Received: August 21, 2013
Accepted: November 22, 2013
Meditter J Hematol Infect Dis 2014, 6(1): e2014002, DOI 10.4084/MJHID.2014.002
This article is available on PDF format at:

Surbhi Goyal, Usha Rani Singh, Usha Rusia
Department of Pathology, University College of Medical Sciences, Dilshad Garden, Delhi, India

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Introduction: Bone marrow examination is an indispensable diagnostic tool to evaluate neoplastic and non neoplastic hematological diseases.Aims: To compare bone marrow aspirate with trephine biopsy in hematological disorders. To determine the optimum trephine preprocessing length in lymphoma infiltration. Methods: Diagnostic comparison was done between simultaneous bone marrow aspirates and trephine biopsies in 449 patients. Biopsies were fixed in formalin, decalcified in 5.5% EDTA and routinely processed. Concordance rates and validity parameters for aspirate were calculated. Three deeper sections of trephine biopsy, cut at 0.1–0.2 mm intervals, were assessed for lymphoma involvement. Proportion of biopsies showing marrow infiltration by lymphoma cells was plotted against trephine length and correlation was assessed. Results: Aspirate had a high sensitivity for acute leukemia (89.4%) and multiple myeloma (88.5%), moderate for NHL (67.6%) and nonhematopoietic metastases (58.3%) and low for aplastic anemia (38.5%) and Hodgkin lymphoma (5%). Aspirate has no role in granulomatous myelitis and myelofibrosis. Lymphoma positivity increased with trephine length, with maximum positivity (68.9%) seen in 17-20 mm group and no further gain beyond 20 mm. (lymphoma positivity ≤ 16mm=40.3% and ≥17mm=66.1%, p=0.0011). Conclusion: Aspirate has a high specificity; its sensitivity depends upon the type of disease. Apart from few conditions, in which aspirate alone is sufficient, biopsy is mandatory in most. Preprocessing trephine length of 17-20 mm examined at multiple deeper levels was found optimal for assessing lymphoma positivity. |
Introduction
Bone marrow examination is an indispensable diagnostic tool in the evaluation of various hematological disorders, non hematological malignancies, pyrexia of unknown origin and infective diseases. [1] It is also valuable for follow up of patients undergoing chemotherapy and bone marrow transplantation. [1,2] Involvement of marrow by metastases has a significant impact on patient management and prognosis. [3] Bone marrow examination serves to establish or confirm a primary diagnosis of lymphoma or to determine the extent of disease dissemination for staging purposes. [4] Rarely, bone marrow examination has been useful in detecting non-hematopoietic malignancy in clinically unsuspected cases. [5] At times, marrow metastases may have normal serum chemistry and hematologic parameters and may even be missed by bone scans and advanced imaging modalities. [6] This fact highlights the importance of using sensitive techniques for the detection of marrow metastasis. Accurate diagnosis of myelitis by disseminated infections is important for timely management. Bone marrow aspiration (BMA) is a simple, reliable and rapid method of marrow evaluation. Trephine biopsy provides more comprehensive information regarding the marrow cellularity, architectural patterns and overall hematopoiesis. But biopsy is a painful procedure and its processing takes at least 48-72 hours. So, to perform trephine biopsies in all patients may not be cost effective in terms of clinician and laboratory personnel time, efforts and patient discomfort. Few studies have analyzed the diagnostic accuracy of bone marrow aspirate in comparison with trephine biopsy. [7-10] Literature on correlation of lymphoma positivity with trephine biopsy length is even sparse. [11,12] With these considerations, a prospective study was conducted with the objectives of comparing the accuracy of BMA with trephine biopsy done simultaneously in the diagnosis of hematological disorders and to determine the optimum trephine preprocessing length to assess lymphoma infiltration.
Materials and methods
This single institution prospective study was approved by the
Institutional Ethics Committee and informed consent was obtained from
all the patients.
Subject population: From January 2011 to February
2012, 514
patients were recruited in the study and underwent both bone marrow
aspirate and biopsy simultaneously. Of these, 65 (12.6%) biopsies were
inadequate for assessment and were excluded from analysis. So, final
study cohort comprised 449 patients who had undergone both aspirate and
biopsy simultaneously. Patient demographic information, clinical
history including physical findings, chemo/radiotherapy, complete blood
count with peripheral smear findings and indication of bone marrow were
collected by one author. Aspirate findings were compared to that of
trephine biopsy. Of these, 382 patients were diagnosed or follow up
cases of hematolymphoid malignancy and their distribution is shown in table
1. Sixty seven patients presented with
pancytopenia/bicytopenia and bone marrow was done to detect the
etiology (Figure1).
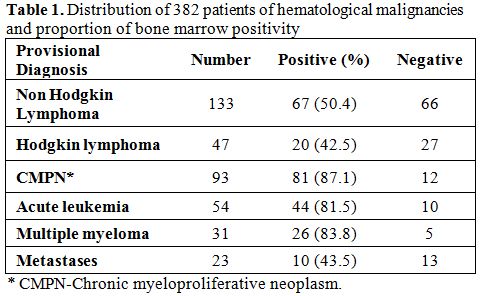 |
Table 1. Distribution of 382 patients of hematological malignancies and proportion of bone marrow positivity. |
Bone marrow aspirate and trephine biopsy:
BMA was done using
Salah’s needle and 0.25 to 0.5 ml of aspirate was withdrawn with a 20
ml plastic syringe from posterior superior iliac spine. Aspirated
material was delivered onto clean glass slides and smears were prepared
immediately. After that, trephine biopsy was performed using Jamshidi’s
needle through the same incision, approximately 0.5–1 cm away from the
site of aspiration to avoid obtaining a hemorrhagic biopsy. Peripheral
smears and marrow aspirate smears were stained by Wright’s stain.
Trephine biopsies fixed in 10% neutral buffered formalin, were
subjected to decalcification in 5.5% EDTA solution for 24 hrs. After
decalcification, the preprocessing length of trephine biopsy was
measured with a metric scale and biopsy was routinely processed in
automated tissue processor and embedded in paraffin blocks. In cases
where a trephine was in several pieces, the total length was recorded.
2-3µm thick sections were cut and stained with hematoxylin &
eosin.
All the smears and sections were reviewed by two experienced
pathologists in consensus. At least three deeper sections, cut at
intervals of 0.1-0.2 mm, were examined to assess marrow involvement in
patients of lymphoma and suspected metastases. While examining the
aspirate, the pathologists were blinded to biopsy findings. BMA and
biopsy findings were compared. Wherever indicated, histochemistry was
performed. Gömöri’s reticulin and Masson’s trichrome were performed to
grade marrow fibrosis according to European Consensus grading system. [13]
In cases where tuberculosis was suspected, cold Ziehl Neelson was
performed to stain for acid fast bacilli (AFB). PAS was done to look
for glycogen and fungal hyphae. For immunohistochemistry, sections on
poly-lysine slides were taken and immunohistochemistry was done by
standard streptavidin biotinylated peroxidise method. In suspicious
cases of marrow infiltration by lymphoma, panel of antibodies (CD45, CD
20, CD 15, CD 30, CD 3, CD 5) was employed for confirmation and further
subtyping. Antibodies against λ and κ light chains were used to
establish the monoclonality in neoplastic plasma cells. To confirm
nonhematopoietic marrow metastases in suspicious cases, antibodies
against cytokeratin, Neuron specific enolase, CD99, S-100 and
epithelial membrane antigen were used wherever required.
Statistical analysis: Results were statistically
analyzed using
SPSS software (version 17.0, SPSS, Chicago, Illinois, USA). Concordance
rates were calculated between aspirate and biopsy. In groups with
sufficient sample size, validity parameters were calculated. Proportion
of trephine biopsies showing lymphoma infiltration was plotted on y
axis and total preprocessing trephine length on x axis, in increments
of 4 mm. Fischer’s exact test was used to analyze the significance in
lymphoma positivity between two groups and P value <0.05 was
considered statistically significant.
Results
Lymphoma: Lymphomas accounted for 40.1% of
all patients. Of
these, 73.9% (133/180) patients were of Non Hodgkin Lymphoma (NHL) and
Hodgkin lymphoma comprised 26.1%. Of 133 trephines being evaluated for
NHL staging, 67 (50.4%) showed marrow infiltration in the form of
paratrabecular nodular, interstitial or diffuse pattern (Figure
2).
Of these, 20 were SLL/CLL, 15 were follicular and mantle cell, 13 were
large cell type and rest could not be further subtyped. 46 out of these
67 aspirates were reported as positive for lymphoma infiltration, 15
were reported as negative and six were inadequate. In 66 cases, both
biopsy and aspirate were negative for lymphoma infiltration.
Chemotherapy induced changes comprised increased vessel density,
necrosis and marked fibrosis of intertrabecular space. These were seen
in four trephine biopsies but not in aspirates.
In Hodgkin
lymphoma, marrow involvement was seen in 42.5% (20/47) patients. Both
biopsy and aspirate were negative for lymphoma in rest 27 patients.
Large binucleate cells with moderate amount of cytoplasm,
vesicular nucleus and prominent eosinophilic nucleolus (classical Reed
Sternberg cells) and mononuclear cells (variant RS) were seen (Figure
3)
and confirmed by bright paranuclear positivity for CD 15/ CD30. Focal
fibrosis and necrosis was seen in 12 cases. Epithelioid cell granulomas
were found in five of them. However, stain for AFB was negative. Only 1
out of 20 aspirate (5%) showed few large atypical mononuclear variants
and occasional classical RS cells, suggestive of marrow involvement. 17
aspirates (85%) were reported as negative for marrow infiltration.
| Figure 2. Trephine biopsy shows nodular, paratrabecular infiltration by atypical lymphoid cells in a patient of small lymphocytic lymphoma (H&Ex100). |
| Figure 3. High magnification of trephine biopsy shows classical binucleate Reed Sternberg cells (Inset with arrow) and mononuclear variant Reed Sternberg cells (Inset) in a polymorphous background comprising of plasma cells, eosinophils and lymphocytes (H&Ex400). |
Chronic myeloproliferative neoplasm (CMPN):
of 81 patients,
70 were of chronic myeloid leukemia (CML), 9 of primary myelofibrosis
(PMF) and 2 of hypereosinophilic syndrome (HES). 28 patients of CML in
chronic phase (CP) had minimal fibrosis on biopsy. 30 CML- CP biopsies
showed increased number of micromegakaryocytes and grade 2 reticulin
fibrosis. 7 of these (23.3%) yielded inadequate aspirates. Trephine
biopsy and aspirate were suggestive of blast crises in 10 patients. In
2 patients, peripheral smear and aspirate showed blasts less than 10%
suggestive of chronic phase, but trephine biopsy showed focal
aggregates of blasts in an entire intertrabecular space, warranting a
diagnosis of blast crisis. 9 patients of PMF had cellular marrow with
grade 3 reticulin fibrosis and collagenisation (Figure 4).
Clusters of atypical megakaryocytes having hyperchromatic bulbous
nuclei, were seen adjacent to vascular sinuses. BMA showed dry tap in 7
of these cases after repeated attempts and cellular marrow particles
having atypical bizarre megakaryocytes in the remaining 2 cases.
Aspirate and biopsy were in agreement in two HES patients.
Acute leukemia: BMA was in agreement with biopsy in
39 patients,
but was inadequate in 5 patients who had tightly packed marrow with
blasts on biopsy. Nine patients were in complete hematological
remission both on aspiration and biopsy.
Metastases: definitive evidence of marrow metastases
was seen in
10/23 patients. Small round cell tumors - Ewing’s/ PNET, neuroblastoma,
Wilm’s tumor and retinoblastoma were the primary in children (Figure
5).
Six adult patients (mean age=59yrs) presented with backache, anemia and
had multiple lytic lesions in vertebral column with differential of
metastases and multiple myeloma. Bone marrow examination revealed
metastatic adenocarcinoma from prostate, breast, gastrointestinal tract
and lung. Of ten marrow metastases, aspirate detected only six. Three
aspirates were negative and one was inadequate. In rest 13 suspected
patients, both aspirate and biopsy were negative for metastases.
| Figure 4. a) Trephine biopsy of Primary myelofibrosis showing cellular marrow with preponderance of myeloid precursors, atypical megakaryocytes and fibrosis (H&Ex200), b) Gomori’s reticulin shows grade 3 fibrosis (Reticulin x200) c) Fibrosis is confirmed by bluestained collagen (Masson trichrome x200). |
| Figure 5. a) Trephine biopsy shows metastatic neuroblastoma in a child (H&Ex 400) b) Bone marrow aspirate of the same patient shows metastatic small round cell tumor (Wright stain x400). |
Multiple myeloma: biopsy and aspirate were concordant in 23/26 (88.5%) patients of multiple myeloma. Three aspirates were hypocellular due to fibrosis with focal aggregation of myeloma cells on biopsy sections. On immunohistochemistry, these cells showed evidence of monoclonality by κ.
| Figure 6. a) Bone marrow aspirate shows diluted marrow with scattered plasma cells in a patient of multiple myeloma, overall percentage 10% (Wright stain x 400) b) Biopsy from same patient shows paratrabecular collection of plasma cells and plasmablasts (H&Ex400) c) On immunohistochemistry, these cells show λ light chain restriction, confirming the monoclonality (Immunostain λ x400). |
| Figure 7. a) Bone marrow aspirate from a pancytopenic patient showing hypocellular marrow particles with entangled lymphocytes and plasma cells and occasional erythroid precursors (Wright stain x 400) b) Biopsy shows markedly hypocellular marrow with increased fat spaces, confirming the diagnosis of aplastic anemia (H&E x 100). |
Marrow metastases from neuroblastoma and small cell carcinoma
along
with secondary myelofibrosis on biopsy presented as pancytopenia, for
which marrow was done.
Comparison of bone marrow aspirate with trephine biopsy:
concordance rates were calculated between BMA and trephine biopsy (Table
2). In larger subgroups we also calculated validity
parameters taking trephine biopsy as gold standard (Table 3).
Correlation of trephine length with lymphoma positivity:
we had
184 adequate biopsies for lymphoma staging in our study. Of these 49.4%
(91) were positive for lymphoma infiltration after examining three
sections at deeper levels. The mean length of trephine core was 14 mm,
ranging from 1-32 mm. We found that lymphoma positivity showed a rising
trend with length of trephine core, with maximum positivity (68.9%)
seen in 17-20 mm group, but no further improvement beyond 20 mm (Table
4, Figure 8).
Based on this two groups were made, taking 16 mm as cut-off. Fischer’s
exact test was applied and the difference in both the groups was found
to be statistically significant (lymphoma positivity ≤16mm=40.3% and
≥17mm=66.1%, p=0.0011).
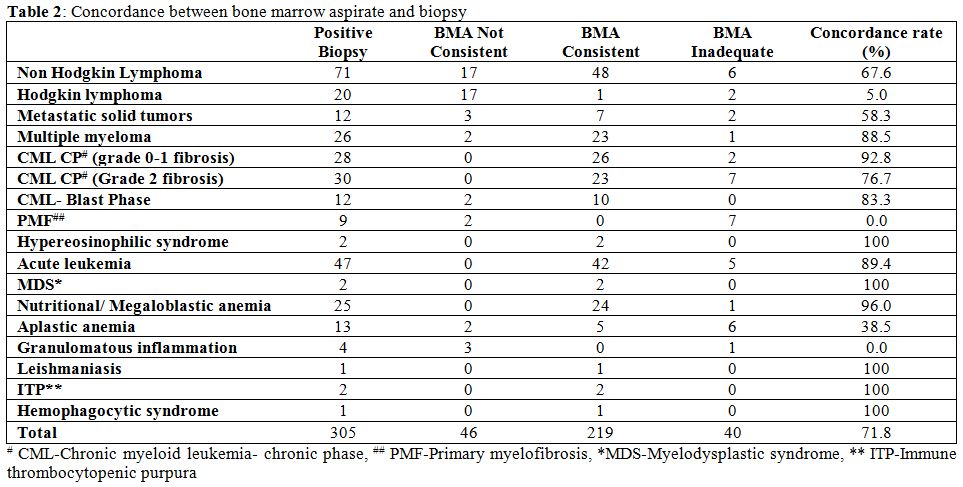 |
Table 2. Concordance between bone marrow aspirate and biopsy. |
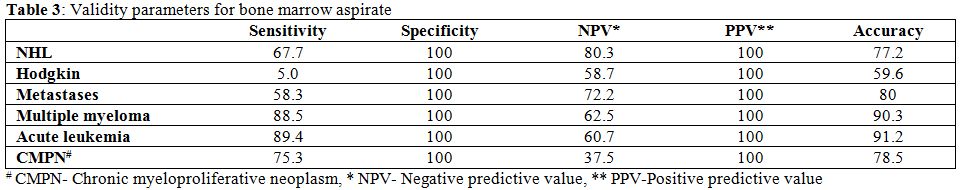 |
Table 3. Validity parameters for bone marrow aspirate. |
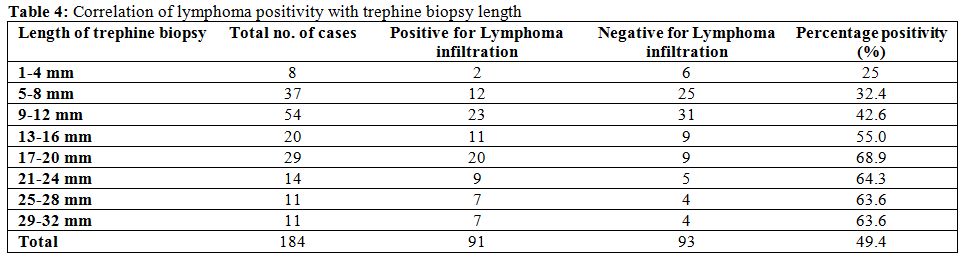 |
Table 4. Correlation of lymphoma positivity with trephine biopsy length. |
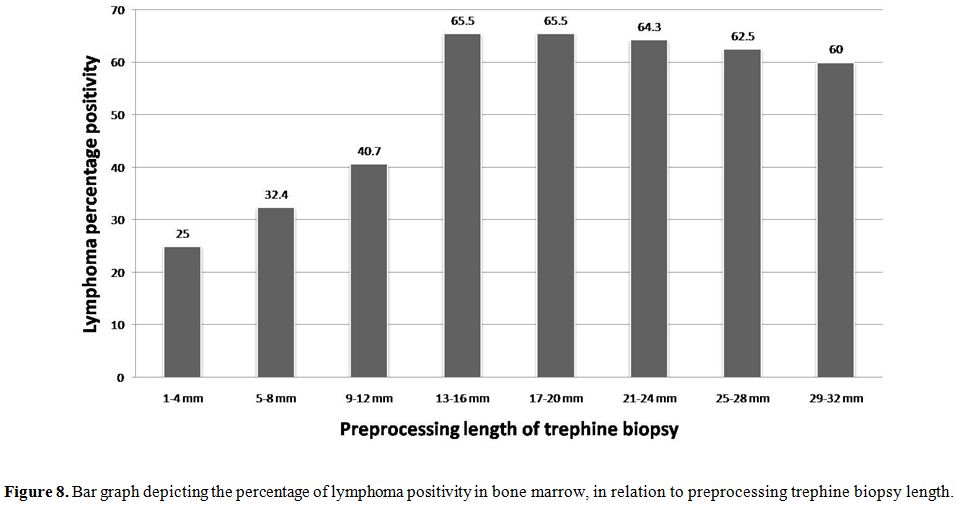 |
Figure 8. Bar graph depicting the percentage of lymphoma positivity in bone marrow, in relation to preprocessing trephine biopsy length. |
Discussion
We have evaluated the role of bone marrow aspirate in
comparison
with trephine biopsy in diagnosis of various hematological disorders.
In 28.2% cases aspirate was nondiagnostic, with an overall sensitivity
of 71.8%. Jamshidi and Swain reported that in 14-16% patients, aspirate
was non diagnostic. [14]
Immunohistochemistry was
diagnostically helpful in our study in case of NHL, Hodgkin lymphoma
and multiple myeloma, where equivocal morphology and low tumor cell
burden posed a dilemma. In biopsies with few suspicious cells or crush
artifact, it can increase the diagnostic accuracy by unmasking the
obscured patterns and morphology. We found bone marrow aspirate to be
100% specific in most of the disorders, but sensitivity and accuracy
depends upon the disease being evaluated. In hematological
malignancies, highest sensitivity was seen in acute leukemias (90%),
multiple myeloma (88.5%) followed by CMPNs (77.2%). In diffuse marrow
pathologies like nutritional anemia, leishmaniasis, ITP and HPS,
diagnostic sensitivity of marrow aspirate was 100%. Trephine biopsy did
not provide any additional information. Therefore, aspirate may obviate
the need of biopsy in such situations.
Frequency of positive BMA in metastatic marrow varies from 23% to 100%
in different studies. [15-18]
In our study, 41.7% of aspirates missed marrow metastases, similar to
results of previous studies. Focal deposit of nonhematopoietic
malignant cells and tumor associated desmoplasia, necrosis are the
cause of dry tap on aspiration. According to Chandra et al, aspirate
along with imprint smear has similar diagnostic accuracy to trephine
biopsy and can avoid the inevitable delay caused by decalcification and
routine histopathological processing of the biopsy. [7]
Overall incidence of marrow involvement by Hodgkin and Non Hodgkin
lymphoma was quite high (42.5% and 51.8% respectively) in our study.
Various studies have reported marrow infiltration in lymphoma ranging
from 27.1 to 55.1%. [19] This
variation can be
attributed to higher incidence of Hodgkin lymphoma in our population
and inclusion of different proportion of patients of early/advanced
stage. Only two third of NHL positive marrows were picked up on
aspirate and 23.9% were missed on aspiration. BM biopsy renders
information which cannot be determined from aspiration, such as spatial
distribution and extent of infiltrates, overall cellularity and
fibrosis. This also implies that trephine biopsy may be more useful in
postchemotherapy patients to assess the residual tumor cell burden and
degree of chemotherapy response. Newer techniques like flow cytometry
can increase the sensitivity BMA in NHL patients, but could not be
evaluated in the present study. Availability of broad panel of
antibodies suitable for paraffin-embedded tissues, enables us to
perform complete immunophenotyping on trephines and allows
classification of lymphoma infiltrates according to established
algorithms. [4] Our finding that
only 5% aspirates
were positive, confirm the fact that BMA does not have much role in
detecting marrow involvement by Hodgkin disease. Our findings are in
agreement with those of Moid and Sharma et al. [17,20]
Although role of biopsy is controversial especially in stage I and IIA
Hodgkin lymphoma, it is still irreplaceable in staging (especially in
stage IIB or III cases) and hence alters the treatment.[21]
We recommend that instead of BMA, trephine biopsy should be done for
staging in Hodgkin lymphoma. Necrosis is usually seen post
chemotherapy, but we found very high incidence of necrosis and fibrosis
(60%) at the time of primary diagnosis. Foci of fibrosis in the absence
of classical or variant RS cells, with Hodgkin lymphoma diagnosed
elsewhere, are highly suspicious of marrow involvement. [22]
BMA was 88.5% sensitive in diagnosis of multiple myeloma. Trephine
biopsy helped to identify focal compact masses of plasma cells without
any stroma in 7.7% patients which were missed on aspirate. Biopsy is
more sensitive method for quantifying plasma cell burden (using CD138
IHC), especially in patients with low percentage of plasma cells on
aspirate. [23] However,
cytomorphological
classification of myeloma is better done on aspirate or imprint
(mature, intermediary, immature and plasmablastic types). [24]
In acute leukemia, aspirate had a high accuracy of 91.2%. 10.6%
aspirates were inadequate in which trephine biopsy showed near total
replacement of marrow by blasts or myeloid precursors and extensive
fibrosis. In MDS, aspirate was 100% sensitive but trephine biopsy
provided additional information such as detection abnormal localization
of immature precursors (ALIP) and aggregates of myeloblasts. Presence
of fibrosis or fatty changes in marrow can make accurate disease
characterization very difficult or impossible on aspirates. [25] Literature suggests the utility of
imprint cytology in providing excellent cytomorphological details in
cases of dry tap, [7] but we did
not evaluate its role in the present study. Peripheral smear and BMA
may show overlapping findings in CMPNs. Role
of trephine biopsy is not only in differentiation of CMPNs, but also to
assess the overall marrow cellularity, histotopography and morphology
of megakaryocytes and blasts (CD34 positive precursors) and degree of
myelofibrosis. [26] Non diagnostic
aspirates in CML
patients, who had grade 2 marrow fibrosis highlights the importance of
trephine biopsy in CML. Also, focal collection of blasts occupying
significant intertrabecular space in biopsy clinched the diagnosis of
blast crises, irrespective of blast count in peripheral smear and BMA
as was seen in our case. [26] BMA
does not have much role in diagnosis of PMF because diffuse
osteomyelosclerosis, intrasinusoidal hematopoiesis and vascular
proliferation, which are characteristic of fibrotic PMF, can be
confirmed and graded on biopsy sections only. [27]
Megaloblastic anemia was the most common (37%) cause of pancytopenia in
our study. High incidence of megaloblastic anemia can be explained by
prevalent malnutrition and infectious diseases, seen in tropical
country as ours. Aplastic anemia was the etiology in 19%, but aspirate
was suggestive in only 38.5% cases. Trephine biopsy gives the
qualitative and quantitative assessment of cellularity, therefore, is
confirmatory in the diagnosis of aplastic anemia and overcomes the
limitation of dry tap. [28] In
addition, biopsy can
provide the number and distribution of megakaryocytes, lymphocytes,
plasma cells in marrow and blasts, all of which are prognostic markers
required in follow up of aplastic anemia. [28]
Rarely, Hodgkin and NHL can present as pan or bicytopenia without any
evidence of lymphadenopathy/ hepatosplenomegaly as was seen in 5.9%
cases. [22] Disseminated
tuberculosis was another
important cause of pancytopenia in our population (5.9%). Aspirate is
not useful in diagnosis of granulomatous myelitis as seen in our study,
confirming the findings of Toi et al. [1]
Granulomatous response can be seen in tuberculosis, Hodgkin lymphoma,
NHL, fungal infections and sarcoidosis. [29]
Though AFB stain was positive in 50% cases, presence of discrete
epithelioid cell caseating granulomas and clinical findings were
suggestive of disseminated tuberculosis in all of them (Figure
9). Very rarely, metastases can present with pancytopenia as
was seen in neuroblastoma and small cell carcinoma (2.9% cases).
| Figure 9. Trephine biopsy shows epithelioid cell granuloma with central necrosis (H&Ex100). |
Amount of assessable marrow included in the biopsy specimen is of more importance than the total length. Logically, the likelihood of lymphoma positivity should rise concomitant with the length of interpretable biopsy specimen and examination of serial deeper sections. However, in our study no diagnostic gain was achieved above a length of 20 mm, with maximum percentage positivity (68.9%) obtained in biopsies 17-20 mm long. Trephine biopsies ≥17mm had significantly higher lymphoma positivity as compared to those of ≤16mm. The National Cancer Institute has recommended a trephine length of ≥20mm for NHL staging. [11] Campbell et al has supported this recommendation and emphasized the role of examining multiple sections. [12] Bain suggested a minimum trephine length of 16 mm, based on the findings of Bishop set al. in which a plateau was achieved in the rate of detection of metastatic tumour after trephine length exceeded 16 mm. [30,31] We found preprocessing trephine biopsy 17-20 mm long, along with examination of multiple deeper sections optimal for detection of lymphoma infiltration. There are few limitations of our study. We did not evaluate the role of flow cytometry and touch imprint cytology in our study, both of which can increase the diagnostic accuracy. There were many subgroups in our study, some of which had small sample size. This is because we did not focus on any single disease, rather prospectively included all the patients presenting to us over a period of one year.
Conclusion
Bone marrow aspirate is a simple and rapid alternative to biopsy, and has high specificity and positive predictive value. Aspirate is especially useful for acute leukemia, multiple myeloma, nutritional anemia, immune thrombocytopenias and other diffuse marrow disorders, where sensitivity and NPV are also equally good. Aspirate has a very limited role as far as Hodgkin lymphoma, granulomatous myelitis, aplastic anemia and myelofibrosis are concerned, making biopsy mandatory. In NHL, metastases and CMPNs, aspirate alone is insufficient and biopsy is complementary. Biopsy provides additional information like marrow fibrosis, pattern of marrow involvement, topographical alterations of hematopoietic cells, and postchemotherapy changes, which are prognostically useful. Trephine biopsies 17-20 mm long, examined at multiple deeper levels, had maximum proportion of lymphoma positivity and are optimal for assessing lymphoma infiltration. Biopsies longer than 20 mm don’t offer any added advantage.
References






























[TOP]