Successful Management of Pregnancy and Hepatic Toxicity in a CML
Female Patient Treated with Nilotinib: a Case Report and a Review
Domenico Santorsola1 and Elisabetta Abruzzese2
1 MD, Servizio Dipartimentale di Ematologia ed Oncologia San Nicola Pellegrino Trani, Italy
2 MD, PhD, Hematology, S. Eugenio Hospital, Tor Vergata University, Rome, Italy
Corresponding author: Domenico Santorsola.
Servizio Dipartimentale di Ematologia ed Oncologia, San Nicola
Pellegrino, Trani -ASL BAT (Barletta, Andria Trani, Viale Padre Pio,
76125, Trani, Tel: 0883483311, Fax: 0883483261. E-mail
d.santorsola@alice.it
Published: February 15, 2015
Received: October 22, 2014
Accepted: January 19, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015020, DOI
10.4084/MJHID.2015.020
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We report a case of a young patient
with chronic viral hepatitis HBV infection, diagnosed with CML in March
2006 and treated with imatinib 400mg/die as first line therapy with
concomitant Lamivudine. Patient obtained a complete hematologic
response (CHR) in 2 months, complete cytogenetic response (CCyR) in six
months and major molecular response (MMR) at 24 months. After three
years of treatment, she became imatinib intolerant and resistant. In
November 2009 patient started nilotinib 400mg/BID. Patient tolerated
well the new molecule never experiencing hepatic impairment. After
switching to nilotinib, she reached in 12 months transcript reduction
more than 3 log (MMR). Even if patient had been informed of the need of
continuous therapy and to use effective methods of contraception during
tyrosine kinase inhibitor (TKI) treatment, in 2012 she decided to plan
a pregnancy. In August 2012 a MR4 was
documented, and treatment discontinued before starting pregnancy. She
was placed on interferon and observed throughout her pregnancy. The
disease remained stable achieving an undetectable transcript level; she
delivered a healthy boy in September 2013. Treatment with nilotinib was
re-started three months after delivery, and she is still in molecular
remission (MR5). A complete discussion of the case and the available
literature is presented.
|
Introduction
Imatinib, the first BCR–ABL1 tyrosine kinase inhibitor (TKI)
approved for the treatment of patients with chronic myeloid leukemia
(CML), has profoundly changed the management of CML improving the
prognosis and the quality of life for such patients.[1,2] The second
and third generation TKIs include Nilotinib (Tasigna, Novartis),
Dasatinib (Sprycel, Bristol Myers Squibb), Bosutinib (Bosulif, Pfizer),
and the recently approved Ponatinib (Iclusig, Ariad Pharma).
Results
with imatinib at 8 years are excellent with rates of complete
cytogenetic remission (CCyR) of 82%, and an estimated overall survival
of 89%, but 1/3 of patients need to change treatment mainly due to
adverse events (AE)/intolerance or unsatisfactory therapeutic
outcome.[3] Nilotinib, as other second generation TKIs, is a safe and
efficient treatment for long-term use in patients with chronic phase
(CP) CML, who are intolerant of, or resistant to imatinib.[4]
We
report a 34-year-old woman with CML and HBV-infection, who became
imatinib resistant/intolerant successfully reaching deep molecular
response with nilotinib as a second line therapy. Her chronic viral
hepatitis B infection was managed without reactivation, and she got
successfully pregnant after nilotinib discontinuation.
Case Report and Literature Review
In April 2006, patient was diagnosed with CP-CML with classic (9;22)
(q34,q11) translocation found in all 20 metaphases and a BCR-ABL p210
b2a2 transcript, intermediate Sokal risk. Past medical history was
unremarkable, except for a HBV-positive infection diagnosed in 1990.
In
May 2006 she started treatment with imatinib 400mg/die. Patient also
started the antiviral therapy with Lamivudine that was well tolerated
and stopped on July 2006 for sustained negative viral load. The patient
achieved complete hematologic response (CHR) within two months and CCyR
in six months. From November 2006 to July 2007 patient underwent
several imatinib dose reduction and suspension due to persistent
leucopenia grade 3 while maintaining a CCyR. After 24 months (June
2008) patient achieved major molecular response (MMR). In December 2008
patient achieved a MR4.
Unfortunately,
after achieving MMR, patient became again imatinib intolerant this time
by experiencing severe dermatological toxicity that was critically
affecting her quality of life (QoL) and did not resolve after dose
reduction and local therapy. For this, in November 2009 patient stopped
imatinib and started nilotinib 400mg/BID as second line therapy.
Biochemical
laboratory abnormalities were described using Nilotinb, including
elevations of alanine aminotransferase and aspartate aminotransferase,
but no cross-intolerance to skin reactions were reported.[5] Since
starting nilotinib, the patient has been strictly monitored every two
weeks for all hepatic functionality markers with no abnormalities
recorded and no reactivation of HBV. The patient re-gained MMR in 12
months and completely recovered from the dermatological toxicity
dramatically improving her QoL.
Even if patient had been
informed of the need of continuous therapy and to use effective methods
of contraception since data on pregnancy during TKIs treatment were
limited in 2012, she decided to plan a pregnancy. At this time,
molecular response was stable (Figure 1).
It
is mandatory to stop TKI when a pregnancy in a female patient on
therapy is planned or just started, due to the teratogenic potential of
all TKIs.[6]
So in July 2012, after having confirmed three stable
molecular results patient discontinued nilotinib and started interferon
with 3 MU sc. three times a week. In August 2012 a BCR–ABL1 transcript
reduction more than 4 log (< MR4)
was documented. Patient and her husband started their pregnancy plan
with all necessary gynecological, and andrologic assessments and
patient got pregnant on December 31st
2012. After all necessary evaluation, patient has been consent for the
collection and preservation of umbilical cord blood, which show no
presence of CML transcript. Patient was observed through the whole
pregnancy period; the disease remained stable achieving also in one
sample an undetectable transcript level. On September 1st, 2013 patient delivered a healthy boy after 42 weeks of gestation with a natural childbirth.
At
birth baby weight was 3.65 Kg., length 52 cm; the baby is healthy,
normally growing and just turned a little more than 1 year old.
Treatment
with nilotinib was re-initiated three months after delivery since
patient expressed the desire to breastfeed that was judged not
contraindicated during interferon therapy. After nilotinib restart,
patient confirmed her molecular remission with MR5
undetectable transcript. Patient restarted therapy in agreement with
his hematologist since there were no trials available at this time on
TKIs interruption, and did not want to continue with Interpheron,
neither was feeling safe in stopping therapy.
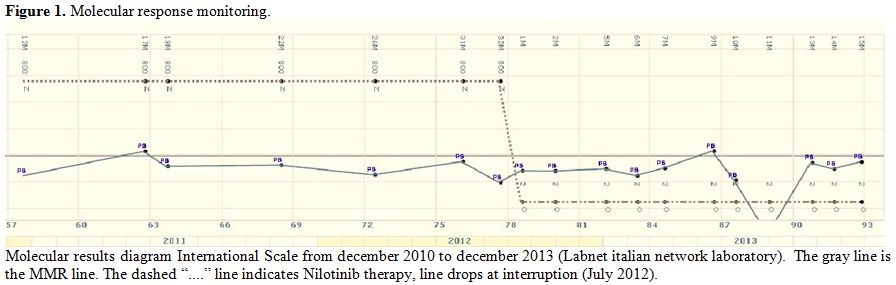 |
Figure
1.Molecular response monitoring. |
Discussion
Imatinib
and the subsequent second and third generation TKIs are recognized to
represent the major advancement in clinical research leading to a
successful targeted therapy with substantial improvement of survival
and quality of life in CML patients. Considering
the significant proportion of female/male patients diagnosed with CML
in reproductive age, and the lifespan of responding patients, issues
relating to fertility and pregnancy are often requested by patients.
For this reason, nowadays the management of fertility should begin at
diagnosis. A patient in reproductive age should be informed about the
risk of unplanned pregnancies in terms of foetal problems and/or the
risk of a loss of response/progression after stopping therapy to carry
the pregnancy. However, it is important to inform that a pregnancy can
be conducted when the treatment has being started, and the response is
optimal.[7]Limited
data are reported concerning either female/male pregnancies conception
while on nilotinib therapy. A recently published complete and updated
review on TKIs and pregnancy of all literature refers to 46 male and 3
female on nilotinb getting pregnant.[8] This should be the 4th case. In
the same review suggestions for the management of a
conception/pregnancy, while on TKIs, are reported. No particular risks
of fertility for male patients taking imatinib or nilotinib are
present, and conception and pregnancy outcomes have been evidenced.
Caution should be used during dasatinib due to the little data
available and the wide range substrate inhibition (other than TKIs) of
this drug.[9] No reports are available for patients taking bosutinib or
ponatinib. For those patients, the possibility to cryopreserve sperm
before starting therapy should be discussed.In
a female patient in reproductive age, effective contraception should be
suggested at diagnosis. A pregnancy should be only planned after the
milestone of a stable MMR, or better (>MR4.5) has been reached from
at list 18-24 months. Obgyn visit for pre-conception tests (including
in some cases male sperm evaluation), ultrasound and planned conception
is highly recommended. Therapy should be stopped immediately before or
right after conception. All TKIs must be avoided during the
organogenesis (post menstrual days 31-71, weeks 5-13). Q-PCR must be
monitored each month/2 months to follow the transcript, depending on
the molecular results.Therapy
during pregnancy should be only considered if a cytogenetic or
hematologic relapse occur. Each case should be individually evaluated
taking into account the rapidity of the relapse, the clinical history
of the patient, and most of all the pregnancy status (weeks of
gestation).Interferon
can be considered safe before and during pregnancy;[10] hydroxyurea can
be used to control leucocytosis after organogenesis.[11] If necessary,
considering the little passage of imatinib and nilotinib in the fetal
compartment, TKIs therapy can be considered after placenta has being
formed, and organogenesis completed.[12] Since dasatinib pass the
placenta, it should be avoided throughout all the pregnancy.[13]After
delivery therapy can be postponed to consent breast feeding, provided a
low level of molecular transcript, or according to the haematologist
judgement. If it is necessary to resume treatment, patient can
breast-feed the baby the first 2-5 days post- partum to give him the
colostrums.[8]Although
this experience is limited to a single patient, the success of the
outcome demonstrates that the management of chronic myeloid leukemia in
women with childbearing potential should be individualized. Based on
the incoming results of the treatment-free remission (TFR) studies,
drugs can be successfully stopped during pregnancy and the same therapy
resumed afterwards. A sustained profound molecular response is an
essential entry criterion for maintaining MMR. All patients were
sensitive to TKI retreatment.[14] Several other TKIs discontinuation
studies are on-going and may serve to provide a new treatment paradigm
for younger patients who will benefit of pregnancy without exposure to
TKIs. References
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal
GM, Fanning S et al. Effects of a selective inhibitor of the Abl
tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996;
2: 561–566. http://dx.doi.org/10.1038/nm0596-561 PMid:8616716

- Hochhaus
A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L et al.
Six-year follow-up of patients receiving imatinib for the first-line
treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061. http://dx.doi.org/10.1038/leu.2009.38 PMid:19282833

- Deininger
M, O'Brien SG, Guilhot F, et al: International Randomized Study of
interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained survival and
low risk for progression or events in patients with newly diagnosed
chronic myeloid leukemia in chronic phase (CML-CP) treated with
imatinib. 51st ASH Annual Meeting and Exposition, New Orleans, LA,
December 5-8, 2009 (abstr 1126).
- Giles
FJ, le Coutre PD, Pinilla-Ibarz J Nilotinib in imatinib-resistant or
imatinib-intolerant patients with chronic myeloid leukemia in chronic
phase: 48-month follow-up results of a phase II study. Leukemia. 2013
Jan;27(1):107-12

- Hagop M. Kantarjian,
Francis J. Giles, Kapil N. Bhalla. Nilotinib is effective in patients
with chronic myeloid leukemia in chronic phase after imatinib
resistance or intolerance: 24-month follow-up results Blood 2011 117:
1141-1145 http://dx.doi.org/10.1182/blood-2010-03-277152 PMid:21098399

- Pye
SM, Cortes J, Ault P, Hatfied A, Kantarjian H, Pilot R, Rosti G,
Apperly J. The effects of imatinib on pregnancy outcome. Blood
2008;111:5505-5508 http://dx.doi.org/10.1182/blood-2007-10-114900 PMid:18322153

- Baccarani
M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al.
European LeukemiaNet recommendations for the management of chronic
myeloid leukemia: 2013. Blood 2013;122:872–84. http://dx.doi.org/10.1182/blood-2013-05-501569 PMid:23803709

- Abruzzese
E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase
inhibitors and pregnancy. 2014 Apr 7;6(1): e2014028. doi:
10.4084/MJHID.2014.028. Mediterr J Hematol Infect Dis.. eCollection
2014. Review http://dx.doi.org/10.4084/mjhid.2014.028

- Shah NP, Tran C, Lee FY, et al. Science. 2004;305:399-401 http://dx.doi.org/10.1126/science.1099480 PMid:15256671

- Pons
JC, Lebon P, Frydman R, Delfraissy JF. Pharmacokinetics of
interferon-alpha in pregnant women and fetoplacental passage. Fetal
Diagn Ther 1995; 10: 7–10. http://dx.doi.org/10.1159/000264183 PMid:7710683

- Patel
M, Dukes IA, Hull JC. Use of hydroxyurea in chronic myeloid leukemia
during pregnancy: A case report. Am J Obstet Gynecol 1991;165: 565–566.
http://dx.doi.org/10.1016/0002-9378(91)90285-Y

- Webb MJ, Jafta D. Imatinib use in pregnancy. Turk J Haematol. 2012, Dec;29(4):405-8. http://dx.doi.org/10.5505/tjh.2012.82542 PMid:24385730 PMCid:PMC3781616

- Berveiller
P, Andreoli A, Mir O, Anselem O, Delezoide AL, Sauvageon H, Chapuis N,
Tsatsaris V. A dramatic fetal outcome following transplacental transfer
of dasatinib. Anticancer Drugs. 2012; Aug;23(7):754-7
http://dx.doi.org/10.1097/CAD.0b013e328352a8fe PMid:22421368 
- Mahon
FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L,
Charbonnier A, Guerci A, Varet B, Etienne G, Reiff J, Rousselot P
Discontinuation of imatinib in patients with chronic myeloid leukaemia
who have maintained complete molecular remission for at least 2 years:
the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol
2010; 11: 1029–35 http://dx.doi.org/10.1016/S1470-2045(10)70233-3

[TOP]















