Received: November 06, 2013
Accepted: February 02, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014011, DOI 10.4084/MJHID.2014.011
This article is available on PDF format at:

Salvatore Giacomo Morano1, Lorenzo Coppola2, Roberto Latagliata1, Paola Berneschi1, Antonio Chistolini1, Alessandra Micozzi1, Corrado Girmenia1, Massimo Breccia1, Gregorio Brunetti1, Fulvio Massaro1, Giovanni Rosa3, Pietro Guerrisi4, Franco Mandelli1, Roberto Foa'1 and Giuliana Alimena1
1 Dipartimento
di Ematologia, Oncologia, Anatomia Patologica e Medicina
Rigenerativa, Azienda Policlinico Umberto I, "Sapienza" Universita' di
Roma
2 Istituto di Ematologia “AOU” Sassari, Italy.
3 Dipartimento Anestesia e Rianimazione, Azienda
Policlinico Umberto I, "Sapienza" Universita' di Roma
4 Dipartimento Scienze Radiologiche, Azienda
Policlinico Umberto I, "Sapienza" Universita' di Roma

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Several severe complications may be associated with the use of central venous catheters (CVC). We retrospectively evaluated on a large cohort of patients the incidence of CVC-related early and late complications. From 7/99 to 12/2005, 1102 CVC have been implanted at our Institution in 881 patients with hematological malignancies (142,202 total day number of implanted CVC). Early mechanic complications were 79 (7.2% - 0.55/1,000 days/CVC). Thirty-nine episodes of early infective complications (<1 week from CVC implant) occurred (3.5% - 0.3/1000 days/CVC): furthermore, 187 episodes of CVC-related sepsis (17% - 1.3/1000 days/CVC) were recorded. There were 29 episodes (2.6%) of symptomatic CVC-related thrombotic complications, with a median interval from CVC implant of 60 days (range 7 – 395). The rate of CVC withdrawal due to CVC-related complications was 26%. The incidence of CVC-related complications in our series is in the range reported in the literature notwithstanding cytopenia often coexisting in hematological patients. |
Introduction
Central
venous catheters (CVC) are commonly employed in the management of
hematological malignancies. Their use is crucial in order to have a
safe and easy venous access, for blood sampling, drug infusions,
supportive iv treatments, blood product administration and parenteral
nutrition all along the course of the disease; in addition, CVC are
helpful in some therapeutic procedures, such as stem cell collection
and apheresis.
However, the use of CVC is often associated to several complications,
that can lead to a device malfunctioning and/or to an increased patient
morbidity with longer hospital admissions and more expensive medical
assistance.[1,2]
CVC-related complications can be divided into early complications
(mechanical and infective) and late complications (mechanical,
infective and thrombotic); early complications are generally secondary
to the insertion procedures, while late complications are more
frequently due to malpractice in the CVC management during the
follow-up.
Several studies have addressed the incidence of infective and
thrombotic CVC-related complications in patients with solid tumors; on
the contrary, at present only few data are available in hematological
malignancies, even if patients in this setting are quite different from
patients with solid tumors due to the occurrence of more severe and
prolonged cytopenia.[3,4]
The aim of this retrospective study was to evaluate the rate of early
and late CVC-related complications in a large cohort of patients with
acute and chronic hematological malignancies, followed at a single
Institution with a specific team dedicated to CVC insertion and
management.
Patients and Methods
Patient
Population.
All consecutive patients with acute and chronic hematological
malignancy followed at our Institution, who received a CVC insertion
between July 1999, and December 2005 were collected in the present
retrospective analysis.
Prior to CVC insertion, all patients were screened with a complete
blood count, coagulation tests and chest radiography to rule out
conditions contraindicating a CVC insertion. Patients with a platelet
count <50 x 109/l received platelet infusion before the
insertion.
CVC
Characteristics and Insertion.
A single type of tunneled CVC (Groshong-Bard, monolumen 7 Fr) was used.
This long-term, silicone, valved CVC was preferred to polyurethane,
non-valved CVCs in consideration of a presumed reduced thrombotic risk
as compared to polyurethane and non-valved devices. All insertion
procedures were performed in a surgical ward under strict aseptic
conditions and maximal barrier precautions: no sterile cap, no sterile
facemask, sterile gloves, sterile body gowns, careful skin antisepsis
and vast draping of the procedure area. Povidone-iodine (10% iodine)
was used as a skin antiseptic for all the procedures; two preliminary
washings were performed, and the solution was kept on the skin for two
minutes in each one. All insertion procedures were performed by the
same clinician with the assistance of a professional nurse, using a
percutaneous route according to the Seldinger-peel-away technique.
Local anesthesia or light sedation was used during the insertion, and
the distal CVC portion was positioned at the atrio-caval junction under
brilliance amplification guide.
The day after the insertion, 2nd
chest radiography was performed to confirm the correct position of the
distal CVC tip and to exclude early complications.
The CVC management and the weekly medications were performed by the
ward nurses when the patients were hospitalized and by the same
dedicated team which inserted the CVC in case of patients discharge.
For each patient, a chart was provided by a dedicated team in which all
data regarding CVC management and related complications were timely
recorded.
Definition
of CVC-related Complications.
The CVC-related complications were evaluated as early complications if
they occurred in the first week since the CVC insertion; all
complications occurring thereafter were considered as late
complications.
The following types of events were considered as mechanical
complications: impaired or unsuccessful venipuncture, pneumothorax,
arterial puncture, hematoma, dislocation, obstruction, pinch-off,
malfunction (defined as persistent pain on infusion and/or inability to
infuse or withdraw after an initial period of a correct CVC function),
accidental removal, and breakage/leakage.
A diagnosis of deep venous thrombosis (DVT) required the direct
visualization of the thrombus at the ultrasound or CT-scan examination
with lack of compressibility by probe pressure and/or absence of
spontaneous flow by Doppler and/or absence of phase flow with
respiration. A diagnosis of superficial thrombophlebitis was made when
at least one of the following signs was evident: local induration or
erythema, warmth, pain or tenderness along the CVC vein.
The criteria according to the guidelines of the Centers for Disease
Control were employed to define infective CVC-related complications.
These criteria required evidence of fever and/or local symptoms
(hyperemia, local pain) associated with positivity of microbiological
cultures (blood cultures, semi-quantitative cultures of the tip) when
the same organism was isolated from the CVC tip and the peripheral
blood sample, with no apparent source of infection other than CVC.
The unit of analysis was the central line within a patient, and CVC
related complication rate was defined as the number of adverse events
divided by number of CVCs and the number of central line-days during
the period from CVC insertion to the onset of the adverse event,
multiplied by 1000.
Results
Patient
Population.
Between July 1999 and December 2005, 1,102 CVC have been inserted in
881 patients (503 males and 378 females, median age 44.4 years, range 1
– 87 years) with hematological malignancies followed at our
Hematological Department and treated with chemotherapy and/or stem cell
transplantation. The distribution of the different hematological
diseases is shown in Table
1. The CVC was inserted more than once during the course
of the disease in 176 patients (range 2-5 insertions).
The CVC insertion was performed in the right subclavian vein, in 883
cases (80.1%), and in the left subclavian vein, in the remaining 219
cases (19.9%).
Mean duration of catheterization was 131.76 days (145,202 CVC-days),
range 5-1.651. The CVC was removed due to the completion of the
treatment plan in 706 (64%) of cases and due to death in 110 (10%) of
cases; CVC-related complications led to the CVC withdrawal in the
remaining 286 (26%) of cases.
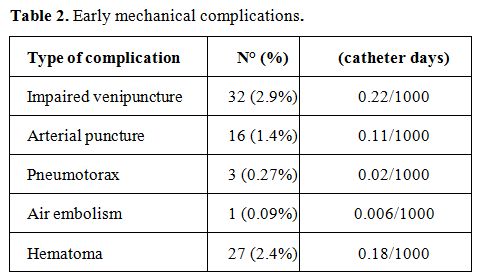
Early CVC-related Complications. There were 79 episodes (7.2% -
0.54/1000 catheter days) of early mechanical complications; the
different types of mechanical complications and their relative
frequencies are reported in Table
2.
Among the 32 episodes of impaired venipuncture, an echo-Doppler
examination revealed stenosis or thrombotic obstruction of the vessel
in 14 cases.
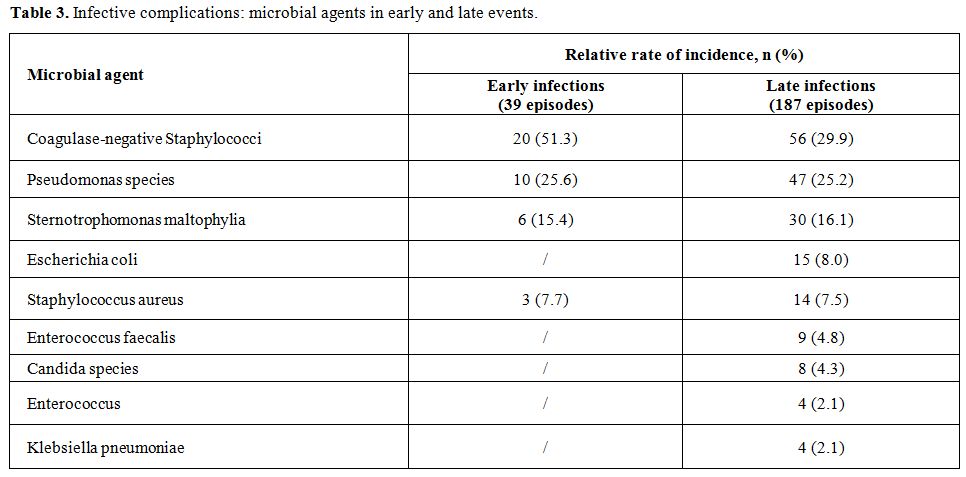
There were 39 episodes (3.5% - 0.3/1000 catheter days) of early
infective complications. A different distribution of such complications
according to the year of CVC insertion was reported, ranging from 9
episodes in 1999 to only 1 episode in 2004; moreover, no episode was
reported in the first 9 months of 2005.
In these cases, the underlying hematological malignancy was AML in 31
patients, ALL in 3 patients, NHL in 3 patients and MM in 2 patients. In
32/39 episodes (82.0%), the absolute number of polymorphonuclear cells
(PMN) was lower than 0.5 x 109/l.
The incidence of different infective agents is reported in the Table 3.
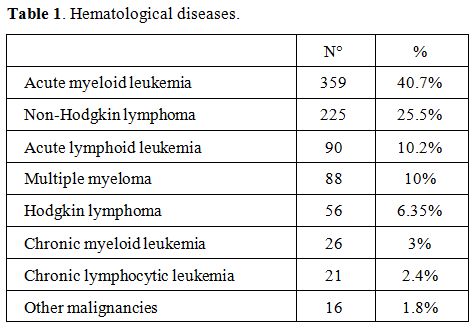 |
Table 1. Hematological diseases. |
 | Table 2. Early mechanical complications. |
 | Table 3. Infective complications: microbial agents in early and late events. |
Discussion
Management of onco-hematological patients has profoundly changed following the introduction in the clinical practice of CVC, which has enabled an easier planning of chemotherapy, transplant procedures, blood sampling and intravenous supportive care. However, their routine use is also linked to a number of CVC-related complications. Among the complications commonly reported in literature on Groshong CVCs, infections are the most frequent (0.1-11.5 per 1000 CVC days),[5,6] although mechanical problems, including thromboembolic accidents, may occur at a non-negligible rate (1.2-13%).[7,8] However, these data primarily refer to adult patient populations with solid tumours.Conclusions
Our data emphasize the safety and efficacy of Groshong CVC all along the treatment course in patients with hematological diseases. Furthermore, the use of a dedicated team with a progressively increasing expertise was capable of reducing the rate of early and late CVC-related complications, also in this subset of patients commonly affected by cytopenia; this seems to translate into a long period of CVC employment that in our experience covered all treatment programs in more than 75% of patients.References















[TOP]