CD4/CD8 ratio. Autoimmune hemolytic anemia
(AIHA) is the most common autoimmune complication of CLL and has been
reported in 10-25% of CLL patients. However, the stage-adjusted
estimated rate of AIHA in CLL is about 5%. Conversely, CLL is three
times more common in patients who present with AIHA. Direct agglutinin
test (DAT) is positive in 7-14% of CLL patients but AIHA may also occur
in DAT negative patients.
Autoimmune thrombocytopenia (AIT) is the second most common complication of CLL and has been reported in 2-3% of patients. DAT is positive in AIT but presence of antiplatelet antibodies is neither diagnostic nor reliable. Autoimmune neutropenia (AIN) and pure red cell aplasia (PRCA) are very rare complications of CLL and like other autoimmune complications of CLL may occur at any clinical stage. It is believed that most case reports of AIN and PRCA in CLL actually belong to large granular lymphocytic leukemia (LGL). Non-hematologic autoimmune complications of CLL including cold agglutinin disease (CAD), paraneoplastic pemphigus (PNP), acquired angioedema, and anti-myelin associated globulin are rare.
Before starting any treatment, clinicians should distinguish between autoimmune cytopenias and massive bone marrow infiltration since autoimmune complications of CLL are not necessarily equal to advanced disease with poor prognosis. According to IWCLL guideline, steroids are the mainstay of treatment of simple autoimmunity. Intravenous immunoglobulin (IVIg), cyclosporine, and rituximab are used in complex, steroid refractory cases. Monotherapy with purine analogues and alkylating agents should be avoided as they may increase CLL associated autoimmune complications.
Autoimmune thrombocytopenia (AIT) is the second most common complication of CLL and has been reported in 2-3% of patients. DAT is positive in AIT but presence of antiplatelet antibodies is neither diagnostic nor reliable. Autoimmune neutropenia (AIN) and pure red cell aplasia (PRCA) are very rare complications of CLL and like other autoimmune complications of CLL may occur at any clinical stage. It is believed that most case reports of AIN and PRCA in CLL actually belong to large granular lymphocytic leukemia (LGL). Non-hematologic autoimmune complications of CLL including cold agglutinin disease (CAD), paraneoplastic pemphigus (PNP), acquired angioedema, and anti-myelin associated globulin are rare.
Before starting any treatment, clinicians should distinguish between autoimmune cytopenias and massive bone marrow infiltration since autoimmune complications of CLL are not necessarily equal to advanced disease with poor prognosis. According to IWCLL guideline, steroids are the mainstay of treatment of simple autoimmunity. Intravenous immunoglobulin (IVIg), cyclosporine, and rituximab are used in complex, steroid refractory cases. Monotherapy with purine analogues and alkylating agents should be avoided as they may increase CLL associated autoimmune complications.
Introduction
Chronic lymphocytic leukemia (CLL), characterized by progressive accumulation of nonfunctional and monoclonal B lymphocytes in the blood, bone marrow and lymphatic system,[1] is the most common leukemia in the western world. CLL accounts for approximately 30 percent of all leukemias.[2] According to the National Cancer Institute-Working Group (NCI-WG) 2008, CLL is presence of greater than 5000 small mature appearing monoclonal B lymphocytes in the peripheral blood. However, the clonality of B lymphocytes has to be confirmed by flow cytometry. CLL is mainly a disease of elderly and the median age at onset is 72 years. As it is evident from data of 18 Surveillance Epidemiology and End Results (SEER) databases, the age-adjusted incidence rate for CLL between the years of 2005-2009 was 4.2 per 100,000 men and women annually.[3]
Autoimmunity secondary to CLL may have hematologic and non-hematologic manifestations.[4,5] Hematologic autoimmune phenomena include hemolytic anemia (AIHA), thrombocytopenia (AIT), and neutropenia (AIN), and pure red blood cell aplasia (PRCA).
Autoimmune cytopenias in CLL may occur at any stages of CLL, respond well to treatment and do not affect the overall survival of CLL patients.[6] Although a number of non-hematologic autoimmune conditions have sporadic associations with CLL, autoimmune paraneoplastic pemphigus, autoimmune glomerulonephritis and autoimmune C1 esterase inhibitor deficiency have been shown to have a definite association[1,6] (Tables 1 and 2).
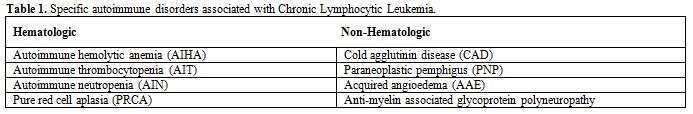
Table 1. Specific autoimmune disorders associated with Chronic Lymphocytic Leukemia.
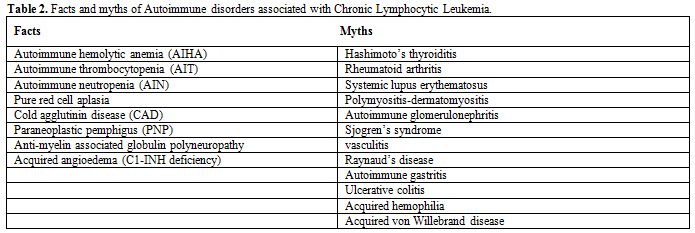
Table 2. Facts and myths of Autoimmune disorders associated with Chronic Lymphocytic Leukemia.
Epidemiology of Autoimmune Cytopenias
The incidence of autoimmune cytopenias varies from 4.3% to 26% in different reports.[6] Since these data has been extracted from tertiary care centers database, the true prevalence and incidence of autoimmune cytopenias in CLL patients is unknown. Prior studies might have overestimated the prevalence owing to the lack of specific diagnostic methods. On the other hand, better management and new drugs that have changed the overall survival of these patients, have affected the prevalence of autoimmune cytopenias in CLL patients. Autoimmune neutropenia might have been over reported in some of previous series since these studies have included large granular lymphocytic leukemia (LGL) in their study.[7] Recent studies estimated that the incidence of autoimmune cytopenias might be in the range of 5 to 10%.[8]
A study of 1750 CLL patients seen over a period of 10 years at Mayo clinic[9] found that 24% of patients had cytopenias. The most common causes of cytopenias were marrow failure (54%), autoimmunity (18%), non CLL related cytopenias (11%), long term complications of treatment (4%) and splenomegaly (3%).
The incidence of AIT was estimated 1-5% in various studies.[9-11] The AIN incidence is much more difficult to assess since neutropenia is frequently not CLL-related, and currently available tests (anti neutrophil antibodies) cannot diagnose AIN with certainty. The incidence of AIN in a large series of CLL patients followed for 10 years at Mayo clinic, was 0.17% (3 out of 1750).[12]
A thorough review of literature using words “auto immune granulocytopenia”, “auto immune leukopenia” “autoimmune neutropenia” in CLL revealed only 4 case reports of CLL associated neutropenia.[13,14] All of these case reports were autoimmunity secondary to monotherapy with purine analogues or alkylating agents.
Pathogenesis of Autoimmune Cytopenias in CLL
A brief overview of autoimmunity. Autoimmune diseases are caused by adaptive immunity where, immune responses are directed towards autologous component(s) of the body. They represent loss of the self-tolerance mechanisms in circulating B and T cells. A defining characteristic of autoimmune diseases is the presence of antibodies and T cells specific for antigens expressed by the target tissue. These antigens are called auto antigens; the effectors of adaptive immunity that recognize them are known as autoantibodies and autoimmune T cells. There are regulatory mechanisms in the T cell repertoire where auto reactive T cells are usually suppressed by regulatory T cells (Treg) and imbalance of these mechanisms can result in autoimmunity.[15-19]
Autoimmune diseases can be classified by the type of adaptive immunological effector mechanism causing the disease. Three kinds of mechanisms are responsible which correspond to three of the four categories of hypersensitivity reactions (types II, III IV). Autoimmune diseases are never caused by immunoglobulin E (IgE), the source of type I hypersensitivity reactions.[15] Description of these effector mechanisms is beyond the scope of this review.
Mechanisms of autoimmunity in CLL. In autoimmune cytopenias, autoimmunity usually corresponds to the type II hypersensitivity reaction in which the antibodies are frequently directed at the antigens on blood cells. Immunoglobulin G and IgM antibodies bind to certain blood cells (red cells, platelets and neutrophils), where they activate complement system by classical pathway, and trigger their destruction. Alternatively, phagocytes in reticuloendothelial system may clear C3b and antibody-coated cells from circulation.[15]
The underlying mechanisms of pathologic auto antibody production in CLL associated cytopenias are not well understood. In AIHA, both the T cell dysfunction and malignant B cells are involved in autoantibody production. Malignant B lymphocytes act as antigen presenting cells (APCs) and present antigen to T Helper cell and lead to their activation. Activated T helper cells mediate antibody production by non-malignant B lymphocytes. These auto antibodies lead to hemolysis by destroying the target red cells. Murine and human studies revealed these antibodies are mostly targeted towards Rh antigens on the surface of red blood cells.[20,21]
Autoimmune thrombocytopenia. There are many proposed mechanisms that by which autoantibodies are produced and interact with antigens on platelets in CLL. Ninety percent of auto antibodies are produced by nonmalignant B cells rather than malignant B cells[22] and are directed towards platelet surface antigens glycoprotein Ib/IX and IIb/IIIa.[18,19] These autoantibodies are IgG type and polyclonal in nature. These circulating autoantibodies attach to the platelet antigens and destroy the platelets via opsonisation and antibody dependent cellular cytotoxicity and are finally cleared in the reticuloendothelial system in the liver and spleen. In 10% of the cases, the mechanism is similar to cold hemagglutinin disease where the IgM monoclonal antibodies are produced by malignant B cells.[23] Besides production of pathologic autoantibodies, there is evidence to suggest that suppression of Treg cells also leads to autoimmunity in CLL which was first reported in post-fludarabine cytopenias.[16-19]
Visco et al. retrospectively studied 463 CLL patients with available immunoglobulin heavy-chain variable (IGHV) gene status and B-cell receptor (BCR) configuration [heavy-chain complementary-determining region 3 (HCDR3)], of whom 36 developed AIT. Unmutated IGHV mutational status, deletion 11q23 and stereotyped BCR were significantly associated with shorter time to AIT development than other factors.[24,25] The risk of AIT has been reported to be increased in CLL patients with positive cells for ZAP70 expression, negative for CD38 and abnormal fluorescence in situ hybridization (FISH).[11,26-28] Positive direct anti globulin test and occurrence of AIHA are other reported risk factors for AIT development.[11]
Anti-platelet antibodies may present in CLL patients without having thrombocytopenia. The importance of this phenomenon is not clear and there is no data on how many of these patients subsequently will develop thrombocytopenia. With combination of autoantibody mediated megakaryocyte destruction and inability of the marrow to produce megakaryocytes secondary to infiltrating leukemic cells AIT develops.[8]
Autoimmune neutropenia. Pathogenesis of AIN is not well studied and our current understanding of autoimmune neutrophil destruction is mainly based on in vitro observations. Agglutination of neutrophils due to anti neutrophil antibodies (ANAs), complement mediated neutrophil destruction and phagocytosis of neutrophils coated with ANAs in spleen and liver is some of the proposed mechanisms.[29]
Autoimmune neutropenia is commonly seen in association with connective tissue disorders, Grave’s disease, hepatitis B and C, human immunodeficiency virus, parvovirus B19, and Helicobacter pylori infections, and malignancies such as large granular lymphocytic leukemia, hairy cell leukemia and Hodgkin’s lymphoma. Some of these conditions may coexist with CLL, and should be considered before labeling CLL as the culprit for AIN.[29] Autoimmune neutropenia has been commonly seen in hairy cell leukemia and Hodgkin’s Lymphoma but is rare in CLL.[23,30] Mono therapy of CLL with medications such as fludarabine, rituximab and alemtuzumab are also associated with AIN.[14,31]
Clinical Presentation
Autoimmune thrombocytopenia. Autoimmune thrombocytopenia is an incidental finding in more than half of CLL patients[12,32] and it is prudent that patients with immune thrombocytopenia be screened for CLL.[33,34] In an Italian series, the median time from diagnosis of CLL to development of AIT was 13 months.[11] Thrombocytopenia due to marrow infiltration is usually seen in later stages but AIT may occur at any time during the course of CLL.[33,34]
Bleeding due to thrombocytopenia is rare in CLL associated AIT unless platelet counts are very low (less than 15,000). Even in rapid AIT, only 50% of patients present with bleeding and less than 10% has clinically significant bleeding.[12]
Autoimmune Neutropenia. Recurrent infections are the only clinical presentation of AIN and should be identified early and treated appropriately. The classic signs and symptoms of infection may be absent in neutropenia. The risk of infection is high with an absolute neutrophil count (ANC) below 500 and it increases exponentially with an ANC below 100 for more than 5 days.[35-37]
Common sites of infection in severe neutropenia (ANC below 500) include skin, oral cavity, and perirectal area in addition to intravenous lines and port-a-catheters. Careful examination of all catheter sites is part of AIN evaluation. In Elting et al. study, gram positive organisms comprise 46% of the infections, while 42% are due to gram negative organisms and 12% are poly microbial; lung (40%), and skin and soft tissues (30%) were the most common sites and urinary tract, sinuses and oropharynx, skeletal, enteric tract, meninges, and endocardium were the remaining cases.[35]
Diagnosis
Gradual cytopenias in CLL are multifactorial. If CLL associated cytopenia occurs acutely, sepsis syndrome should be considered since CLL patients are at increased risk of opportunistic infections owing to nonfunctional lymphocytes and related hypogammaglobulinemia.
A high degree of suspicion is required to diagnose autoimmunity as a cause of cytopenias given the multiple etiologies. Cytopenias might present before CLL requires therapy, however, the highest risk is in patients with advanced disease.[38] Clinicians should suspect AIT when there is more than 50% drop in platelet count to lower than 100000. If platelet counts were low from the beginning, then a careful examination of the peripheral blood smear is required.[39] An assay for EDTA dependent pseudo thrombocytopenia and inspection of platelet volume curve to rule out inherited macro thrombocytopenia may also be required. Furthermore, a careful review of recent changes in medications including prescribed and over the counter drugs is useful to exclude drug induced thrombocytopenia. Platelet antibodies are neither sensitive nor specific for AIT[18,40] and their confirmation is not necessary for diagnosis of CLL associated AIT.[41] Increased mean platelet volume and platelet distribution width are suggestive of AIT in some patients.
In advanced CLL, isolated thrombocytopenia without anemia is more likely to have autoimmune etiology.[33,34,42] Because thrombocytopenia commonly seen with infections such as Hepatitis C, Helicobacter pylori and human immunodeficiency virus, it is prudent to investigate for these infections, and sometimes management of these infections may increase platelet counts without using corticosteroids[43] (Table 3).
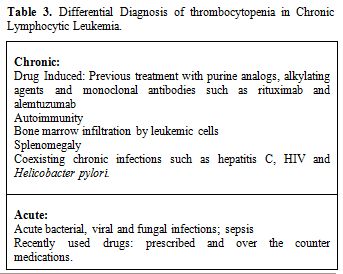
Table 3. Differential Diagnosis of thrombocytopenia in Chronic Lymphocytic Leukemia.
Demonstration of increased and reticulated megakaryocytes in bone marrow in response to thrombocytopenia confirms the diagnosis. Bone marrow biopsy shows normal or increased megakaryocytes with immature forms. Sometimes heavily infiltrated bone marrow with leukemic cells makes it difficult to assess the megakaryocyte numbers. Response to the trial of intravenous immunoglobulin or steroids may confirm the diagnosis. Thirty percent of AIT cases also have simultaneous AIHA (Evans Syndrome).[44,45]
In contrast, AIN is extremely rare and difficult to diagnose with certainty.[29] AIN is a diagnosis of exclusion and only should be suspected when there is persistent and prolonged absolute neutropenia accompanied with failure of neutrophil production or maturation arrest in bone marrow.[5] Antineutrophil antibodies are neither sensitive nor specific and should not be used as a tool for diagnosis. It should be emphasized that it is difficult to appreciate neutrophil precursors in a heavily infiltrated marrow by leukemic cells. It is crucial to recognize marrow infiltration as soon as possible since these patients are at risk for overwhelming infections and sepsis[5] (Table 4).
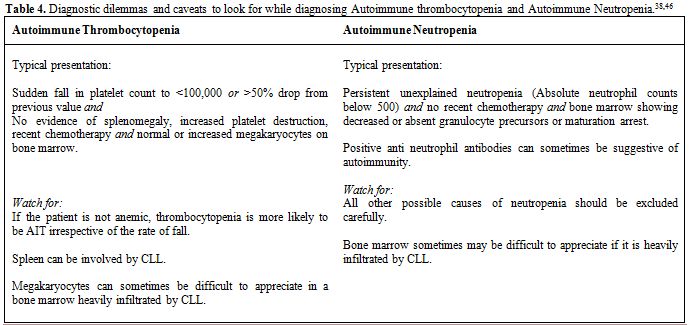
Table 4. Diagnostic dilemmas and caveats to look for while diagnosing Autoimmune thrombocytopenia and Autoimmune Neutropenia.[38,46]
Management
Autoimmune thrombocytopenia. There is no randomized controlled clinical trial for management of CLL associated autoimmune thrombocytopenia, and treatment is mainly based on small case series. Whether patient requires CLL chemotherapy or autoimmune disorder management is the first question that clinicians should raise.
In patients with non-progressive CLL, management is similar to primary immune thrombocytopenic purpura with corticosteroids, Intravenous immunoglobulin, rituximab, thrombopoietin receptor agonists, and splenectomy in refractory cases. In a life threatening hemorrhage condition that requires a rapid intervention, intravenous immunoglobulin 1 gram per kilogram per day[43] or methyl prednisone 1 gram per day for 3 doses, followed by platelet transfusion may be lifesaving.[4] Intravenous immunoglobulin effect does not last for more than a few weeks in the body.
According to the American society of hematology guideline, if thrombocytopenia is an incidental finding and there is no significant hemorrhage, there is no need for treatment. However, treatment is indicated when platelet count drops below 30,000, and is low enough to constitute a risk of bleeding.[43]
Corticosteroids. Oral prednisone, dexamethasone and intravenous methyl prednisolone have shown equal efficacy in the treatment of AIT. Oral prednisone at a dose of 0.5 to 2 mg per kilogram body weight should be initiated and tapered after a response has been achieved. It usually takes 1-2 weeks to observe a response. However, if there is no response after 4-6 weeks, it is highly unlikely to respond to steroids and alternative treatments should be sought. Pulse therapy with high dose dexamethasone (40 milligrams per day for 4 days which can be repeated every couple of weeks) is another option.[47]
More than two third of patients will completely respond to steroids, but there is no response in the remained one third of patients. In such a condition, any of the other therapies described below can be tried. Among responders, the median duration of response may have about 2 years.[12]
Immunosuppressive agents other than corticosteroids. Low dose cyclosporine,[48] mycophenolate mofetil,[49,50] cyclophosphamide[51,52] or azathioprine are alternative immunosuppressive therapy[53] and may be considered in patients who fail to respond to corticosteroids, require higher maintenance doses, relapse quickly or who develop side effects (Table 5).
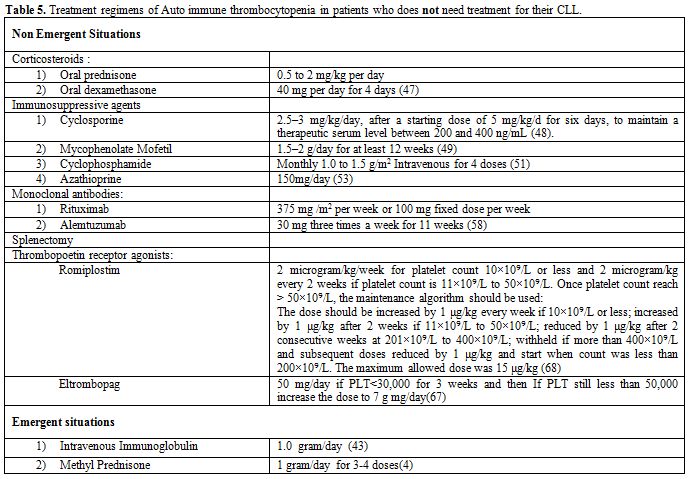
Table 5. Treatment regimens of Auto immune thrombocytopenia in patients who does not need treatment for their CLL.
Autoimmune thrombocytopenia refractory to immunosuppression. The goal of treatment for CLL associated AIT refractory to corticosteroids is to maintain a safer platelet count above 20000 to 50000. A reevaluation with a bone marrow biopsy/aspiration may be required to check for marrow failure. Patients with progressive marrow failure secondary to leukemic infiltration may benefit from CLL specific therapy. Also cytomegalovirus (CMV) reactivation has to be considered in the differential diagnosis in these patients as it is common in advanced stage of CLL and destroys platelets and megakaryocytes.[8]
Single agent rituximab or splenectomy may be tried in refractory cases. However, there is no randomized clinical trial which directly compares these two approaches. Rituximab is used in patients in whom splenectomy cannot be safely performed.[54,55] Although rituximab is well tolerated, only 20% of patients achieved a sustained response.[55] Low dose mycophenolate mofetil may be used after rituximab to prolong the response in selected patients.[56]
Single agent monoclonal antibody therapy. Rituximab,[57] Alemtuzumab[58] and Veltuzumab[59] have been reported to be effective in CLL associated AIT.[60,61] Most of the published literature about rituximab is in primary immune thrombocytopenic purpura. Rituximab may be used in steroid refractory disease as a single agent, when treatment for CLL is not required. It may also be used in fludarabine induced autoimmunity.[61] The dose of rituximab is 375 mg per square meter given weekly for 4 weeks. A lower fixed dose of 100mg per week also has been studied, and has similar response rates.[62,63]
Thrombopoietin agonist. Thrombopoietin (TPO), a ligand for c-mpl, stimulates committed megakaryocytic progenitors proliferation and induces maturation of megakaryocytes. Two thrombopoietin receptor agonists, romiplostim[64] and eltrombopag[65] were studied in multiple studies and are the only agents approved by FDA for the treatment of primary immune thrombocytopenic purpura. In a Danish retrospective registration study,[66] Seven patients of secondary ITP (CLL is one of them, the others included Systemic lupus erythematosus, Evans syndrome, Human Immunodeficiency Virus infection and celiac disease) were treated with TPO agonists. 57% responded with a platelet counts greater than 30,000 after 4 weeks.
TPO agonists are used for thrombocytopenia refractory to other therapies such as corticosteroids, intravenous immunoglobulin (IV Ig), single agent rituximab and splenectomy or when splenectomy is contraindicated.[67,68] It has been suggested that these therapeutic modules should be used when a durable response is not obtained with other therapies and the platelet count continues to decline below 50,000.
The European Medicines Evaluation Agency approved TPOs for splenectomised patients who are refractory to other treatments. The American Society of Hematology recommendation for TPOs includes patients at risk of bleeding, patients in whom splenectomy is contraindicated, and patients who have failed at least one of therapies other than glucocorticoids.[43] Thrombopoietins do not cause remission and continuous administration is required. Although TPOs are well-tolerated and no significant side effects are generally reported, studies have shown that they can increase bone marrow reticulin fibrosis and have increased risk of thrombocytosis.[69] There are no trials comparing romiplostim and eltrombopag. However, higher incidence of portal vein thrombosis reported with eltrombopag in thrombocytopenia secondary to chronic liver disease. We suggest that the etiology of thrombocytopenia should be sought before using TPOs in patients with liver disease.[67]
Splenectomy. Antibody and complement bound blood cells still have the ability to function normally. Spleen clears opsonized cells from circulation, and by reducing its rate, splenectomy may be an effective treatment. In a large series published by Vianelli et al. in 2013 with a minimum of ten years follow up,[70] there was a durable response to splenectomy. Out of 233 patients followed for 10 years, there was 88% response to splenectomy with 77% complete response. The response was well-maintained, and free of any treatment in 59% of patients. Splenic irradiation may be an alternative for patients in whom surgery is contraindicated.
Patients will require vaccination against encapsulated organisms including Streptococcus pneumonia, Hemophilus influenza b, and Neisseria meningitides at least 2-3 weeks before splenectomy.[71-73] CLL patients are generally at increased risk of infections owing to defects in both cellular and humoral immune systems and qualitative and quantitative defects in B, T, and natural killer (NK) cells, neutrophils and the monocyte/macrophage system.[74] Splenectomised patients have variable response to vaccination and immunization is most useful if used earlier in the disease process, and when immunoglobulin levels are better preserved.[74] Considering that protein or conjugated vaccines are preferred for better immune response,[75-78] it may be appropriate to routinely immunize all patients at the time of diagnosis, and before extensive immunosuppressive treatment has been given.
Supportive care. In AIT patients with active bleeding, supportive care including platelet transfusions, antifibrinolytic agents such as tranexamic acid and eventually emergent surgical intervention may be required.
Patients on long term steroids are at risk of Pneumocystis jiroveci infection and prophylaxis is required. It is not clear that above which dose of steroids patients are considered to be at risk, but there is a general agreement that any dose above 20 milligrams per day has a higher risk for Pneumocystis jiroveci.[8]
Autoimmune thrombocytopenia in the setting of advanced CLL. There is no standard treatment for this subgroup of patients. As described above, purine analogues and alkylating agents have a risk of inducing autoimmunity and should be avoided.
Two mostly studied chemo immunotherapy regimens in these patients include rituximab, cyclophosphamide and dexamethasone (RCD)[79-82] and rituximab, cyclophosphamide, vincristine and prednisone (R-CVP).[83] These two regimens have not been compared with each other in any randomized clinical trials.
In 2009, Kaufman et al. reported RCD reported usage of RCD in 21 patients with progressive CLL and coexisting autoimmune cytopenias.[84] Three Out of 21 patients had steroid resistant AIT and their nadir platelet counts were 1000, 1000 and 14000. The platelet counts increased to 408000, 161000 and 135000 after treatment with RCD in 9, 15, and 29 months respectively. The first patient who relapsed at 9 months was retreated with RCD and had 15 months’ response without any second relapse.
In a case control study of R-CVP by Bowen et al., 6 out of 20 studied patients had AIT.[83] The median increase in platelet count was 106000 (range from 31000 to 216000), and median time to next treatment was 21.7 months. Patients with no response to R-CVP subsequently had a sustained complete response to splenectomy.
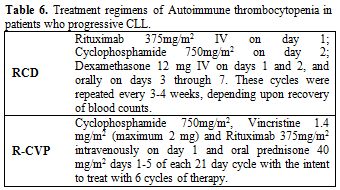
Table 6. Treatment regimens of Autoimmune thrombocytopenia in patients who progressive CLL.
Autoimmune Neutropenia
Autoimmune neutropenia in CLL requires a multidisciplinary management plan as these patients are at increased risk of atypical infections. Owing to both qualitative and quantitative defects in B, T, and NK cells both cellular and humoral arms of the adaptive immune systems are impaired in CLL. Monocyte/macrophage lineage is affected resulting in impairment of the innate immunity.[74]
When the absolute neutrophil count reaches below 500, risk of infection is increased, and it is important to consider treatment of AIN. AIN accompanied by fever of more than 100.4 F should be treated as neutropenic sepsis. Granulocyte – colony stimulating factor (G-CSF) and prophylactic antibiotics are indicated in persistent neutropenia without fever. However, normalization of neutrophil count does not last long after discontinuation of G-CSF. Splenectomy was commonly used before the availability of G-CSF but the results are not encouraging.[85]
Sirolimus, cyclosporine and IVIg are reported to be helpful in AIN in several case reports.[85-87] Rituximab and Alemtuzumab can provide long lasting remissions but the evidence is limited.[88,89]
Finally, it is very import to offer age appropriate vaccination to all CLL patients to prevent life threatening infections.
Conclusion
Compared to cytopenias from the leukemic marrow involvement, autoimmune cytopenias have a better prognosis. The pathogeneses of AIT and AIN are not well understood, and lack of confirmatory tests is a diagnostic challenge. Since CLL associated cytopenias are not common, there is no randomized clinical trial on management of these conditions. Multicenter randomized clinical trials are necessary to enroll more patients in therapeutic trials.
Chronic lymphocytic leukemia (CLL), characterized by progressive accumulation of nonfunctional and monoclonal B lymphocytes in the blood, bone marrow and lymphatic system,[1] is the most common leukemia in the western world. CLL accounts for approximately 30 percent of all leukemias.[2] According to the National Cancer Institute-Working Group (NCI-WG) 2008, CLL is presence of greater than 5000 small mature appearing monoclonal B lymphocytes in the peripheral blood. However, the clonality of B lymphocytes has to be confirmed by flow cytometry. CLL is mainly a disease of elderly and the median age at onset is 72 years. As it is evident from data of 18 Surveillance Epidemiology and End Results (SEER) databases, the age-adjusted incidence rate for CLL between the years of 2005-2009 was 4.2 per 100,000 men and women annually.[3]
Autoimmunity secondary to CLL may have hematologic and non-hematologic manifestations.[4,5] Hematologic autoimmune phenomena include hemolytic anemia (AIHA), thrombocytopenia (AIT), and neutropenia (AIN), and pure red blood cell aplasia (PRCA).
Autoimmune cytopenias in CLL may occur at any stages of CLL, respond well to treatment and do not affect the overall survival of CLL patients.[6] Although a number of non-hematologic autoimmune conditions have sporadic associations with CLL, autoimmune paraneoplastic pemphigus, autoimmune glomerulonephritis and autoimmune C1 esterase inhibitor deficiency have been shown to have a definite association[1,6] (Tables 1 and 2).

Table 1. Specific autoimmune disorders associated with Chronic Lymphocytic Leukemia.

Table 2. Facts and myths of Autoimmune disorders associated with Chronic Lymphocytic Leukemia.
Epidemiology of Autoimmune Cytopenias
The incidence of autoimmune cytopenias varies from 4.3% to 26% in different reports.[6] Since these data has been extracted from tertiary care centers database, the true prevalence and incidence of autoimmune cytopenias in CLL patients is unknown. Prior studies might have overestimated the prevalence owing to the lack of specific diagnostic methods. On the other hand, better management and new drugs that have changed the overall survival of these patients, have affected the prevalence of autoimmune cytopenias in CLL patients. Autoimmune neutropenia might have been over reported in some of previous series since these studies have included large granular lymphocytic leukemia (LGL) in their study.[7] Recent studies estimated that the incidence of autoimmune cytopenias might be in the range of 5 to 10%.[8]
A study of 1750 CLL patients seen over a period of 10 years at Mayo clinic[9] found that 24% of patients had cytopenias. The most common causes of cytopenias were marrow failure (54%), autoimmunity (18%), non CLL related cytopenias (11%), long term complications of treatment (4%) and splenomegaly (3%).
The incidence of AIT was estimated 1-5% in various studies.[9-11] The AIN incidence is much more difficult to assess since neutropenia is frequently not CLL-related, and currently available tests (anti neutrophil antibodies) cannot diagnose AIN with certainty. The incidence of AIN in a large series of CLL patients followed for 10 years at Mayo clinic, was 0.17% (3 out of 1750).[12]
A thorough review of literature using words “auto immune granulocytopenia”, “auto immune leukopenia” “autoimmune neutropenia” in CLL revealed only 4 case reports of CLL associated neutropenia.[13,14] All of these case reports were autoimmunity secondary to monotherapy with purine analogues or alkylating agents.
Pathogenesis of Autoimmune Cytopenias in CLL
A brief overview of autoimmunity. Autoimmune diseases are caused by adaptive immunity where, immune responses are directed towards autologous component(s) of the body. They represent loss of the self-tolerance mechanisms in circulating B and T cells. A defining characteristic of autoimmune diseases is the presence of antibodies and T cells specific for antigens expressed by the target tissue. These antigens are called auto antigens; the effectors of adaptive immunity that recognize them are known as autoantibodies and autoimmune T cells. There are regulatory mechanisms in the T cell repertoire where auto reactive T cells are usually suppressed by regulatory T cells (Treg) and imbalance of these mechanisms can result in autoimmunity.[15-19]
Autoimmune diseases can be classified by the type of adaptive immunological effector mechanism causing the disease. Three kinds of mechanisms are responsible which correspond to three of the four categories of hypersensitivity reactions (types II, III IV). Autoimmune diseases are never caused by immunoglobulin E (IgE), the source of type I hypersensitivity reactions.[15] Description of these effector mechanisms is beyond the scope of this review.
Mechanisms of autoimmunity in CLL. In autoimmune cytopenias, autoimmunity usually corresponds to the type II hypersensitivity reaction in which the antibodies are frequently directed at the antigens on blood cells. Immunoglobulin G and IgM antibodies bind to certain blood cells (red cells, platelets and neutrophils), where they activate complement system by classical pathway, and trigger their destruction. Alternatively, phagocytes in reticuloendothelial system may clear C3b and antibody-coated cells from circulation.[15]
The underlying mechanisms of pathologic auto antibody production in CLL associated cytopenias are not well understood. In AIHA, both the T cell dysfunction and malignant B cells are involved in autoantibody production. Malignant B lymphocytes act as antigen presenting cells (APCs) and present antigen to T Helper cell and lead to their activation. Activated T helper cells mediate antibody production by non-malignant B lymphocytes. These auto antibodies lead to hemolysis by destroying the target red cells. Murine and human studies revealed these antibodies are mostly targeted towards Rh antigens on the surface of red blood cells.[20,21]
Autoimmune thrombocytopenia. There are many proposed mechanisms that by which autoantibodies are produced and interact with antigens on platelets in CLL. Ninety percent of auto antibodies are produced by nonmalignant B cells rather than malignant B cells[22] and are directed towards platelet surface antigens glycoprotein Ib/IX and IIb/IIIa.[18,19] These autoantibodies are IgG type and polyclonal in nature. These circulating autoantibodies attach to the platelet antigens and destroy the platelets via opsonisation and antibody dependent cellular cytotoxicity and are finally cleared in the reticuloendothelial system in the liver and spleen. In 10% of the cases, the mechanism is similar to cold hemagglutinin disease where the IgM monoclonal antibodies are produced by malignant B cells.[23] Besides production of pathologic autoantibodies, there is evidence to suggest that suppression of Treg cells also leads to autoimmunity in CLL which was first reported in post-fludarabine cytopenias.[16-19]
Visco et al. retrospectively studied 463 CLL patients with available immunoglobulin heavy-chain variable (IGHV) gene status and B-cell receptor (BCR) configuration [heavy-chain complementary-determining region 3 (HCDR3)], of whom 36 developed AIT. Unmutated IGHV mutational status, deletion 11q23 and stereotyped BCR were significantly associated with shorter time to AIT development than other factors.[24,25] The risk of AIT has been reported to be increased in CLL patients with positive cells for ZAP70 expression, negative for CD38 and abnormal fluorescence in situ hybridization (FISH).[11,26-28] Positive direct anti globulin test and occurrence of AIHA are other reported risk factors for AIT development.[11]
Anti-platelet antibodies may present in CLL patients without having thrombocytopenia. The importance of this phenomenon is not clear and there is no data on how many of these patients subsequently will develop thrombocytopenia. With combination of autoantibody mediated megakaryocyte destruction and inability of the marrow to produce megakaryocytes secondary to infiltrating leukemic cells AIT develops.[8]
Autoimmune neutropenia. Pathogenesis of AIN is not well studied and our current understanding of autoimmune neutrophil destruction is mainly based on in vitro observations. Agglutination of neutrophils due to anti neutrophil antibodies (ANAs), complement mediated neutrophil destruction and phagocytosis of neutrophils coated with ANAs in spleen and liver is some of the proposed mechanisms.[29]
Autoimmune neutropenia is commonly seen in association with connective tissue disorders, Grave’s disease, hepatitis B and C, human immunodeficiency virus, parvovirus B19, and Helicobacter pylori infections, and malignancies such as large granular lymphocytic leukemia, hairy cell leukemia and Hodgkin’s lymphoma. Some of these conditions may coexist with CLL, and should be considered before labeling CLL as the culprit for AIN.[29] Autoimmune neutropenia has been commonly seen in hairy cell leukemia and Hodgkin’s Lymphoma but is rare in CLL.[23,30] Mono therapy of CLL with medications such as fludarabine, rituximab and alemtuzumab are also associated with AIN.[14,31]
Clinical Presentation
Autoimmune thrombocytopenia. Autoimmune thrombocytopenia is an incidental finding in more than half of CLL patients[12,32] and it is prudent that patients with immune thrombocytopenia be screened for CLL.[33,34] In an Italian series, the median time from diagnosis of CLL to development of AIT was 13 months.[11] Thrombocytopenia due to marrow infiltration is usually seen in later stages but AIT may occur at any time during the course of CLL.[33,34]
Bleeding due to thrombocytopenia is rare in CLL associated AIT unless platelet counts are very low (less than 15,000). Even in rapid AIT, only 50% of patients present with bleeding and less than 10% has clinically significant bleeding.[12]
Autoimmune Neutropenia. Recurrent infections are the only clinical presentation of AIN and should be identified early and treated appropriately. The classic signs and symptoms of infection may be absent in neutropenia. The risk of infection is high with an absolute neutrophil count (ANC) below 500 and it increases exponentially with an ANC below 100 for more than 5 days.[35-37]
Common sites of infection in severe neutropenia (ANC below 500) include skin, oral cavity, and perirectal area in addition to intravenous lines and port-a-catheters. Careful examination of all catheter sites is part of AIN evaluation. In Elting et al. study, gram positive organisms comprise 46% of the infections, while 42% are due to gram negative organisms and 12% are poly microbial; lung (40%), and skin and soft tissues (30%) were the most common sites and urinary tract, sinuses and oropharynx, skeletal, enteric tract, meninges, and endocardium were the remaining cases.[35]
Diagnosis
Gradual cytopenias in CLL are multifactorial. If CLL associated cytopenia occurs acutely, sepsis syndrome should be considered since CLL patients are at increased risk of opportunistic infections owing to nonfunctional lymphocytes and related hypogammaglobulinemia.
A high degree of suspicion is required to diagnose autoimmunity as a cause of cytopenias given the multiple etiologies. Cytopenias might present before CLL requires therapy, however, the highest risk is in patients with advanced disease.[38] Clinicians should suspect AIT when there is more than 50% drop in platelet count to lower than 100000. If platelet counts were low from the beginning, then a careful examination of the peripheral blood smear is required.[39] An assay for EDTA dependent pseudo thrombocytopenia and inspection of platelet volume curve to rule out inherited macro thrombocytopenia may also be required. Furthermore, a careful review of recent changes in medications including prescribed and over the counter drugs is useful to exclude drug induced thrombocytopenia. Platelet antibodies are neither sensitive nor specific for AIT[18,40] and their confirmation is not necessary for diagnosis of CLL associated AIT.[41] Increased mean platelet volume and platelet distribution width are suggestive of AIT in some patients.
In advanced CLL, isolated thrombocytopenia without anemia is more likely to have autoimmune etiology.[33,34,42] Because thrombocytopenia commonly seen with infections such as Hepatitis C, Helicobacter pylori and human immunodeficiency virus, it is prudent to investigate for these infections, and sometimes management of these infections may increase platelet counts without using corticosteroids[43] (Table 3).

Table 3. Differential Diagnosis of thrombocytopenia in Chronic Lymphocytic Leukemia.
Demonstration of increased and reticulated megakaryocytes in bone marrow in response to thrombocytopenia confirms the diagnosis. Bone marrow biopsy shows normal or increased megakaryocytes with immature forms. Sometimes heavily infiltrated bone marrow with leukemic cells makes it difficult to assess the megakaryocyte numbers. Response to the trial of intravenous immunoglobulin or steroids may confirm the diagnosis. Thirty percent of AIT cases also have simultaneous AIHA (Evans Syndrome).[44,45]
In contrast, AIN is extremely rare and difficult to diagnose with certainty.[29] AIN is a diagnosis of exclusion and only should be suspected when there is persistent and prolonged absolute neutropenia accompanied with failure of neutrophil production or maturation arrest in bone marrow.[5] Antineutrophil antibodies are neither sensitive nor specific and should not be used as a tool for diagnosis. It should be emphasized that it is difficult to appreciate neutrophil precursors in a heavily infiltrated marrow by leukemic cells. It is crucial to recognize marrow infiltration as soon as possible since these patients are at risk for overwhelming infections and sepsis[5] (Table 4).

Table 4. Diagnostic dilemmas and caveats to look for while diagnosing Autoimmune thrombocytopenia and Autoimmune Neutropenia.[38,46]
Management
Autoimmune thrombocytopenia. There is no randomized controlled clinical trial for management of CLL associated autoimmune thrombocytopenia, and treatment is mainly based on small case series. Whether patient requires CLL chemotherapy or autoimmune disorder management is the first question that clinicians should raise.
In patients with non-progressive CLL, management is similar to primary immune thrombocytopenic purpura with corticosteroids, Intravenous immunoglobulin, rituximab, thrombopoietin receptor agonists, and splenectomy in refractory cases. In a life threatening hemorrhage condition that requires a rapid intervention, intravenous immunoglobulin 1 gram per kilogram per day[43] or methyl prednisone 1 gram per day for 3 doses, followed by platelet transfusion may be lifesaving.[4] Intravenous immunoglobulin effect does not last for more than a few weeks in the body.
According to the American society of hematology guideline, if thrombocytopenia is an incidental finding and there is no significant hemorrhage, there is no need for treatment. However, treatment is indicated when platelet count drops below 30,000, and is low enough to constitute a risk of bleeding.[43]
Corticosteroids. Oral prednisone, dexamethasone and intravenous methyl prednisolone have shown equal efficacy in the treatment of AIT. Oral prednisone at a dose of 0.5 to 2 mg per kilogram body weight should be initiated and tapered after a response has been achieved. It usually takes 1-2 weeks to observe a response. However, if there is no response after 4-6 weeks, it is highly unlikely to respond to steroids and alternative treatments should be sought. Pulse therapy with high dose dexamethasone (40 milligrams per day for 4 days which can be repeated every couple of weeks) is another option.[47]
More than two third of patients will completely respond to steroids, but there is no response in the remained one third of patients. In such a condition, any of the other therapies described below can be tried. Among responders, the median duration of response may have about 2 years.[12]
Immunosuppressive agents other than corticosteroids. Low dose cyclosporine,[48] mycophenolate mofetil,[49,50] cyclophosphamide[51,52] or azathioprine are alternative immunosuppressive therapy[53] and may be considered in patients who fail to respond to corticosteroids, require higher maintenance doses, relapse quickly or who develop side effects (Table 5).

Table 5. Treatment regimens of Auto immune thrombocytopenia in patients who does not need treatment for their CLL.
Autoimmune thrombocytopenia refractory to immunosuppression. The goal of treatment for CLL associated AIT refractory to corticosteroids is to maintain a safer platelet count above 20000 to 50000. A reevaluation with a bone marrow biopsy/aspiration may be required to check for marrow failure. Patients with progressive marrow failure secondary to leukemic infiltration may benefit from CLL specific therapy. Also cytomegalovirus (CMV) reactivation has to be considered in the differential diagnosis in these patients as it is common in advanced stage of CLL and destroys platelets and megakaryocytes.[8]
Single agent rituximab or splenectomy may be tried in refractory cases. However, there is no randomized clinical trial which directly compares these two approaches. Rituximab is used in patients in whom splenectomy cannot be safely performed.[54,55] Although rituximab is well tolerated, only 20% of patients achieved a sustained response.[55] Low dose mycophenolate mofetil may be used after rituximab to prolong the response in selected patients.[56]
Single agent monoclonal antibody therapy. Rituximab,[57] Alemtuzumab[58] and Veltuzumab[59] have been reported to be effective in CLL associated AIT.[60,61] Most of the published literature about rituximab is in primary immune thrombocytopenic purpura. Rituximab may be used in steroid refractory disease as a single agent, when treatment for CLL is not required. It may also be used in fludarabine induced autoimmunity.[61] The dose of rituximab is 375 mg per square meter given weekly for 4 weeks. A lower fixed dose of 100mg per week also has been studied, and has similar response rates.[62,63]
Thrombopoietin agonist. Thrombopoietin (TPO), a ligand for c-mpl, stimulates committed megakaryocytic progenitors proliferation and induces maturation of megakaryocytes. Two thrombopoietin receptor agonists, romiplostim[64] and eltrombopag[65] were studied in multiple studies and are the only agents approved by FDA for the treatment of primary immune thrombocytopenic purpura. In a Danish retrospective registration study,[66] Seven patients of secondary ITP (CLL is one of them, the others included Systemic lupus erythematosus, Evans syndrome, Human Immunodeficiency Virus infection and celiac disease) were treated with TPO agonists. 57% responded with a platelet counts greater than 30,000 after 4 weeks.
TPO agonists are used for thrombocytopenia refractory to other therapies such as corticosteroids, intravenous immunoglobulin (IV Ig), single agent rituximab and splenectomy or when splenectomy is contraindicated.[67,68] It has been suggested that these therapeutic modules should be used when a durable response is not obtained with other therapies and the platelet count continues to decline below 50,000.
The European Medicines Evaluation Agency approved TPOs for splenectomised patients who are refractory to other treatments. The American Society of Hematology recommendation for TPOs includes patients at risk of bleeding, patients in whom splenectomy is contraindicated, and patients who have failed at least one of therapies other than glucocorticoids.[43] Thrombopoietins do not cause remission and continuous administration is required. Although TPOs are well-tolerated and no significant side effects are generally reported, studies have shown that they can increase bone marrow reticulin fibrosis and have increased risk of thrombocytosis.[69] There are no trials comparing romiplostim and eltrombopag. However, higher incidence of portal vein thrombosis reported with eltrombopag in thrombocytopenia secondary to chronic liver disease. We suggest that the etiology of thrombocytopenia should be sought before using TPOs in patients with liver disease.[67]
Splenectomy. Antibody and complement bound blood cells still have the ability to function normally. Spleen clears opsonized cells from circulation, and by reducing its rate, splenectomy may be an effective treatment. In a large series published by Vianelli et al. in 2013 with a minimum of ten years follow up,[70] there was a durable response to splenectomy. Out of 233 patients followed for 10 years, there was 88% response to splenectomy with 77% complete response. The response was well-maintained, and free of any treatment in 59% of patients. Splenic irradiation may be an alternative for patients in whom surgery is contraindicated.
Patients will require vaccination against encapsulated organisms including Streptococcus pneumonia, Hemophilus influenza b, and Neisseria meningitides at least 2-3 weeks before splenectomy.[71-73] CLL patients are generally at increased risk of infections owing to defects in both cellular and humoral immune systems and qualitative and quantitative defects in B, T, and natural killer (NK) cells, neutrophils and the monocyte/macrophage system.[74] Splenectomised patients have variable response to vaccination and immunization is most useful if used earlier in the disease process, and when immunoglobulin levels are better preserved.[74] Considering that protein or conjugated vaccines are preferred for better immune response,[75-78] it may be appropriate to routinely immunize all patients at the time of diagnosis, and before extensive immunosuppressive treatment has been given.
Supportive care. In AIT patients with active bleeding, supportive care including platelet transfusions, antifibrinolytic agents such as tranexamic acid and eventually emergent surgical intervention may be required.
Patients on long term steroids are at risk of Pneumocystis jiroveci infection and prophylaxis is required. It is not clear that above which dose of steroids patients are considered to be at risk, but there is a general agreement that any dose above 20 milligrams per day has a higher risk for Pneumocystis jiroveci.[8]
Autoimmune thrombocytopenia in the setting of advanced CLL. There is no standard treatment for this subgroup of patients. As described above, purine analogues and alkylating agents have a risk of inducing autoimmunity and should be avoided.
Two mostly studied chemo immunotherapy regimens in these patients include rituximab, cyclophosphamide and dexamethasone (RCD)[79-82] and rituximab, cyclophosphamide, vincristine and prednisone (R-CVP).[83] These two regimens have not been compared with each other in any randomized clinical trials.
In 2009, Kaufman et al. reported RCD reported usage of RCD in 21 patients with progressive CLL and coexisting autoimmune cytopenias.[84] Three Out of 21 patients had steroid resistant AIT and their nadir platelet counts were 1000, 1000 and 14000. The platelet counts increased to 408000, 161000 and 135000 after treatment with RCD in 9, 15, and 29 months respectively. The first patient who relapsed at 9 months was retreated with RCD and had 15 months’ response without any second relapse.
In a case control study of R-CVP by Bowen et al., 6 out of 20 studied patients had AIT.[83] The median increase in platelet count was 106000 (range from 31000 to 216000), and median time to next treatment was 21.7 months. Patients with no response to R-CVP subsequently had a sustained complete response to splenectomy.

Table 6. Treatment regimens of Autoimmune thrombocytopenia in patients who progressive CLL.
Autoimmune Neutropenia
Autoimmune neutropenia in CLL requires a multidisciplinary management plan as these patients are at increased risk of atypical infections. Owing to both qualitative and quantitative defects in B, T, and NK cells both cellular and humoral arms of the adaptive immune systems are impaired in CLL. Monocyte/macrophage lineage is affected resulting in impairment of the innate immunity.[74]
When the absolute neutrophil count reaches below 500, risk of infection is increased, and it is important to consider treatment of AIN. AIN accompanied by fever of more than 100.4 F should be treated as neutropenic sepsis. Granulocyte – colony stimulating factor (G-CSF) and prophylactic antibiotics are indicated in persistent neutropenia without fever. However, normalization of neutrophil count does not last long after discontinuation of G-CSF. Splenectomy was commonly used before the availability of G-CSF but the results are not encouraging.[85]
Sirolimus, cyclosporine and IVIg are reported to be helpful in AIN in several case reports.[85-87] Rituximab and Alemtuzumab can provide long lasting remissions but the evidence is limited.[88,89]
Finally, it is very import to offer age appropriate vaccination to all CLL patients to prevent life threatening infections.
Conclusion
Compared to cytopenias from the leukemic marrow involvement, autoimmune cytopenias have a better prognosis. The pathogeneses of AIT and AIN are not well understood, and lack of confirmatory tests is a diagnostic challenge. Since CLL associated cytopenias are not common, there is no randomized clinical trial on management of these conditions. Multicenter randomized clinical trials are necessary to enroll more patients in therapeutic trials.
References
- Dameshek W. Chronic lymphocytic
leukemia--an accumulative disease of immunolgically incompetent
lymphocytes. Blood. 1967 Apr;29(4):Suppl:566-84.

- Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the
diagnosis and treatment of chronic lymphocytic leukemia: A report from
the international workshop on chronic lymphocytic leukemia updating the
national cancer institute-working group 1996 guidelines. Blood. 2008
Jun 15;111(12):5446-56. http://dx.doi.org/10.1182/blood-2007-06-093906 PMid:18216293 PMCid:PMC2972576

- Howlader N, Noone AM, Krapcho M, Neyman N,
Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H,
Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER
cancer statistics review, 1975-2009 (vintage 2009 populations),
National Cancer Institute. Bethesda.
- Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol. 2006 Apr;33(2):230-9. http://dx.doi.org/10.1053/j.seminoncol.2006.01.011 PMid:16616070

- Hamblin TJ, Oscier DG, Young BJ. Autoimmunity in chronic lymphocytic leukaemia. J Clin Pathol. 1986 Jul;39(7):713-6. http://dx.doi.org/10.1136/jcp.39.7.713 PMid:3488334 PMCid:PMC500029

- Hamblin TJ. Non-hemic autoimmunity in CLL. Leuk Res. 2009 Mar;33(3):366-7. http://dx.doi.org/10.1016/j.leukres.2008.09.011 PMid:18930543

- Viny AD, Lichtin A, Pohlman B, Loughran T,
Maciejewski J. Chronic B-cell dyscrasias are an important clinical
feature of T-LGL leukemia. Leuk Lymphoma. 2008 May;49(5):932-8. http://dx.doi.org/10.1080/10428190801932635 PMid:18452068

- Zent CS, Kay NE. Autoimmune complications
in chronic lymphocytic leukaemia (CLL). Best Pract Res Clin Haematol.
2010 Mar;23(1):47-59. http://dx.doi.org/10.1016/j.beha.2010.01.004 PMid:20620970 PMCid:PMC2909690

- Zent CS, Ding W, Schwager SM, Reinalda MS,
Hoyer JD, Jelinek DF, et al. The prognostic significance of cytopenia
in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br J
Haematol. 2008 May;141(5):615-21. http://dx.doi.org/10.1111/j.1365-2141.2008.07086.x PMid:18373706 PMCid:PMC2675611

- Mauro FR, Foa R, Cerretti R, Giannarelli
D, Coluzzi S, Mandelli F, et al. Autoimmune hemolytic anemia in chronic
lymphocytic leukemia: Clinical, therapeutic, and prognostic features.
Blood. 2000 May 1;95(9):2786-92. PMid:10779422

- Visco C, Ruggeri M, Laura Evangelista M,
Stasi R, Zanotti R, Giaretta I, et al. Impact of immune
thrombocytopenia on the clinical course of chronic lymphocytic
leukemia. Blood. 2008 Feb 1;111(3):1110-6. http://dx.doi.org/10.1182/blood-2007-09-111492 PMid:17986663

- Zent CS, Ding W, Reinalda MS, Schwager SM,
Hoyer JD, Bowen DA, et al. Autoimmune cytopenia in chronic lymphocytic
leukemia/small lymphocytic lymphoma: Changes in clinical presentation
and prognosis. Leuk Lymphoma. 2009 Aug;50(8):1261-8. http://dx.doi.org/10.1080/10428190903026492 PMid:19811329

- Stern SC, Shah S, Costello C. Probable
autoimmune neutropenia induced by fludarabine treatment for chronic
lymphocytic leukaemia. Br J Haematol. 1999 Sep;106(3):836-7. http://dx.doi.org/10.1046/j.1365-2141.1999.01682.x PMid:10469479

- Roberts L, Lucas G, Green L, Lindsay J,
Bhattacharyya S, Thornton P, et al. Autoimmune neutropenia following
therapy for chronic lymphocytic leukaemia: A report of three cases. Br
J Haematol. 2007 Jan;136(2):348-9. http://dx.doi.org/10.1111/j.1365-2141.2006.06436.x PMid:17156393

- Parham P. The immune system. New York: Garland Pub.: Current Trends; 2000.
- Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005 Dec;17(6):638-42. http://dx.doi.org/10.1016/j.coi.2005.09.002 PMid:16209918

- Beyer M, Kochanek M, Darabi K, Popov A,
Jensen M, Endl E, et al. Reduced frequencies and suppressive function
of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic
leukemia after therapy with fludarabine. Blood. 2005 Sep
15;106(6):2018-25. http://dx.doi.org/10.1182/blood-2005-02-0642 PMid:15914560

- Johnsen J. Pathogenesis in immune
thrombocytopenia: New insights. Hematology Am Soc Hematol Educ Program.
2012;2012:306-12.

- Semple JW, Provan D, Garvey MB, Freedman
J. Recent progress in understanding the pathogenesis of immune
thrombocytopenia. Curr Opin Hematol. 2010 Nov;17(6):590-5. http://dx.doi.org/10.1097/MOH.0b013e32833eaef3 PMid:20739879

- Barker RN, Hall AM, Standen GR, Jones J,
Elson CJ. Identification of T-cell epitopes on the rhesus polypeptides
in autoimmune hemolytic anemia. Blood. 1997 Oct 1;90(7):2701-15.
PMid:9326237

- Hall AM, Vickers MA, McLeod E, Barker RN.
Rh autoantigen presentation to helper T cells in chronic lymphocytic
leukemia by malignant B cells. Blood. 2005 Mar 1;105(5):2007-15. http://dx.doi.org/10.1182/blood-2003-10-3563 PMid:15284121

- Kipps TJ, Carson DA. Autoantibodies in
chronic lymphocytic leukemia and related systemic autoimmune diseases.
Blood. 1993 May 15;81(10):2475-87. PMid:8490163

- Cartron J, Fior R, Boue F, Tertian G, Gane
P, Cartron JP. Non hodgkin's lymphoma presenting as neutropenia related
to an IgM monoclonal anti-i antibody. Hematol Cell Ther. 1996
Apr;38(2):225-30. http://dx.doi.org/10.1007/s00282-996-0225-3 PMid:8932012

- Visco C, Giaretta I, Ruggeri M, Madeo D,
Tosetto A, Rodeghiero F. Un-mutated IgVH in chronic lymphocytic
leukemia is associated with a higher risk of immune thrombocytopenia.
Leukemia. 2007 May;21(5):1092-3. PMid:17301811

- Visco C, Maura F, Tuana G, Agnelli L,
Lionetti M, Fabris S, et al. Immune thrombocytopenia in patients with
chronic lymphocytic leukemia is associated with stereotyped B-cell
receptors. Clin Cancer Res. 2012 Apr 1;18(7):1870-8. http://dx.doi.org/10.1158/1078-0432.CCR-11-3019 PMid:22322667

- Bosch F, Muntanola A, Gine E, Carrio A,
Villamor N, Moreno C, et al. Clinical implications of ZAP-70
expressionin chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2006
Jul 15;70(4):214-7. http://dx.doi.org/10.1002/cyto.b.20131 PMid:16906580

- Crespo M, Bosch F, Villamor N, Bellosillo
B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for
immunoglobulin-variable-region mutations in chronic lymphocytic
leukemia. N Engl J Med. 2003 May 1;348(18):1764-75. http://dx.doi.org/10.1056/NEJMoa023143 PMid:12724482

- Stamatopoulos B, Meuleman N, De Bruyn C,
Pieters K, Anthoine G, Mineur P, et al. A molecular score by
quantitative PCR as a new prognostic tool at diagnosis for chronic
lymphocytic leukemia patients. PLoS One. 2010 Sep 16;5(9). http://dx.doi.org/10.1371/journal.pone.0012780

- Akhtari M, Curtis B, Waller EK. Autoimmune neutropenia in adults. Autoimmun Rev. 2009 Sep;9(1):62-6. http://dx.doi.org/10.1016/j.autrev.2009.03.006 PMid:19293004

- Burks EJ, Loughran TP,Jr. Pathogenesis of
neutropenia in large granular lymphocyte leukemia and felty syndrome.
Blood Rev. 2006 Sep;20(5):245-66. http://dx.doi.org/10.1016/j.blre.2006.01.003 PMid:16530306

- Voog E, Morschhauser F, Solal-Celigny P.
Neutropenia in patients treated with rituximab. N Engl J Med. 2003 Jun
26;348(26):2691,4; discussion 2691-4.

- Zent CS, Shanafelt T. Management of
autoimmune cytopenia complicating chronic lymphocytic leukemia. Leuk
Lymphoma. 2009 Jun;50(6):863-4. http://dx.doi.org/10.1080/10428190902919226 PMid:19455465

- Binet JL, Auquier A, Dighiero G, Chastang
C, Piguet H, Goasguen J, et al. A new prognostic classification of
chronic lymphocytic leukemia derived from a multivariate survival
analysis. Cancer. 1981 Jul 1;48(1):198-206. http://dx.doi.org/10.1002/1097-0142(19810701)48:1<198::AID-CNCR2820480131>3.0.CO;2-V

- Rai KR, Sawitsky A, Cronkite EP, Chanana
AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic
leukemia. Blood. 1975 Aug;46(2):219-34. PMid:1139039

- Elting LS, Rubenstein EB, Rolston KV,
Bodey GP. Outcomes of bacteremia in patients with cancer and
neutropenia: Observations from two decades of epidemiological and
clinical trials. Clin Infect Dis. 1997 Aug;25(2):247-59. http://dx.doi.org/10.1086/514550 PMid:9332520

- Bodey GP, Buckley M, Sathe YS, Freireich
EJ. Quantitative relationships between circulating leukocytes and
infection in patients with acute leukemia. Ann Intern Med. 1966
Feb;64(2):328-40. http://dx.doi.org/10.7326/0003-4819-64-2-328 PMid:5216294

- Brown AE. Neutropenia, fever, and infection. Am J Med. 1984 Mar;76(3):421-8. http://dx.doi.org/10.1016/0002-9343(84)90661-2

- Hodgson K, Ferrer G, Pereira A, Moreno C,
Montserrat E. Autoimmune cytopenia in chronic lymphocytic leukaemia:
Diagnosis and treatment. Br J Haematol. 2011 Jul;154(1):14-22. http://dx.doi.org/10.1111/j.1365-2141.2011.08707.x PMid:21534942

- Hodgson K, Ferrer G, Montserrat E, Moreno
C. Chronic lymphocytic leukemia and autoimmunity: A systematic review.
Haematologica. 2011 May;96(5):752-61. http://dx.doi.org/10.3324/haematol.2010.036152 PMid:21242190 PMCid:PMC3084923

- Beardsley DS, Ertem M. Platelet autoantibodies in immune thrombocytopenic purpura. Transfus Sci. 1998 Sep;19(3):237-44. http://dx.doi.org/10.1016/S0955-3886(98)00037-X

- Provan D, Stasi R, Newland AC, Blanchette
VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on
the investigation and management of primary immune thrombocytopenia.
Blood. 2010 Jan 14;115(2):168-86. http://dx.doi.org/10.1182/blood-2009-06-225565 PMid:19846889

- Binet JL. Treatment of chronic lymphocytic
leukaemia. french co-operative group on CLL. Baillieres Clin Haematol.
1993 Dec;6(4):867-78. http://dx.doi.org/10.1016/S0950-3536(05)80180-5

- Neunert C, Lim W, Crowther M, Cohen A,
Solberg L,Jr, Crowther MA, et al. The american society of hematology
2011 evidence-based practice guideline for immune thrombocytopenia.
Blood. 2011 Apr 21;117(16):4190-207. http://dx.doi.org/10.1182/blood-2010-08-302984 PMid:21325604

- SILVERSTEIN MN, HECK FJ. Acquired
hemolytic anemia and associated thrombocytopenic purpura: With special
reference to evans' syndrome. Proc Staff Meet Mayo Clin. 1962 Feb
28;37:122-8. PMid:13912960

- GUENTHER FW, BUBE FW. On the diagnosis and
therapy of peracute hemolytic anemia associated with thrombocytopenia
caused by autoimmune bodies (evans-syndrome). Arch Kinderheilkd. 1962
Nov;167:155-9. PMid:13951082

- Ding W, Zent CS. Diagnosis and management
of autoimmune complications of chronic lymphocytic leukemia/ small
lymphocytic lymphoma. Clin Adv Hematol Oncol. 2007 Apr;5(4):257-61.
PMid:17607284

- Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY,
Chan NP, et al. Initial treatment of immune thrombocytopenic purpura
with high-dose dexamethasone. N Engl J Med. 2003 Aug 28;349(9):831-6. http://dx.doi.org/10.1056/NEJMoa030254 PMid:12944568

- Emilia G, Luppi M, Morselli M, Forghieri
F, Potenza L, Torelli G. A possible role for low-dose cyclosporine in
refractory immune thrombocytopenic purpura. Haematologica. 2008
Jul;93(7):1113-5. http://dx.doi.org/10.3324/haematol.12741 PMid:18508788

- Colovic M, Suvajdzic N, Colovic N, Tomin
D, Vidovic A, Palibrk V. Mycophenolate mophetil therapy for chronic
immune thrombocytopenic purpura resistant to steroids,
immunosuppressants, and/or splenectomy in adults. Platelets.
2011;22(2):153-6. http://dx.doi.org/10.3109/09537104.2010.520372 PMid:21142405

- Provan D, Moss AJ, Newland AC, Bussel JB.
Efficacy of mycophenolate mofetil as single-agent therapy for
refractory immune thrombocytopenic purpura. Am J Hematol. 2006
Jan;81(1):19-25. http://dx.doi.org/10.1002/ajh.20515 PMid:16369979

- Reiner A, Gernsheimer T, Slichter SJ.
Pulse cyclophosphamide therapy for refractory autoimmune
thrombocytopenic purpura. Blood. 1995 Jan 15;85(2):351-8. PMid:7811992

- Verlin M, Laros RK,Jr, Penner JA.
Treatment of refractory thrombocytopenic purpura with cyclophosphamine.
Am J Hematol. 1976;1(1):97-104. http://dx.doi.org/10.1002/ajh.2830010111 PMid:988746

- Quiquandon I, Fenaux P, Caulier MT,
Pagniez D, Huart JJ, Bauters F. Re-evaluation of the role of
azathioprine in the treatment of adult chronic idiopathic
thrombocytopenic purpura: A report on 53 cases. Br J Haematol. 1990
Feb;74(2):223-8. http://dx.doi.org/10.1111/j.1365-2141.1990.tb02569.x PMid:2317458

- Cooper N, Evangelista ML, Amadori S, Stasi
R. Should rituximab be used before or after splenectomy in patients
with immune thrombocytopenic purpura? Curr Opin Hematol. 2007
Nov;14(6):642-6. http://dx.doi.org/10.1097/MOH.0b013e3282c8ca50 PMid:17898569

- Aleem A, Alaskar AS, Algahtani F, Rather
M, Almahayni MH, Al-Momen A. Rituximab in immune thrombocytopenia:
Transient responses, low rate of sustained remissions and poor response
to further therapy in refractory patients. Int J Hematol. 2010
Sep;92(2):283-8. http://dx.doi.org/10.1007/s12185-010-0635-4 PMid:20640541

- Bruserud O, Havardstein K. Should low-dose
mycophenolate mofetil be used to prolong the response after rituximab
therapy in patients with immune thrombocytopenic purpura? A case
report. Hematology. 2009 Aug;14(4):224-6. http://dx.doi.org/10.1179/102453309X439782 PMid:19635186

- Aleem A. Rituximab therapy in a patient
with autoimmune hemolytic anemia and immune thrombocytopenia associated
with chronic lymphocytic leukemia. Ann Saudi Med. 2008
Sep-Oct;28(5):382-5. http://dx.doi.org/10.4103/0256-4947.51696 PMid:18779631

- Osterborg A, Karlsson C, Lundin J.
Alemtuzumab to treat refractory autoimmune hemolytic anemia or
thrombocytopenia in chronic lymphocytic leukemia. Curr Hematol Malig
Rep. 2009 Jan;4(1):47-53. http://dx.doi.org/10.1007/s11899-009-0007-4 PMid:20425438

- Milani C, Castillo J. Veltuzumab, an
anti-CD20 mAb for the treatment of non-hodgkin's lymphoma, chronic
lymphocytic leukemia and immune thrombocytopenic purpura. Curr Opin Mol
Ther. 2009 Apr;11(2):200-7. PMid:19330725

- D'Arena G, Capalbo S, Laurenti L, Del
Poeta G, Nunziata G, Deaglio S, et al. Chronic lymphocytic
leukemia-associated immune thrombocytopenia treated with rituximab: A
retrospective study of 21 patients. Eur J Haematol. 2010
Dec;85(6):502-7. http://dx.doi.org/10.1111/j.1600-0609.2010.01527.x PMid:20846302

- Fernandez MJ, Llopis I, Pastor E, Real E,
Grau E. Immune thrombocytopenia induced by fludarabine successfully
treated with rituximab. Haematologica. 2003 Feb;88(2):ELT02.
PMid:12604433

- Zaja F, Vianelli N, Volpetti S, Battista
ML, Defina M, Palmieri S, et al. Low-dose rituximab in adult patients
with primary immune thrombocytopenia. Eur J Haematol. 2010
Oct;85(4):329-34. http://dx.doi.org/10.1111/j.1600-0609.2010.01486.x PMid:20546023

- Zaja F, Volpetti S, Chiozzotto M, Puglisi
S, Isola M, Buttignol S, et al. Long-term follow-up analysis after
rituximab salvage therapy in adult patients with immune
thrombocytopenia. Am J Hematol. 2012 Sep;87(9):886-9. http://dx.doi.org/10.1002/ajh.23272 PMid:22718483

- D'Arena G, Cascavilla N. Romiplostim for
chronic lymphocytic leukemia-associated immune thrombocytopenia. Leuk
Lymphoma. 2011 Apr;52(4):701-4. http://dx.doi.org/10.3109/10428194.2010.542598 PMid:21171868

- S.Koehrer MK, WG.Wierda. <br
/>Eltrombopag, a second-generation thrombopoietin receptor agonist,
for chronic lymphocytic leukemia-associated ITP. <br /> leukemia.
2010 may;24(5):1096-8.epub 2010 mar 25. http://dx.doi.org/10.1038/leu.2010.45

- Gudbrandsdottir S, Frederiksen H,
Hasselbalch H. Thrombopoietin-receptor agonists in haematological
disorders: The danish experience. Platelets. 2012;23(6):423-9. http://dx.doi.org/10.3109/09537104.2011.634931 PMid:22185370

- Bussel JB, Provan D, Shamsi T, Cheng G,
Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts
and bleeding during treatment of chronic idiopathic thrombocytopenic
purpura: A randomised, double-blind, placebo-controlled trial. Lancet.
2009 Feb 21;373(9664):641-8. http://dx.doi.org/10.1016/S0140-6736(09)60402-5

- Kuter DJ, Bussel JB, Lyons RM, Pullarkat
V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in
patients with chronic immune thrombocytopenic purpura: A double-blind
randomised controlled trial. Lancet. 2008 Feb 2;371(9610):395-403. http://dx.doi.org/10.1016/S0140-6736(08)60203-2

- Bussel JB, Kuter DJ, Pullarkat V, Lyons
RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with
romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009
Mar 5;113(10):2161-71. http://dx.doi.org/10.1182/blood-2008-04-150078 PMid:18981291

- Vianelli N, Palandri F, Polverelli N,
Stasi R, Joelsson J, Johansson E, et al. Splenectomy as a curative
treatment for immune thrombocytopenia: A retrospective analysis of 233
patients with a minimum follow up of 10 years. Haematologica. 2013
Jun;98(6):875-80. http://dx.doi.org/10.3324/haematol.2012.075648 PMid:23144195 PMCid:PMC3669442

- Uhnoo I, Lepp T. Life-threatening
infection in splenectomised patients is preventable. but this requires
better vaccination routines, education and antibiotic prophylaxis.
Lakartidningen. 2012 Aug 8-21;109(32-33):1406-10. PMid:22953428

- Kim HS, Kriegel G, Aronson MD. Improving the preventive care of asplenic patients. Am J Med. 2012 May;125(5):454-6. http://dx.doi.org/10.1016/j.amjmed.2011.11.009 PMid:22386974

- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011 Jul 2;378(9785):86-97. http://dx.doi.org/10.1016/S0140-6736(10)61493-6

- Dearden C. Disease-specific complications of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:450-6. http://dx.doi.org/10.1182/asheducation-2008.1.450 PMid:19074125

- Sinisalo M, Aittoniemi J, Oivanen P,
Kayhty H, Olander RM, Vilpo J. Response to vaccination against
different types of antigens in patients with chronic lymphocytic
leukaemia. Br J Haematol. 2001 Jul;114(1):107-10. http://dx.doi.org/10.1046/j.1365-2141.2001.02882.x PMid:11472353

- Sinisalo M, Aittoniemi J, Kayhty H, Vilpo
J. Vaccination against infections in chronic lymphocytic leukemia. Leuk
Lymphoma. 2003 Apr;44(4):649-52. http://dx.doi.org/10.1080/1042819031000063408 PMid:12769342

- Sinisalo M, Vilpo J, Itala M, Vakevainen
M, Taurio J, Aittoniemi J. Antibody response to 7-valent conjugated
pneumococcal vaccine in patients with chronic lymphocytic leukaemia.
Vaccine. 2007 Dec 21;26(1):82-7. http://dx.doi.org/10.1016/j.vaccine.2007.10.053 PMid:18053620

- Sinisalo M, Vilpo J, Itala M, Vakevainen
M, Taurio J, Aittoniemi J. Efficacy of pneumococcal vaccination on
chronic lymphocytic leukemia: Should we rely on surrogate markers?
Vaccine. 2008 Jul 29;26(32):3959. http://dx.doi.org/10.1016/j.vaccine.2008.04.063 PMid:18514977

- Gupta N, Kavuru S, Patel D, Janson D,
Driscoll N, Ahmed S, et al. Rituximab-based chemotherapy for
steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic
leukemia. Leukemia. 2002 Oct;16(10):2092-5. http://dx.doi.org/10.1038/sj.leu.2402676 PMid:12357362

- Kaufman M, Limaye SA, Driscoll N, Johnson
C, Caramanica A, Lebowicz Y, et al. A combination of rituximab,
cyclophosphamide and dexamethasone effectively treats immune cytopenias
of chronic lymphocytic leukemia. Leuk Lymphoma. 2009 Jun;50(6):892-9. http://dx.doi.org/10.1080/10428190902887563 PMid:19391041

- Michallet AS, Rossignol J, Cazin B,
Ysebaert L. Rituximab-cyclophosphamide-dexamethasone combination in
management of autoimmune cytopenias associated with chronic lymphocytic
leukemia. Leuk Lymphoma. 2011 Jul;52(7):1401-3. http://dx.doi.org/10.3109/10428194.2011.591005 PMid:21699387

- Rossignol J, Michallet AS, Oberic L,
Picard M, Garon A, Willekens C, et al.
Rituximab-cyclophosphamide-dexamethasone combination in the management
of autoimmune cytopenias associated with chronic lymphocytic leukemia.
Leukemia. 2011 Mar;25(3):473-8. http://dx.doi.org/10.1038/leu.2010.278 PMid:21127498

- Bowen DA, Call TG, Shanafelt TD, Kay NE,
Schwager SM, Reinalda MS, et al. Treatment of autoimmune cytopenia
complicating progressive chronic lymphocytic leukemia/small lymphocytic
lymphoma with rituximab, cyclophosphamide, vincristine, and prednisone.
Leuk Lymphoma. 2010 Apr;51(4):620-7. http://dx.doi.org/10.3109/10428191003682767 PMid:20302386 PMCid:PMC3448550

- Kaufman M, Limaye SA, Driscoll N, Johnson
C, Caramanica A, Lebowicz Y, et al. A combination of rituximab,
cyclophosphamide and dexamethasone effectively treats immune cytopenias
of chronic lymphocytic leukemia. Leuk Lymphoma. 2009 Jun;50(6):892-9. http://dx.doi.org/10.1080/10428190902887563 PMid:19391041

- Rose NR, Mackay IR. The autoimmune diseases. 4th ed. Amsterdam; Boston: Elsevier / Academic Press; 2006.
- Martino R, Sureda A, Ayats R, Muniz-Diaz
E, Altes A, Brunet S. Successful treatment of chronic autoimmune
neutropenia with cyclosporin A. Haematologica. 1994 Jan-Feb;79(1):66-9.
PMid:15378951

- Ghnaya H, Bekov K, Jira M, Kettaneh A,
Mejri O, Tiev KP, et al. Primary chronic autoimmune neutropenia
successfully treated with sirolimus. Rev Med Interne. 2008
Nov;29(11):940-2. http://dx.doi.org/10.1016/j.revmed.2008.02.005 PMid:18400338

- Dungarwalla M, Marsh JC, Tooze JA, Lucas
G, Ouwehand W, Pettengell R, et al. Lack of clinical efficacy of
rituximab in the treatment of autoimmune neutropenia and pure red cell
aplasia: Implications for their pathophysiology. Ann Hematol. 2007
Mar;86(3):191-7. http://dx.doi.org/10.1007/s00277-006-0202-5 PMid:17123083

- Marsh JC, Gordon-Smith EC. CAMPATH-1H in the treatment of autoimmune cytopenias. Cytotherapy. 2001;3(3):189-95. http://dx.doi.org/10.1080/146532401753174133 PMid:12171725
