Received: June 3, 2013
Accepted: January 7, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014017, DOI 10.4084/MJHID.2014.017
This article is available on PDF format at:

Ruben Fernandez-Alvarez1, ME. Gonzalez1, Almudena Fernandez1, AP. Gonzalez-Rodriguez3, JM Sancho4, Francisco Dominguez2 and Carmen Fernandez1
1Department
of Hematology, Hospital de Cabueñes,
Gijon, Spain.
2Department of Pathology, Hospital de Cabueñes, Gijon, Spain.
3Stem Cell Transplant Unit, Hospital
Universitario Central de Asturias, Oviedo, Spain.
4Clinical Hematology Department, ICO-Hospital
Germans Trias i Pujol, Barcelona, Spain.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Lymphomatoid
granulomatosis (LYG) is a very rare Epstein-Barr virus (EBV) associated
B-cell lymphoproliferative disorder. We report the case of a
41-year-old man who presented with fever and respiratory symptoms.
Computed tLymphomatoid
granulomatosis (LYG) is a very rare Epstein-Barr virus (EBV) associated
B-cell lymphoproliferative disorder. We report the case of a
41-year-old man who presented with fever and respiratory symptoms.
Computed tomography showed multiple nodules in both lung fields.
Polymerase chain reaction (PCR) analysis for EBV was positive in
bronchoalveolar lavage and biopsy of lung node yielded a diagnosis of
LYG, grade III. Shortly after initiation of treatment with agressive
chemotherapy, neurological deterioration appeared. Neuroimaging
findings revealed hydrocephalus and PCR analysis of the cerebrospinal
fluid (CSF) was positive for EBV. Treatment with intravenous rituximab
led to rapid reduction of EBV load in CSF, along with clinical and
radiological improvement. After completion of treatment with
immunochemotherapy, an autologous stem cell transplantation was
performed. Patient stays in remission 18 months after diagnosis.
|
Introduction
Lymphomatoid
granulomatosis (LYG) is a rare B-cell lymphoproliferative disorder
characterized by an angiocentric and angiodestructive lymphoid
proliferation. Cytological composition consists of large atypical B
cells (which are Epstein-Barr [EBV]-positive) accompanied by reactive T
cells.[1] Progression to a diffuse
large B-cell lymphoma (DLBCL) occurs in up to 15% of cases,[2] and distinction between high grade
LYG and lymphoma can be subtle.
Clinical presentation is heterogeneous. Pulmonary involvement is almost
always present, while other common sites include central nervous system
(CNS) and skin.[2]
Currently, LYG is considered an EBV related B-lymphoproliferative
disorder,[3]
similar to other lymphoproliferative disorders such as
post-transplantation lymphoproliferative disease. Although LYG is most
commonly diagnosed in patients without over immunodeficiency, it is
probable that most cases display an immunologic defect that is unable
to adequately control EBV-infected B-cells.[4]
Disease course is unpredictable and there is no clearly established
treatment. After the recognition of EBV-positive B cells involvement,
rituximab has been considered as a promising treatment.[5]
We report here a case of an agressive LYG involving skin, lungs and
CNS, with documented active EBV replication, and effective treatment
with chemotherapy plus rituximab.
Case Report
A 41-year-old male presented with fever, weight loss and cough.
Physical examination revealed multiple erythematous plaque lesions on
his back. Laboratory results revealed elevated serum lactate
dehydrogenase (LDH) level, mild anemia and elevated C reactive protein
level. Computed tomography (CT) scan showed ground glass infiltrates at
lower lobes. Antibiotics were started, without improvement. Serologic
tests for Mycoplasma, hepatitis B, hepatitis C and Human
Immunodeficiency virus (HIV) were negative, whereas Cytomegalovirus
(CMV) and EBV were positive, indicating previous infections. Additional
workup showed negative antinuclear antibodies and antineutrophil
cytoplasmic antibodies (ANCA). Two skin biopsies were performed,
showing a dense T-cell lymphoid infiltrate, EBV-ISH negative. Steroid
treatment was started (1 mg/kg/day), and fever and skin lesions
disappeared rapidly. However, patient developed confusion, gait
imbalance and diplopia.
Cerebrospinal fluid (CSF) analysis revealed: 10 white blood cells (WBC,
all mononuclear), protein 1.11 g/L, and glucose 71 mg/dl. CSF flow
cytometry detected a polyclonal T-cell population. Cranial magnetic
resonance imaging (MRI) showed diffuse hyperintense lesions involving
white matter around ventricles, without contrast enhancement (Figure 1[a]).
Two weeks later, respiratory symptoms worsened and a second CT revealed
multiple pulmonary nodules in middle and lower fields, some of them
with cavitation (Figure 2).
Microbiological tests of bronchoalveolar lavage (BAL) were negative,
except amplification of EBV-DNA by polymerase chain reaction (PCR),
that yielded 4080 copies/mL. Biopsy of lung node showed a highly
polymorphic infiltrate of large and atypical lymphoid cells, displaying
an angiocentric and angiodestructive distribution (Figure 3[a]).
Immunostains showed B-cell proliferation (PAX-5+, MUM-1+ -Figure 3[b]-) with
very intense EBER expression (Figure
3[c]), together with T-cell proliferation (Figure 3[d]).
These findings led to suspicion of T-cell lymphoma, but after later
review, final diagnosis was LYG, grade III. Complete staging revealed
mild hepatosplenomegaly, while bone marrow was normal.
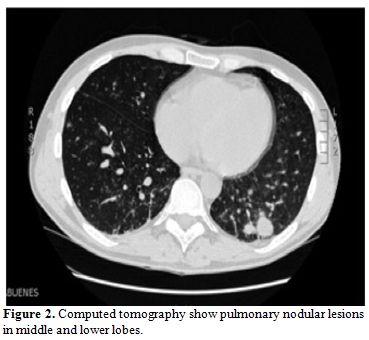 | Figure 2. Computed tomography show pulmonary nodular lesions in middle and lower lobes. |
Because
of suspicion of T-cell lymphoma with probable CNS involvement,
treatment was started with a high-dose methotrexate (MTX) based
protocol (ifosfamide, 1500 mg/m2
daily for 5 days plus mesna; etoposide, 150 mg/m2
daily for 3 days; cytarabine, 100 mg/m2
daily for 3 days; and MTX, 3 g/m2
on Day 5, with leucovorin rescue) plus intrathecal application of MTX,
Ara-C, and hydrocortisone. Shortly after, the patient developed
somnolence, incoherent speech, and nystagmus. Cranial CT scan revealed
important hydrocephalus with worsening of previous findings (Figure 4).
An Ommaya reservoir was placed and examination of CSF showed a WBC
count of 8 cells/mL, with normal glucose and protein levels. The Gram
staining and culture were negative. PCR analysis for EBV was positive,
yielding 43700 copies/mL. HHV-6, CMV, HSV, VZV and JCV were negative by
PCR assay.
At this stage, treatment with rituximab (375 mg/m2/iv)
was added, once weekly for 4 doses. EBV-DNA copies in CSF rapidly
declined in the following weeks (Figure
5).
A cranial MRI performed after completion of rituximab demonstrated
significant improvement, and pulmonary CT scan showed decreasing size
of lung nodules.
Once patient improved, he received one further cycle of the same
high-dose MTX-protocol, together with rituximab and intrathecal
chemotherapy. Autologous stem cell transplantation was scheduled. The
preparative regimen consisted of total body irradiation (10 Gy) and
cyclophosphamide (100 mg/Kg) without major complications. On follow-up,
both cerebral MRI and pulmonary CT scan have not shown progression. 18
months after the diagnosis, patient remains in remission.
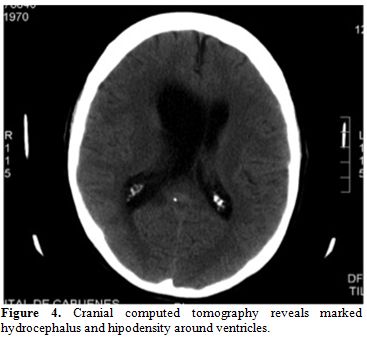 | Figure 4. Cranial computed tomography reveals marked hydrocephalus and hipodensity around ventricles. |
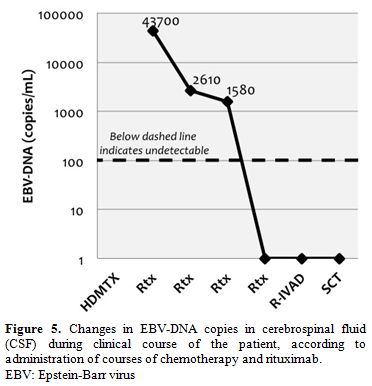 | Figure 5. Changes in EBV-DNA copies in cerebrospinal fluid (CSF) during clinical course of the patient, according to administration of courses of chemotherapy and rituximab. EBV: Epstein-Barr virus |
Discussion
LYG was first described by Liebow et al.[6]
in 1972,
and originally was considered a form of T-cell lymphoma, based on the
predominant T-cell infiltrate. It was later demonstrated that LYG is an
EBV-associated B-cell lymphoproliferative disorder, accompanied by a
large number of reactive T-cells.[7]
The distinctive
morphologic feature of LYG is an angiocentric and angiodestructive
lymphoid infiltrate, with infiltration of the vascular wall and
variable degree of necrosis. Currently, LYG is considered a
lymphoproliferative disorder with a broad pathological and clinical
spectrum. Lipford et al.[8]
classified LYG lesions
into 3 histologic grades on the basis of the degree of cytologic atypia
and necrosis. The number of EBV-positive cells correlates with
histologic grade.1 In grade 1 lesions, EBV-positive cells are
infrequent whereas they are readily detected in grade 2 and 3 lesions.
It is important to note that the number of EBV-cells may vary over time
or between sites, as reflected in our case (skin, grade 1; lungs, grade
3).
Clinically, LYG demonstrates predilection for men, most often in adults
between the 4th and 6th decades of life. The lungs are virtually always
affected in LYG.2 Radiologically, pulmonary lesions are heterogeneous,
from diffuse infiltrates to nodules.[9]
According to its angiodestructive nature, occasional
cavitation/necrosis is seen, as in our case.
Skin is affected in approximately 45% of patients. Cutaneous lesions
may appear before the pulmonary disorder,[10]
as in our case. Dermatologic manifestations are diverse, but most
typically appear as nodules. The clinical pattern of the cutaneous
lesions correlates with histological grade. Beaty et al.[10]
showed that the plaque lesions were negative for EBV and demonstrated
IgH polyclonal patterns. In contrast, angiodestruction, cellular atypia
and EBV positivity are more common in nodules. Our case presented
erythematous plaques, without EBV, which is consistent with grade 1 LYG.
Central nervous system (CNS) involvement occurs in 25-35% of cases,
usually accompanying pulmonary lesions.[2]
Neuroimaging findings are variable. Mizuno et al.[11]
reviewed 45 LYG cases involving CNS, and classified them as tumorous
lesions and non-tumorous lesions (multiple focal lesions or diffuse
involvement, as in our case). Most common finding consists of multiple
focal lesions involving the white matter, deep grey matter, or the
brainstem,[12] showing high signal
intensity (on
T2-weighted images) and contrast enhancement; however it's possible
that some lesions only manifest T2 prolongation. Our case presented
diffuse involvement of white matter, with typical T2 hyperintensity,
but did not show contrast enhancement. Histologic documentation of
brain lesions is not usually available and CSF analysis presents low
sensitivity for the diagnosis of LYG in the brain,[12]
as in our case. High EBV-DNA levels were found in our case, which is
consistent with the biology of the process. No previous reports exist
on the detection of EBV-DNA in CSF of patients with cerebral LYG, so
significance of this finding is unknown. We think measurement could be
clinically useful for diagnosis support and for monitoring treatment
response. In our case, virologic response in CSF correlated with
radiological and clinical response.
LYG is considered an EBV driven process. Its implication was first
described in 1990 when EBV DNA was found in tissue samples of 21 out of
29 patients with LYG.[3] Evidence
of subtle immune deficits has been found in otherwise immunocompetent
individuals with the disease.[4]
Disease hypothesis suggests LYG arise in the setting of a dysregulation
of EBV surveillance (deficit of EBV-specific CD8 T-cells). Progressive
oncogenic events transform lower grade to higher grade disease: grades
1 and 2 are polyclonal or oligoclonal and immune dependent; grade 3
disease is monoclonal and immune independent.[13]
Therapy of LYG includes corticosteroids, chemotherapy or observation,
generally with poor outcomes,[14]
as these therapies can potentially worsen the intrinsic immunologic
defect. In our case, neurological condition deteriorated after steroids
and chemotherapy. We hypothesized that decline in cellular immunity
caused an increase of EBV infected cells, as we found a strikingly high
viral load of EBV in CSF.
A risk-adapted treatment strategy has been developed by the US National
Cancer Institute (NCI),[13]
with the objective of improving immunologic defect and eradicating
EBV-positive B cells. They use interpheron alpha for low-grade LYG, and
immunochemotherapy (rituximab plus CHOEP regimen) for high-grade LYG.
Rituximab is considered a promising treatment for LYG, after the
recognition of EBV-positive B-cells involvement. Recently, Hernandez et
al.[5] reviewed the use of
rituximab in LYG. To date,
a total of 22 cases have been reported: 11 patients had improvement (6
in monotherapy, 5 in combination with other drugs), whereas 7 patients
progressed. The role of rituximab for CNS lesions is also unclear: of
22 patients reported, only 7 had CNS involvement.[5,15-19] Responses were successful in 3
cases, all of them received rituximab as monotherapy.[15-17]
In conclusion, our case presented asynchronous manifestations of LYG at
skin, lungs and CNS, with detection of EBV-DNA at sites of involvement.
Rituximab was effective in the treatment, and we demonstrate that
clearance of EBV viral load in CSF correlated with clinical and
radiological improvement.
References

















[TOP]