Received: August 30, 2013
Accepted: January 29, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014020, DOI 10.4084/MJHID.2014.020
This article is available on PDF format at:

Andrea Tendas, Luca Cupelli, Agostina Siniscalchi, Laura Scaramucci, Marco Giovannini, Teresa Dentamaro, Alessio Perrotti, Tommaso Caravita, Paolo de Fabritiis and Pasquale Niscola
Hematology
Division, Sant'Eugenio Hospital, Rome, Italy.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Chronic
myelomonocytic leukemia (CMML) is an uncommon neoplastic hematological
disorder, typically affecting the elderly and characterized by a marked
clinical heterogeneity and a remarkable propensity for transformation
into acute myeloid leukemia. Hypomethylating agents represent the most
innovative management approach in this difficult setting. At our
institution, between 2010 and 2012, we have treated with azacitidine 10
CMML patients with a median age of 75 (62–86) years. The overall
response rate of 70% was achieved without remarkable toxicities; in
particular, most therapy-induced side effects were managed on
outpatient basis. With a median follow-up of 12,5 (2–27) months, 6
patients are alive, and 4 of them continue to receive the treatment;
the median survival from the start of therapy was not reached. In
conclusion, also in the light of our encouraging experience,
azacitidine can offer new chances of treatment also in the difficult
setting of elderly CMML.
|
Introduction
Chronic
myelomonocytic leukemia (CMML) is an uncommon neoplastic hematological
disorder, typically affecting the elderly and characterized by marked
clinical heterogeneity and remarkable propensity for transformation
into acute myeloid leukemia (AML).[1]
Although CMML portrays a worse outcome when compared to myelodysplastic
syndromes (MDS),[2] it shares
several features similar to MDS.[1,2]
In particular, affected patients usually are elderly and may present
with frailty, comorbidities,[3]
and several forms of disability,[4]
being all factors hampering in most cases the access to aggressive
treatment approaches, such as allogeneic hemopoietic stem cells
transplantation (HSCT), which still remains the only potentially
curative measure in this setting,[1,4]
However, the potential disease-modifying activity of hypomethylating
agents, such as azacitidine and decitabine, has been recently reported
and nowadays these agents represent the most innovative management
approach in suitable CMML patients.[1]
Patients and Methods
At our institution, between 2010 and 2012, we have treated with
azacitidine 10 CMML patients (6 males) with a median age of 75 (62–86)
years. According to WHO 2008 criteria,[5]
6, 2 and 2
patients had CMML-2, CMML-1 and AML progressed from CMML respectively.
Four patients had proliferative CMML (MPR-CMML) whereas the remaining
six presented myelodysplastic CMML (MD-CMML); 2 out of 10 patients had
an abnormal karyotype (46, XY, Inv12 and 45, X,-Y, respectively).
Details are reported on Table
1.
Two patients had secondary likely therapy-related CMML; the first has
undergone radio-chemotherapy for a solid tumor 3 years before[6] whereas the second was an unusual
case of aggressive CMML transformed from a 7- years lasting MDS
(refractory anemia)[7].
The median time from the diagnosis to the start of azacitidine was 3
(1-21) months. Prior therapies included cytoreductive therapy and
erythroid stimulating agents; 4 patients were transfusion dependent at
some time point of disease course before starting azacitidine. At the
time of treatment, the M.D. Anderson Prognostic Scoring System (MDAPS)[8]
was low, intermediate-1, intermediate-2 and high in 1, 2, 4 and 1 CMML
patients, respectively. Criteria for initiation of azacitidine were
represented by aggressive disease features, such as splenomegaly,
transfusion dependence, cytopenia- related syndromes (bleeding and
recurrent infections), an increasing number of peripheral and/or bone
marrow blasts, and by patient’s symptomatology, such as general
malaise, weight loss. In particular, 2 patients with MPR-CMML-2 were
treated because of high bone marrow blasts count (16-19%), 1
patient with MPR-CMML-1 presented severely symptomatic thrombocytopenia
and 1 patient with MD-CMML-1 presented severe neutropenia. After
written consent had been obtained, all patients received azacitidine
(75 mg/m2
x 7 days, 5+2+2 schedule,
every four weeks, subcutaneously). In MPR-CMML patients undergoing
cytoreductive treatment, hydroxyurea was discontinued at the moment of
azacitidine start; no cytoreduction was added during treatment and at
response assessment. Supportive care was given as required. Bone marrow
response was assessed in 9 patients (following the sixth cycle in 6
patients and the fourth in 3); response was not assessed in 1 patient,
due to early sudden death (multi-organ failure), which occurred after
the second cycle. Responses were classified according to the modified
IWG response criteria in myelodysplasia[9]
for MD-CMML and IWG consensus criteria for treatment response in
myelofibrosis[10] for MPR-CMML.
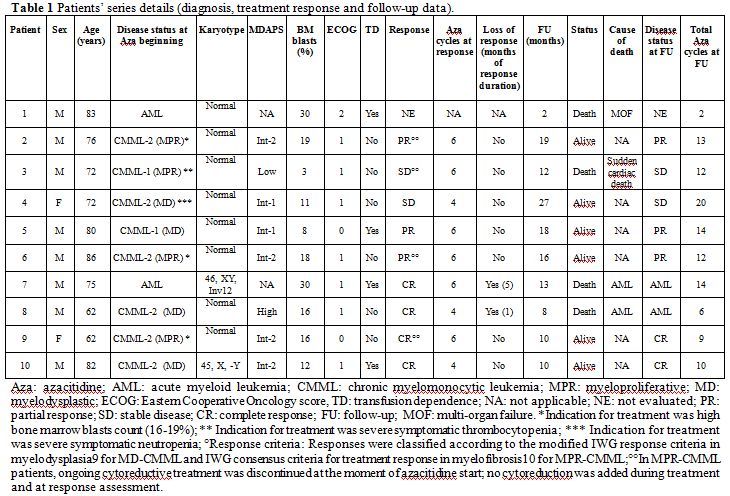 | Table 1. Patients’ series details (diagnosis, treatment response and follow-up data). |
Results
Out of 9 evaluable patients, 4 (44.4%) patients achieved complete remission (CR) and 3 (33.3%) partial remissions (PR) with an overall response rate (ORR) of 77.7%; 2 (22, 3%) patients maintained a stable disease. At the time of writing (August 2013), the median follow-up was of 12.5 (2 – 27) months; 6 (66,6%) out of 9 evaluable patients were alive, and 4 of them were on azacitidine. Median overall survival (OS) was not reached. Responding patients continued the treatment up to disease progression or intolerance. Two (22.3%) out of 9 CMML patients progressed to AML following the sixth and the fourteenth cycle respectively, after having obtained a CR. Overall, 4 (40%) out of 10 patients initially treated with azacitidine have deceased: 2, 1 and 1 because of AML progression, multi-organ failure (before response assessment) and sudden cardiac death (being the patient with stable CMML). Treatment outcome was similar in MPR-CMML, when compared with MD-CMML; intermediate-2 / high MDAPS CMML and AML patients exhibited a better response rate (CR in 4 and PR in 2 patients, respectively), when compared with low / intermediate-1 MDAPS CMML patients (PR in 1 and stable disease in 2 patients, respectively); treatment outcome details are reported on Table 1. Treatment was well-tolerated, and no remarkable side effects directly attributable to the agent were recorded; in particular, no hospitalization was needed for treatment complications and all of them were managed in the ambulatory setting.
Conclusion
Our experience was encouraging, mainly due to the
following reasons.
Firstly, the use of azacitidine in our hands achieved good responses in
70% of the treated patients, similar to response rate reported in the
literature;[11-13] furthermore
this result was obtained in high risk patients with
unfavorable prognostic profile.
Secondly, this agent was particularly safe and manageable in our
experience, and then can be offered also to very old patients.[13]
In conclusion, CMML is a rare disorder and thus there is a paucity of
randomized trials utilizing different agents. Until now, most of these
patients have received only palliative cytoreduction, being very few of
them eligible for more aggressive treatments, such as HSCT. In this
view, we have reported a real life experience regarding a little series
of CMML patients treated outside a clinical trial. Our experience adds
further data to the scanty and scarce evidences supporting the
effective activity and the significant benefits provided by azacitidine
in this setting. Although our not controlled experience was limited to
a small group of patients, our results are encouraging and confirmed
previously published papers which reported the possibility of CR with
an ORR ranging from 39% to 60% and a median OS from 12 to 37 months.[12-13] Thus, this agent can offer new
chances of treatment also in the difficult setting of elderly CMML
patients.
References














[TOP]