Received: August 20, 2014
Accepted: Septmebr 11, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014065, DOI 10.4084/MJHID.2014.065This article is available on PDF format at:

Federico Lussana1 and Alessandro Rambaldi1
1 Hematology and Bone Marrow Transplant Unit, Azienda Ospedaliera Papa Giovanni XXIII, Bergamo, Italy.
Accepted: Septmebr 11, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014065, DOI 10.4084/MJHID.2014.065
|
This
is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract Adult acute lymphoblastic leukemia
(ALL) is a heterogeneous disease, due to the expression of different
biological and clinical risk factors, for which allogeneic stem cell
transplantation (alloHSCT) is an effective consolidation therapy. The
non-relapse mortality of alloHSCT remains significantly higher compared
with that of conventional chemotherapy; therefore, one of the main
challenges in the care of ALL is to establish a more precise prognostic
definition to select patients who could take advantage from an
alloHSCT. Currently, the use of minimal residual disease following
induction and early consolidation therapy has improved the prognostic
accuracy in defining ALL risk class. In Philadelphia-positive ALL, the
introduction of tyrosine kinase inhibitors pre and post alloHSCT
appears to improve outcomes significantly and, in the absence of
specifically designed clinical trials, alloHSCT remains the most
effective post- remission therapy. Nowadays, alloHSCT can be performed
according to different modalities encompassing the use of different
conditioning regimens, as well as different donors and stem cell
source, with a significant accessibility to transplant.
|
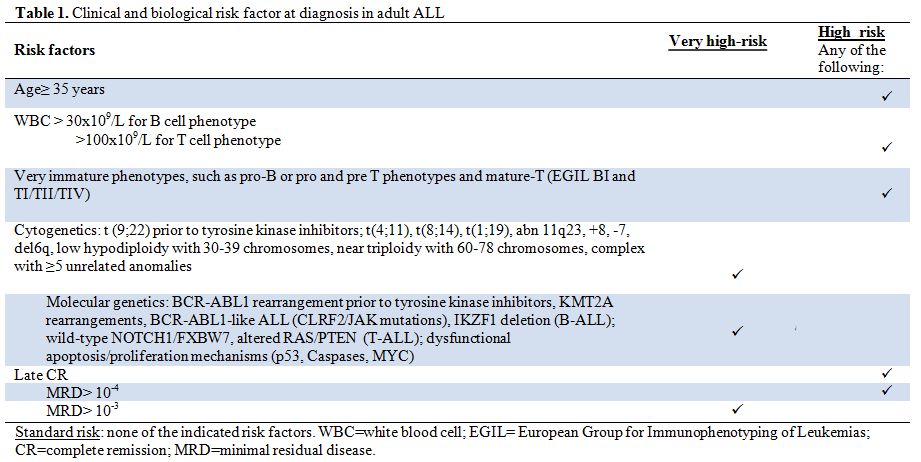 |
Table 1. Clinical and biological risk factor at diagnosis in adult ALL. |
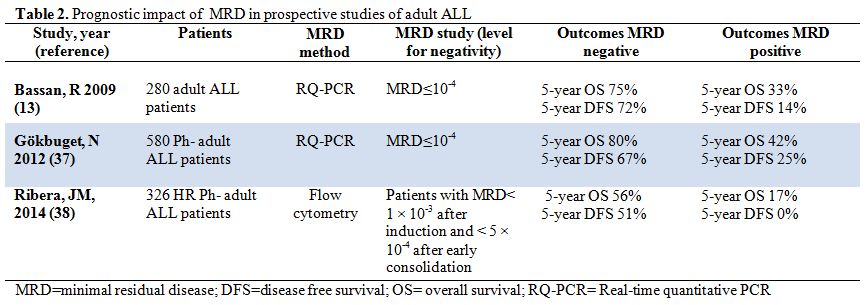 |
Table 2. Prognostic impact of MRD in prospective studies of adult ALL. |
| Figure 1. Summary of our suggestions for the treatment of adult acute lymphoblastic leukemia. |




























































































