Received: January 15, 2014
Accepted: March 10, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014028, DOI 10.4084/MJHID.2014.028
This article is available on PDF format at:

Elisabetta Abruzzese, Malgorzata Monika Trawinska, Alessio Pio Perrotti and Paolo De Fabritiis
Hematology,
S. Eugenio Hospital, Tor Vergata University.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract The
management of patients with chronic myeloid leukemia (CML) during
pregnancy has become recently a matter of continuous debate. The
introduction of the Tyrosine Kinase Inhibitors (TKIs) in clinical
practice has dramatically changed the prognosis of CML patients; in
fact, patients diagnosed in chronic phase can reasonably expect many
years of excellent disease control and good quality of life, as well as
a normal life expectancy, including the necessity to address issues
relating to fertility and pregnancy. Physicians are frequently being
asked for advice regarding the need for, and/or the appropriateness of,
stopping treatment in order to conceive. In this report, we will review
the data published in terms of fertility, conception, pregnancy,
pregnancy outcome and illness control for TKI treated CML patients, as
well as how to manage a planned and/or unplanned pregnancy.
|
Introduction
The
hybrid BCR-ABL gene and its tyrosine kinase constitutionally active
recombinant fusion protein (p210 BCR-ABL) deriving from the reciprocal
translocation between chromosomes 9 and 22 is associated with the
clinical development of chronic myeloid leukemia (CML).[1-2]
This
fusion results in the expression of two forms of protein-tyrosine
kinases: p190 (BCR-ABL) and p210 (BCR-ABL) with subsequent
dysregulation of intracellular signaling that drive cells to enhanced
proliferative capability and resistance to apoptosis.
The presence of this well defined pathogenetic defect at the molecular
level led to the development of Imatinib, a tyrosin kinase inhibitor
able to block the BCR-ABL aberrant molecule, thus shutting down the
leukemia phenotype.[3-4]
Imatinib (Glivec, Novartis), is the first of a series of tyrosin kinase
inhibitors (TKIs), a group of drugs used to manage patients with
chronic myeloid leukemia (CML) through the competitive ATP inhibition
at the catalytic binding site of the bcr-abl protein.[5]
The second and
third generation TKIs include Nilotinib (Tasigna, Novartis), Dasatinib
(Sprycel, Bristol Myers Squibb), Bosutinib (Bosulif, Pfizer), and the
recently approved Ponatinib (Iclusig, Ariad Pharma).
The introduction of TKIs in clinical practice has dramatically changed
the prognosis of CML patients. Data derived from first line therapy
(IRIS Study) at 7 years follow up, confirmed one year later, reports
cumulative best rates of complete cytogenetic remission (CCR) of 82%,
and an estimated overall survival of 89%.[6-8]
Patients diagnosed in chronic phase can reasonably expect many years of
excellent disease control and good quality of life (QoL); furthermore,
patients in an optimal response can reach a life expectancy similar to
the non-leukemic, same age, population.[9]
Even if a higher median age at diagnosis (55-60 y.o.) were reported,
the GIMEMA registry of CML has reported that approximately 50% of
patients at diagnosis are in reproductive age (Figure 1).
This has addressed issues relating fertility and pregnancy and
physicians are frequently asked for advice regarding the need and/or
the appropriateness of stopping treatment in order to conceive.
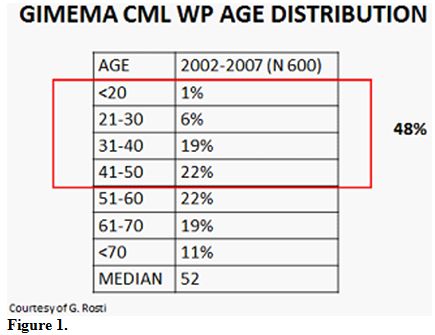 | Figure 1 |
TKIs in Animal Model
Imatinib:
Studies on either males or females rats and mice have shown that
Imatinib administered to fertile animals has a teratogenic but not
gonadotoxic activity.
However, when male rats were given Imatinib at dosages between 20 and
60mg/kg (corresponding to the human dose of 200-600mg/d) lower
testicular weight and reduction of sperm mobility were observed at
higher dosage. If similar dosages were given to immature rats,
interference with the normal process of testis maturation was noticed,
while, at sexual maturity, a normal number of sperm counts, motility,
maturation and development and higher levels of FSH and LH were
registered.[10] Effects of Imatnib
on ovary were unremarkable, so that
the fertility of male and female rats was not affected.
The effects on gestation differed by the dosage utilized: teratogenic
effects of skull and bone formation (exencephaly, encephalocele, absent
or reduced frontal bones and absent parietal bones) were seen when
Imatinib was administered during organogenesis at 100mg/kg
(corresponding to 1000 mg in humans), while, at higher dosages, total
fetal loss was seen in all animals. Fetal loss was not observed at
dosages <=30 mg (Novartis: Imatinib investigator brochure).
Nilotinib:
As reported with Imatinib, genotoxicity studies in bacterial in vitro
and in vivo mammalian systems did not reveal evidences for a mutagenic
potential of Nilotinib.
In pharmacokinetic distribution studies in rats at dosages up to
180mg/kg per day, Nilotinib showed minimal brain and testis
penetration. A significant decrease in total epididymal weight was
observed at the maximum dose level, while all other male reproductive
parameters, including sperm count and sperm motility, were unaffected.
Reproductive and developmental studies have been completed in rats and
rabbit. No effects on fertility were noticed in males or females rats,
while at doses >20mg/kg/d the embryos died. Compared to
Imatinib, no
evidence of teratogenicity in rabbits or rats was seen, while the drug
was embryo- and fetotoxic in rats and rabbits at dosage producing
maternal toxicity. The oral administration of Nilotinib in female rats
from d6 gestation to d21 post-partum resulted in only maternal effects,
as observed after longer gestational period, reduced food consumption
and lower body weight gain at 60mg/kg. The maternal dose of 60mg was
also associated with decreased pup body weight and changes in some
minor physical, developmental parameters (earlier tooth eruptions and
eye opening).
Following a single dose of 20 mg/kg oral dose of C14 Nilotinib in
pregnant rats, the higher tissue concentration compared to blood were
observed in the maternal liver, kidney, uterus heart and amnion. In the
fetus, the tissue concentration was lower than that observed in the
maternal organs, except for the liver, which had 1.6 fold higher
levels. After oral daily dosing between 30 and 100 mg/kg, Nilotinib
concentration was below the limit of quantification in rabbit’s
fetuses, while in the group treated with 300mg/kg; fetus concentration
was only 8% of the maternal serum, indicating a low absorption of
Nilotinib in the fetus. The transfer of drug and metabolites to milk
was observed following an oral dose of C14 Nilotinb to lactating rats.
It is estimated that, in 1L of human milk, the infant can be exposed to
0.26% of a 400mg adult dose. (Novartis: Nilotinib investigator brochure)
Dasatinib:
Dasatinib did not appear to affect fertility in male rats at dosage
<10 mg/kg/day and was not toxic to the offspring at these doses,
while effects included reduced size and secretion of seminal vesicles
and testis and an immature prostate.
In female rats, Dasatinib was observed to be teratogenic, extensively
distributed in maternal tissues, and secreted into milk.[11]
At plasma concentrations below those observed in humans receiving
therapeutic dosage of Dasatinib, embryo-fetal toxicities were observed
in pregnant rabbit and rats. Fetal deaths were observed in rats. In
both rats and rabbits, the lowest doses of Dasatinib tested resulted in
embryo-fetal toxicities. These doses produced maternal AUCs 0.3 fold
the human AUC in females at dose of 70 mg twice daily, and 0.1 fold the
human AUC in rats and rabbits. Embryo-fetal toxicities included
skeletal malformations at multiple sites (scapula and all long bones,
including ribs), reduced ossification, generalized oedema and
microhepatia. (Dasatinib Investigator Brochure; Sprycel® (Dasatinib)
tablets, Summary of Product Characteristics).
It is not known whether Dasatinib is excreted in human milk.
Other
TKIs:
Little is known concerning the recently approved Bosutinib (Bosulif,
Pfizer) and Ponatinib (Iclusig, Aria Pharma), although for both of them
preclinical studies of mutagenesis seem to be negative, and
teratogenity is similar to the other described TKIs with effects on
osteogenesis and vasculogenesis.
TKIs in Pregnancies and Conception
Imatinib
in man:
Since the first reports of unplanned conception in male taking Imatinib
at standard and higher dosages, no increased risk of congenital
malformations or increased abortion have been reported.[12-14]
In a series of female and male patients treated with Imatinib, Ault et
al[15] described 8 male patients
who conceived, with an exposure of 18
months (range 4-48 months) to Imatinib. Taking into consideration the
72 days for male gonads to come to maturation, the majority of those
patients have been fully exposed to the drug at conception. In those 8
patients, conception resulted in the birth of 8 babies (1 spontaneous
abortion and one twin pregnancy). One of those babies was born with a
mild malrotation of the small intestine, surgically fixed. No problems
were observed after the birth in terms of growth and development in 38
months follow up.
More than 150 cases have been described so far and except for the
malrotation of the intestine reported earlier and one stillbirth with
malformations in the previous series, the other pregnancies/deliveries
resulted uneventfully.
Imatinib
in women: Completely different is the outcome when the
woman is taking Imatinib, and the embryo-fetus is exposed to the drug.
Different reports have been published in the past years reporting
patients treated with Imatinib who conceived and or became pregnant
during therapy. In the series of Ault et al., together with the 8 male
patients, 10 female were presented (1 in CCR, 1 in accelerated phase
and 8 in an advanced phase of the disease) who received Imatinib at
standard dose (9 patients) or 800mg (1 patient) until pregnancy was
identified, with exposure between 4 and 9 weeks. Two patients had a
spontaneous abortion after discontinuation of Imatinib, while one
patient had a therapeutic abortion. The remaining seven pregnancies
were carried to term resulting in the birth of 8 babies (twin girls).
One baby had a hypospadias that was surgically corrected, while the
remaining 7 were healthy and presented with a normal growth and
development after 53 month follow up.
The first systematic review of pregnancies was reported by Pye et
al,[16] who collected information
from different Institutions,
describing 180 women exposed to Imatinib during pregnancy. Of these
pregnancies, outcome data are available for only 125 (69%). Women were
exposed to Imatinib during all pregnancy (38 patients), during 1st trimester
(103 patients), during both 1st
and 2nd
trimester (4 patients) or after the 1st
trimester (4 patients). Of those with known outcomes, 50% delivered
normal infants and 28% underwent elective terminations, 3 following the
identification of abnormalities. Of 12 infants with abnormalities
identified in carried pregnancies, 8 were live births, 1 stillbirth,
and 3 terminations. A total of 10 of the 12 infants with abnormalities
have been exposed to Imatinib during the first trimester (information
unavailable for the remaining 2 infants). Because of both tyrosine
kinases and bcr-abl inhibition by Imatinib, it is conceivable that the
congenital abnormalities result from inhibition of members of this
extensive family. Furthermore, the abnormalities evidenced were similar
to those observed in preclinical studies (exencephaly, encephalopathies
and abnormalities of the skull bones observed in the rodent studies).
Recently, more attention is given to the possibility of a
planned/unplanned pregnancy in CML patients of both sexes. The majority
of the events described, including 2 cases from our institution
(unpublished data), are carefully followed, pregnancies are possibly
planned, and Imatinib interrupted early or right before conception, so
all of the pregnancies resulted in normal babies. The behavior of both
physicians and patients may be different in other Countries where
discontinuation of the drug is not systematic, and most of the
pregnancies were not even reported to the hematologist when
discovered.[18-19] Of the 28
pregnancies from 21 female patients in
remission reported by the Pakistan group, 27 assumed Imatinib. Of the
27 exposed pregnancies, 6 were exposed during 1st
trimester (wks 6-12),
12 during 1st
and 3rd,[14-31] and 9
throughout the pregnancy until delivery. One stillbirth presented with
congenital malformations and one baby died without malformations after
one week after an apparently uneventful pregnancy. In this series, the
authors reported as adverse event 2 babies born prematurely with low
birth weight. Finally, three spontaneous abortion and 3 elective
abortions were recorded.
Table 1
summarizes 167 of
a total 210 Imatinib female pregnancies published and/or observed in
our Institution with sufficient data and follow up. Within those 167
pregnancies, 128 were uneventful (77%), while 24 ended in spontaneous
abortion (14%), a percentage slightly higher compared to the normal
population (10-12%).[17]
Fifteen/167 (9%) presented with abnormalities,
including one referred to a concomitant drug (warfarin syndrome). All
patients in this group were exposed to Imatinib during organogenesis
(>5wk gestation).
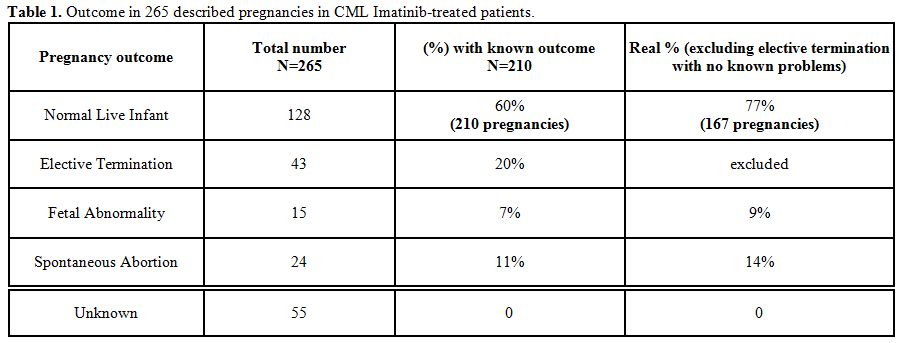 | Table 1. Outcome in 265 described pregnancies in CML Imatinib-treated patients. |
Finally, although
most pregnancies exposed to Imatinib may have a
successful outcome, a significant proportion of drug-related serious
fetal malformations and a slightly higher risk of spontaneous abortion
remain at risk. For this reason, pregnancy should not be avoided, but
planned.
Nilotinib
in men:
Little is reported regarding conception during Nilotinib treatment. One
case from our Institute (unpublished data) regards a 33 years male
patient with CML enrolled in the GIMEMA NILIM trial (alternated
Nilotinib/Imatinib), who wanted to conceive his 2nd
child. He discussed the opportunity to delay therapy but, taking into
consideration the 72 days necessary to complete gonads maturation
process in male, we suggested conceiving in the first two months on
Nilotinib therapy. He conceived after 40 days and had a healthy boy
born at term. He is now 5 years old, regularly growing and healthy.
The investigator brochure, ed.8 (June 2012) refers to a total of 36
cases of drug exposure via the father. One of these cases presented
with fetal abnormalities ended in a therapeutic abortion.
Nilotinib
in woman:
As for men, very little is reported for women getting pregnant while
exposed to Nilotinib. Two cases were published and indexed, and one
more case was reported by an Italian institution. The firstly published
case regards a 30-year-old woman with chronic myeloid leukemia who
became pregnant twice successfully. Philadelphia-positive CML in its
chronic phase was diagnosed at 16 weeks of her first gestation and
received no treatment throughout her pregnancy. At 38 weeks of
gestation, a normal infant was delivered by cesarean section. Two years
later, while, in major molecular response (MMR) on Nilotinib 200 mg
bid, she became pregnant again. The unplanned pregnancy was identified
during her first trimester of gestation after the patient had
experienced 7.4 weeks of amenorrhea. The patient was informed of the
potential fetal toxicities of therapy, but decided to carry on her
pregnancy. Nilotinib was stopped, and no further treatment was given
until delivery. A follow-up with ultrasound scans during the course of
the pregnancy was unremarkable. At gestational week 33, she delivered
via cesarean section a healthy male baby weighing 3.2 kg. He was
breast-fed for 2 months and at 5 months post-partum, the child was
healthy and normally developing.[20]
The second case was reported by the French intergroup of CML.[21] It regarded a 38 years female who
got her 5th
pregnancy while on Nilotinib. Treatment was stopped when pregnancy was
discovered, and replaced by Interpheron-a. Three months ultrasound
showed a big omphalocele ending into the pregnancy interruption.
The other case, unpublished, was reported in Italy: a 41 years female
resistant to Imatinib, switched to Nilotinib 400 bid, achieving MMR
after 3 months, and complete molecular remission (CMR) with bcr-abl
transcript undetectable at 9 months. After 11 months she became
pregnant. Fetus was exposed for 5 weeks before stopping therapy. The
pregnancy was unremarkable. She lost CMR, but PCR stayed <0.19%
IS
until delivering a healthy baby girl.
In the Nilotinib investigator’s brochure, 45 cases have been reported
of drug exposure during pregnancy. There was only one case with fetal
abnormalities (probably the same already described by the French
group). In addition, there was one female exposed pregnant of twins
with one twin experiencing congenital transposition of great vessels
resulting in death, and the other twin experiencing a non-serious heart
murmur.
Dasatinib
in men:
Cortes et al evaluated the effect of Dasatinib on pregnant partners of
9 male patients who conceived children while receiving Dasatinib (22).
Normal newborns were reported in 7 cases, with the outcome of the other
cases unknown. All male patients remained on treatment during and after
the pregnancies. In 1 case, the mother experienced pre-eclampsia but
delivered a healthy newborn at 37 weeks, without birth defects or
neonatal complications.
Dasatinib
in woman.
Published information regarding the use of Dasatinib in pregnancy is
limited to 17 cases describing the outcome of female patients with CML,
who became pregnant while receiving Dasatinib. In all but 1 case,
Dasatinib was stopped upon confirmation of pregnancy in patients
electing to give birth. Data from 16 patients with CML enrolled in
phase 1 to 3 Dasatinib clinical trials and 6 voluntarily submitted
reports were used to complete the study described by Cortes et al..[22]
A total of 13 pregnant female patients were identified: of these, 1
patient gave birth to a normal newborn, 1 to a premature newborn, 4 had
induced abortions, 2 had spontaneous abortions and 5 were pregnant at
last reported follow-up. The normal infant was born to a 36-year-old
female patient with CML in chronic phase (CP). Pregnancy was identified
after 7 weeks of gestation when the patient was under treatment with 70
mg of Dasatinib twice daily. The premature infant was born to a
29-year-old female patient with CML in accelerated phase treated with
Dasatinib 70 mg twice daily; he was delivered by caesarian section
after 7 months' gestation, was “small for dates” but had no defects.
Kroll et al[23] reported the use
of Dasatinib in a 23-year-old female
patient with CP-CML, who became pregnant while undergoing treatment.
When pregnancy was identified after approximately 5 weeks of gestation,
she was being treated with Dasatinib 70 mg once daily and was in CCR.
Treatment was discontinued immediately. While the patient was closely
monitored and off therapy, she developed leukocytosis and a mild
thrombocytosis. Subsequently, she was given hydroxyurea, but developed
progressive leukocytosis and increased levels of lactic dehydrogenase
levels. Cytarabine was administered to reduce the white blood cells
(WBC) count to normal within several days. To avoid further fetal
exposure to chemotherapy and resume more definitive therapy for the
mother’s CML, doctors induced labor at 35 weeks. The patient delivered
a healthy female baby without any developmental delay or structural and
functional anomalies. Three days post delivery, Dasatinib 70 mg daily
was restarted concurrently with a 2-week hydroxyurea tapering. She
reached CCR in the peripheral blood 5 months after re-initiating
Dasatinib.
Conchon et al[24] reported the use
of Dasatinib in a 25-year-old female
patient with CP-CML, who became pregnant while undergoing treatment
with 70 mg twice daily. Pregnancy was identified during the first
trimester, while she was in hematologic remission and Dasatinib was
discontinued immediately. Hematologic relapse occurred, and the patient
was, therefore, treated with INF-α (although complete hematologic
response was not achieved). The patient delivered a male baby at 33
weeks with no sequelae or malformations. A few days following delivery,
the patient was treated with hydroxyurea for 4 months, and then
restarted TKI.
Berveiller et al[25] reported a
23-year-old woman with CML, who became
pregnant after the switch to Dasatinib following Imatinib failure. When
pregnancy was identified at 9 weeks of gestation, she was treated with
Dasatinib 100 mg once daily for 4 weeks achieving complete hematologic
response. Dasatinib was not discontinued due to the high-risk
characteristics of the patient, and an obstetric ultrasonography at 16
weeks of gestation revealed a fetal hydrops with subcutaneous edema,
pleural effusion, and ascites. Pregnancy was terminated due to the poor
perinatal prognosis after 17 weeks of gestation. The patient delivered
a eutrophic male fetus with no organ malformations except for
microretrognathia and hypertelorism. Fetal karyotype was also normal.
Fetopathologic examination revealed a subcutaneous edema in the nuchal
and dorsal regions. Transplacental transfer of Dasatinib was observed
with drug concentrations of 4 ng/mL in maternal plasma, 3 ng/mL in
fetal plasma, and 2 ng/mL in amniotic fluid.
Bayraktar et al[26] reported a
normal pregnancy outcome of a
25-year-old patient with CML, who was treated with Dasatinib 100 mg
once daily for the first 6 weeks of gestation. Once the pregnancy was
confirmed, Dasatinib was stopped, and the patient was managed
conservatively with close observation of her disease. During her
pregnancy, hematologic relapse occurred with mild leukocytosis and
thrombocytosis that did not require treatment. The infant was delivered
at 37 weeks without any documented birth defects. The patient was
restarted on Dasatinib 100 mg daily shortly after her delivery and did
not breastfeed. At 2 years’ follow-up post delivery, the patient had
molecular remission and her daughter met all developmental milestones.
Other
TKIs:
As far as we know, no conception/pregnancies were described while
taking Bosutinib or Ponatinib. In our Institution, we followed a male
patient diagnosed with CML and enrolled in a Bosutinib trial that
cryopreserved his sperm before starting therapy and conceived with an
intra-uterine insemination (IUI) a healthy baby girl who is now 3 years
old and normally developing.
Discussion
Imatinib and the subsequent second and third generation TKIs has
represented and represents, a major advance leading to a successful
targeted therapy with substantial improvement of survival and quality
of life in CML patients.
Considering the significant proportion of female/male patients
diagnosed with CML in reproductive age, and the substantial normal
lifespan of those patients when treated and responding, it became
mandatory to address issues relating to fertility and pregnancy. The
management of fertility begins at diagnosis. In fact, the patient in
reproductive age should be informed about the risk of unplanned
pregnancies in terms of fetal problems and/or the risk of uncontrolled
disease in the case of stopping therapy, but also on the possibility
that a controlled pregnancy can be carried out when the treatment has
being started, and the response is optimal.
There are no consensus/guidelines regarding the best behaviour in case
of pregnancy.
While it seems that there are no problems in terms of fertility,
conception and delivery of female partners of male patients (even if
for newer TKIs not enough data are available), female patients should
not be exposed to TKIs during pregnancy.
Based on the published data, 10-20% of maternal exposure during the 1st
trimester to TKIs ends in fetal problems or spontaneous abortion. The
problems consist mainly in skeletal malformations, soft tissue
abnormalities (especially involving the vessels and organs formation)
and small for date babies, and are similar to the one described in
animal studies.[27]
An independent algorithm by Kumar et al. for the management of CML
during pregnancy recommended discontinuation of TKIs. If patients are
in the first or second trimester, interferon alpha (IFN-α) can be
given; leukapheresis can regulate white blood cell (WBC) counts as
required and hydroxyurea considered for patients not responding to
IFN-α. Patients in their third trimester and not responding to IFN-α or
hydroxyurea can be treated with Imatinib, and if still not responding,
with second generation TKIs.[28]
Shapira et al suggested an algorithm in which pregnancies discovered at
diagnosis should be considered for Imatinib treatment only if
unresponsive to IFN-α and, possibly, after 1st trimester.[29]
Imatinib is a compound that highly binds to plasma proteins and has a
high molecular weight that should limit placental transfer. Two studies
addressed the possibility to administer Imatinib later in the course of
pregnancy, evaluating the concentration of this drug and its active
metabolites in the different maternal/fetus compartments. In two cases
presented by Russell at al, Imatinib appears to cross the human
placenta poorly. In two pregnancies taking Imatinib during the 3rd
trimester, concentration of drug and his metabolite CGP74588 was
measured at delivery in maternal blood, placenta, and cord blood.
Little or no drug was found in the cord blood, while it was present at
high concentration in maternal blood and placental tissue, confirming
this hypothesis.[30] In a
different pregnancy with exposure from 21st
to 39th
week of gestation, Imatinib was present at 338ng/mL in the cord blood
and 478 ng/mL in the peripheral blood infant (1/3 range) vs 1562 ng/mL
in maternal blood.[31]
In contrast, an high concentration of drug was found in breast milk in
both studies, as confirmed in other works and described in animal
models.[32]
These reports suggest that in a male patient taking Imatinib or
Nilotinib, no particular risks of fertility; conception or pregnancy
have been evidenced. Caution should be used when patient is taking
Dasatinib, due to the very few data available. No reports are available
for patients taking Bosutinib or Ponatinib. For those patients, the
possibility to cryopreserve sperm before starting therapy should be
discussed.
In a female patient, in reproductive age, effective contraception
should be suggested at diagnosis. A pregnancy should be planned only
after the milestone of a stable MMR (or better, e.g. >MR4.5)
reached
more than 18-24 months earlier. Ob-gyn visit for pre-conception tests
(including in some cases male sperm evaluation), ultrasound and planned
conception is highly recommended.
Therapy should be stopped immediately before or soon after conception.
All drugs must be avoided during the organogenesis (post menstrual days
31-71, weeks 5-13), Q-PCR must be monitored each month/2 months to
follow the transcript. Therapy should be considered only if a
cytogenetic or hematologic relapse occurs.[33]
Each single patient
should be individually evaluated taking into account the rapidity of
the relapse, the clinical CML history, and most of all, the pregnancy
status (weeks of gestation).
Interferon can be considered safe throughout pregnancy[34]
and
hydroxyurea can be used to control leucocytosis after
organogenesis.[35] When necessary,
TKIs therapy with Imatinib or
Nilotinib could be considered after placenta has been formed and
organogenesis completed,[36]
although high Nilotinib concentration has
been found in fetal liver, in animal models. Dasatinib, on the
contrary, seems to pass the barrier and should be avoided.
All patients can breast feed the first 2-5 days postpartum to give the
baby the colostrum. Newborns have very immature digestive systems, and
colostrum delivers its nutrients in a very concentrated low-volume
form. It has a mild laxative effect, encouraging the passing of the
baby's first stool; that helps to clear excess bilirubin, contains
immune cells and many antibodies, immune substances and a series of
cytokines and growth factor.[37]
Considering the few days of delay to
resume treatment, it could be important for the newborn to access this.
After delivery and providing a good molecular transcript, therapy can
be postponed to consent full breast feeding, according to the
haematologist judgement.
All cases with good control of the illness at conception and a good
response to a specific TKI stopped during pregnancy and resumed after
delivery, have re-reached MMR within 3-6 months, confirming the
possibility of a safe therapy manipulation during pregnancy.[38-40]
In conclusion, we can resume all those informations updating a table
earlier presented by Apperly (Table
2).
It is important to take into consideration that each case should be
considered as a single case in which many factors may play a crucial
role: the biology of the illness, the response to treatment, the
outcome during the pregnancy, and the willing of the patient should be
clearly considered and discussed.[41-42]
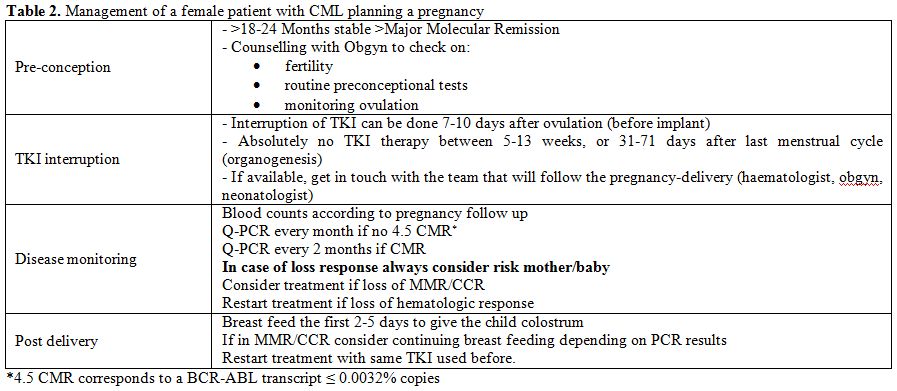 | Table 2. Management of a female patient with CML planning a pregnancy. |
In our Institution,
we have a team composed by haematologists,
urologists, ob-gyn and neonatologists, that work together during the
planning, the conception, the pregnancy, the delivery and the immediate
post-delivery, to assure the best care to mother and child. We have
followed 3 CML female conception/pregnancies and 6 male conceptions,
all with normal children (1 female patient pregnancy is ongoing) plus
many pregnancies of patients affected by lymphomas or acute leukemia.
The preservation of fertility is part of our routine when a female/male
patient is diagnosed as having a hematologic problem needing therapy.
Through the GIMEMA CML working party, there is an ongoing observational
retrospective and prospective multicenter study to register all female
pregnancies/male conception in TKIs era and a similar registry is in
preparation through the European Leukemia Network of CML Working Party,
coordinated by Italy and Russia. We hope that an extensive report of
such events will help in managing the possibility to conceive in CML
patients in order to give our patients not only a normal lifespan, but
also a normal life.
Acknowledgements
To my daughters Flavia and Valeria.
References









































[TOP]