Received: June 18, 2014
Accepted: October 10, 2014
Meditter J Hematol Infect Dis 2014, 6(1): e2014.068, DOI 10.4084/MJHID.2014.068
This article is available on PDF format at:

Daniel Olson1, Abraham T. Yacoub2, Anita D. Gjini2, Gelenis Domingo3 and John N. Greene1,2
1 University of South Florida Morsani College of Medicine. 12901 Bruce B Downs Blvd, Tampa, FL 33612
2 H. Lee Moffitt Cancer Center and Research Institute. 12902 Magnolia Drive. Tampa, Florida 33612-9497
3 Moffitt Cancer Center and Research Institute. 12902 Magnolia Drive. Tampa, FL 33612-9497

| This is an Open Access article distributed under the
terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: Escherichia coli (E. coli)
is a pathogen of great concern in immunosuppressed patients. While
antimicrobial prophylactic therapy has become the standard, the
emergence of resistant pathogens has some questioning its use. This
study describes our experience with E.coli
as a pathogen in neutropenic patients with a hematologic malignancy,
and addresses future directions of treatment for this patient
population.
Methods: A retrospective chart review of 245 E.coli bacteremia patients at Moffitt Cancer Center from 05/18/02 – 05/15/12 was conducted. Out of 245 patients, 169 did not meet the criteria due to non-neutropenic status, or not diagnosed with a hematologic malignancy, or due to having insufficient medical records. Thus, they were excluded from the study. As a result, 76 patients were involved in this study. Patients were identified via microbiology laboratory computerized records. Results: The included patients experienced clinically significant E.coli bacteremia resulting in a median hospital stay of 14.7 days. Several patients developed severe sepsis requiring the use of pressor and ventilator therapy. Conclusions: E.coli is a major pathogen in these patient populations resulting in extended hospital stays and specialized treatment to overcome their E.coli bacteremia. The data supports the use of fluoroquinolone prophylactic therapy, however, earlier detection and treatment of neutropenic infection is needed. |
Introduction
Neutropenia is a blood disorder characterized by an abnormally low
number of white blood cells called neutrophils. Neutrophils are
essential in the defense against bacterial and fungal pathogens, thus
neutropenic patients are highly susceptible to these pathogens.[1] Hematological cancer patients are already at an increased risk of infection due to chemotherapy-induced immunosuppression.[2]
Therefore, when patients with hematologic malignancies become
neutropenic, they are at an even higher risk for developing infectious
complications.
These infections can result in significant morbidity and mortality due
to the development of febrile neutropenia and bacteremia.[3]
Several organisms are responsible for causing infection in hematologic
patients with neutropenia. Various fungi, as well as, Gram-negative and
Gram-positive bacteria were found to be causes of infection in
neutropenic patients with hematological malignancies.[4]
In recent years, E.coli sequence
type ST131 has been given much worldwide attention as an emerging
multi-drug resistant (MDR) pathogen. Data suggests that this sequence
of E.coli may be the main explanation for recent increases in antimicrobial resistance prevalence in E.coli.[7] Serious extra-intestinal infections with this MDR E.coli ST131
often leave physicians with limited treatment options, higher costs,
and increased usage of “last resort” antimicrobials, such as
carbapenems.[7]
The use of antimicrobial prophylaxis in neutropenic patients has shown
some effect in reducing infectious complications. In particular,
fluoroquinolones, are widely used to protect patients against
Gram-negative bacteremia.[8] However, the use of these
drugs has to be weighed against the emerging possibility of producing
antibiotic-resistant bacterial strains such as E.coli ST131.[2]
Still, the true impact of fluoroquinolone prophylaxis in regards to
treatment efficacy and adverse effects is only partially known.[2]
The next frontier for treating neutropenic patients with hematologic
malignancies may deal with monitoring microbial gut diversity through
treatment. As part of their treatment regimens, such patients may
undergo hematopoietic stem cell transplantation (HSCT), also known as
bone marrow transplantation (BMT). They are exposed to chemotherapy,
radiation, and antimicrobials in a short time period as part of their
treatment.[9] As a result, the equilibrium between the
intestinal microbiota and mucosal epithelium is disrupted, causing
large shifts in bacterial populations inhabiting the gut thus making
the patient susceptible to bloodstream infections.[9]
While studies have assessed that this microbial shift does occur, we
have yet to find data answering the following question. Can monitoring
these microbial shifts help us treat, or even prevent, MDR E.coli bacteremia infections in neutropenic patients with hematologic malignancies?
Methods
This study used a retrospective chart review design and was approved
by the IRB prior to data collection and analysis. A data list was
obtained from Moffitt Cancer Center records containing the names of all
E.coli bacteremia patients
from 05/18/02 – 05/15/12. Patients were identified through review of
Moffitt’s institutional databases: Cerner/PowerChart and Emageon at
Moffitt Cancer Center. Patients were included in the study if they met
the following criteria: at least 18 years of age, diagnosed with
hematological malignancy, and neutropenic at time of E.coli bacteremia (defined as absolute neutrophil count < 500 cells/μL).
Using the E.coli bacteremia
data list obtained from Moffitt Cancer Center records, 245 E.coli
bacteremia patients were examined for possibility of study inclusion.
Out of the 245 original patients, 169 did not meet the inclusion
criteria, and thus were excluded from the study. Of the 169 excluded
patients, 40 patients were not neutropenic at time of positive E.coli blood
culture, 119 patients did not have a hematologic malignancy diagnosis,
9 patients had insufficient records, and 1 patient was found to not
have E.coli bacteremia.
Therefore, 76 patients met eligibility and are included in the data
analysis. Two patients developed multiple neutropenic E.coli bacteremia
episodes separated by at least 6 months, which were treated as separate
subject events for data analysis. If any patient produced multiple
positive E.coli blood cultures within the same month, the earliest culture was used for study analysis.
The survival rate post E.coli
bacteremia was measured in order to evaluate the long-term prognosis of
these patients. Patient records were used to determine how many days
each patient lived after their positive E.coli blood culture. A Kaplan-Meier survival curve was generated to show survival at various time intervals.
Information on all study patients was stored in a password-protected
database, maintained by investigators at Moffitt Cancer center.
Patients’ data was kept on file until the regulated time (per IRB
requirements). We then compiled the research findings in an excel file
that contained the medical record numbers (MRNs). Upon completion of
data analysis, all direct identifiers (e.g. MRN, DOB, etc) were removed
from the Excel sheet. All files are password protected and only
accessed by the research team. No paper records were kept for this
study and no patient identifiers were disclosed to anyone besides the
investigative team.
Results
Patient Demographics. Of the 76 subjects included in the data analysis, 23 had undergone a BMT at the time of their E.coli bacteremia,
whereas 53 had not. The BMT-Group patients ranged from 35-75 with a
median age of 60 years, and the Non-BMT patients ranged from 22-82
years with a median age of 55. Males outnumbered females in both
groups, making up 73.91% of the BMT-Group, and 62.26 % of the Non-BMT
Group. Of the 23 patients in the BMT-Group, 15 had undergone an
autologous BMT, whereas 8 had undergone an allogenic BMT.
Regarding hematologic malignancies, the BMT-Group had 12% Acute myeloid
leukemia (AML), 12% Acute lymphocytic (or lymphoblastic) leukemia
(ALL), 4% Chronic myelogenous leukemia (CML), 8% Hodgkin’s lymphoma
(HL), 24% Non-Hodgkin’s lymphoma (NHL), 28% Multiple myeloma (MM), 8%
AML + myelodysplastic syndrome (MDS), 4% HL + NHL, and 0% ALL+ CML. In
comparison, the Non-BMT Group had 13% ALL, 33% AML, 2% CML, 0% HD, 26%
NHL, 7% MM, 2% MDS, 7% AML + MDS, 2% ALL + CML, 0% HD + NHL, and 7%
with some other hematologic malignancy.
Severity of E. coli bacteremia infection.
Each patient was classified as having bacteremia without septic
syndrome, having sepsis, severe sepsis, or septic shock as outlined by
current clinical practice definitions.[10] Several parameters were also used to assess the overall severity of the E.coli bacteremia infections in each group. These parameters included: hospital stay length in days when E.coli bacteremia occurred, days of neutropenia at time of positive E.coli
blood culture, whether patient was placed on pressor therapy, whether
patient was placed on ventilator, and whether patient required
hemodialysis.
The following results were yielded when looking at the total patient
population. The median hospital stay was 14.7 days; while the median
length of neutropenia at time of positive E.coli
blood culture was 4 days. In terms of bacteremia severity, 50% were
classified as aseptic, 26% were septic, 9% were severe sepsis, and 15%
were in septic shock. In addition, pressor treatment was required in
19%, ventilator treatment was required in 14%, and hemodialysis was
required in 2% (Figure 1, Table 1).
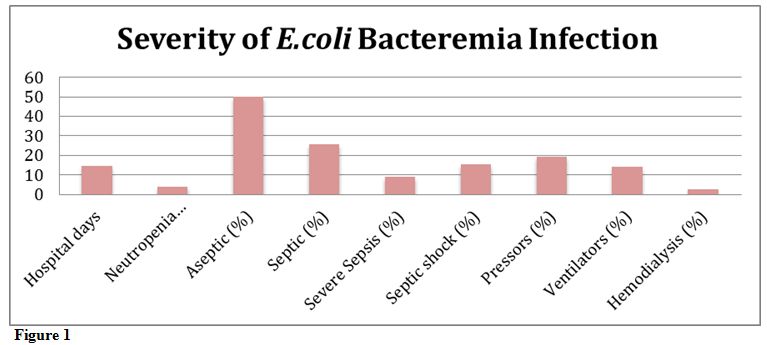 |
Figure 1 |
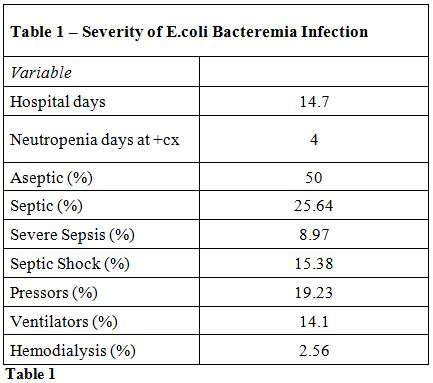 |
Table 1 |
Survival after E. coli Bacteremia.
The survival curve showed that the BMT-group patients had better
survival outcomes in both the short and long-term when compared to the
non-BMT group (Figure 2).
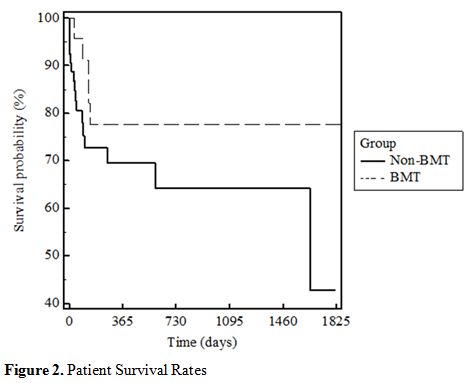 |
Figure 2. Patient Survival Rates |
E. coli Antibiotic Resistance. Antibiotic resistance was measured using the E.coli bacteremia microbiology reports obtained from PowerChart at Moffitt Cancer Center. The top 5 antibiotics the E.coli
was resistant to were: ampicillin, ciprofloxacin, levofloxacin,
trimethoprim and sulfamethoxazole, and ampicillin/sulbactam. Of the
total patient population, 81.58% were resistant to ciprofloxacin,
80.26% were resistant to levofloxacin, 80.26% were resistant to
ampicillin, 59.21% were resistant to trimethoprim and sulfamethoxazole,
and 42.11% were resistant to ampicillin/sulbactam (Figure 3).
The BMT-Group had 91.67% of its patients on a fluoroquinolone
prophylaxis, with 95.83% of patients developing fluoroquinolone
resistant E.coli bacteremia,
whereas the No-BMT group had fewer patients, 66.67%, on fluoroquinolone
prophylaxis, with 72.22% developing fluoroquinolone resistant E.coli bacteremia (Table 2, Figure 4).
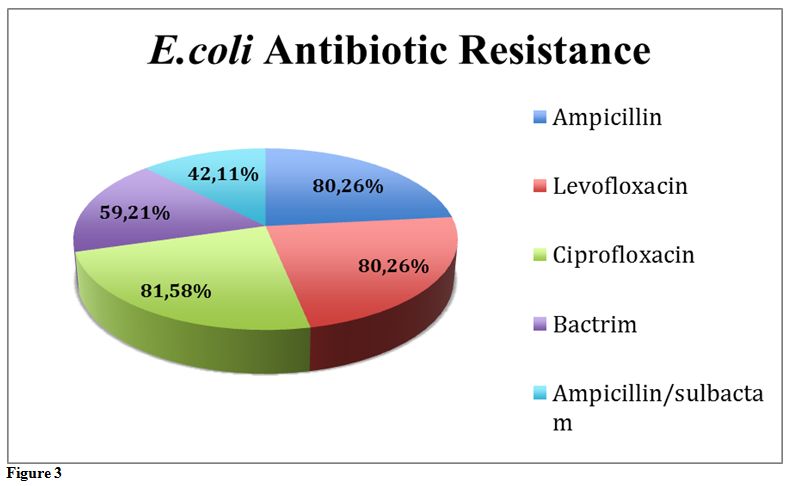 |
Figure 3. |
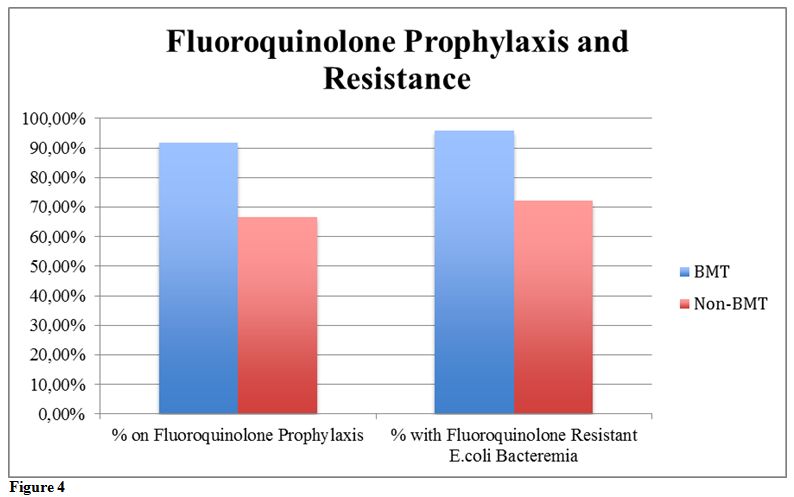 |
Figure 4. |
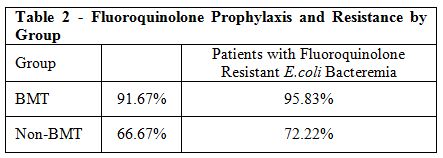 |
Table 2. |
Discussion
We support that fluoroquinolone resistance in this population is a
growing problem. The etiology of bacteria in the fecal flora changes
dramatically after quinolone prophylaxis.[11]
Existing studies have shown that fluoroquinolone prophylaxis in
hematologic cancer patients has been linked to an increase the
incidence of Gram-negative fluoroquinolone resistant bacteria such as E. coli. Quinolone resistant E. coli have caused breakthrough bacteremia during prophylaxis with quinolones.[11]
However, it has been stated that this increase in antibiotic resistant
bacteria is not necessarily responsible for increased morbidity.[2]
Our study found similar results. The BMT-Group had a much higher
percentage of its subjects on a fluoroquinolone prophylactic therapy
than the non-BMT group. However, the BMT-Group had less hospital days
and shorter neutropenia duration at time of positive E.coli blood culture than the Non-BMT group. Also, the BMT-Group patients had less severe E.coli
bacteremias as measured by our aseptic, septic, severe sepsis, and
septic shock categories. Thus, the data supports the claims made by
existing studies, in that the higher occurrence of fluoroquinolone
resistant bacteria does not always mean increased morbidity.[2]
We support the use of fluoroquinolone prophylaxis for neutropenic
patients with hematologic malignancies. If patients develop an E.coli bacteremia while on these drugs, there is a higher chance of the E.coli being fluoroquinolone resistant. Previous analysis demonstrated that E. coli
resistance to fluoroquinolones is significantly related to previous
prophylaxis with these agents, also that is closely associated with
extended-spectrum-B-lactamase (ESBL) production. This finding could
suggest a potential indirect influence of fluoroquinolones resistance
on clinical outcome in hematological cancer patients, related to ESBL
production.[12] However, fluoroquinolone resistance
was not associated with worse outcomes in this study. In addition to
the use of quinolone prophylaxis, we feel there needs to be a better
way of effectively adjusting treatment regimens for neutropenic
infections. The current practice of changing antimicrobials when the
patient develops neutropenic fever puts the patient at a high risk for
developing serious bacteremia infections.
Prophylactic regimens of quinolones may predispose the patient to
dangerous bloodstream infections with multi-drug resistant pathogens
such as E.coli ST1317 or ESBL-producing E. coli strains.
Another concern is the rate of relapsing bacteremia in patients who are
on treatment for a hematological malignancy and also on fluoroquinilone
prophylaxis. Gram-negative bacteria are significantly more frequent
among relapsing bacteremia compared to non-relapsing cases and this
phenomenon may be due to an imbalance of enteric microflora induced by
fluoroquinolone prophylaxis.[13] Cattaneo et al demonstrated that ESBL-producing E. coli was
found to be present in more than a 25 percent of cases at the first
episode of bacteremia and at relapses. Fluoroquinolone resistance was
recorded in all episodes of relapsing E. coli bacteremia.[13]
Instead of changing their prophylactic antimicrobials when they develop
neutropenic fever, perhaps we can get ahead of the game. Recent studies
have shown the ability to monitor microbial gut diversity in
allogenic-HSCT patients throughout treatment via fecal sampling
methods.[9] This monitoring was able to predict which
patients were more prone to develop bloodstream infections as a result
of intestinal domination by certain toxins.[9] Perhaps
these methods can be applied to the treatment of this population. Being
able to identify infection with dangerous strains of microbes, such as E.coli
ST131, may allow us to adjust antimicrobial treatment before serious
infectious complications can occur. In doing so, we may be able to more
effectively treat, or even prevent serious E.coli bacteremia infections that are experienced in this subject population.
The Kaplan-Meier survival analysis showed that the BMT group had better
short-term as well as long-term survival than the non-BMT group. This
data does not support the suggestion from previous studies that
neutropenia in BMT patients is associated with higher mortality when
compared to neutropenia in Non-BMT patients.[4] In
regards to BMT therapy, the data is positive. It shows that undergoing
a BMT procedure may not put the patient at risk for worse outcomes
regarding neutropenic E.coli
bacteremia. We understand that many factors were not controlled for
when comparing these two patient groups, thus further research needs to
be done to evaluate survival differences.
We realize that our study is limited in various ways. The data could
potentially be limited by the relatively small sample size as well as
taking place at a single institution. Also, it was unfeasible for us to
control for the individual differences in the physicians that treated
these subject patients. We acknowledge that our study was limited to a
relatively small group of patients. Further research should attempt to
achieve larger subject populations at multiple institutions to fully
assess E.coli bacteremia infections in this patient population.
Conclusion
In summary, E.coli is a
major pathogen in hematologic malignancy patients with neutropenia.
Patients in this population can experience extended hospital stays and
specialized treatment to overcome their, often serious, E.coli
bacteremia infections. We support the use of fluoroquinolones in
neutropenic patients with hematologic malignancies. These drugs were
associated with a higher chance of the E.coli being fluoroquinolone resistant; however, this was not associated with poorer outcomes.
Still, further research needs to be done to improve treatment of
neutropenic infection in this patient population. Current treatment
guidelines leave patients at risk for developing potentially serious
bacteremia infections. We believe that future research should examine
the efficacy of fecal microbiota monitoring to adjust treatment
guidelines. Perhaps these methods will one day allow us to prevent
serious E.coli bacteremia infections in this patient population.
References













[TOP]