Received: October 23, 2013
Accepted: March 20, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014031, DOI 10.4084/MJHID.2014.031
This article is available on PDF format at:

Abraham T. Yacoub1, Mitsuya Katayama2, JoAnn Tran3, Ronit Zadikany3, Manasa Kandula1 and John Greene4
1
H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia
Drive, Tampa, Florida 33612-9497.
2 University of South Florida, Division of
Infectious Diseases and
International Medicine, 1 Tampa General Circle, G323 Tampa, FL 33606.
3 University of South Florida Morsani College of
Medicine, 12901 Bruce
B. Down Blvd, Tampa, Fl 33612-4742.
4 H. Lee Moffitt Cancer Center and Research
Institute, University of
South Florida College of Medicine, 12902 Magnolia Drive, Tampa, Florida
33612-9497.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Organisms
that are not known to cause serious infection in the immunocompetent
population can, in fact, cause devastating illness in immunosuppressed
neutropenic populations especially those who are undergoing
hematopoietic stem cell transplantation (HSCT), and solid organ
transplantation or a history of malignancy. One organism of interest
isolated from immunosuppressed patients at our institution was
Bordetella bronchiseptica. It is known to cause respiratory tract
disease in the animal population which includes dogs, cats, and
rabbits. This organism rarely causes serious infection in the
immunocompetent population. However; in immunosuppressed patients, it
can cause serious pulmonary disease. We present three cases of B.
bronchiseptica pneumonia in patients with a history of malignancy.
|
Introduction
Bordetella
bronchiseptica
is an aerobic, motile, gram-negative rod most commonly viewed as a
commensal organism that inhabits the upper respiratory tract of various
domestic and feral mammals. Such animals include guinea pigs, rats,
mice, ferrets, horses, chicken, mice, primates and koala bears. The
spectrum of illnesses caused by B.
bronchiseptica
in susceptible animals includes tracheobronchitis of rabbits and guinea
pigs (snuffles) and dogs (kennel cough), as well as turbinate atrophy
in swine.[1-3]
While B. bronchiseptica
infection has been found in this broad range of different hosts, it has
two closely related Bordetella
species, B. pertussis
and B. parapertussis,
that naturally infect humans.[4]
Despite potentially frequent exposure to zoonotic sources of this
opportunistic agent, human infections are rare. As of 2006 fifty-five
cases of human infection have been reported.[5-7]
We present 3 cases and literature review B. bronchiseptica
in the immunosuppressed population.
Case Series
Case
One:
A 43-yr-old female presented with a 2-day history of increasing
swelling of the face and right upper extremity. In addition, she had
one week history of fever, progressive shortness of breath, and cough
with yellow sputum production. Four months prior to admission, she was
diagnosed with superior vena cava syndrome and malignant thymoma. She
was treated with concomitant radiation and chemotherapy followed by
radical excision of the thymoma and reconstruction of the right
subclavian vein with a Gortex graft. She was admitted to the hospital
and diagnosed with superior vena cava syndrome due to thrombosis of the
venous graft. An unsuccessful attempt was made to lyse the thrombus
with urokinase. She had been an active smoker of one pack of cigarettes
a day for 20 years. She did not own a pet and denied any contact with
animals but she had a history of cat scratch disease. Laboratory
results revealed an elevated WBC count of 21 cells/ml. Sputum culture
obtained at admission produced moderate growth (2+) of B. bronchiseptica
and C. albicans. In vitro testing indicated sensitivity to amikacin,
gentamicin, tobramycin and piperacillin. Blood and urine cultures were
negative. Chest X-ray demonstrated bilateral pleural effusions and
lower lung infiltrates. Intravenous cefotaxime 1gm thrice daily was
begun empirically along with 1 dose of intravenous tobramycin 90 mg. On
the fourth day of hospitalization, the dyspnea worsened. Chest x-ray
demonstrated a left greater than right pleural effusion. CT-guided
thoracentesis of the left pleural effusion drained 1 liter of a
transudate-like fluid which was negative for acid fast bacilli,
bacteria, fungi, and malignant cells.
One week after admission, the patient developed severe respiratory
distress. Cefotaxime was changed to intravenous piperacillin 3g every
four hours and tobramycin 100mg every eight hours. Sputum cultures
again grew C. albicans as well as 4+ B. bronchiseptica
sensitive to amikacin, ceftazidime, gentamicin, tobramycin and
piperacillin. On day 9 of admission, the patient developed fever of
39.5°C, septic shock and multi-organ system failure. Sputum culture
grew 2+ C. albicans and 1+ B.
bronchiseptica again sensitive to the same antibiotics
tested previously. Blood culture grew S. epidermidis. She
expired the following day.
Case
Two:
A 51-yr old male presented to the emergency room with headache and
confusion. CT of the head revealed four discrete intracerebral tumors
consistent with brain metastases. Biopsy of the brain revealed
adenocarcinoma of unknown origin. CT of the chest and abdomen and bone
scan showed no abnormality. He had a 60 pack-year tobacco smoking
history. He was treated with concomitant radiation and chemotherapy.
One week after completing a third course of chemoradiation, the patient
complained of left groin pain and swelling for 3 days, recurrent fevers
with chills, productive cough, confusion, dysuria and multiple skin
excoriations in the gluteal area. CT scan of abdomen and pelvis showed
incarcerated left scrotal hernia with perforation and retroperitoneal
abscess. Chest x-ray was consistent with emphysema and consolidation in
bilateral lower lung fields. Blood and urine culture showed no growth.
He underwent exploratory laparotomy for incarcerated hernia with
resection of sigmoid diverticulitis and drainage of a
retroperitoneal abscess with loop colostomy. He was treated with
intravenous ampicillin 2gm four times daily, gentamicin 160mg thrice
daily, and metronidazole 500mg thrice daily. Two days after admission,
chest x-ray showed increased left basilar infiltrates. Blood and urine
cultures were negative. Culture from the perineum abscess grew Bacteroides fragilis,
Clostridium
perfringens, viridans
Streptococcus, E.
coli, and Enterococcus
spp. Intravenous ampicillin/sulbactam 3g four times daily and
fluconazole 200 mg once daily were added, and ampicillin was
discontinued. Five days after admission, the patient became
increasingly confused and combative. Chest x-ray showed diffuse
bilateral infiltrates. Blood culture and urine culture were negative.
Sputum cultures grew Candida; Aspergillus flavus and 2+ Bordetella bronchiseptica
sensitive to cephalothin, ceftazidime, mezlocillin, amikacin,
gentamicin, tobramycin, and piperacillin. Fluconazole was discontinued,
and intravenous amphotericin B lipid complex 3mg/kg daily was added. On
day 8 of hospitalization, CT of the chest revealed diffuse pulmonary
interstitial infiltrates and consolidation at the left lung base. After
a prolonged hospitalization, on day 26 his condition deteriorated and
he expired.
Case
Three:
A 54-yr-old moderately obese male presented for a routine follow up
chest x-ray. Two years prior, he had undergone surgery followed by
chemoradiation for right lung and supraglottal cancer with no evidence
of recurrence. The chest x-ray showed a new left lower lobe
consolidation with a right pleural effusion. CT of the chest confirmed
the presence of the consolidation and was suspicious for malignancy. He
underwent bronchoscopy with video-assisted thoracoscopic surgery with
wedge resection of the lesion. Histopathology of the tissue
demonstrated multiple necrotizing granulomas negative for acid fast
bacilli and fungus. Tissue culture grew B. bronchiseptica.
On admission, he was hoarse with a chronic cough. He had a tobacco
smoking history of 70 pack-years. There was no fever, shortness of
breath, or chest pain and breath sounds were clear. He remained stable
and was discharged five days after surgery. The treatment history was
unavailable.
Discussion
Bordetella
bronchiseptica
infections mainly occur in immunocompromised patients and can cause a
variety of respiratory symptoms, ranging from severe to asymptomatic.[1,2] In a review of the literature, the
majority of patients infected with B.
bronchiseptica had at least one predisposing disease such
as acute lymphocytic leukemia,[3]
chronic lymphocytic leukemia,[4,5]
lymphopenia associated with temolozolide treatment for glioblastoma,[6] cystic fibrosis[7]
or had undergone hematopoietic stem cell transplantation (HSCT),[8,9] or lung transplantation.[10] Our study adds three new cases of B. bronchiseptica
pneumonia to the current literature, which includes a patient with
malignant thymoma, a patient with adenocarcinoma of unknown primary
origin with brain metastases, and a patient with a history of lung and
supraglottic cancer.
It has been suggested that other microorganisms and pathogens often
accompany B.
bronchiseptica pneumonia such as Aspergillus fumigatus,
Klebsiella
pneumonia, Stenotrophomonas
maltophilia, Mycobacterium
tuberculosis, Staphylococcus
aureus, Rhodococcus equi.[9-13]
In two of our patients, Aspergillus flavus and Candida albicans were
isolated along with B.
bronchiseptica.
Some common pulmonary infections rather have a similar presentation to B. bronchiseptica
including Streptococcus
pneumoniae, Haemophilus
influenzae, Mycoplasma
pneumoniae and Chlamydia
pneumonia. Initial misdiagnoses of B. bronchiseptica
have included tuberculosis, pneumocystis,[13]
Legionella[14] and brucellosis.[15] It is important to consider B. bronchiseptica
infections when symptoms of B.
pertussis and B.
parapertussis are displayed. Patients infected with B. bronchiseptica
typically present with classic symptoms of pneumonia and in some cases,
present with acute sinusitis and bronchitis. They may also exhibit a
non-productive “whooping cough” which is also characteristic of B. pertussis,
leading to misdiagnosis.[16]
B. bronchiseptica
and B. pertussis
both possess the gene for the pertussis toxin but the toxin is only
expressed in B.
pertussis. Alterations in the promoter region of the ptx
operon in B.
bronchiseptica lead to transcriptional silencing of the
pertussis toxin gene although the gene is biologically active. The ptx
genes of B.
bronchiseptica have a different DNA sequence than that of B. pertussis and
lacks expression due to mutations in the promoter regions.[17]
The current literature does not suggest that cigarette smoking is a
risk factor for B.
bronchiseptica
pneumonia. However, each of our three patients reported a history of
smoking for 20, 60 and 70 pack years, respectively. One of our patients
was diagnosed with emphysema, and a second was being followed up for a
history of lung and supraglottic cancer. Shimoni[14]
also reported a case of fatal B.
bronchiseptica
pneumonia in lung cancer patient who had smoked for more than 30 years.
The appearance of infections in patients with a mild bronchiectasis,
cystic fibrosis and emphysema suggests that the lung diseases,
especially those that lead to structural changes, may predispose
patients to B.
bronchiseptica infection.[19]
Virulence factors promoting colonization of B. bronchiseptica in
animals include filamentous hemagglutinin, fimbriae, and pertactin
which help the organisms adhere to the cilia of the respiratory
epithelial cells resulting in stasis and difficulty of clearing mucous.
In addition, production of adenylate cyclase toxin may interfere with
the host immune response.[21-25]
In many cases, the origin of the zoonotic infection in
immunocompromised hosts is usually through animal contact. Common
patient histories include recent contact with ill cats and dogs,
healthy dogs,[24] and contact with
newly vaccinated dogs.[27]
Therefore, a history of contact with animals is very important in
immunocompromised individuals and such patients should be counseled on
how to minimize zoonotic infections. They should be strongly cautioned
to seek veterinary consultation for treatment and vaccination of sick
pets and to minimize contact with animals when they are ill. Nosocomial
transmission of B.
bronchiseptica has also been reported in the literature.[28] This suggests that the animal
contact is not the only sole route of transmission of B. bronchiseptica
in immunocompromised patients. Therefore, physicians should be aware of
the potential of immunocompromised patients acquiring B. bronchiseptica
in a healthcare setting.
Previous reports suggest that this organism also exists as a human
commensal.[29-31]
Diagnosis is based on positive cultures or polymerase chain reaction
from a patient with a history of exposure to infected animals.[32] In cases of pneumonia, cultures
from the blood or bronchoalveolar lavage colony counts greater than 104 are useful
for diagnosis rather than sputum cultures, as it would be difficult to
determine whether B.
bronchiseptica
had any role in the infection or if it were just colonizing the airway.
Gram stain of this medium straight rod organism should be reviewed
carefully. A good quality sputum gram stain indicating a good number of
white blood cells and the presence of gram negative coccobacillary
organisms increases the likelihood of B. bronchiseptica
pneumonia. The organism can be cultured in 48 hours on simple nutritive
media at 35°C where it forms small circular colonies. It can be
distinguished from other phenotypically similar organisms using
biochemical tests. B.bronchiseptica
is positive for catalase, urease and oxidase activity, citrate
utilization, motility, tetrazolium reduction and growth on
salmonella-shigella agar. It fails to grow on potassium tellurite agar.
The identification can be confirmed with commercially available tests
like Rapid NFT, API-ZYM and Corning N/F system.[33]
There are no lab values or radiographic findings specific for B.bronchiseptica.
Previous reports in the radiologic literature have described various
findings like multifocal cavitary nodules, ground glass opacities,
consolidation, bronchiectasis, mosaic attenuation and interstitial
pneumonia.[34]
The response to various antimicrobials is similar to that expected of a
gram-negative non-fermentative organism, but it is essential to choose
one that has good intracellular penetration.[3,4]
Though B. bronchiseptica
is an extracellular organism, recent studies have shown that this
organism is able to invade and persist in eukaryotic cells, like
phagocytes and even epithelial cells.[35,36]
This
invasive property is responsible for chronic or recurrent infection in
a host. The pervasive disparity between antibiograms and clinical
benefit can be because of patient factors, like the severe underlying
disease or immunocompromised state, and different properties of B. bronchiseptica
like the adenylate cyclase penetration into the polymorphonuclear cells
and macrophages leading to inhibition of bacteria killing. Likewise,
Kadlec et al found that the beta lactamase gene blaoxa-2 conferred
ampicillin resistance to porcine B.
bronchiseptica isolates while low susceptibility to
cephalosporins was based on the low membrane permeability of B. bronchiseptica.[37]
The various antibiotics that can be used are aminoglycosides,
quinolones, anti-pseudomonal penicillins, tetracycline and TMP-SMZ
depending on the susceptibility, though in vitro susceptibility does
not reflect in many cases clinical response, because of reasons
discussed earlier. Some treatment successes have incorporated
combinations of erythromycin, ciprofloxacin and rifampin,[33] and imipenem.[39]
The duration of treatment has not been established in immunocompromised
patients. It may extend anywhere between 2 weeks to 6 weeks depending
on the immune status of the patient. Severely neutropenic patients and
those with GVHD may require 6 weeks of therapy or even more.[40-42]
Chronic or recurrent infection, even after the patient is not in
contact with infected animals, suggest epithelial invasion or
persistence of the bacterium in macrophages and these cases require an
even longer duration of therapy.[43]
Conclusion
B. bronchiseptica,
found
commonly in the upper respiratory tract of animals, is also a human
commensal in the immunocompetent population. However, it can lead to
life-threatening infection in those with underlying debilitation or
impaired immunity (like patients with neutropenia, diabetes,
malnutrition or transplant patients). B.bronchiseptica
should be considered, in the differential diagnosis, in
immunocompromised patients presenting with respiratory symptoms,
especially those with known contact with animals. Nosocomial
transmission has also occurred, and physicians should be aware that
patients with impaired immunity in the healthcare setting may also
acquire an infection with B.
bronchiseptica.
The duration and choice of antibiotic is determined on a case by case
basis, but treatment with one that has good intracellular penetration
is essential because of the organism’s ability to invade epithelial
cells and phagocytes.
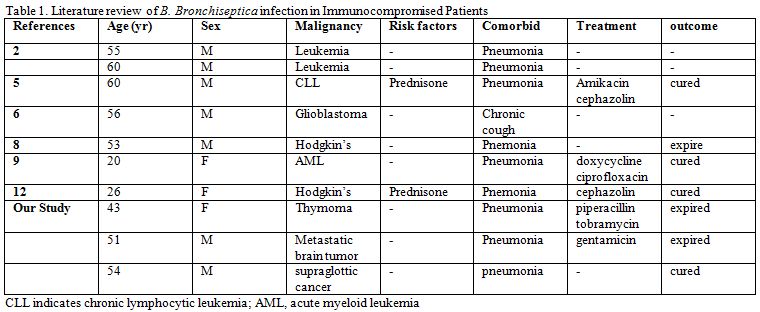 | Table 1. Literature review of B. Bronchiseptica infection in Immunocompromised Patients |
References











































[TOP]