Received: February 24, 2014
Accepted: June 16, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014048, DOI 10.4084/MJHID.2014.048
This article is available on PDF format at:

Massimiliano Salati1, Marina Cesaretti1, Matteo Macchia1, Mufid El Mistiri2 and Massimo Federico1
1
Department of Diagnostic, Clinical and Public Health Medicine, Modena
Cancer Center, Italy.
2 Hamad Medical Corporation, National Center for
Cancer Care and Research (NCCCR), Qatar.

|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract The
epidemiology of Hodgkin lymphoma (HL) has always been a source of
fascination to researchers due to its heterogeneous characteristics of
presentation. HL is an uncommon neoplasm of B-cell origin with an
incidence that varies significantly by age, sex, ethnicity, geographic
location and socioeconomic status. This complex pattern was also found
to be replicated among Mediterranean basin populations. HL incidence
rates progressively decreased from industrialized European countries
such as France (ASR=2.61) and Italy (ASR=2.39) to less developed
nations such as Albania (ASR=1.34) and Bosnia Herzegovina (ASR=1.1).
Regarding HL mortality we have found that countries with the lowest
incidence rates show the highest number of deaths from this cancer and
viceversa. Finally, a wide gap in terms of survival was showed across
the Mediterranean basin with survival rates ranged from 82.3% and 85.1%
among Italian men and women, to 53.3 % and 59.3% among Libyan men and
women, respectively. Factors such as the degree of socio-economic
development, the exposure to risk factors westernization-related, the
availability of diagnostic practices along with different genetic
susceptibilities to HL may explain its variation across Mediterranean
countries. Furthermore, the lack of health resources decisively
contribute to the poor prognosis recorded in less developed region. In
the future, the introduction of appropriate and accessible treatment
facilities along with an adequate number of clinical specialists in the
treatment of HL and other cancers are warranted in order to improve the
outcomes of affected patients and treat a largely curable type of
cancer in disadvantaged regions.
|
Introduction
Hodgkin
lymphoma (HL) is a lymphoid malignancy of B-cell origin which is
classified into either nodular lymphocyte predominant Hodgkin lymphoma
(NLPHL) or classical Hodgkin lymphoma (CHL) in accordance with 2008 WHO
classification. Although they have characteristics in common, these two
disease entities differ in their clinical features and behavior as well
their cellular properties i.e. morphology, immunophenotype and the
preservation or extinction of the B-cell gene expression program. CHL
accounts for 95% of all HLs and can be further subdivided into four
histological subtypes: lymphocyte-rich (LR), nodular sclerosis (NS),
mixed cellularity (MC) or lymphocyte-depleted (LD).[1]
HL is an uncommon neoplasm with an incidence that varies significantly
by age, sex, ethnicity, geographic location and socioeconomic status.
Incidence rates are higher in more developed regions and among males
and lower in Asia. In the United States, about 9,290 new HL cases are
estimated for 2013, an incidence of 2.8 per 100,000 people per year.[2]
The hallmark of HL epidemiology is its variation in occurrence by age
at diagnosis. This is represented in industrialized countries by the
well-known bimodal curve showing two peaks: the most significant for
young-adults (15-34 years of age) and the second one occurring in later
life (above the age of 50). As already reported by Cozen et al, these
peaks are composed mainly of different subtypes with the NS pathology
being predominantly represented in the earlier age peak and the MC
disease being predominant in the later age peak.[3]
Despite its relatively low incidence and its low lifetime risk, HL
accounts for 15% of all cancers in young adults with a high impact on
quality of life. Since its earliest description in the first half of
the 19th
century, HL has proved to be
a difficult form of neoplasm to understand because of its unusual
histopathological aspects i.e. its resemblance to an infectious
process, the variability of B-cell antigen expression among its
subtypes and its occurrence in childhood and young adults. The etiology
and pathogenesis of HL thus remain poorly understood.
Epidemiological studies of HL to date have elucidated only some aspects
of this heterogeneous disorder. Future studies of wider populations are
thus necessary to more fully clarify the complexity of HL. In this
review, we provide a comprehensive description of the known
epidemiological features of HL, focusing on populations in the
Mediterranean basin, and discuss the geographic, ethnic,
socio-demographic and economic factors that impinge upon these
properties. Regional comparisons of patterns and trends for HL between
and within countries were also performed in support our meta-analysis.
Materials and Methods
For the purposes of this study, we defined the Mediterranean area as
the region incorporating countries around the Mediterranean Sea in
Europe, Asia and Africa. We thus collated epidemiologic data on HL from
Spain, France, Italy, Croatia, Bosnia Herzegovina, Albania, Greece,
Turkey, Syria, Lebanon, Israel, Cyprus, Egypt, Libya, Tunisia, Algeria,
and Morocco (specifically the Maghreb region in Northwestern Africa).
We derived the most recent estimates on HL incidence and mortality from
the updated International Agency for Research on Cancer (IARC) online
database, GLOBOCAN 2012.[4]
Furthermore, more accurate
statistics from the latest volume of Cancer Incidence in five
continents (CI5, X version) were used to improve the quality of these
data.[5] Additional information was
obtained from various local cancer registry reports available.
Data regarding HL mortality and time trends for the specified
Mediterranean countries were derived from the World Health Organization
(WHO) mortality database online.[6]
Most of the
countries included in our analysis have previously published relevant
data on HL epidemiology, though the level of coverage, accuracy and
updating varies considerably both within and between countries. For
this reason, temporal trends for HL incidence, mortality and survival
are reported only for those countries in which the collection activity
of cancer registries covered a sufficiently wide period.
Hereafter, we refer to classical HL in this report.
Etiologic Epidemiology
Role
of Epstein-Barr virus (EBV) and other viruses.
Based on either epidemiological findings (e.g. bimodal age-incidence
curve, social-class risk factors, role of protected childhood
environment) or clinical features (e.g. fever, night sweat, weight
loss, elevated erythrocyte sedimentation rate or IL-6 in serum) it has
long been hypothesized a viral etiology for HL.[7-8]
The ubiquitous B-lymphotropic oncogenic Herpesvirus, EBV, has been
proposed as the major candidate for a pathogenetic role due to at least
three pieces of evidence: the biological plausibility of EBV-mediated B
cell transformation, the presence of clonal EBV genomes within HL tumor
cells and three-fold elevated risk of HL in persons with a history of
infectious mononucleosis.[9-11]
Globally, EBV-positive HLs account for up to 40% of all HL cases, and
they have been shown to vary substantially by patient demographic and
tumors characteristics. The presence of EBV in HL is strongly
associated with specific epidemiological features including male
gender, Hispanic ethnicity, mixed cellularity subtype, children and
older adults, lower socio-economic status.
The rate of EBV-positive among HLs differs markedly worldwide
especially with respect to geography: in North America and Western
Europe, EBV was detected in 30 to 50% of HL patients, while in some
parts of Latin America, Africa and Asia the percentage is much higher,
reaching roughly 100% in children.[12]
For instance,
in Peru and Mexico incidence of EBV positivity among HLs ranged from 50
to 95%, in China was 65% and in Kenya reached 92%.[13-17]
Previous mentioned clinicopathological features of EBV-associated HLs
were also maintained across Mediterranean countries, where the
frequency of EBV-positivity was 90% in Greece, 61.5% in Turkey, 50% in
Egypt, 48% in Italy and 30% in Israel (even if Bedouin patients showed
a 66.7% rate of EBV infection).[18-20]
The scenario is quite different for EBV-negative cases, in which a
delayed exposure to common childhood virus other than EBV, such as
other Herpesviruses and Polyomaviruses, has been postulated having a
causative role in the development of HL. However, to date, no
consistent association between any virus and EBV-negative HLs has been
described.
Conditions characterized by immune dysregulation and an
immunodeficiency status may cause a predisposition to the development
of this malignancy. In addition, the main cause of immunodeficiency
relies on HIV infection in developed as well in developing countries.[21] As a result, the risk of developing
HL in HIV patients was estimated at 11-18-fold higher than in the
general population.[22]
Given the remarkable 2012 estimated number of people newly infected
with HIV of 32.000 and 29.000 in Western and Central Europe and Middle
East and Northern Africa respectively, such infectious disease is like
to continue to be responsible for a proportion of HL cases in this
area.[23]
Tubercolosis
and Hodgkin lymphoma.
Another interesting causative relationship is that existing between HL
and tuberculosis (TBC). Nevertheless, apart from some case reports, the
literature lacks epidemiologic studies aimed at investigating such
association.
Both diseases share a pathobiology closely related to the loss of
immune-surveillance of the host and, at the same time, side effects of
anti-lymphoma treatment include immunosuppression, a well-known
predisposing factor for TBC.
Consequently, the risk of TBC is generally higher in HL patients,
especially across endemic areas for TBC infection, such as the African
and the Asian continents. In such regions, TBC has been described to
precede but also to be concomitant or subsequent to HL.[24-26]
Additionally, in some cases, HL and TBC can show similarities in terms
of clinical presentation and course, laboratory tests and imaging
findings.[27]
Thus, the opportunity to distinguish between these two disorders
represents a diagnostic challenge in countries with high prevalence of
TBC.
Familial
aggregation and Hodgkin lymphoma.
Early reports from familial cases of HL provided initial evidence
towards a possible genetic predisposition as well as a role of shared
environmental risk factors in the pathogenesis of HL. Moreover, the
variability of HL incidence across different races, with rates higher
in Jews and lower in Asians, further support this thesis.
HL risk was found to be nearly 100-times higher in identical than in
fraternal twins[28]
and over time multiple case-control and cohort studies have reported a
threefold to ninefold higher risk of disease in first-degree relatives
of HL patients.[29-30] These
findings have been
recently confirmed by linkages of population-based cancer and family
record registries, in which considered large sample size and are less
vulnerable to biases with respect to previous studies. Among them, a
large study using data from the Swedish Family-Cancer Database and the
Danish Cancer Registry, found a significantly increased risk of HL in
first-degree relatives of patients with HL in both populations, with
relative risks of 3.47 (95% CI, 1.77– 6.80) in Sweden and 2.55 (95% CI,
1.01– 6.45) in Denmark and a pooled estimate of 3.11 (95% CI,
1.82–5.29).[31]
The risk of familial HL presented a heterogeneity of effect since it
has been shown to vary by age, sex and degree of familial relationship.
The greatest risk was seen for siblings than for parents of HL
probands, for families of probands under 40 years (RR=4.25), for male
relatives of patients. In addition, an earlier onset for familial than
non-familial cases was found.[32]
Increasingly, recent genome-wide analyses of candidate susceptibility
genes identified various HLA class II polymorphisms (i.e. DRB5-0101
allele, DRB1*1501-DQA1*0102-DQB1*0602 haplotype and TAP 1 allele) as
well as polymorphisms of several cytokine genes (e.g. IL6, IL1R1, IL10,
IL4R) which have been linked to risk of HL.[33-36]
Furthermore, a genome-wide linkage screen performed in 44 high risk HL
families showed the strongest linkage finding on chromosome 4p near the
marker D4S394.[37]
In summary, these findings support a multifactorial disease model for
the pathogenesis of HL involving both genetic and environmental risk
factors.
Descriptive epidemiology
HL
incidence patterns and time trends.
Based on its epidemiological features, including in Mediterranean
countries, HL incidence rates are higher in southern Europe, with the
exception of Israel and Lebanon. The highest incidence of HL was in
fact recorded in Israel in 2012 with an estimated incidence
age-standardized rate (ASR) of 3.71 per 100,000, followed by Lebanon
(ASR=3.67), Croatia (ASR=3.09), France (ASR=2.61) and Italy (ASR=2.39).
The lowest incidence was recorded in Albania with an ASR of 1.1 per
100,000. Other countries with a low HL incidence included Bosnia
Herzegovina (ASR=1.34), Egypt (ASR=1.51), Morocco (ASR=1.7) and Algeria
(ASR=1.83).[4] These data are
reported in more detail in Table
1.
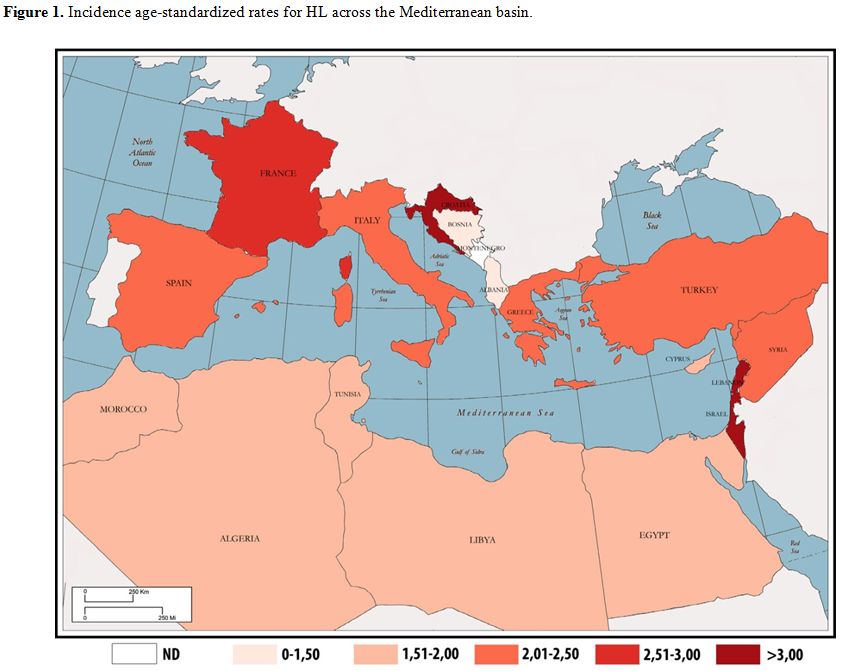 | Figure 1. Incidence age-standardized rates for HL across the Mediterranean basin. |
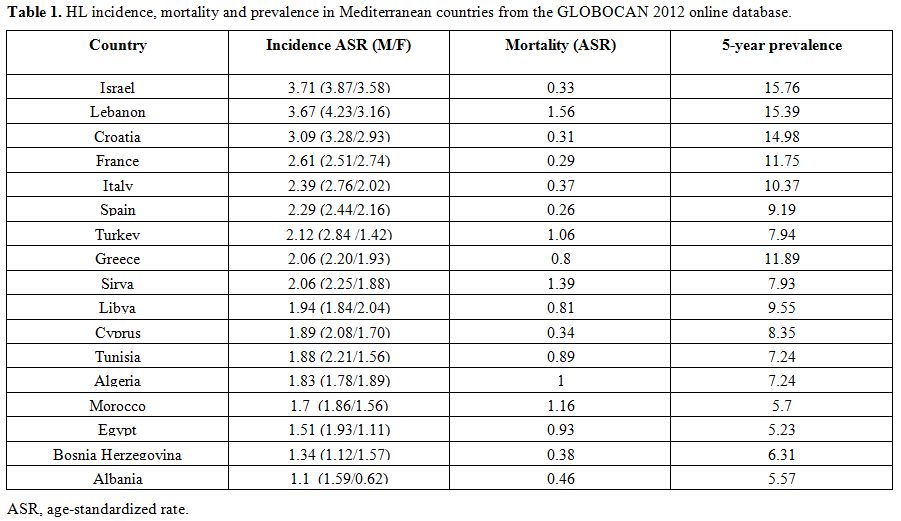 | Table 1. HL incidence, mortality and prevalence in Mediterranean countries from the GLOBOCAN 2012 online database.Incidence age-standardized rates for HL across the Mediterranean basin. |
GLOBOCAN
2012 estimates of HL incidence were found to be globally in line with
the above population-based data, although some interesting differences
were observed. In this regard, we found consistent variation in the
recorded HL incidence rates between Globocan and published cancer
registries data in Israel, Cyprus, Croatia and also among Algerian men.
Of particular note, the most updated incidence data from the Israel
National Cancer Registry reported lower rates than GLOBOCAN 2012 i.e.
ASRs of 3.14 and 3.21 reported in Jewish men and women, respectively,
and an ASR of 2.62 reported for non-Jewish women differed from the
GLOBOCAN estimates; the only comparable rate was for non-Jewish men
(ASR=3.87).[38] Estimates and
population-based data
from the more developed Mediterranean countries on HL were comparable
to those of other industrialized regions worldwide, such as the United
States which reports an incidence of 2.8 per 100,000 men and women per
year.[2]
In Italy, the ASR for HL reported by Associazione Italiana Registro
Tumori (AIRTUM) was 3.4 per 100,000 in both sexes in 2008. More updated
data have revealed a slight geographic gradient in HL incidence across
the Italian peninsula from north to south but this was not a
statistically significant variation. The 2006-2009 European ASRs for HL
by sex and geographic area were as follows: north (4.1), center (3.8)
and south/islands (3.8) in men and north (3.3), center (3.5) and
south/islands (2.8) in women.[39]
About time trends,
the HL incidence rates remained relatively stable among industrialized
countries, comparable to the recorded rates in the United States which
changed minimally over time; a significant increase in HL rates has
only been seen only among blacks (APC=1.0) and Asians/Pacific Islanders
(APC=2.2) since 1992.[2] Recent
epidemiologic analysis
conducted in Spain has revealed no significant frequency variations in
HL incidence between 2000 and 2009.[40]
Some
exceptions have been found in other part of Europe however:
statistically significant 2.6% and 2.2% annual percentage increases in
the HL rates in Italy have been documented by the AIRTUM in men and
women, respectively, during the 1996-2010 period.[41]
About the incidence in other countries bordering the Mediterranean Sea
in recent years, a population-based study from the Israel Cancer
Registry reported a significant and persistent rise in the ASR in both
sexes between 1960 and 2005 in the Jewish population. Of particular
note, in Israeli-born young Jewish adults, the ASR rose from 2.27 per
100,000 during the 1960-1969 period to 3.61 during the years 1997-2005.[42] Thus far, no HL incidence trends
have been available for low-income countries in the Mediterranean basin.
Mortality
patterns and time trends.
Mortality rates due to HL vary markedly across the Mediterranean
region; the estimated 2012 mortality ASRs range from 0.26 per 100,000
in Israel to 1.56 per 100,000 in Lebanon. A correlation between
socio-economic status and death from HL has also emerged within this
region. High income countries such as Spain, France, Israel and Italy
showed mortality rates of 0.26, 0.29, 0.33, and 0.37 per 100,000,
respectively. On the other hand, higher mortality rates have been
recorded in Lebanon (ASR=1.56), Syria (ASR=1.39), Morocco (ASR=1.16)
and Turkey (ASR=1.06).[4] Data on
HL mortality in Mediterranean regions are summarized in more detail in Table 1.
Over the past 40 years, trends in mortality have progressively
decreased worldwide, though they have been variable across countries.
Since the late 1950s, mortality rates have steadily declined in
industrialized countries. ASRs have plummeted from 2.27 to 0.44 in men
and from 1.40 to 0.29 in women in Italy, from 1.47 to 0.37 in men and
from 0.88 to 0.24 in women in France and from 1.04 to 0.41 in men and
from 0.55 to 0.22 in women in Spain. In Italy, the same progressive
downward trend has also been reported by AIRTUM which has recorded a
drop in mortality rates for HL from 0.77 and 0.48 in 1992 to 0.40 and
0.27 in 2007 in men and women, respectively.[39]
In
contrast to the above data, no similar trends seem to have emerged from
low-income countries: the only available mortality time trends in this
regard are from Egypt and show no change over time.
Survival
patterns and time trends. Up to the 1960s, the 5-year
survival rate for HL was less than 10% worldwide.[43]
The outcome for patients diagnosed with HL has progressively improved
since then and the majority of cancer registries around the world
report current 5-year overall survival (OS) rates of up to 80% for
patients with advanced and more than 90% for limited stage disease.
Hence, HL may be currently considered to be one of the most curable
cancers worldwide.
Overall, the highest survival rates for HL patients have been recorded
in western countries. In southern Europe the 5-year relative survival
(RS) rates are comparable to those of the United States and other
European countries. An international geographic comparison performed by
AIRTUM in 2011 has reported 5-RS rates for men of 82.3% in Italy, as
compared to 79.1% in the United States (SEER-17) and 82.5% in some
European countries (EUROCARE-4); among females, these rates were
slightly higher (85.1, 83.7% and 84%, respectively).[41]
In recent years, a Europe-wide study analyzing the survival of patients
with lymphoid neoplasms has indicated a 5-year RS of 84.5% for HL
patients diagnosed from 2000-2002. These rates were highest for
patients with LR (5-year RS, 93.1%) and lowest for LDC cases (5-year
RS, 54.4%). No statistically significant differences were found for CHL
between men and women (82.5% vs. 86%). That study also suggested that
previously noted differences in HL survival rates between regions have
tended to decrease, ranging from 81.4% in eastern Europe to 90.6% in
northern Europe.[44] Moreover, in
this study, the
outcome for patients with all lymphoid neoplasms, including HL, was
reported to decrease substantially with age, as has been well
documented for most types of cancer. Nevertheless, a large
population-based study from Sweden incorporating the results of
long-term follow-ups has recorded a significant 5-year and 10-year RS
improvement in all age categories, including the elderly but with the
exception of the very old (> 80 years).[45]
Furthermore, a relevant increase in survival for HL patients aged 45 to
59 years, and 60 years and older, was recently documented in a study
from the United States (increases in the 10-year RS by 24.8% and 23.3%
between 1980-84 and 2000-04, respectively).[46]
In contrast, the scenario is quite different in more economically
disadvantaged Mediterranean regions. For example, the Libyan Benghazi
Cancer Registry has reported a 5-year overall survival (OS) for HL
patients of only 53.3% in men and 59.3% in women during the period
2003-2005.[47] Turkey has reported
rates that halfway
between this and the outcomes in developed nations i.e. a 5-year RS of
69% for all ages and both sexes combined.[48]
A comparison of outcomes among three groupings of Mediterranean nations
is provided in Table 2.
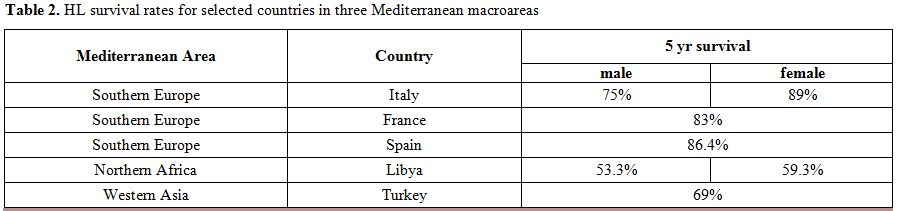 | Table 2. HL survival rates for selected countries in three Mediterranean macroareas. |
Discussion
The epidemiology of HL has always been of interest to cancer
researchers because of its heterogeneous patterns of presentation, and
several etiopathogenetic theories have emerged over time. This complex
pattern was also found to be replicated among Mediterranean basin
populations, and several factors have been shown to exert their
influence on this phenomenon. Although data regarding HL incidence,
mortality, survival and time trends across this region are available
mainly for European countries and are sparse or absent for the majority
of other countries bordering the Mediterranean Sea, we have highlighted
below several points of discussion for incidence time trends in less
developed nations.
First, it emerged from our current investigation that there is a
substantial variation in HL occurrence by geographic area: HL incidence
rates progressively decreased from industrialized European countries
such as France and Italy to less developed nations such as Albania and
Bosnia Herzegovina. This is clearly consistent with the earliest
findings of Correa and O'Connor, who first described the positive
correlation between degree of socio-economic development and risk of
HL.[49] There is a remarkable
differences in the
global occurrence of HL, with incidence rates higher in southern Europe
than in northern Africa and western Asia. That seem to be largely due
to a higher exposure in southern Europe to lifestyle and environmental
risk factors associated with economic transition (including smoking,
obesity, physical inactivity, and reproductive behaviors), as well as
availability of diagnostic practices and awareness of disease. In fact,
although little is currently known about HL pathogenesis, there is
accumulating evidence that the adoption of western world-associated
risk factors by low income countries, the so-called westernization, is
responsible for increasing the number of HL cases in such areas.[50]
A recent analysis performed by InterLymph has found that cigarette
smoking should be added to the few modifiable HL risk factors
identified to date, with a reported odds ratio (OR) of 1.10 in ever
smokers compared to never smokers. This increased risk was also
associated with MC (OR=1.60) and EBV-positive CHL (OR=1.80) among
current smokers.[51]
On the other hand, different genetic susceptibilities to HL between
different races may play an important role in explaining its variation
across countries. As already reported, Asians have a lower incidence of
HL than Caucasians and Blacks, which may indicate a genetic resistance
to this disease that is possibly related to HLA type.[52]
Nevertheless, in Israel, which belongs to western Asia, the HL rates
were found to be the highest of the Mediterranean region. In
particular, Israeli Jews had incidence rates of 4.17 and 5.57 in men
and women, respectively, aged between 15 and 34 years. These rates were
lower among Israeli non-Jews with ASRs of 3.02 in males and 1.57 in
females aged 15-34. These findings may suggest an influence of genetic
background on HL incidence, but environmental influence must also be
taken into account. In fact, Israeli Jews born in America and Europe
have shown the highest HL rates (4.16 and 6.51 in men and women,
respectively) at an equivalent age.[53]
Moreover, Au
et al. have previously corroborated the role of both genetic and
environmental factors in the occurrence of HL: Chinese immigrants in
British Columbia presented a significantly lower 25-year incidence for
HL compared to the rest of the population in this region (standardized
incidence ratio, SIR =0.34) but a still higher rate than would be
expected for the Hong Kong Chinese population (SIR=2.81).[54]
With regard to incidence trends, these have remained relatively stable
over time in western countries but have been increasing in regions
experiencing improved standards of living. This reported rise in HL
rates in less developed countries since the mid-1990s[55]
may be explained by westernization,[50]
the aging and growth of the population, or the adoption of behaviors
and lifestyles associated with economic development such as smoking,
less healthy diets and physical inactivity.
Over the coming decades, these factors will contribute to an increased
overall burden of cancer, especially in low income countries, where HL
is expected to rise among young adults by nearly 57% by 2035. Keeping
in mind all aspects of bias related to predictions, in the same year in
the East Mediterranean region, the estimated number of new HL cases
will rise from 8,374 to 13,110 per year.
Among European countries, the increase in incidence will be less with
the estimated number of new cases expected to rise from 20,410 to
21,076 annually in both sexes by 2035, with 76% (16,010) of these
patients aged below 65 years.
In southern Europe as well as in the United States, no consistent
patterns have been shown to date, with stable or slightly downward
trends recorded in men and less favorable rates in women, reflecting
the absence of any new identified causes of HL over the last few
decades.[56] The only exception to
this trend in
southern Europe has been in Italy, where a significant annual increase
in HL rates by 2.6% in men and 2.2% in women has been reported over the
period from 1996 to 2010. This increase seems not to have been due to
new risk factors in the Italian population, as evidenced by the
temporal stability of HL incidence seen in neighboring countries (i.e.
France and Spain).[57] A greater
attention to diagnostic procedures may in fact explain this trend.
The pronounced and isolated increase in the HL incidence in western
Asia among Israeli-born young Jewish adults from 1960 to 2005 is
currently a subject of investigation in which it has been hypothesized
that as yet identified factors are responsible. Regarding HL mortality,
as expected, we have found that countries with the lowest incidence
rates show the highest number of deaths from this cancer and vice
versa. The past few decades have been characterized by a significant
progress in the management of HL and the introduction of more effective
and less toxic front-line treatments within risk-adapted strategies
have made this a largely curable disease. In most western and northern
European countries, HL mortality has continued to steadily decline
since the late 1960s. However, in central and eastern European
countries this decrease has been relatively recent and in fact up to
the 1990s central and eastern countries were characterized by
unfavorable trends.[58]
Nevertheless, the clinical
outcomes for HL patients have been variable across the Mediterranean
basin. By comparing survival rates, we have found a particularly poor
prognosis for HL patients in northern Africa, where the rates for HL
are similar to those reported for non-Hodgkin lymphoma in more
developed countries (5-year RS=57%).
As reported by several authors, morphology strongly influences the
prognosis of HL patients. In particular MC, LD and not otherwise
specified (NOS) subtypes had significantly poorer outcome compared with
NS or NLPHL[59] At the same time,
HL subtype
distributions seem to vary across the Mediterranean basin. For example,
MC and NOS are more frequent in Algeria, Egypt, Libya and Turkey where
mortality rates are among the highest in this area (Table 3).
Unfortunately, no histological data are available from Lebanon, Syria
or Morocco, where the estimated HL mortality ASR peaks at 1.56, 1.39
and 1.16, respectively (Table
1).
However, differences in morphology explain only some of the geographic
variations observed in survival outcome. A study coordinated by Ben
Lakhal has suggested the presence of an intrinsically more aggressive
HL affecting less developed countries including Tunisia,[60]
but further evidence is needed to confirm this finding. More
importantly, a wide disparity in the availability of health resources
may better explain the difference in HL mortality recorded within the
Mediterranean basin.
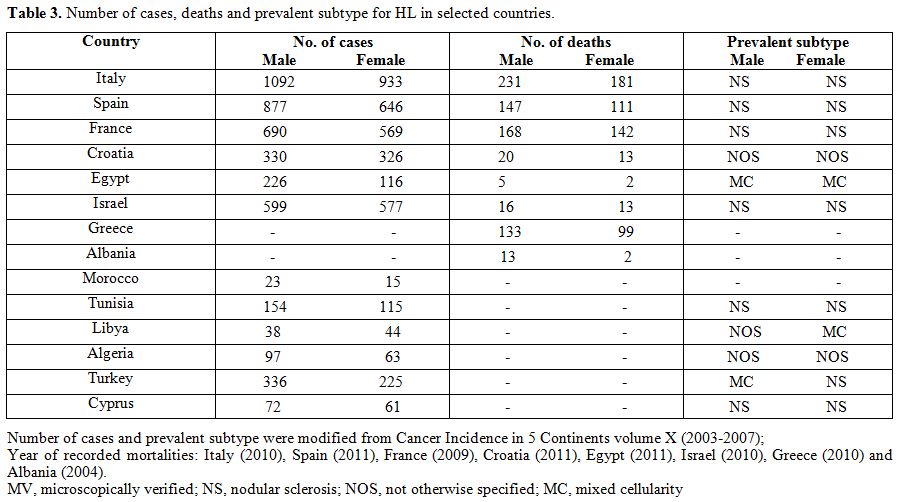 | Table 3. Number of cases, deaths and prevalent subtype for HL in selected countries. |
Radiotherapy
represents an important resource for treating HL patients, particularly
for cases of limited stage disease, but its availability is still low
among Mediterranean low- and middle-income countries. In the Maghreb
area, only Egypt with 85% coverage and Morocco with 89% coverage can
provide an acceptable level of radiotherapy treatment. Libya has an
adequate number of machines but not all are utilized due to a lack of
cancer clinicians. In Albania, the issue is insufficient maintenance
support despite this country’s serious efforts to provide adequate
cancer care services to its citizens. The annual maintenance bill for
these machines of US$110,000 and requirement to replace one in three
machines at a cost of US$150,000 is beyond the budget of the Albanian
health system. In Syria the situation is not much better with only 2
radiotherapy centers operating at present serving 22.4 million people.[61]
In addition, the ever increasing costs of new and innovative therapies
may also contribute to the gap in survival outcomes that exist within
the Mediterranean region.
In conclusion, the provision of appropriate and accessible treatment
facilities along with an adequate number of clinical specialists in the
treatment of HL and other cancers represents a future challenge that
must be overcome to improve the outcomes of affected patients and treat
a largely curable type of cancer in disadvantaged regions.
References


















































[TOP]