Targeting Dormant Bacilli to Fight Tuberculosis
Lanfranco Fattorini1, Giovanni Piccaro1, Alessandro Mustazzolu1, Federico Giannoni1
1 Dipartimento di Malattie Infettive, Parassitarie e Immunomediate, Istituto Superiore di Sanitŕ, Roma
Correspondence
to:
Lanfranco Fattorini. Dipartimento di Malattie Infettive, Parassitarie e
Immunomediate. Istituto Superiore di Sanitŕ, Viale Regina Elena
299, 00161 Roma. Tel. +39 06 49903167; E-mail: lanfranco.fattorini@iss.it
Published: November 19, 2013
Received: November 14, 2013
Accepted: November 15, 2013
Meditter J Hematol Infect Dis 2013, 5(1): e2013072, DOI 10.4084/MJHID.2013.072
This article is available on PDF format at:

This is an Open Access article
distributed under the terms of the
Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits
unrestricted use, distribution, and reproduction in any medium,
provided the original work is properly cited
Abstract
Tuberculosis
(TB) is an infectious disease caused by Mycobacterium tuberculosis
(Mtb), which kills about 2 million people annually. Furthermore, 2
billion people worldwide are latently infected with this organism, with
10% of them reactivating to active TB due to re-growth of
nonreplicating (dormant) Mtb residing in their tissues. Because of the
huge reservoir of latent TB it is important to find novel drugs/drug
combinations killing dormant bacilli (microaerophiles, anaerobes and
drug-tolerant persisters) surviving for decades in a wide spectrum of
granulomatous lesions in the lungs of TB patients. Antibiotic treatment
of drug-susceptible TB requires administration of isoniazid, rifampin,
pyrazinamide, ethambutol for 2 months, followed by isoniazid and
rifampin for 4 months. To avoid reactivation of dormant Mtb to active
pulmonary TB, up to 9 months of treatment with isoniazid is required.
Therefore, a strategy to eliminate dormant bacilli needs to be
developed to shorten therapy of active and latent TB and reduce the
reservoir of people with latent TB. Finding drugs with high rate of
penetration into the caseous granulomas and understanding the biology
of dormant bacilli and in particular of persister cells, phenotypically
resistant to antibiotics, will be essential to eradicate Mtb from
humans. In recent years unprecedented efforts have been done in TB drug
discovery, aimed at identifying novel drugs and drug combinations
killing both actively replicating and nonreplicating Mtb in vitro, in
animal models and in clinical trials in humans.
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis (Mtb), a microorganism firstly described by Robert Koch in 1882. After more than one century the disease has not yet been eradicated and in 2011 the World Health Organization (WHO) estimated an incidence of 8.7 million new cases (13% co-infected with HIV) and 1.4 million deaths from TB.[1]
Active TB is quite well controlled by antibiotic treatment, but therapy lasts 6 months including 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), ethambutol (EMB), and 4 months of INH and RIF.[2-3] The WHO makes great efforts to facilitate drug treatment in Africa, Asia and in the former Soviet Union by the so-called directly observed therapy (DOT) ensuring the patients actually take medications. Indeed, poor adherence to therapy is one of the reasons why particularly in low-income countries is increasing the number of TB patients harboring multidrug-resistant (MDR) Mtb strains (i.e. resistant to at least INH and RIF, the two most powerful drugs) and extensively drug-resistant (XDR) strains [i.e. MDR strains resistant to any fluoroquinolone and to at least one injectable second-line drug (amikacin, kanamycin, capreomycin)].[4] In many industrialized countries the majority of MDR/XDR cases occurs in foreign-born people emigrated from regions where drug-resistant TB is endemic.[4-5] Several studies are currently underway to search for new drugs reducing length of TB therapy to 3-4 months or less, and some new molecules [Bedaquiline (TMC-207), Delamanid (OPC-67683), PA-824 and others] or antibiotics used for other infections [e.g. moxifloxacin (MXF), gatifloxacin (GTF), rifapentine (RPT), linezolid (LZ), clofazimine (CFZ)], are currently being evaluated in appropriate combinations in phases II or III clinical trials or in observational studies.[2-3] Vaccine development is also in progress, with at least 13 preparations being evaluated in phases I or II clinical trials.[2,6] Currently, the only vaccine in use is the Bacillus Calmette-Guérin (BCG) vaccine, which protects children in the first 5-10 years of life especially against miliary and meningeal TB, but that does protect adults neither from pulmonary TB nor from reactivation of latent TB to active TB.[2,6] Indeed, besides active TB about 2 billion people (one third of humanity) are estimated to be latently infected with Mtb, i.e. they harbor this organism in a nonreplicating (NR) (dormant) persistent stage somewhere in their tissues, as revealed by positivity to a skin test performed with a protein extract of Mtb known as tuberculin or purified protein derivative (PPD).[7-9] It is estimated that 5% of tuberculin skin test (TST)-positive people develop pulmonary TB within 2 years after infection and that another 5% becomes ill lifetime for reactivation of dormant Mtb to an actively replicating (AR) stage. Because of the huge reservoir of latently infected individuals, it is important to know more about the biology of dormant Mtb in order to develop new therapeutic tools for active and latent TB with the ultimate goal of eradicating the tubercle bacillus from human beings.[2,7-8,10]
Pathogenesis of TB
Although a single Mtb cell is potentially sufficient to infect a person, it is likely that only prolonged exposure to aerosols (1-10 µm in diameter) produced during coughing by patients with pulmonary TB causes transmission of the organism from sick people to healthy contacts. By microscopic observation of the sputum of the TB-patients using the Ziehl-Neelsen stain, also known as the acid-fast stain, Mtb cells are visualized as red bacilli, 3-4 µm in length. TB can affect any organ but in most cases (~ 80%) typically attacks the lungs. Mtb is transmitted through the air, and if it reaches pulmonary alveoli it is phagocytosed by macrophages and transported into the lung parenchyma where other cells are recalled to form granulomas, the hallmark tissue reaction of TB.[7-8,10] These lesions contain activated macrophages and multinucleated giant cells surrounded by a rim of lymphocytes and by a fibrous capsule to circumscribe the field of battle between the immune system and Mtb. Different granulomas types (cellular, necrotic, caseous) may coexist within the same individual during active TB, due to different maturation stages of the lesion.[8,10] The centre of the caseous granulomas, found in active and latent TB, is formed by a hypoxic and necrotic material (caseum) likely consisting of dead macrophages and other cells.[8,10] Analysis of the lipid composition of caseum showed that it contains cholesterol, cholesteryl esters, triacylglycerols and lactosylceramide, and that development of the human TB granuloma to caseation correlated with pathogen-mediated disregulation of host lipid metabolism.[11]
Primary granulomas are formed mainly at the base of lungs (primary TB) and are caused by very low doses of infection (1-5 tubercle bacilli). In most of primarily infected people lesions resolve spontaneously with no symptoms while in the remaining 5-10% (more often children) local or systemic disease (menigeal or even miliary TB) develop within 1-2 years.[7,12] In 90-95% of primary cases, Mtb infection evolves in latent infection with no symptoms; the TST become positive after 3-8 weeks and positivity is maintained throughout the entire lifetime likely due to persistence of NR Mtb in the tissues for many years. Mtb can also migrate via the lymphatics and the bloodstream from primary lesions to secondary sites located at the apical zones of the lungs with formation of post-primary granulomas (post-primary TB). For unknown reasons in about 10% of post-primary cases the immune system is unable to control the infection, allowing dormant bacilli to reactivate by multiplying to high density and increasing their concentration in the granulomas of the apical zones of the lungs. It is assumed that interaction with high amounts of Mtb antigens activates the immune response leading to the occurrence of caseous necrosis, liquefaction, cavity formation and release of the tubercle bacilli into airways of highly contagious pulmonary TB patients.[7,12] Thus, the infection-disease-infection cycle mediated by reactivation of dormant Mtb in about 10% of TST-positive people with latent TB is the mechanism by which Mtb perpetuates its survival. In humans, a minimal size of around 0.1 mm3 was found to be essential for the initial formation of central necrosis.[13-14] In patients with tuberculomas with latent TB the distant parts of lung tissue showed strong vascularization and proliferative activity, which lacked in cavitary TB lung lesions. Immune insulation of caseous granulomas may favor caseum liquefaction and cavity formation.[13-15]
Dormant Mtb
In the hypoxic core of poorly vascularized necrotic and/or caseous granulomas the low oxygen pressure restricts the growth of aerobic AR Mtb to microaerophilic/anaerobic NR Mtb allowing bacilli to transit into a dormant state, i.e. a condition characterized by low metabolic activity that renders the bacteria resistant to killing by host immune response and antibiotics.
Initial response to hypoxia is regulated by the two-component response regulator DosR which, after phosphorylation by either of two sensor kinases (DosT, a hypoxia sensor, and DosS, a redox sensor) leads to induction of a set of 48 genes.[16] Besides hypoxia, the DosR regulon is also induced in response to nitric oxide, nutrient starvation, and following infection of macrophages, mice, and guinea pigs. DosR regulon induction is transient, with about half genes returning to baseline within 24 hours. A second wave of gene expression then occurs after the initial DosR-mediated hypoxic response, consisting of 230 genes (Enduring Hypoxic Response, EHR) involved in the control of the regulatory factors and enzymatic machines of the long-term bacteriostasis program of NR Mtb.[17]
Several in vitro models to obtain NR Mtb in vitro have been developed over the years, based on reduced oxygen availability, nutrient starvation or the use of standing cultures. One of the most popular ways is the Wayne model of hypoxia (Figure 1)[18-19] in which dormant bacilli are obtained by gradual adaptation of stirred cultures of aerobic Mtb to anaerobiosis through the self-generated formation of an oxygen gradient. In this model at least two stages of nonreplicating persistence designated NRP-1 and NRP-2 occurs, with 1% (microaerophilic stage) and 0.06% (anaerobic stage) dissolved oxygen levels, respectively. Dormancy models including combined stresses of low oxygen, high CO2, low nutrients and acidic pH were also described.[20] All these models are useful to screen drugs/drug combinations that can kill NR Mtb.
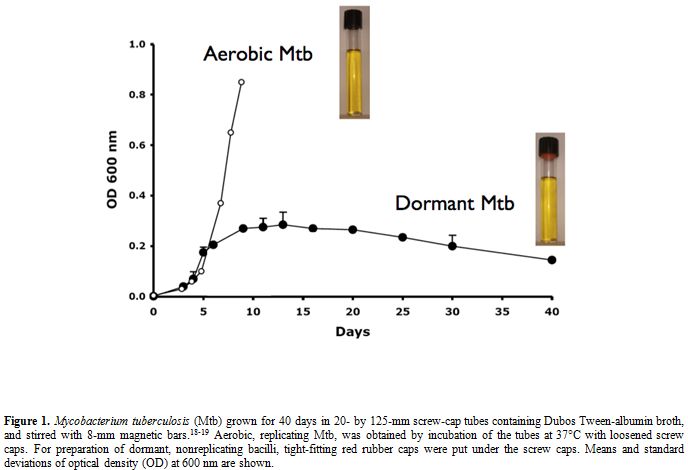
Figure 1. Mycobacterium tuberculosis (Mtb) grown for 40 days in 20- by 125-mm screw-cap tubes containing Dubos Tween-albumin broth, and stirred with 8-mm magnetic bars.[18-19] Aerobic, replicating Mtb, was obtained by incubation of the tubes at 37°C with loosened screw caps. For preparation of dormant, nonreplicating bacilli, tight-fitting red rubber caps were put under the screw caps. Means and standard deviations of optical density (OD) at 600 nm are shown.
Dormant Mtb cells collected from lung tissues can be difficult to grow in culture media. In old studies examining surgically removed specimens from closed cavities of lungs of drug-treated patients, acid-fast bacilli were observed on microscopic sections but no colony growth was seen in solid media during the normal 8 weeks of incubation.[21] However, by prolonging the incubation of cultures up to 3 to 10 months it was possible to obtain Mtb colonies, demonstrating the existence of few slowly growing tubercle bacilli surviving after drug treatment. Results from open cavities were in startling contrast to those from closed cavities, showing bacillary growth within 8 weeks. Differences in bacterial growth were also reported in more recent investigations, showing that Mtb was always cultivable from active cavitary TB but not from nonprogressive tuberculomas of healthy patients.[22] To evade host responses, stresses bacteria can enter various reversible NR states characterized by impaired culturability.[23] The peptidoglycan structure plays an important role in the maintenance of bacterial dormancy and a variation in a specific cross-link occurs during stationary-phase adaptation of Mtb. Five resuscitation-promoting factors (Rpf) similar to lyzozyme and lytic transglycosylases and sharing sequences with the Rpf of Micrococcus luteus were described and investigated for their capacity to resuscitate dormant mycobacteria.[24] These studies indicated that activation of dormant cells by Rpf requires peptidoglycan hydrolysis facilitating cell division and/or the release of products acting as anti-dormancy signals.
There is strong evidence that Mtb uses lipids as a major energy source for persistence in the host. Fatty acids are stored as triacylglycerol in seed oils of plants and in the adipose tissues of mammals for use as energy during dormancy/hibernation. Mtb uses host triacylglycerol to accumulate lipid droplets intracellularly, and acquires a dormancy-like phenotype in lipid-loaded macrophages.[25] Wax exter synthesis and utilization of host cholesterol are also required for Mtb to enter dormancy.[26-27] Interestingly, intracellular lipid inclusion bodies were also observed in several Mtb cells isolated from the sputum of TB patients, as expected from the possibility that after tuberculoma disintegration a mixture of AR and NR Mtb is released into the airways.[28] Mtb can also enter within adipocytes, where it accumulates intracytoplasmic lipid inclusions for survival in a NR state.[29] Given the wide distribution of the adipose tissue throughout the body, Mtb may persist inside this tissue for long periods of time. Overall, due to the close relationship between lipid metabolism and dormancy, and the ability of NR Mtb to survive in lipid-reach caseous granulomas,[11] cure and eradication of TB by drugs will be difficult.
Where does dormant Mtb live exactly? After treatment of guinea pigs with the novel drug TMC-207, Mtb was almost completely eradicated from the tissues, but few acid-fast bacilli were found to be extracellular within the central core of caseous necrosis and surrounding acellular rim of the primary granulomas.[30-31] This zone was hypoxic and morphologically similar to that described for human lung lesions. The acellular rim may then be a primary location of persisting Mtb after drug treatment, highlighting the importance of developing new drugs/drug combinations able to eradicate extracellular Mtb remaining within necrotic lesions. In contrast, in drug-treated mice a homogenous reduction in viable counts was observed, indicating that the mouse model, which fails to show significant necrosis and hypoxia in lung lesions, is not suitable for the study of hypoxic responses of Mtb.[30-31] Other investigators confirmed that TB granulomas of guinea pigs, rabbits and nonhuman primates, but not mice, are hypoxic.[32] Indeed, measurements of pO2 in granulomas of Mtb-infected rabbits using pimonidazole labeling and a fiber optic oxygen probe revealed pO2 values lower than 2 mm Hg, in comparison with 151 mm Hg in the air and 37 mm Hg in murine Mtb lesions.[15,32]
The low efficiency of TB therapy is due to different reasons, including slow growth of Mtb related to periods of dormancy, low penetration of drugs in different granulomatous lesions, generation of drug-tolerant dormant persisters. In recent investigations Dartois et al compared by high-pressure liquid chromatography the distribution of INH, RIF, PZA and MXF in pulmonary lesions and plasma of Mtb-infected rabbits and found that MXF showed the best partitioning into lung and granulomas.[33] However, more sophisticated measurements performed by MALDI mass spectrometry imaging showed that MXF and PA-824 do not diffuse effectively through caseum.[15,34] Preliminary studies of this group suggested that PZA preferentially accumulates in caseum versus inflammatory cells.[15]
The prolonged treatment necessary to cure TB is also due to drug-tolerant dormant persisters i.e. a subpopulation of NR or slowly replicating bacilli that survive the cidal action of antibiotics.[35] These cells have noninheritable phenotypic resistance or tolerance to antibiotics, but their progeny is fully susceptible to drugs. Mtb persisters likely comprise different subpopulations and consist of a very small proportion of Mtb. To give an example in vivo, in the lungs of guinea pigs treated for 6 weeks with 15 mg/kg of TMC-207, 1 persisting Mtb out of about 20,000 bacilli was recovered after treatment.[30] In vitro, low numbers of Mtb persisters are present in early exponential phase, but their number increases sharply up to 1% of the population at late exponential and stationary phases.[36] Thus, their proportion likely depends by specific conditions including the age of a culture, the length of drug exposure, the type and concentration of antibiotics.[35] It is not yet clear while their formation can be promoted in a stochastic or deterministic (for instance by induction) manner but it is possible that both mechanisms are involved.[35-37] A study of the transcriptome of Mtb persisters identified a small number of genes upregulated by different stresses [antibiotic exposure, increasing hypoxia (Wayne model), hypoxia by continuous flow of low oxygen (EHR), nutrient starvation in phosphate buffered saline] which may represent a core dormancy response.[36] Recently it was reported that mycobacterial persisters may be eradicated in vitro with antibiotic-generated hydroxyl radicals, suggesting that stimulation of reactive oxygen species provides a potential strategy to managing persistent infections.[38] This observation is in keeping with the knowledge that bactericidal antibiotics may stimulate the production of highly deleterious hydroxyl radicals in Gram-negative and Gram-positive bacteria.[39]
Activity of Drugs Against Dormant Mtb
The most important objective for discovery of new anti-TB drugs is to find new drugs/drug combinations able to effectively eradicate in vivo both AR (aerobic) bacilli and NR (microaerophilic, anaerobic and persisters) bacilli to shorten therapy of active TB below 6 months, and effectively reduce the reservoir of latently infected individuals. Using the Wayne model and other models it was shown that NR bacilli were insensitive to INH, while being inhibited by RIF, PZA, fluoroquinolones (e.g. MXF), aminoglycosides [e.g. amikacin (AK)], capreomycin (CP) and nitrocompounds [e.g. metronidazole (MZ), niclosamide (NC), nitazoxanide (NTZ), PA-824 and other drugs].[3,19,35] Among nitrocompounds, MZ strongly reduced Mtb viable counts under anaerobic conditions, but showed no activity under aerobic conditions.[3,18] Using the Wayne model we found that the combination MZ+RIF sterilized long-term (26-days-old) dormant Mtb cultures and that some combinations (RIF+MXF+MZ+AK or RIF+MXF+MZ+CP) killed both AR and NR Mtb, as measured by a test of residual viability more sensitive than colony forming units (CFU) based on the lack of re-growth after 100 days of incubation in liquid medium (MGIT 960).[40-41] In vivo activity of MZ was reported to be different in various animal models, and was related to the presence of caseous necrosis, or to drug toxicity. Indeed, MZ had no activity in mice and was toxic in a combination treatment in guinea pigs but showed good efficacy in rabbits and prevented reactivation of latent TB in macaques.[42] When administered to MDR-TB patients in a controlled trial, MZ increased early sputum smear and culture conversion, but was too neurotoxic to use over the long term.[43] Due to MZ toxicity, novel and better tolerated nitroimidazoles with activity against both aerobic and anaerobic Mtb are presently investigated in vitro and in vivo with promising results, indicating the importance of including at least a drug with anaerobic activity in newly designed regimens. The novel nitroimidazole PA-824 demonstrated anti-TB activity in combination with other drugs in mice and in human early bactericidal activity (EBA) studies.[43] The drug inhibits the mycolic acid biosynthesis of aerobic Mtb and kills NR Mtb by intracellular nitric oxide release.[3,44] In the Wayne model we showed that the combination (RIF+MXF+AK+PA-824) was more efficient than the combination currently used in human therapy (RIF+INH+PZA+EMB) and killed both AR and NR Mtb in 14 days.[19] Also TMC-207, a diarylquinoline recently approved with restraint by the Food and Drug Administration for treatment of MDR-TB, is effective against both AR and NR Mtb.[45-47] The drug acts specifically by targeting the membrane-bound c-subunit of ATP synthase of AR Mtb and the residual ATP synthase enzymatic activity of NR Mtb.
Since anti-TB treatment requires activity of drugs against both AR and NR bacilli living in a wide spectrum of lung lesions, use of drug combinations is essential to cover all of these requirements. Understanding where a bacterial population resides and evaluating the ability of novel molecules to penetrate a site allows a more rational approach to the design of new combinations. Traditional Minimum Inhibitory Concentration (MIC) measures the activity of a compound only against AR aerobic cells, representing a non-physiological situation in low or no vascularized necrotic (caseous) granulomas.[15] Thus, NR or slowly replicating Mtb assays have been developed including low oxygen (Wayne model), nutrient starvation and nitric-oxide release assays using MZ and INH as positive (growth) and negative (no growth) controls, respectively.[48] The necrotic tissue has a pH of about 6.549 while the pH of active TB lesions is between 5.5 and 6.0 or lower.[19] The standard Wayne model of hypoxia at pH 6.6 or the modified version at pH 5.8 we recently set up[18-19,40-41] may mimic the microenvironment of necrotic and cellular granulomas, respectively, and be suitable for measuring the activity of new compounds under different hypoxic conditions. To eradicate Mtb from the tissues we need to kill both AR and NR Mtb that are not eliminated with current therapy. A complication in the discovery of new drugs/combinations is the lack of a sensitive test determining when Mtb is dead.[8] Considering that NR Mtb may not form colonies on agar, we found that re-growth of drug-exposed Mtb in a liquid medium (MGIT 960) was a test much more sensitive than CFU measurements to demonstrate the ‘cidal’ activity of a combination.[19,41] We think that this test should be used together with the CFUs when studying the sterilizing activity of new drugs or combinations.
Besides in vitro tests the sterilizing activity of a combination against AR and NR Mtb can be determined in animal models reaching different stages of granuloma formation ranging from mouse model (lacking organized granulomatous structures) to guinea pig, rabbit and macaque models, showing necrosis, caseation, liquefaction and cavity formation.[15] No one animal model fully mimics the complete spectrum of lesions shown by humans. However, in the practice, the most popular model for determining the sterilizing activity of drug combinations is still the mouse model which, due to low costs and drug requirements, is widely used by monitoring CFUs in organs and the relapse rate 3 months after discontinuation of treatment. Recently, some combinations sterilizing Mtb-infected mice were reported, including TMC-207+PZA+CFZ+RPT (6 weeks), TMC-207+PZA+CFZ (8 weeks), TMC-207+PZA+RPT (8 weeks).[50-51] However, due to lack of hypoxia and necrosis in mouse lungs, efficacy of single drugs and combinations not necessarily predicts the clinical feature in humans.[15] Interestingly, the use of a hypoxic mouse model (C3HeB/FeJ mice) developing lesions with liquefactive necrosis is under validation for testing new drugs and combinations.[52] In the guinea pigs model, the combination PA-824+MXF+PZA killed Mtb from lungs in 8 weeks and showed the highest EBA values in humans.[53-54] This and at least other 37 drug combinations containing high-doses INH, RIF, RPT or fluoroquinolones, TMC-207, PA-824, OPC-67683, and other drugs (SQ-109, PNU-100480, AZD-5847) are in Phases I, II or III clinical trials against drug-susceptible and -resistant TB, and results are expected to be reported in the next few years.[2-3]
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis (Mtb), a microorganism firstly described by Robert Koch in 1882. After more than one century the disease has not yet been eradicated and in 2011 the World Health Organization (WHO) estimated an incidence of 8.7 million new cases (13% co-infected with HIV) and 1.4 million deaths from TB.[1]
Active TB is quite well controlled by antibiotic treatment, but therapy lasts 6 months including 2 months of isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), ethambutol (EMB), and 4 months of INH and RIF.[2-3] The WHO makes great efforts to facilitate drug treatment in Africa, Asia and in the former Soviet Union by the so-called directly observed therapy (DOT) ensuring the patients actually take medications. Indeed, poor adherence to therapy is one of the reasons why particularly in low-income countries is increasing the number of TB patients harboring multidrug-resistant (MDR) Mtb strains (i.e. resistant to at least INH and RIF, the two most powerful drugs) and extensively drug-resistant (XDR) strains [i.e. MDR strains resistant to any fluoroquinolone and to at least one injectable second-line drug (amikacin, kanamycin, capreomycin)].[4] In many industrialized countries the majority of MDR/XDR cases occurs in foreign-born people emigrated from regions where drug-resistant TB is endemic.[4-5] Several studies are currently underway to search for new drugs reducing length of TB therapy to 3-4 months or less, and some new molecules [Bedaquiline (TMC-207), Delamanid (OPC-67683), PA-824 and others] or antibiotics used for other infections [e.g. moxifloxacin (MXF), gatifloxacin (GTF), rifapentine (RPT), linezolid (LZ), clofazimine (CFZ)], are currently being evaluated in appropriate combinations in phases II or III clinical trials or in observational studies.[2-3] Vaccine development is also in progress, with at least 13 preparations being evaluated in phases I or II clinical trials.[2,6] Currently, the only vaccine in use is the Bacillus Calmette-Guérin (BCG) vaccine, which protects children in the first 5-10 years of life especially against miliary and meningeal TB, but that does protect adults neither from pulmonary TB nor from reactivation of latent TB to active TB.[2,6] Indeed, besides active TB about 2 billion people (one third of humanity) are estimated to be latently infected with Mtb, i.e. they harbor this organism in a nonreplicating (NR) (dormant) persistent stage somewhere in their tissues, as revealed by positivity to a skin test performed with a protein extract of Mtb known as tuberculin or purified protein derivative (PPD).[7-9] It is estimated that 5% of tuberculin skin test (TST)-positive people develop pulmonary TB within 2 years after infection and that another 5% becomes ill lifetime for reactivation of dormant Mtb to an actively replicating (AR) stage. Because of the huge reservoir of latently infected individuals, it is important to know more about the biology of dormant Mtb in order to develop new therapeutic tools for active and latent TB with the ultimate goal of eradicating the tubercle bacillus from human beings.[2,7-8,10]
Pathogenesis of TB
Although a single Mtb cell is potentially sufficient to infect a person, it is likely that only prolonged exposure to aerosols (1-10 µm in diameter) produced during coughing by patients with pulmonary TB causes transmission of the organism from sick people to healthy contacts. By microscopic observation of the sputum of the TB-patients using the Ziehl-Neelsen stain, also known as the acid-fast stain, Mtb cells are visualized as red bacilli, 3-4 µm in length. TB can affect any organ but in most cases (~ 80%) typically attacks the lungs. Mtb is transmitted through the air, and if it reaches pulmonary alveoli it is phagocytosed by macrophages and transported into the lung parenchyma where other cells are recalled to form granulomas, the hallmark tissue reaction of TB.[7-8,10] These lesions contain activated macrophages and multinucleated giant cells surrounded by a rim of lymphocytes and by a fibrous capsule to circumscribe the field of battle between the immune system and Mtb. Different granulomas types (cellular, necrotic, caseous) may coexist within the same individual during active TB, due to different maturation stages of the lesion.[8,10] The centre of the caseous granulomas, found in active and latent TB, is formed by a hypoxic and necrotic material (caseum) likely consisting of dead macrophages and other cells.[8,10] Analysis of the lipid composition of caseum showed that it contains cholesterol, cholesteryl esters, triacylglycerols and lactosylceramide, and that development of the human TB granuloma to caseation correlated with pathogen-mediated disregulation of host lipid metabolism.[11]
Primary granulomas are formed mainly at the base of lungs (primary TB) and are caused by very low doses of infection (1-5 tubercle bacilli). In most of primarily infected people lesions resolve spontaneously with no symptoms while in the remaining 5-10% (more often children) local or systemic disease (menigeal or even miliary TB) develop within 1-2 years.[7,12] In 90-95% of primary cases, Mtb infection evolves in latent infection with no symptoms; the TST become positive after 3-8 weeks and positivity is maintained throughout the entire lifetime likely due to persistence of NR Mtb in the tissues for many years. Mtb can also migrate via the lymphatics and the bloodstream from primary lesions to secondary sites located at the apical zones of the lungs with formation of post-primary granulomas (post-primary TB). For unknown reasons in about 10% of post-primary cases the immune system is unable to control the infection, allowing dormant bacilli to reactivate by multiplying to high density and increasing their concentration in the granulomas of the apical zones of the lungs. It is assumed that interaction with high amounts of Mtb antigens activates the immune response leading to the occurrence of caseous necrosis, liquefaction, cavity formation and release of the tubercle bacilli into airways of highly contagious pulmonary TB patients.[7,12] Thus, the infection-disease-infection cycle mediated by reactivation of dormant Mtb in about 10% of TST-positive people with latent TB is the mechanism by which Mtb perpetuates its survival. In humans, a minimal size of around 0.1 mm3 was found to be essential for the initial formation of central necrosis.[13-14] In patients with tuberculomas with latent TB the distant parts of lung tissue showed strong vascularization and proliferative activity, which lacked in cavitary TB lung lesions. Immune insulation of caseous granulomas may favor caseum liquefaction and cavity formation.[13-15]
Dormant Mtb
In the hypoxic core of poorly vascularized necrotic and/or caseous granulomas the low oxygen pressure restricts the growth of aerobic AR Mtb to microaerophilic/anaerobic NR Mtb allowing bacilli to transit into a dormant state, i.e. a condition characterized by low metabolic activity that renders the bacteria resistant to killing by host immune response and antibiotics.
Initial response to hypoxia is regulated by the two-component response regulator DosR which, after phosphorylation by either of two sensor kinases (DosT, a hypoxia sensor, and DosS, a redox sensor) leads to induction of a set of 48 genes.[16] Besides hypoxia, the DosR regulon is also induced in response to nitric oxide, nutrient starvation, and following infection of macrophages, mice, and guinea pigs. DosR regulon induction is transient, with about half genes returning to baseline within 24 hours. A second wave of gene expression then occurs after the initial DosR-mediated hypoxic response, consisting of 230 genes (Enduring Hypoxic Response, EHR) involved in the control of the regulatory factors and enzymatic machines of the long-term bacteriostasis program of NR Mtb.[17]
Several in vitro models to obtain NR Mtb in vitro have been developed over the years, based on reduced oxygen availability, nutrient starvation or the use of standing cultures. One of the most popular ways is the Wayne model of hypoxia (Figure 1)[18-19] in which dormant bacilli are obtained by gradual adaptation of stirred cultures of aerobic Mtb to anaerobiosis through the self-generated formation of an oxygen gradient. In this model at least two stages of nonreplicating persistence designated NRP-1 and NRP-2 occurs, with 1% (microaerophilic stage) and 0.06% (anaerobic stage) dissolved oxygen levels, respectively. Dormancy models including combined stresses of low oxygen, high CO2, low nutrients and acidic pH were also described.[20] All these models are useful to screen drugs/drug combinations that can kill NR Mtb.

Figure 1. Mycobacterium tuberculosis (Mtb) grown for 40 days in 20- by 125-mm screw-cap tubes containing Dubos Tween-albumin broth, and stirred with 8-mm magnetic bars.[18-19] Aerobic, replicating Mtb, was obtained by incubation of the tubes at 37°C with loosened screw caps. For preparation of dormant, nonreplicating bacilli, tight-fitting red rubber caps were put under the screw caps. Means and standard deviations of optical density (OD) at 600 nm are shown.
Dormant Mtb cells collected from lung tissues can be difficult to grow in culture media. In old studies examining surgically removed specimens from closed cavities of lungs of drug-treated patients, acid-fast bacilli were observed on microscopic sections but no colony growth was seen in solid media during the normal 8 weeks of incubation.[21] However, by prolonging the incubation of cultures up to 3 to 10 months it was possible to obtain Mtb colonies, demonstrating the existence of few slowly growing tubercle bacilli surviving after drug treatment. Results from open cavities were in startling contrast to those from closed cavities, showing bacillary growth within 8 weeks. Differences in bacterial growth were also reported in more recent investigations, showing that Mtb was always cultivable from active cavitary TB but not from nonprogressive tuberculomas of healthy patients.[22] To evade host responses, stresses bacteria can enter various reversible NR states characterized by impaired culturability.[23] The peptidoglycan structure plays an important role in the maintenance of bacterial dormancy and a variation in a specific cross-link occurs during stationary-phase adaptation of Mtb. Five resuscitation-promoting factors (Rpf) similar to lyzozyme and lytic transglycosylases and sharing sequences with the Rpf of Micrococcus luteus were described and investigated for their capacity to resuscitate dormant mycobacteria.[24] These studies indicated that activation of dormant cells by Rpf requires peptidoglycan hydrolysis facilitating cell division and/or the release of products acting as anti-dormancy signals.
There is strong evidence that Mtb uses lipids as a major energy source for persistence in the host. Fatty acids are stored as triacylglycerol in seed oils of plants and in the adipose tissues of mammals for use as energy during dormancy/hibernation. Mtb uses host triacylglycerol to accumulate lipid droplets intracellularly, and acquires a dormancy-like phenotype in lipid-loaded macrophages.[25] Wax exter synthesis and utilization of host cholesterol are also required for Mtb to enter dormancy.[26-27] Interestingly, intracellular lipid inclusion bodies were also observed in several Mtb cells isolated from the sputum of TB patients, as expected from the possibility that after tuberculoma disintegration a mixture of AR and NR Mtb is released into the airways.[28] Mtb can also enter within adipocytes, where it accumulates intracytoplasmic lipid inclusions for survival in a NR state.[29] Given the wide distribution of the adipose tissue throughout the body, Mtb may persist inside this tissue for long periods of time. Overall, due to the close relationship between lipid metabolism and dormancy, and the ability of NR Mtb to survive in lipid-reach caseous granulomas,[11] cure and eradication of TB by drugs will be difficult.
Where does dormant Mtb live exactly? After treatment of guinea pigs with the novel drug TMC-207, Mtb was almost completely eradicated from the tissues, but few acid-fast bacilli were found to be extracellular within the central core of caseous necrosis and surrounding acellular rim of the primary granulomas.[30-31] This zone was hypoxic and morphologically similar to that described for human lung lesions. The acellular rim may then be a primary location of persisting Mtb after drug treatment, highlighting the importance of developing new drugs/drug combinations able to eradicate extracellular Mtb remaining within necrotic lesions. In contrast, in drug-treated mice a homogenous reduction in viable counts was observed, indicating that the mouse model, which fails to show significant necrosis and hypoxia in lung lesions, is not suitable for the study of hypoxic responses of Mtb.[30-31] Other investigators confirmed that TB granulomas of guinea pigs, rabbits and nonhuman primates, but not mice, are hypoxic.[32] Indeed, measurements of pO2 in granulomas of Mtb-infected rabbits using pimonidazole labeling and a fiber optic oxygen probe revealed pO2 values lower than 2 mm Hg, in comparison with 151 mm Hg in the air and 37 mm Hg in murine Mtb lesions.[15,32]
The low efficiency of TB therapy is due to different reasons, including slow growth of Mtb related to periods of dormancy, low penetration of drugs in different granulomatous lesions, generation of drug-tolerant dormant persisters. In recent investigations Dartois et al compared by high-pressure liquid chromatography the distribution of INH, RIF, PZA and MXF in pulmonary lesions and plasma of Mtb-infected rabbits and found that MXF showed the best partitioning into lung and granulomas.[33] However, more sophisticated measurements performed by MALDI mass spectrometry imaging showed that MXF and PA-824 do not diffuse effectively through caseum.[15,34] Preliminary studies of this group suggested that PZA preferentially accumulates in caseum versus inflammatory cells.[15]
The prolonged treatment necessary to cure TB is also due to drug-tolerant dormant persisters i.e. a subpopulation of NR or slowly replicating bacilli that survive the cidal action of antibiotics.[35] These cells have noninheritable phenotypic resistance or tolerance to antibiotics, but their progeny is fully susceptible to drugs. Mtb persisters likely comprise different subpopulations and consist of a very small proportion of Mtb. To give an example in vivo, in the lungs of guinea pigs treated for 6 weeks with 15 mg/kg of TMC-207, 1 persisting Mtb out of about 20,000 bacilli was recovered after treatment.[30] In vitro, low numbers of Mtb persisters are present in early exponential phase, but their number increases sharply up to 1% of the population at late exponential and stationary phases.[36] Thus, their proportion likely depends by specific conditions including the age of a culture, the length of drug exposure, the type and concentration of antibiotics.[35] It is not yet clear while their formation can be promoted in a stochastic or deterministic (for instance by induction) manner but it is possible that both mechanisms are involved.[35-37] A study of the transcriptome of Mtb persisters identified a small number of genes upregulated by different stresses [antibiotic exposure, increasing hypoxia (Wayne model), hypoxia by continuous flow of low oxygen (EHR), nutrient starvation in phosphate buffered saline] which may represent a core dormancy response.[36] Recently it was reported that mycobacterial persisters may be eradicated in vitro with antibiotic-generated hydroxyl radicals, suggesting that stimulation of reactive oxygen species provides a potential strategy to managing persistent infections.[38] This observation is in keeping with the knowledge that bactericidal antibiotics may stimulate the production of highly deleterious hydroxyl radicals in Gram-negative and Gram-positive bacteria.[39]
Activity of Drugs Against Dormant Mtb
The most important objective for discovery of new anti-TB drugs is to find new drugs/drug combinations able to effectively eradicate in vivo both AR (aerobic) bacilli and NR (microaerophilic, anaerobic and persisters) bacilli to shorten therapy of active TB below 6 months, and effectively reduce the reservoir of latently infected individuals. Using the Wayne model and other models it was shown that NR bacilli were insensitive to INH, while being inhibited by RIF, PZA, fluoroquinolones (e.g. MXF), aminoglycosides [e.g. amikacin (AK)], capreomycin (CP) and nitrocompounds [e.g. metronidazole (MZ), niclosamide (NC), nitazoxanide (NTZ), PA-824 and other drugs].[3,19,35] Among nitrocompounds, MZ strongly reduced Mtb viable counts under anaerobic conditions, but showed no activity under aerobic conditions.[3,18] Using the Wayne model we found that the combination MZ+RIF sterilized long-term (26-days-old) dormant Mtb cultures and that some combinations (RIF+MXF+MZ+AK or RIF+MXF+MZ+CP) killed both AR and NR Mtb, as measured by a test of residual viability more sensitive than colony forming units (CFU) based on the lack of re-growth after 100 days of incubation in liquid medium (MGIT 960).[40-41] In vivo activity of MZ was reported to be different in various animal models, and was related to the presence of caseous necrosis, or to drug toxicity. Indeed, MZ had no activity in mice and was toxic in a combination treatment in guinea pigs but showed good efficacy in rabbits and prevented reactivation of latent TB in macaques.[42] When administered to MDR-TB patients in a controlled trial, MZ increased early sputum smear and culture conversion, but was too neurotoxic to use over the long term.[43] Due to MZ toxicity, novel and better tolerated nitroimidazoles with activity against both aerobic and anaerobic Mtb are presently investigated in vitro and in vivo with promising results, indicating the importance of including at least a drug with anaerobic activity in newly designed regimens. The novel nitroimidazole PA-824 demonstrated anti-TB activity in combination with other drugs in mice and in human early bactericidal activity (EBA) studies.[43] The drug inhibits the mycolic acid biosynthesis of aerobic Mtb and kills NR Mtb by intracellular nitric oxide release.[3,44] In the Wayne model we showed that the combination (RIF+MXF+AK+PA-824) was more efficient than the combination currently used in human therapy (RIF+INH+PZA+EMB) and killed both AR and NR Mtb in 14 days.[19] Also TMC-207, a diarylquinoline recently approved with restraint by the Food and Drug Administration for treatment of MDR-TB, is effective against both AR and NR Mtb.[45-47] The drug acts specifically by targeting the membrane-bound c-subunit of ATP synthase of AR Mtb and the residual ATP synthase enzymatic activity of NR Mtb.
Since anti-TB treatment requires activity of drugs against both AR and NR bacilli living in a wide spectrum of lung lesions, use of drug combinations is essential to cover all of these requirements. Understanding where a bacterial population resides and evaluating the ability of novel molecules to penetrate a site allows a more rational approach to the design of new combinations. Traditional Minimum Inhibitory Concentration (MIC) measures the activity of a compound only against AR aerobic cells, representing a non-physiological situation in low or no vascularized necrotic (caseous) granulomas.[15] Thus, NR or slowly replicating Mtb assays have been developed including low oxygen (Wayne model), nutrient starvation and nitric-oxide release assays using MZ and INH as positive (growth) and negative (no growth) controls, respectively.[48] The necrotic tissue has a pH of about 6.549 while the pH of active TB lesions is between 5.5 and 6.0 or lower.[19] The standard Wayne model of hypoxia at pH 6.6 or the modified version at pH 5.8 we recently set up[18-19,40-41] may mimic the microenvironment of necrotic and cellular granulomas, respectively, and be suitable for measuring the activity of new compounds under different hypoxic conditions. To eradicate Mtb from the tissues we need to kill both AR and NR Mtb that are not eliminated with current therapy. A complication in the discovery of new drugs/combinations is the lack of a sensitive test determining when Mtb is dead.[8] Considering that NR Mtb may not form colonies on agar, we found that re-growth of drug-exposed Mtb in a liquid medium (MGIT 960) was a test much more sensitive than CFU measurements to demonstrate the ‘cidal’ activity of a combination.[19,41] We think that this test should be used together with the CFUs when studying the sterilizing activity of new drugs or combinations.
Besides in vitro tests the sterilizing activity of a combination against AR and NR Mtb can be determined in animal models reaching different stages of granuloma formation ranging from mouse model (lacking organized granulomatous structures) to guinea pig, rabbit and macaque models, showing necrosis, caseation, liquefaction and cavity formation.[15] No one animal model fully mimics the complete spectrum of lesions shown by humans. However, in the practice, the most popular model for determining the sterilizing activity of drug combinations is still the mouse model which, due to low costs and drug requirements, is widely used by monitoring CFUs in organs and the relapse rate 3 months after discontinuation of treatment. Recently, some combinations sterilizing Mtb-infected mice were reported, including TMC-207+PZA+CFZ+RPT (6 weeks), TMC-207+PZA+CFZ (8 weeks), TMC-207+PZA+RPT (8 weeks).[50-51] However, due to lack of hypoxia and necrosis in mouse lungs, efficacy of single drugs and combinations not necessarily predicts the clinical feature in humans.[15] Interestingly, the use of a hypoxic mouse model (C3HeB/FeJ mice) developing lesions with liquefactive necrosis is under validation for testing new drugs and combinations.[52] In the guinea pigs model, the combination PA-824+MXF+PZA killed Mtb from lungs in 8 weeks and showed the highest EBA values in humans.[53-54] This and at least other 37 drug combinations containing high-doses INH, RIF, RPT or fluoroquinolones, TMC-207, PA-824, OPC-67683, and other drugs (SQ-109, PNU-100480, AZD-5847) are in Phases I, II or III clinical trials against drug-susceptible and -resistant TB, and results are expected to be reported in the next few years.[2-3]
References
- World Health Organization. Global tuberculosis report 2012. WHO/HTM/TB/2012.6
- Zumla A, Raviglione M, Hafner R, von Reyn
CF. Tuberculosis. N Engl J Med. 2013; 368:745-55.
http://dx.doi.org/10.1056/NEJMra1200894 PMid:23425167

- Zumla A, Nahid P, Cole ST. Advances in the
development of new tuberculosis drugs and treatment regimens. Nat Rev
Drug Discov. 2013;12:388-404. http://dx.doi.org/10.1038/nrd4001 PMid:23629506

- Ahuja SD, Ashkin D, Avendano M, Banerjee R,
Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan
ED, Chiang CY, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D,
Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM,
Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim
HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li
J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M,
O'Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Pe-a J, Pérez-Guzmán
C, Quelapio MI, Ponce-de-Leon A, Riekstina V, Robert J, Royce S, Schaaf
HS, Seung KJ, Shah L, Shim TS, Shin SS, Shiraishi Y, Sifuentes-Osornio
J, Sotgiu G, Strand MJ, Tabarsi P, Tupasi TE, van Altena R, Van der
Walt M, Van der Werf TS, Vargas MH, Viiklepp P, Westenhouse J, Yew WW,
Yim JJ; Collaborative Group for Meta-Analysis of Individual Patient
Data in MDR-TB. Multidrug resistant pulmonary tuberculosis treatment
regimens and patient outcomes: an individual patient data meta-analysis
of 9,153 patients. PLoS Med. 2012;9(8):e1001300. PMid:22952439
PMCid:PMC3429397

- Fattorini L, Mustazzolu A, Piccaro G,
Pardini M, Filippini P, Giannoni F, Migliori GB, Sotgiu G, Borroni E,
Cirillo DM; Italian Multicentre Study on Resistance to Antituberculosis
Drugs Group.Drug-resistant tuberculosis among foreign-born persons in
Italy. Eur Respir J. 2012;40:497-500. http://dx.doi.org/10.1183/09031936.00021012 PMid:22855470

- Ottenhoff TH, Kaufmann SH.Vaccines against
tuberculosis: where are we and where do we need to go? PLoS Pathog.
2012;8(5):e1002607. http://dx.doi.org/10.1371/journal.ppat.1002607 PMid:22589713 PMCid:PMC3349743

- Locht C, Rouanet C, Hougardy JM, Mascart F.
How a different look at latency can help to develop novel diagnostics
and vaccines against tuberculosis. Expert Opin Biol Ther.
2007;7:1665-77. http://dx.doi.org/10.1517/14712598.7.11.1665 PMid:17961090

- Barry CE 3rd, Boshoff HI, Dartois V, Dick
T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D.The spectrum
of latent tuberculosis: rethinking the biology and intervention
strategies. Nat Rev Microbiol. 2009;7:845-55. PMid:19855401

- Fox GJ, Barry SE, Britton WJ, Marks GB.
Contact investigation for tuberculosis: a systematic review and
meta-analysis. Eur Respir J. 2013;41:140-56. http://dx.doi.org/10.1183/09031936.00070812 PMid:22936710 PMCid:PMC3533588

- Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36:514-32. http://dx.doi.org/10.1111/j.1574-6976.2012.00331.x PMid:22320122 PMCid:PMC3319523

- Kim MJ, Wainwright HC, Locketz M, Bekker
LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U,
Tsenova L, Kaplan G, Russell DG. Caseation of human tuberculosis
granulomas correlates with elevated host lipid metabolism. EMBO Mol
Med. 2010;2:258-74. http://dx.doi.org/10.1002/emmm.201000079 PMid:20597103 PMCid:PMC2913288

- Cardona PJ, Ruiz-Manzano J. On the nature of Mycobacterium tuberculosis-latent bacilli. Eur Respir J. 2004;24:1044-51. http://dx.doi.org/10.1183/09031936.04.00072604 PMid:15572551

- Ulrichs T, Kosmiadi GA, Trusov V, Jörg S,
Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human
tuberculous granulomas induce peripheral lymphoid follicle-like
structures to orchestrate local host defence in the lung. J Pathol.
2004;204:217-28. http://dx.doi.org/10.1002/path.1628 PMid:15376257

- Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261-9. http://dx.doi.org/10.1002/path.1906 PMid:16362982

- Dartois V, Barry CE 3rd. A medicinal
chemists' guide to the unique difficulties of lead optimization for
tuberculosis. Bioorg Med Chem Lett. 2013;23:4741-50. http://dx.doi.org/10.1016/j.bmcl.2013.07.006 PMid:23910985

- Kumar A, Toledo JC, Patel RP, Lancaster JR
Jr, Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and
DosT is a hypoxia sensor. Proc Natl Acad Sci U S A. 2007;104:11568-73. http://dx.doi.org/10.1073/pnas.0705054104 PMid:17609369 PMCid:PMC1906723

- Rustad TR, Harrell MI, Liao R, Sherman DR.
The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One.
2008;30;3(1):e1502.

- Wayne, L.G., and L.G. Hayes. An in vitro
model for sequential study of shiftdown of Mycobacterium tuberculosis
through two stages of nonreplicating persistence. Infect Immun. 1996;
64:2062-9. PMid:8675308 PMCid:PMC174037

- Piccaro G, Giannoni F, Filippini P,
Mustazzolu A, Fattorini L. Activities of drug combinations against
Mycobacterium tuberculosis grown in aerobic and hypoxic acidic
conditions. Antimicrob Agents Chemother. 2013;57:1428-33. http://dx.doi.org/10.1128/AAC.02154-12 PMid:23295931 PMCid:PMC3591868

- Deb C, Lee CM, Dubey VS, Daniel J,
Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. A novel in
vitro multiple-stress dormancy model for Mycobacterium tuberculosis
generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One.
2009 Jun 29;4(6):e6077. http://dx.doi.org/10.1371/journal.pone.0006077 PMid:19562030 PMCid:PMC2698117

- Vandiviere HM, Loring WE, Melvin I, Willis
S. The treated pulmonary lesion and its tubercle bacillus. II. The
death and resurrection. Am J Med Sci. 1956;232:30-7. http://dx.doi.org/10.1097/00000441-195607000-00006 PMid:13326887

- Ulrichs T, Kosmiadi GA, Jörg S, Pradl L,
Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Differential
organization of the local immune response in patients with active
cavitary tuberculosis or with nonprogressive tuberculoma. J Infect Dis.
2005;192:89-97. http://dx.doi.org/10.1086/430621 PMid:15942898

- Kana BD, Mizrahi V.
Resuscitation-promoting factors as lytic enzymes for bacterial growth
and signaling. FEMS Immunol Med Microbiol. 2010;58:39-50. http://dx.doi.org/10.1111/j.1574-695X.2009.00606.x PMid:19799629

- Gupta RK, Srivastava BS, Srivastava R.
Comparative expression analysis of rpf-like genes of Mycobacterium
tuberculosis H37Rv under different physiological stress and growth
conditions. Microbiology. 2010;156:2714-22. http://dx.doi.org/10.1099/mic.0.037622-0 PMid:20522500

- Daniel J, Maamar H, Deb C, Sirakova TD,
Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to
accumulate lipid droplets and acquires a dormancy-like phenotype in
lipid-loaded macrophages. PLoS Pathog. 2011;7(6):e1002093. http://dx.doi.org/10.1371/journal.ppat.1002093 PMid:21731490 PMCid:PMC3121879

- Sirakova TD, Deb C, Daniel J, Singh HD,
Maamar H, Dubey VS, Kolattukudy PE. Wax ester synthesis is required for
Mycobacterium tuberculosis to enter in vitro dormancy. PLoS One.
2012;7(12):e51641. http://dx.doi.org/10.1371/journal.pone.0051641 PMid:23272127 PMCid:PMC3522743

- Pandey AK, Sassetti CM. Mycobacterial
persistence requires the utilization of host cholesterol. Proc Natl
Acad Sci USA. 2008;105:4376-80. http://dx.doi.org/10.1073/pnas.0711159105 PMid:18334639 PMCid:PMC2393810

- Garton NJ, Waddell SJ, Sherratt AL, Lee
SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS,
Butcher PD, Barer MR. Cytological and transcript analyses reveal fat
and lazy persister-like bacilli in tuberculous sputum. PLoS Med.
2008;5(4):e75. http://dx.doi.org/10.1371/journal.pmed.0050075 PMid:18384229 PMCid:PMC2276522

- Neyrolles O, Hernandez-Pando R,
Pietri-Rouxel F, et al. Is Adipose Tissue a Place for Mycobacterium
tuberculosis Persistence? PLoS ONE. 2006;1:e43. http://dx.doi.org/10.1371/journal.pone.0000043 PMid:17183672 PMCid:PMC1762355

- Lenaerts AJ, Hoff D, Aly S, et al.
Location of persisting mycobacteria in a Guinea pig model of
tuberculosis revealed by r207910. Antimicrob Agents Chemother.
2007;51:3338-45. http://dx.doi.org/10.1128/AAC.00276-07 PMid:17517834 PMCid:PMC2043239

- Hoff DR, Ryan GJ, Driver ER, Ssemakulu CC,
De Groote MA, Basaraba RJ, Lenaerts AJ. Location of intra- and
extracellular Mycobacterium tuberculosis populations in lungs of mice
and guinea pigs during disease progression and after drug treatment.
PLoS One. 2011; 21;6(3):e17550.

- Via LE, Lin PL, Ray SM, et al. Tuberculous
granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates.
Infect Immun. 2008;76:2333-40. http://dx.doi.org/10.1128/IAI.01515-07 PMid:18347040 PMCid:PMC2423064

- Kjellsson MC, Via LE, Goh A, Weiner D, Low
KM, Kern S, Pillai G, Barry CE 3rd, Dartois V. Pharmacokinetic
evaluation of the penetration of antituberculosis agents in rabbit
pulmonary lesions. Antimicrob Agents Chemother. 2012;56:446-57. http://dx.doi.org/10.1128/AAC.05208-11 PMid:21986820 PMCid:PMC3256032

- Prideaux B, Dartois V, Staab D, Weiner DM,
Goh A, Via LE, Barry CE 3rd, Stoeckli M. High-sensitivity MALDI-MRM-MS
imaging of moxifloxacin distribution in tuberculosis-infected rabbit
lungs and granulomatous lesions. Anal Chem. 2011;83:2112-8. http://dx.doi.org/10.1021/ac1029049 PMid:21332183 PMCid:PMC3158846

- Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56:2223-30. http://dx.doi.org/10.1128/AAC.06288-11 PMid:22391538 PMCid:PMC3346619

- Keren I, Minami S, Rubin E, Lewis K.
Characterization and transcriptome analysis of Mycobacterium
tuberculosis persisters. MBio. 2011;2(3):e00100-11.

- Dörr T, Vulic M, Lewis K. Ciprofloxacin
causes persister formation by inducing the TisB toxin in Escherichia
coli. PLoS Biol. 2010;8(2):e1000317.

- Grant SS, Kaufmann BB, Chand NS, Haseley
N, Hung DT. Eradication of bacterial persisters with
antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A.
2012;109:12147-52. http://dx.doi.org/10.1073/pnas.1203735109 PMid:22778419 PMCid:PMC3409745

- Kohanski MA, Dwyer DJ, Hayete B, Lawrence
CA, Collins JJ. A common mechanism of cellular death induced by
bactericidal antibiotics. Cell. 2007;130:797-810. http://dx.doi.org/10.1016/j.cell.2007.06.049 PMid:17803904

- Iona E, Giannoni F, Pardini M, Brunori L,
Orefici G, Fattorini L. Metronidazole plus Rifampin Sterilizes
Long-Term Dormant Mycobacterium tuberculosis. Antimicrob Agents
Chemother. 2007; 51:1537-40. http://dx.doi.org/10.1128/AAC.01468-06 PMid:17242153 PMCid:PMC1855463

- Filippini P, Iona E, Piccaro G, Peyron P,
Neyrolles O, Fattorini L. Activity of drug combinations against dormant
Mycobacterium tuberculosis. Antimicrob Agents Chemother.
2010;54:2712-5. http://dx.doi.org/10.1128/AAC.01736-09 PMid:20350948 PMCid:PMC2876400

- Lin PL, Dartois V, Johnston PJ, Janssen C,
Via L, Goodwin MB, Klein E, Barry CE 3rd, Flynn JL. Metronidazole
prevents reactivation of latent Mycobacterium tuberculosis infection in
macaques. Proc Natl Acad Sci U S A. 2012;109:14188-93. http://dx.doi.org/10.1073/pnas.1121497109 PMid:22826237 PMCid:PMC3435210

- Carroll MW, Jeon D, Mountz JM, Lee JD,
Jeong YJ, Zia N, Lee M, Lee J, Via LE, Lee S, Eum SY, Lee SJ, Goldfeder
LC, Cai Y, Jin B, Kim Y, Oh T, Chen RY, Dodd LE, Gu W, Dartois V, Park
SK, Kim CT, Barry CE 3rd, Cho SN. Efficacy and safety of metronidazole
for pulmonary multidrug-resistant tuberculosis. Antimicrob Agents
Chemother. 2013;57:3903-9. http://dx.doi.org/10.1128/AAC.00753-13 PMid:23733467

- Singh R, Manjunatha U, Boshoff HI, Ha YH,
Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S,
Keller TH, Jiricek J, Barry CE 3rd. PA-824 kills nonreplicating
Mycobacterium tuberculosis by intracellular NO release. Science.
2008;322:1392-5. http://dx.doi.org/10.1126/science.1164571 PMid:19039139 PMCid:PMC2723733

- Cohen J. Infectious disease. Approval of novel TB drug celebrated--with restraint. Science. 2013;339:130. http://dx.doi.org/10.1126/science.339.6116.130 PMid:23307714

- Koul A, Vranckx L, Dendouga N, Balemans W,
Van den Wyngaert I, Vergauwen K, Göhlmann HW, Willebrords R, Poncelet
A, Guillemont J, Bald D, Andries K. Diarylquinolines are bactericidal
for dormant mycobacteria as a result of disturbed ATP homeostasis. J
Biol Chem. 2008;283:25273-80. http://dx.doi.org/10.1074/jbc.M803899200 PMid:18625705

- Rao SP, Alonso S, Rand L, Dick T, Pethe K.
The protonmotive force is required for maintaining ATP homeostasis and
viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc
Natl Acad Sci U S A. 2008;105:11945-50. http://dx.doi.org/10.1073/pnas.0711697105 PMid:18697942 PMCid:PMC2575262

- Franzblau SG, DeGroote MA, Cho SH, Andries
K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois
V, Lenaerts AJ. Comprehensive analysis of methods used for the
evaluation of compounds against Mycobacterium tuberculosis.
Tuberculosis (Edinb). 2012;92:453-88. http://dx.doi.org/10.1016/j.tube.2012.07.003 PMid:22940006

- Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139-63. http://dx.doi.org/10.1146/annurev.micro.55.1.139 PMid:11544352

- Williams K, Minkowski A, Amoabeng O,
Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL.
Sterilizing activities of novel combinations lacking first- and
second-line drugs in a murine model of tuberculosis. Antimicrob Agents
Chemother. 2012;56:3114-20. http://dx.doi.org/10.1128/AAC.00384-12 PMid:22470112 PMCid:PMC3370712

- Tasneen R, Li SY, Peloquin CA, Taylor D,
Williams KN, Andries K, Mdluli KE, Nuermberger EL. Sterilizing activity
of novel TMC207- and PA-824-containing regimens in a murine model of
tuberculosis. Antimicrob Agents Chemother. 2011;55:5485-92. http://dx.doi.org/10.1128/AAC.05293-11 PMid:21930883 PMCid:PMC3232786

- Driver ER, Ryan GJ, Hoff DR, Irwin SM,
Basaraba RJ, Kramnik I, Lenaerts AJ. Evaluation of a mouse model of
necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs
against Mycobacterium tuberculosis. Antimicrob Agents Chemother.
2012;56:3181-95. http://dx.doi.org/10.1128/AAC.00217-12 PMid:22470120 PMCid:PMC3370740

- Diacon AH, Dawson R, von
Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C,
Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 14-day
bactericidal activity of PA-824, bedaquiline, pyrazinamide, and
moxifloxacin combinations: a randomised trial. Lancet. 2012;380:986-93.
http://dx.doi.org/10.1016/S0140-6736(12)61080-0

- Dutta NK, Alsultan A, Gniadek TJ, Belchis
DA, Pinn ML, Mdluli KE, Nuermberger EL, Peloquin CA, Karakousis PC.
Potent rifamycin-sparing regimen cures Guinea pig tuberculosis as
rapidly as the standard regimen. Antimicrob Agents Chemother.
2013;57:3910-6. http://dx.doi.org/10.1128/AAC.00761-13 PMid:23733473
